The Influence of the Mineral–Microbial Deodorizing Preparation on Ammonia Emission and Growth Performance in Turkey Production
Abstract
1. Introduction
2. Experiments
2.1. Broiler Turkeys and Production Premisses
2.2. Deodoric® Biopreparation
2.3. Measurements
2.4. Bacteriological Identification Methods
2.5. Quantitative Microbiological Analyses
2.6. Statistical Analysis
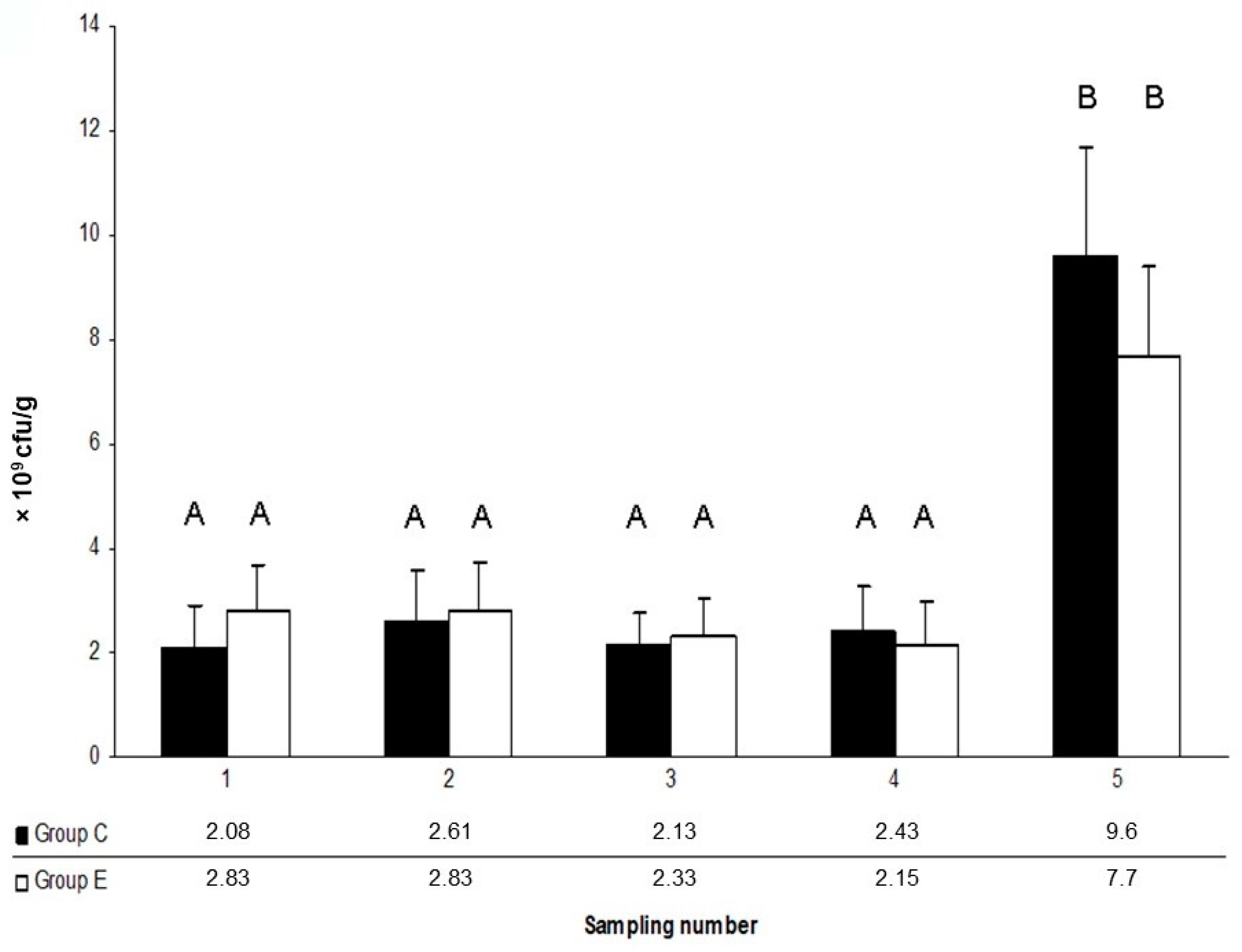
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leytem, A.B.; Dungan, R.S.; Bjorneberg, D.L.; Koehn, A.C. Greenhouse Gas and Ammonia Emissions from an Open-Freestall Dairy in Southern Idaho. J. Environ. Qual. 2013, 42, 10–20. [Google Scholar] [CrossRef]
- Miner, J.R. Nuisance concerns and odor control. J. Dairy Sci. 1997, 80, 2667–2672. [Google Scholar] [CrossRef]
- Lacey, R.; Mukhtar, S.; Carey, J.B.; Ullman, J.L. A Review of Literature Concerning Odors, Ammonia, and Dust from Broiler Production Facilities: 1. Odor Concentrations and Emissions. J. Appl. Poult. Res. 2004, 13, 500–508. [Google Scholar] [CrossRef]
- He, Z.; Pagliari, P.H.; Waldrip, H.M. Applied and Environmental Chemistry of Animal Manure: A Review. Pedosphere 2016, 26, 779–816. [Google Scholar] [CrossRef]
- He, Z. Environmental Chemistry of Animal Manure; Nova Science Publishers: New York, NY, USA, 2011; pp. 458–459. [Google Scholar]
- Jacobsen, B.H.; Latacz-Lohmann, U.; Luesink, H.; Michels, R.; Ståhl, L. Costs of regulating ammonia emissions from livestock farms near Natura 2000 areas-analyses of case farms from Germany, Netherlands and Denmark. J. Environ. Manag. 2019, 246, 897–908. [Google Scholar] [CrossRef]
- Clean Air Directive. Directive 2016/2284/EU Clean Air Program. Available online: http://ec.europa.eu/environment/air/clean_air/index.htm (accessed on 20 April 2020).
- European Economic Community. Reference Document on Best Available Techniques for Intensive Rearing of Poultry and Pigs. JRC Science for Policy Report. 2017. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/IRPP/JRC107189_IRPP_Bref_2017_published.pdf (accessed on 20 April 2020).
- European Economic Community. Clean Air Program. The European Commission. 2013. Available online: http://ec.europa.eu/environment/air/clean_air/index.htm (accessed on 20 April 2020).
- European Economic Community. Facts Figures on Agriculture Reductions as Proposed under the Commission’s NECD Proposal. 2015. Available online: http://ec.europa.eu/environment/air/pdf/review/Facts%20and%20figures%20agriculture%20under%20the%20NEC.pdf (accessed on 20 April 2020).
- UNEC. Framework Code for Good Agricultural Practice for Reducing Ammonia Emissions; United Nations Economic Commission for Europe; European Commission: Geneva, Switzerland, 2015. [Google Scholar]
- Bouwman, A.F.; Lee, D.S.; Asman, W.A.H.; Dentener, F.J.; Van Der Hoek, K.W.; Olivier, J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar] [CrossRef]
- Beusen, A.H.; Bouwman, A.F.; Heuberger, P.; Van Drecht, G.; Van Der Hoek, K. Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos. Environ. 2008, 42, 6067–6077. [Google Scholar] [CrossRef]
- European Environment Agency. Ammonia (NH3) Emissions. 2015. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea-32-ammonia-nh3-emissions-1/assessment-4 (accessed on 20 April 2020).
- Sommer, S.G.; Webb, J.; Hutchings, N.D. New Emission Factors for Calculation of Ammonia Volatilization From European Livestock Manure Management Systems. Front. Sustain. Food Syst. 2019, 3, 101. [Google Scholar] [CrossRef]
- Van Der Hoek, K. Estimating ammonia emission factors in Europe. Atmos. Environ. 1998, 32, 315–316. [Google Scholar] [CrossRef]
- Koerkamp, P.G.; Metz, J.; Uenk, G.; Phillips, V.; Holden, M.; Sneath, R.; Short, J.; White, R.; Hartung, J.; Seedorf, J.; et al. Concentrations and Emissions of Ammonia in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Koziel, J.A.; Aneja, V.P.; Baek, B.-H. Gas-to-Particle Conversion Process Between Ammonia, Acid Gases, and Fine Particles in the Atmosphere. In Animal Agriculture and the Environment, National Center for Manure & Animal Waste Management White Papers; American Society of Agricultural and Biological Engineers: St Joseph, MI, USA, 2006; pp. 201–224. [Google Scholar] [CrossRef]
- Maurer, D.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air management practices assessment tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Menzi, H.; Pain, B.; Misselbrook, T.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Eurich-Menden, B.; Döhler, H.; Dämmgen, U. Ammoniak-Emissionen der deutschen Landwirtschaft-technische Minderungspotenziale. Landtech.–Agric. Eng. 2004, 59, 162–163. [Google Scholar]
- Lin, X.-J.; Cortus, E.; Zhang, R.; Jiang, S.; Heber, A. Ammonia, hydrogen sulfide, carbon dioxide and particulate matter emissions from California high-rise layer houses. Atmos. Environ. 2012, 46, 81–91. [Google Scholar] [CrossRef]
- Pagliari, P.; Wilson, M.; He, Z. Animal Manure Production and Utilization: Impact of Modern Concentrated Animal Feeding Operations. In Nitrification Inhibitors-Potentials and Limitations; American Society of Agronomy: Madison, WI, USA, 2020; pp. 1–14. [Google Scholar]
- Anderson, D.P.; Beard, C.W.; Hanson, R.P. The Adverse Effects of Ammonia on Chickens Including Resistance to Infection with Newcastle Disease Virus. Avian Dis. 1964, 8, 369. [Google Scholar] [CrossRef]
- Miles, D.M.; Branton, S.L.; Lott, B.D.; Simmons, J.D. Quantified detriment of ammonia to broilers. Poult. Sci. 2002, 81, 54–55. [Google Scholar]
- Beker, A.; VanHooser, S.L.; Swartzlander, J.H.; Teeter, R.G. Atmospheric Ammonia Concentration Effects on Broiler Growth and Performance. J. Appl. Poult. Res. 2004, 13, 5–9. [Google Scholar] [CrossRef]
- Donham, K.J.; Cumro, D.; Reynolds, S.J.; Merchant, J.A. Dose-Response Relationships Between Occupational Aerosol Exposures and Cross-Shift Declines of Lung Function in Poultry Workers. J. Occup. Environ. Med. 2000, 42, 260–269. [Google Scholar] [CrossRef]
- Golbabaei, F.; Islami, F. Evaluation of Workers’ Exposure to Dust, Ammonia and Endotoxin in Poultry Industries at the Province of Isfahan, Iran. Ind. Health 2000, 38, 41–46. [Google Scholar] [CrossRef]
- Kirychuk, S.P.; Senthilselvan, A.; A Dosman, J.; Juorio, V.; Feddes, J.J.R.; Willson, P.; Classen, H.; Reynolds, S.J.; Guenter, W.; Hurst, T.S. Respiratory symptoms and lung function in poultry confinement workers in Western Canada. Can. Respir. J. 2003, 10, 375–380. [Google Scholar] [CrossRef]
- Viegas, S.; Faísca, V.M.; Dias, H.; Clérigo, A.; Carolino, E.; Viegas, S. Occupational Exposure to Poultry Dust and Effects on the Respiratory System in Workers. J. Toxicol. Environ. Health Part A 2013, 76, 230–239. [Google Scholar] [CrossRef]
- Nowak, A.; Bakuła, T.; Matusiak, K.; Gałęcki, R.; Borowski, S.; Gutarowska, B. Odorous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells. Int. J. Environ. Res. Public Health 2017, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Matusiak, K.; Borowski, S.; Bakuła, T.; Opaliński, S.; Kołacz, R.; Gutarowska, B. Cytotoxicity of Odorous Compounds from Poultry Manure. Int. J. Environ. Res. Public Health 2016, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J. Respiratory Disease Hazards to Workers in Livestock and Poultry Confinement Structures. Semin. Respir. Crit. Care Med. 1993, 14, 49–59. [Google Scholar] [CrossRef]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic Effects of Dust and Ammonia on the Occupational Health Effects of Poultry Production Workers. J. Agromed. 2002, 8, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Carvalheiro, M.F. Airways inflammation among workers in poultry houses. Int. Arch. Occup. Environ. Health 2006, 79, 487–490. [Google Scholar] [CrossRef]
- Walker, J.; Robarge, W.P.; Shendrikar, A.; Kimball, H. Inorganic PM2.5 at a U.S. agricultural site. Environ. Pollut. 2006, 139, 258–271. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of February 15, 2010 on the requirements and conduct of behavior in keeping species of farm animals for which protection standards have been defined in the European Union legislation. J. Laws 2010, 56, 344.
- American Conference of Governmental Industrial. Hygienists Threshold Limit Values (TLVs) and Biological and Exposure Indices (BEIs); ACGIH: Cincinnati, OH, USA, 2001; pp. 72–75. [Google Scholar]
- Ritz, C.W.; Fairchild, B.D.; Lacy, M.P. Implications of Ammonia Production and Emissions from Commercial Poultry Facilities: A Review. J. Appl. Poult. Res. 2004, 13, 684–692. [Google Scholar] [CrossRef]
- Reece, F.N.; Lott, B.D.; Deaton, J.W. Low Concentrations of Ammonia During Brooding Decrease Broiler Weight. Poult. Sci. 1981, 60, 937–940. [Google Scholar] [CrossRef]
- Deaton, J.W.; Reece, F.N.; Lott, B.D. Effect of Atmospheric Ammonia on Pullets at Point of Lay. Poult. Sci. 1984, 63, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.M.; Branton, S.L.; Lott, B.D. Atmospheric Ammonia is Detrimental to the Performance of Modern Commercial Broilers. Poult. Sci. 2004, 83, 1650–1654. [Google Scholar] [CrossRef]
- Vučemilo, M.; Matković, K.; Vinković, B.; Jakšić, S.; Granic, K.; Mas, N. The effect of animal age on air pollutant concentration in a broiler house. Czech J. Anim. Sci. 2008, 52, 170–174. [Google Scholar] [CrossRef]
- Carlile, F.S. Ammonia in Poultry Houses: A Literature Review. World’s Poult. Sci. J. 1984, 40, 99–113. [Google Scholar] [CrossRef]
- Giannadaki, D.; Giannakis, E.; Pozzer, A.; Lelieveld, J. Estimating health and economic benefits of reductions in air pollution from agriculture. Sci. Total. Environ. 2018, 622, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Whyte, R.T. Aerial pollutants and the health of poultry farmers. World’s Poult. Sci. J. 1993, 49, 139–156. [Google Scholar] [CrossRef]
- Hartung, E.; Jungbluth, T.; Bascher, W. Reduction of Ammonia and Odor Emissions from a Piggery with Biofilters. Trans. ASAE 2001, 44, 113–118. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R.; Miller, D.M. Evaluation of Chemical Amendments to Reduce Ammonia Volatilization from Poultry Litter. Poult. Sci. 1996, 75, 315–320. [Google Scholar] [CrossRef]
- Moore, P.A. Improving the sustainability of animal agriculture by treating manure with alum. In Environmental Chemistry of Animal Manure; He, Z., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 349–381. [Google Scholar]
- DeLaune, P.B.; Moore, P.A.; Daniel, T.C.; Lemunyon, J.L. Effect of chemical and microbial amendments on ammonia volatilization from composting poultry litter. J. Environ. Qual. 2004, 33, 728–734. [Google Scholar] [CrossRef]
- Li, H.; Xin, H.; Liang, Y.; Burns, R.T. Reduction of Ammonia Emissions from Stored Laying Hen Manure Through Topical Application of Zeolite, Al+ Clear, Ferix-3, or Poultry Litter Treatment. J. Appl. Poult. Res. 2008, 17, 421–431. [Google Scholar] [CrossRef]
- Anderson, K.; Moore, J.P.A.; Martin, J.; Ashworth, A.J. Effect of a New Manure Amendment on Ammonia Emissions from Poultry Litter. Atmosphere 2020, 11, 257. [Google Scholar] [CrossRef]
- Santoso, U.; Ohtani, S.; Tanaka, K.; Sakaida, M. Dried Bacillus subtilis Culture Reduced Ammonia Gas Release in Poultry House. Asian-Australas. J. Anim. Sci. 1999, 12, 806–809. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.; Melear, N.; Lakly, D. Reducing Nitrogen Loss during Poultry Litter Composting Using Biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef]
- Maeda, K.; Toyoda, S.; Shimojima, R.; Osada, T.; Hanajima, D.; Morioka, R.; Yoshida, N. Source of Nitrous Oxide Emissions during the Cow Manure Composting Process as Revealed by Isotopomer Analysis of and amoA Abundance in Betaproteobacterial Ammonia-Oxidizing Bacteria. Appl. Environ. Microbiol. 2010, 76, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Gutarowska, B.; Brycki, B.; Kołacz, R. Method of Preparation of Biopreparation for Deodorization of Poultry Manure. Patent P-393863, 7 February 2011. [Google Scholar]
- Matusiak, K.; Borowski, S.; Opaliński, S.; Bakuła, T.; Kołacz, R.; Gutarowska, B. Impact of a microbial-mineral biopreparation on microbial community and deodorization of manures. Acta Biochim. Pol. 2015, 62, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Matusiak, K.; Powałowski, S.; Pielech-Przybylska, K.; Makowski, K.; Nowak, A.; Rosowski, M.; Komorowski, P.; Gutarowska, B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeterior. Biodegrad. 2017, 119, 299–308. [Google Scholar] [CrossRef]
- Gałęcki, R.; Dąbrowski, M.; Bakuła, T.; Obremski, K.; Nowak, A.; Gutarowska, B. The Influence of the Mineral-Microbial Preparation on Ammonia Concentration and Productivity in Laying Hens Houses. Atmosphere 2019, 10, 751. [Google Scholar] [CrossRef]
- National Institutes of Health. National Research Council Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2010; pp. 1–246. [Google Scholar]
- Hill, L.; Vernon, H.M.; Hargood-Ash, D. The kata-thermometer as a measure of ventilation. Proc. R. Soc. Lond. Ser. B 1922, 93, 198–206. [Google Scholar]
- Mochida, T. A measurement method of air movement and radiant temperature with dry and wet Kata thermometers. Res. Rep. Dep. Engineet. Hokkaido Univ. 1978, 87, 1–10. [Google Scholar]
- Kośla, T. Metodyka badań z higieny zwierząt i prewencji weterynaryjnej; Wydawnictwo SGGW: Warszawa, Poland, 2011; pp. 5–118. [Google Scholar]
- Winnicki, S.; Woyke, W.; Pleskot, K. Kodeks zaleceń i praktyk; IBMER: Warszawa, Poland, 2003; p. 46. [Google Scholar]
- The National Research Institute of Animal Production. Karty Informacyjne do założeń technologicznych produkcji zwierzęcej; Nr Karty 1.01.04; IBMER publisher: Warsaw, Poland, 1977. [Google Scholar]
- Gutarowska, B.; Matusiak, K.; Borowski, S.; Rajkowska, A.; Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite—bentonite carrier. J. Environ. Manag. 2014, 141, 70–76. [Google Scholar] [CrossRef]
- Dawkins, M.S.; A Donnelly, C.; Jones, T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Natural 2004, 427, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S. Ammonia affects performance and thermoregulation of male broiler chickens. Anim. Res. 2004, 53, 289–293. [Google Scholar] [CrossRef]
- Deaton, J.W.; Reece, F.N.; Lott, B.D. Effect of Atmospheric Ammonia on Laying Hen Performance. Poult. Sci. 1982, 61, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.P.; Beard, C.W.; Hanson, R.P. Influence of Poultry House Dust, Ammonia, and Carbon Dioxide on the Resistance of Chickens to Newcastle Disease Virus. Avian Dis. 1966, 10, 177. [Google Scholar] [CrossRef]
- Kleven, S.H. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult. Sci. 1998, 77, 1146–1149. [Google Scholar] [CrossRef]
- Glisson, J.R. Bacterial respiratory disease of poultry. Poult. Sci. 1998, 77, 1139–1142. [Google Scholar] [CrossRef]
- Nahm, K. Feed formulations to reduce N excretion and ammonia emission from poultry manure. Bioresour. Technol. 2007, 98, 2282–2300. [Google Scholar] [CrossRef]
- Adrizal, A.; Silaban, R.; Sumiati, S.; Yusrizal, Y.; Sumadja, W.A.; Yatno, Y.; Noferdiman, N.; Koh, K.; Rahman, M. Nitrogen and Ammonia Mitigation on Laying Hen Farms: Effects of Low-protein Diet and Manure Filtering. Int. J. Poult. Sci. 2017, 16, 125–131. [Google Scholar] [CrossRef]
- Nimmermark, S.; Gustafsson, G. Influence of temperature, humidity and ventilation rate on the release of odour and ammonia in a floor housing system for laying hens. Agric. Engineer. Int. CIGR Ejournal 2005, 7, 1–4. [Google Scholar]
- Elliott, H.A.; Collins, N.E. Factors Affecting Ammonia Release in Broiler Houses. Trans. ASAE 1982, 25, 0413–0418. [Google Scholar] [CrossRef]
- Ni, J. Mechanistic Models of Ammonia Release from Liquid Manure: A Review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Zhang, G.; Bannink, A.; Chadwick, D.; Misselbrook, T.; Harrison, R.; Hutchings, N.; Menzi, H.; Monteny, G.; Ni, J.; et al. Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores. Adv. Agron. 2006, 89, 261–335. [Google Scholar] [CrossRef]
- Koerkamp, P.G. Ammonia Emission from Aviary Housing Systems for Laying Hens: Inventory, Characteristics and Solutions. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 1998. [Google Scholar]
- Demmers, T.G.M.; Hissink, M.G.; Uenk, G.H. The Drying of Poultry Manure in a Drying Tunnel and the Effect on Ammonia Emission; Rapport; IMAG-DLO: Wageningen, The Netherlands, 1992. [Google Scholar]
- Kroodsma, W.; Scholtens, R.; Huis in’t Veld, J. Ammonia admissions from poultry housing systems. In Proceedings of the Seminar of the 2nd and 3rd Technical Section of the CIGR, Uppsala, Sweden, 20–22 September 1988. JTI Rapport 96(2): 7.1–7.13. [Google Scholar]
- Ahmed, M.; Ahmad, S.; Waldrip, H.M.; Ramin, M.; Raza, M.A. Whole Farm Modeling: A Systems Approach to Understanding and Managing Livestock for Greenhouse Gas Mitigation, Economic Viability and Environmental Quality. In Nitrification Inhibitors-Potentials and Limitations; ASA Special Publication 67; American Society of Agronomy: Madison, WI, USA, 2020; pp. 345–371. [Google Scholar]

| Measured Parameters n = 56 | Control Group | Experimental Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | CI95% | ME | MO | V | SD | CI95% | ME | MO | V | |||
| NH3 concentration (ppm) | 25.68 | 11.65 | 7.96–14.01 | 24.00 | m | 135.71 | 14.43 | 5.14 | 3.96–6.83 | 17.00 | 18.00 | 25.14 |
| NH3 emission (g/h) | 3.58 | 1.70 | 1.26–2.58 | 3.36 | m | 135.71 | 2.02 | 0.68 | 0.50–1.04 | 2.38 | 2.52 | 25.14 |
| Temperature (°C) | 20.88 | 1.17 | 0.87–1.82 | 21.20 | 21.40 | 1.39 | 21.28 | 0.98 | 0.72–1.52 | 21.40 | m | 0.96 |
| Humidity (%) | 55.79 | 6.12 | 4.52–9.48 | 55.40 | m | 37.50 | 52.80 | 4.43 | 3.27–6.86 | 52.60 | m | 19.62 |
| Air speed (m/s) | 0.052 | 0.031 | 0.02–0.05 | 0.05 | 0.06 | 0.001 | 0.05 | 0.048 | 0.02–0.05 | 0.065 | 0.05 | 0.0002 |
| Cooling (W/m2) | 1.85 | 0.18 | 0.13–0.28 | 1.87 | 1.98 | 0.032 | 1.98 | 0.17 | 0.12–0.26 | 1.93 | 1.84 | 0.027 |
| Correlations Between Environmental Parameters | Control Group | Experimental Group | ||
|---|---|---|---|---|
| p-value | r Coefficient | p-value | r Coefficient | |
| NH3/temperature | 0.0009 | 0.74 | 0.19 | 0.44 |
| NH3/humidity | 0.01 | 0.59 | 0.1 | 0.61 |
| NH3/air speed | 0.015 | 0.60 | 0.1 | 0.53 |
| NH3/cooling | 0.005 | 0.66 | 0.09 | 0.42 |
| NH3/age | 0.11 | 0.75 | 0.35 | −0.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałęcki, R.; Dąbrowski, M.; Bakuła, T.; Obremski, K.; Baranowski, M.; Nowak, A.; Gutarowska, B. The Influence of the Mineral–Microbial Deodorizing Preparation on Ammonia Emission and Growth Performance in Turkey Production. Atmosphere 2020, 11, 743. https://doi.org/10.3390/atmos11070743
Gałęcki R, Dąbrowski M, Bakuła T, Obremski K, Baranowski M, Nowak A, Gutarowska B. The Influence of the Mineral–Microbial Deodorizing Preparation on Ammonia Emission and Growth Performance in Turkey Production. Atmosphere. 2020; 11(7):743. https://doi.org/10.3390/atmos11070743
Chicago/Turabian StyleGałęcki, Remigiusz, Michał Dąbrowski, Tadeusz Bakuła, Kazimierz Obremski, Mirosław Baranowski, Adriana Nowak, and Beata Gutarowska. 2020. "The Influence of the Mineral–Microbial Deodorizing Preparation on Ammonia Emission and Growth Performance in Turkey Production" Atmosphere 11, no. 7: 743. https://doi.org/10.3390/atmos11070743
APA StyleGałęcki, R., Dąbrowski, M., Bakuła, T., Obremski, K., Baranowski, M., Nowak, A., & Gutarowska, B. (2020). The Influence of the Mineral–Microbial Deodorizing Preparation on Ammonia Emission and Growth Performance in Turkey Production. Atmosphere, 11(7), 743. https://doi.org/10.3390/atmos11070743







