Historical Radial Growth of Chinese Torreya Trees and Adaptation to Climate Change
Abstract
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Methods
3. Results
3.1. Tree Age and Growth
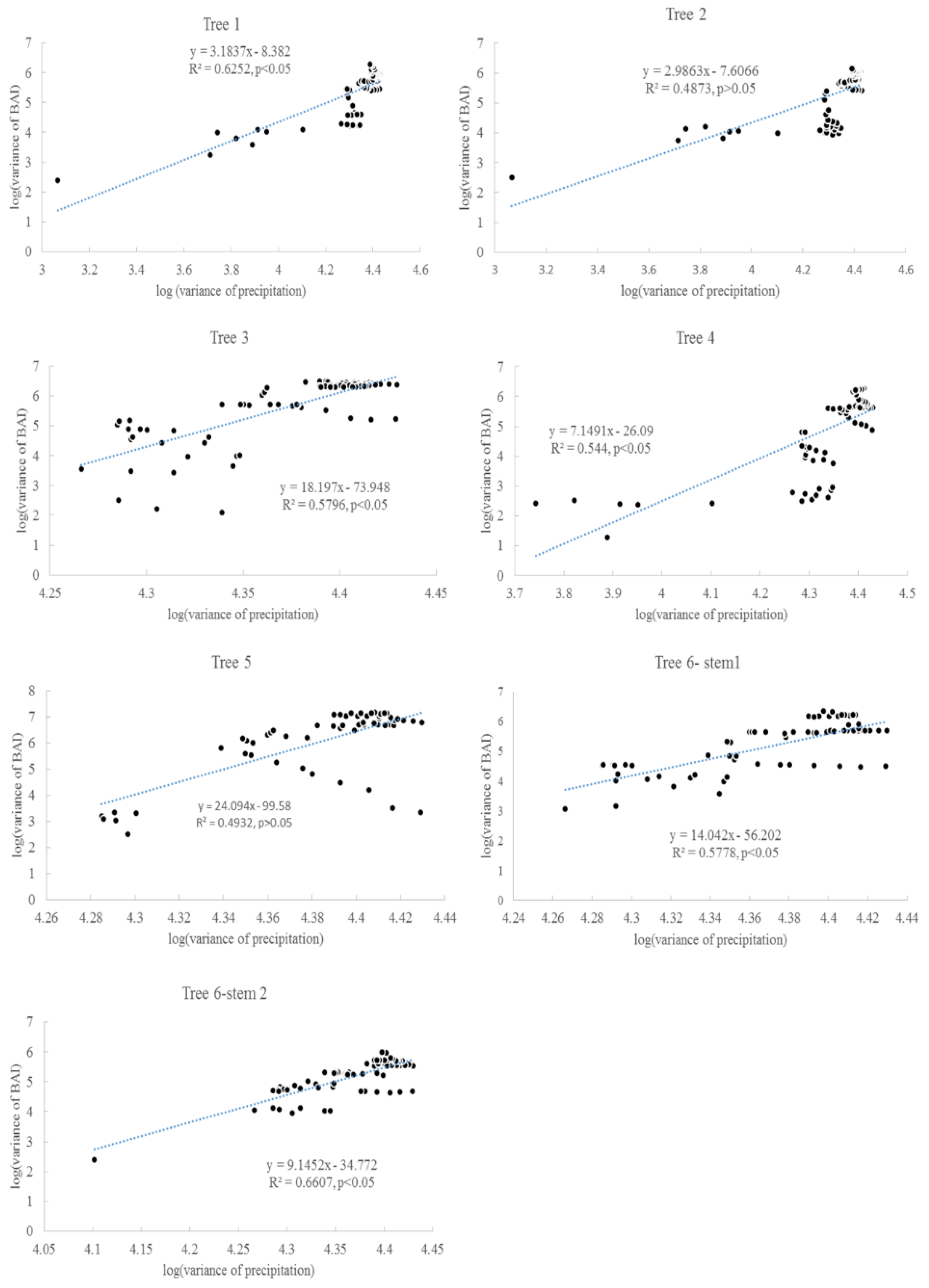
3.2. Taylor’s Power Law
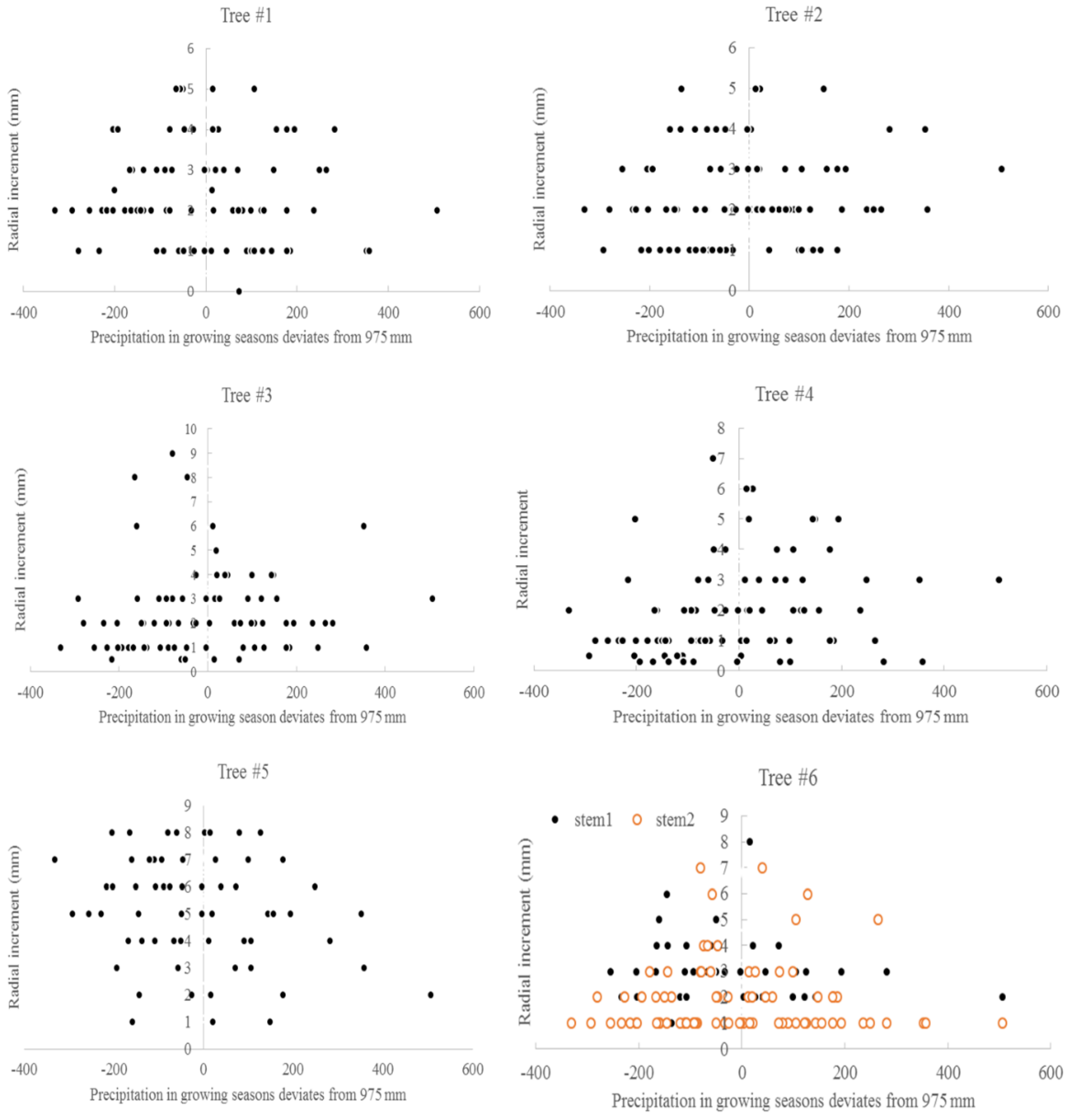
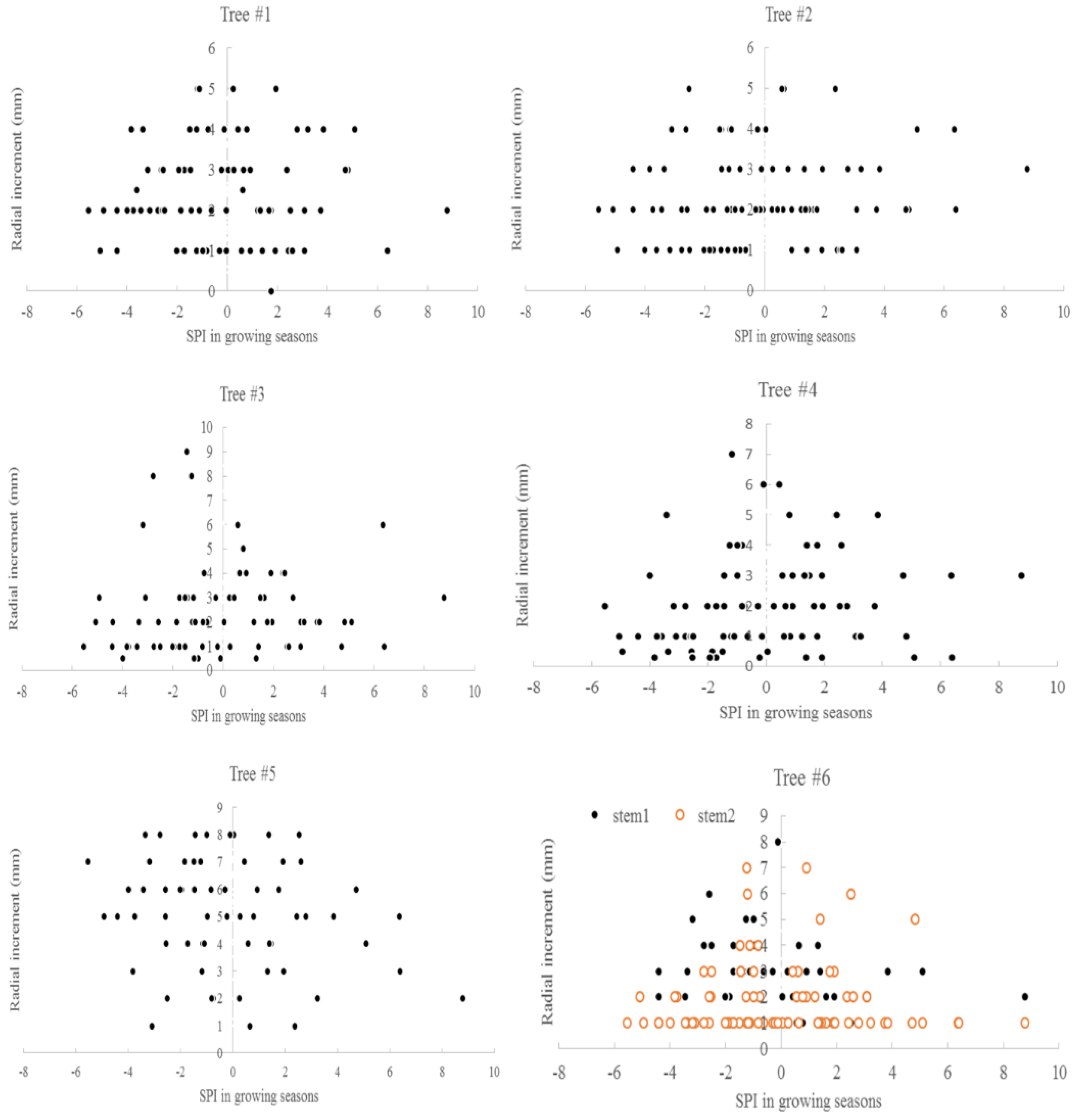
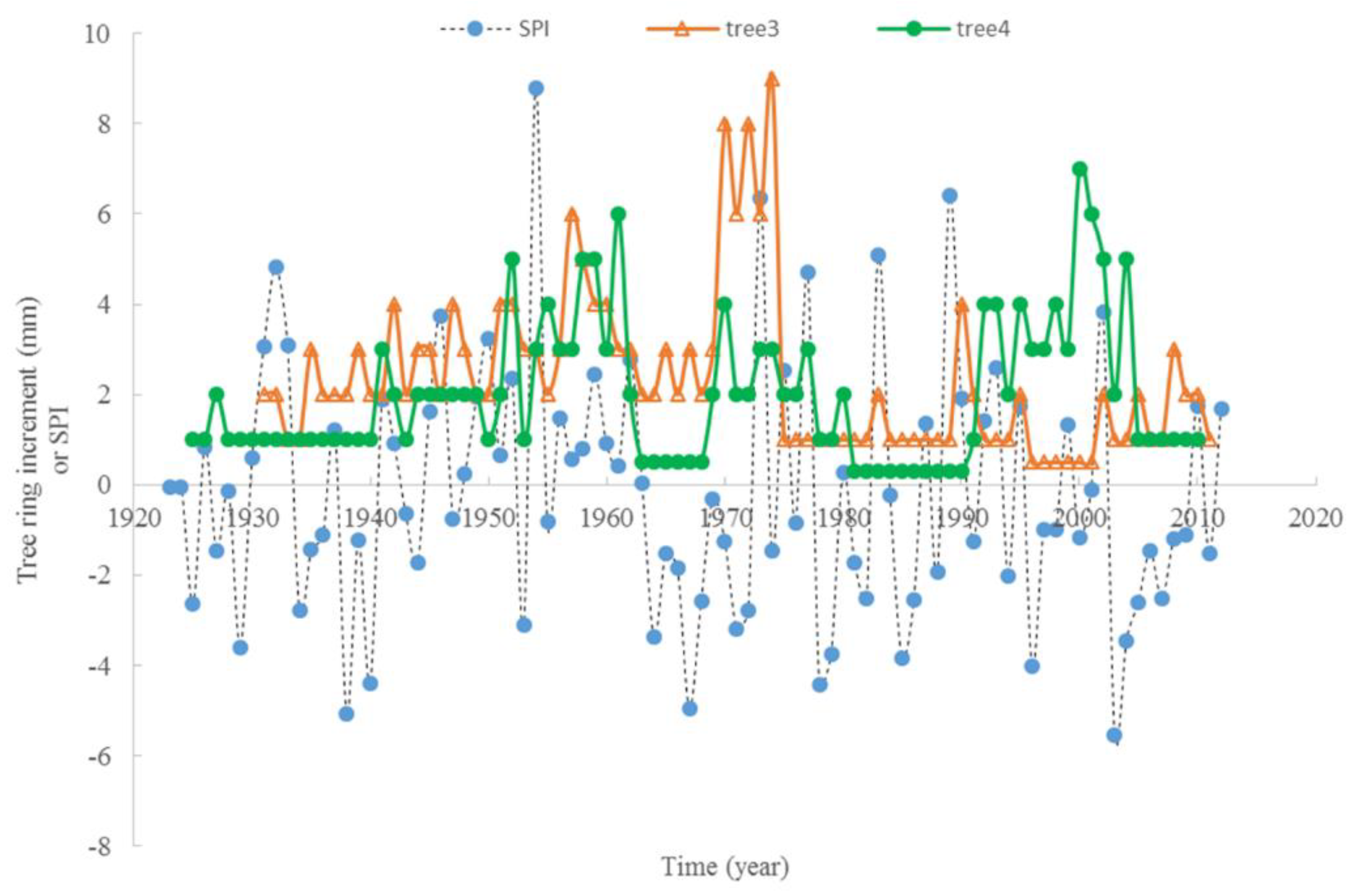
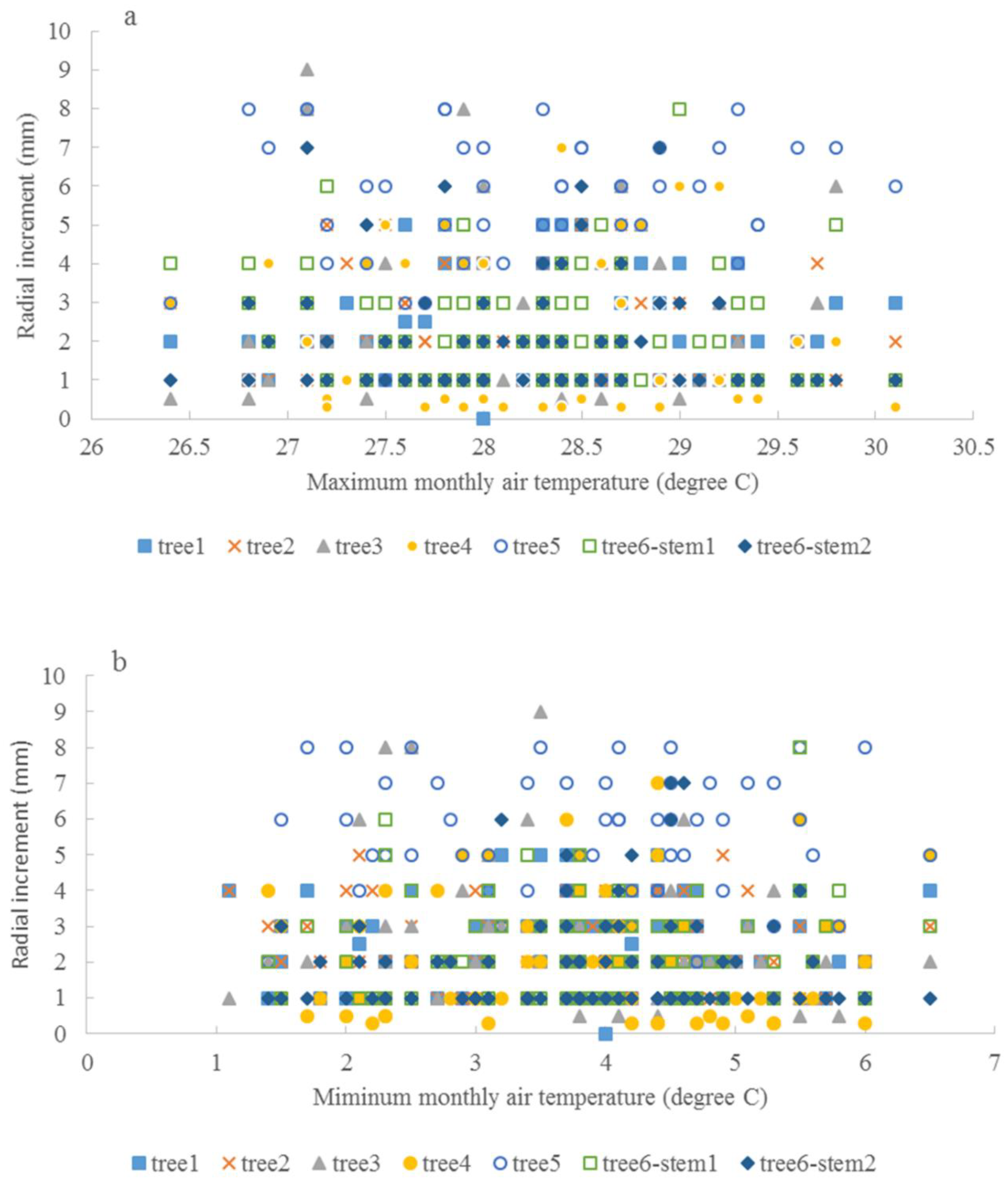
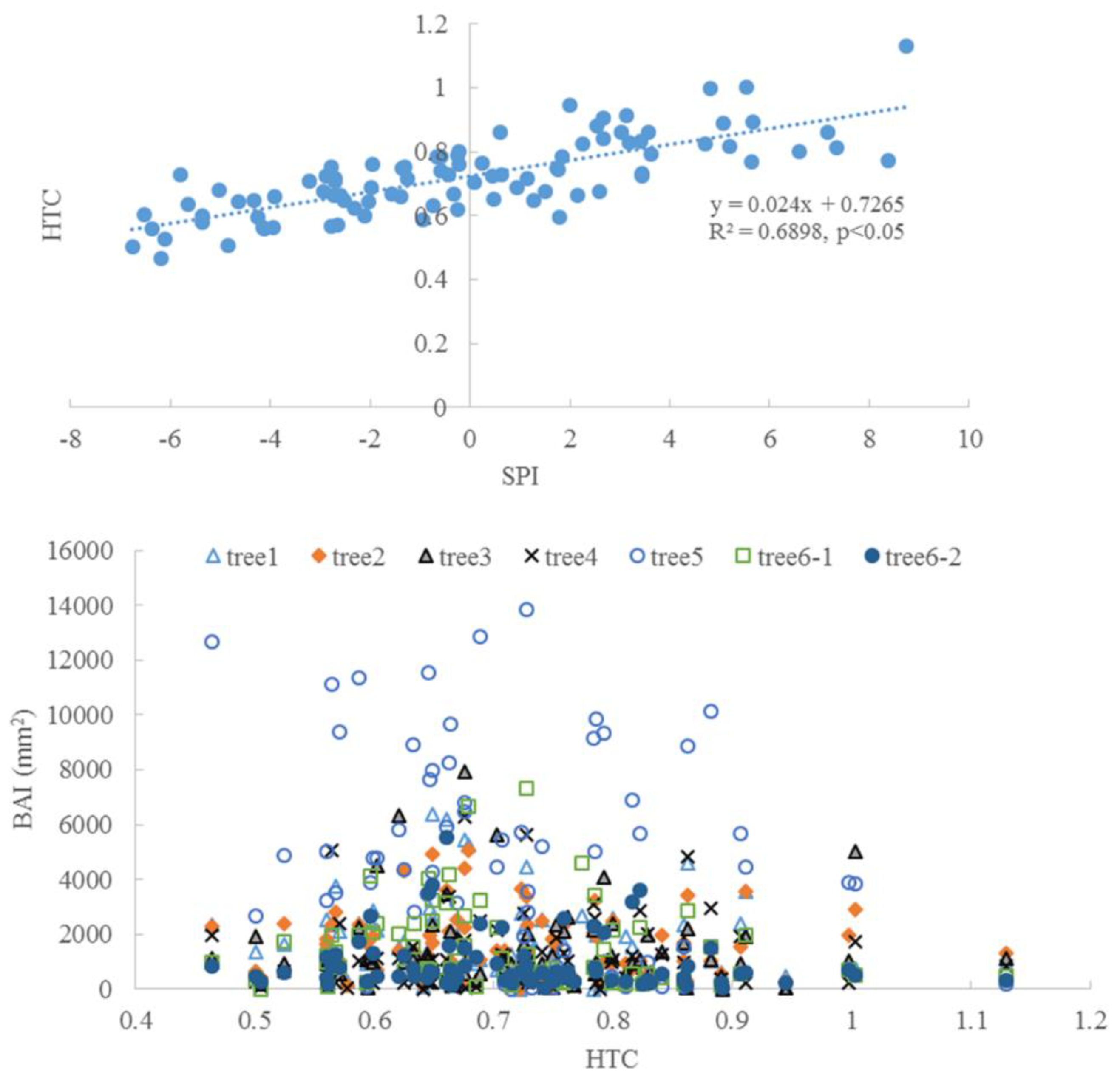
3.3. Climate and Radial Growth
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef]
- Brandl, S.; Paul, C.; Knoke, T.; Falk, W. The influence of climate and management on survival probability for Germany’s most important tree species. For. Ecol. Manag. 2020, 458, 117652. [Google Scholar] [CrossRef]
- Vossen, P. Olive oil: History, production, and characteristics of the world’s classic oils. HortScience 2007, 42, 1093–1100. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.C.; Chakraborty, S.; Dreccer, M.F.; Howden, S.M. Plant adaptation to climate change — opportunities and priorities in breeding. Crop. Pasture Sci. 2012, 63, 251–268. [Google Scholar] [CrossRef]
- van der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.R.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2014, 8, 4. [Google Scholar] [CrossRef]
- Maryinez-Feria, R.A.; Basso, B. Unstable crop yields reveal opportunities for site-specific adaptations to climate variability. Sci. Rep. 2020, 10, 2885. [Google Scholar] [CrossRef]
- Chen, X.; Brockway, D.G.; Guo, Q. Characterizing the dynamics of cone production for longleaf pine forests in the southeastern United States. For. Ecol. Manag. 2018, 429, 1–6. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Telbaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Hammer, G.L.; McLean, G.; Chapman, S.; Zheng, B.; Doherty, A.; Harrison, M.T.; van Oosterom, E.; Jordan, D. Crop design for specific adaptation in variable dryland production environments. Crop. Pasture Sci. 2014, 65, 614–626. [Google Scholar] [CrossRef]
- Chen, X.; Niu, J. Evaluating the adaptation of Chinese Torreya plantations to climate change. Atmosphere 2020, 11, 176. [Google Scholar] [CrossRef]
- Chen, X.; Jin, H. Review of cultivation and development of Chinese torreya in China. For. Trees Liveli. 2019, 28, 68–78. [Google Scholar] [CrossRef]
- Li, Z.; Dai, W. Chinese Torreya; Science Press: Beijing, China, 2007. (in Chinese) [Google Scholar]
- Roy, D.F. Torreya Arn. Torreya. In Seeds of Woody Plants in the United States; Schopmeyer, C.S., Ed.; Agriculture Handbook No. 450 USDA Forest Service: Washington, DC, USA, 1974. [Google Scholar]
- Shin, S.; Lee, S.G.; Kang, H. Spatial distribution patterns of old-growth forest of dioecious tree Torreya nucifera in rocky Gotjawal terrain of Jeju Island, South Korea. J. Ecol. Environ. 2017, 41, 31. [Google Scholar] [CrossRef]
- Farjon, A. A Handbook of the World′s Conifers; Brill Academic Publishing: Leiden, Netherlands, 2010. [Google Scholar]
- Wang, B.; Ming, Q.-W. Zhejiang Shaoxing Kuaijishan Guxiangfeiqun; China Agricultural Press: Beijing, China, 2015. (in Chinese) [Google Scholar]
- Hu, H.-H. Synoptical study of Chinese Torreyas: With supplemental notes on the distribution and habitat by R. C. Ching. Contrib. Biol. Lab. Sci. Soc. China 1927, 3, 1–37. [Google Scholar]
- Cheng, X.; Li, Z.; Yu, W.; Dai, W.; Fu, Q. Distribution and ecological characteristics of Torreya grandis in China. J. Zhejiang For. Coll. 2007, 24, 383–388, (in Chinese with English abstract). [Google Scholar]
- Chen, X.; Jin, H. A case study of enhancing sustainable intensification of Chinese torreya forest in Zhuji of China. Environ. Nat. Res. Res. 2019, 9, 53–60. [Google Scholar] [CrossRef]
- People’s Government of Shaoxing City. Kuanjishan Ancient Chinese Torreya Community; Proposal for Global Important Agricultural Heritage System Initiative: Shaoxing, China, 2013.
- Chen, X.; Chen, H. Dynamics in production of four heritage foods at the mountainous region of Shaoxing City, China. Emir. J. Food Agric. 2019, 31, 645–653. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Li, G.; Zheng, Z.; Su, W. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Li, B.-L. The possible response of life zones in China under global climate change. Glob. Planet. Chang. 2003, 38, 323–337. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, R.; Hu, Y.; Song, Y.; Hanninen, H.; Wu, J. Interactive effects of drought and shading on Torreya grandis seedlings: Physiological and growth responses. Trees 2019, 33, 951–961. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.-Z. Seedling cultivation of Chinese Torreya and observation on height growth in one-year-old seelings. Appl. For. Tech. 2006, 4, 18–19. (in Chinese). [Google Scholar]
- Boden, S.; Kahle, H.P.; Wilpert, K.V.; Spiecker, H. Resilience of Norway spruce (Picea abies (L.) Karst) growth to changing climatic conditions in Southwest Germany. For. Ecol. Manag. 2014, 315, 12–21. [Google Scholar] [CrossRef]
- Costa, M.S.; Ferreira, K.E.B.; Botosso, P.C.; Callado, C.H. Growth analysis of five Leguminosae native tree species from a seasonal semidecidual lowland forest in Brazil. Dendrochronologia 2015, 36, 23–32. [Google Scholar] [CrossRef]
- Worbes, M.; Staschel, R.; Roloff, A.; Junk, W.J. Tree ring analysis reveals age structure, dynamics and wood production of a natural forest stand in Cameroon. For. Ecol. Manag. 2003, 173, 105–123. [Google Scholar] [CrossRef]
- Mbow, C.; Chhin, S.; Sambou, B.; Skole, D. Potential of dendrochronology to assess annual rates of biomass productivity in savanna trees of West Africa. Dendrochronologia 2012, 31, 41–51. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M.C.M. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic Forest. For. Ecol. Manag. 2014, 329, 1–9. [Google Scholar] [CrossRef]
- Granato-Souza, D.; Adenesky-Filho, E.; Esemann-Quadros, K. Dendrochronology and climatic signals in the wood of Nectandra oppositifolia from a dense rain forest in southern Brazil. J. For. Res. 2018, 30, 545–553. [Google Scholar] [CrossRef]
- Natalini, F.; Correia, A.C.; Vazquez-Pique, J.; Alejano, R. Tree rings reflect growth adjustments and enhanced synchrony among sites in Iberian stone pine (Pinus pinea L.) under climate change. Ann. For. Sci. 2015, 72, 1023–1033. [Google Scholar] [CrossRef]
- Prestes, A.; Klausner, V.; Silva, I.R.; Ojeda-Gonzalez, A.; Lorensi, C. Araucaria growth response to solar and climate variability in South Brazil. Ann. Geophys. Dis. 2018, 36, 717–719. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Islam, M. Long-term growth decline in Toona ciliata in a moist tropical forest in Bangladesh: Impact of global warming. Acta Oecol. 2017, 80, 8–17. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Corlett, R.T. The impacts of droughts in tropical forests. Trends Plant. Sci. 2016, 21, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H. Comparing environmental impacts of Chinese Torreya plantations and regular forests using remote sensing. Environ. Dev. Sustain. 2020, in press. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, P.; Niu, J.; Chen, X. Evaluating soil and nutrients (C, N, and P) loss in Chinese Torreya plantations. Environ. Pollut. 2020, in press. [Google Scholar] [CrossRef]
- Pearse, I.S.; LaMontagne, J.M.; Koenig, W.D. Inter-annual variation in seed production has increased over time (1900–2014). Proc. Royal Soc. B 2017, 284, 20171666. [Google Scholar] [CrossRef]
- Taylor, L.R. Aggregation, variance and the mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Lucas, C.; Puchi, P.; Profumo, L.; Ferreira, A.; Muñoz, A. Effect of climate on tree growth in the Pampa biome of Southeastern South America: First tree-ring chronologies from Uruguay. Dendrochronologia 2018, 52, 113–122. [Google Scholar] [CrossRef]
- McKee, T.B.; Doesken, N.J.; Kliest, J. The relationship of drought frequency and duration to time scales. In Proceedings of the 8th Conference of Applied Climatology, Anaheim, CA, USA, 17–22 January 1993; American Meteorological Society: Boston, MA, USA, 1993; pp. 179–184. [Google Scholar]
- Evarte-Bundere, G.; Evarts-Bunders, P. Using of the hydrothermal coefficient (HTC) for interpretation of distribution of non-native tree species in Latvia on example of cultivated species of genus Tilia. Acta Biol. Univ. Daugavp. 2012, 12, 135–148. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Academic: San Diego, CA, USA, 1976. [Google Scholar]
- Black, B.A.; Colbert, J.J.; Pederson, N. Relationships between radial growth rates and lifespan within North American tree species. Ecoscience 2008, 15, 349–357. [Google Scholar] [CrossRef]
- Chen, X. Carbon storage traits of main tree species in natural forests in Northeast China. J. Sustain. For. 2006, 23, 67–84. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Q.; Brockway, D.G. Power laws in cone production of longleaf pine across its native range in the United States. Sustain. Agric. Res. 2017, 4, 64–73. [Google Scholar] [CrossRef][Green Version]
- Chen, X. Will more tree diversity bring back more income from timber? A case study from Alabama of USA. For. Lett. 2017, 110, 20–25. [Google Scholar]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Becker, G.S.; Beeckman, H.; Boanerges, S.D.; Botosso, P.C.; et al. Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef]
- Baker, P.J.; Palmer, J.G.; D’Arrigo, R. The dendrochronology of Callitris intratropica in northern Australia: Annual ring structure, chronology development and climate correlations. Aust. J. Bot. 2008, 56, 311–320. [Google Scholar] [CrossRef]
- Trouet, V.; Coppin, P.; Beeckman, H. Annual growth ring patterns in Brachystegia spiciformis reveal influence of precipitation on tree growth. Biotropica 2006, 38, 375–382. [Google Scholar] [CrossRef]
- McCullough, I.M.; Davis, F.W.; Williams, A.P. A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For. Ecol. Manag. 2017, 402, 223–233. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; Hurt, G.C.; et al. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Chen, X. Diverse scaling relationships of tree height and diameter in five tree species. Plant. Ecol. Divers. 2018, 11, 147–155. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H. Analyzing patterns of seed production for Chinese Torreya. HortScience 2020, 55, 778–786. [Google Scholar] [CrossRef]
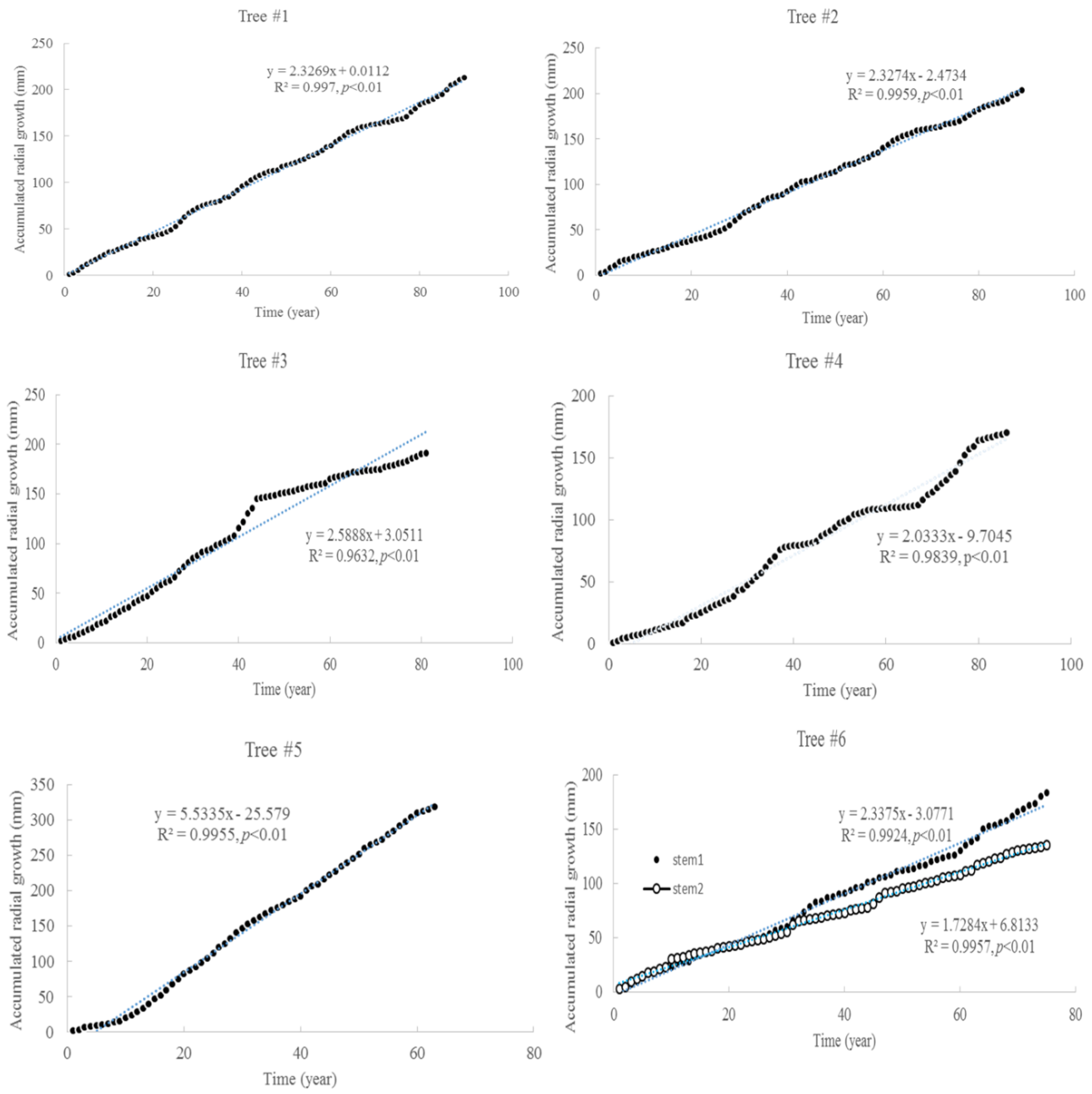
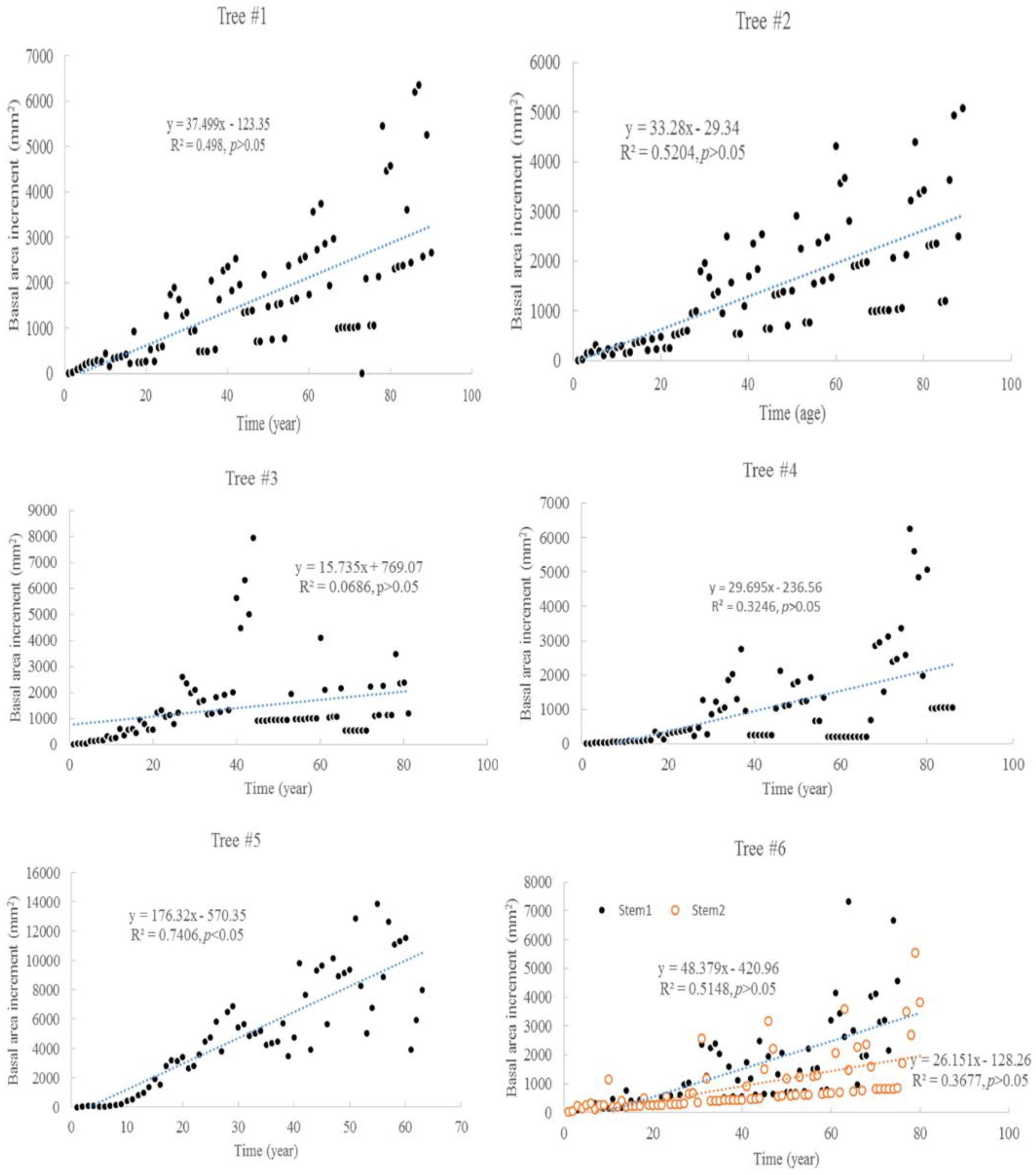

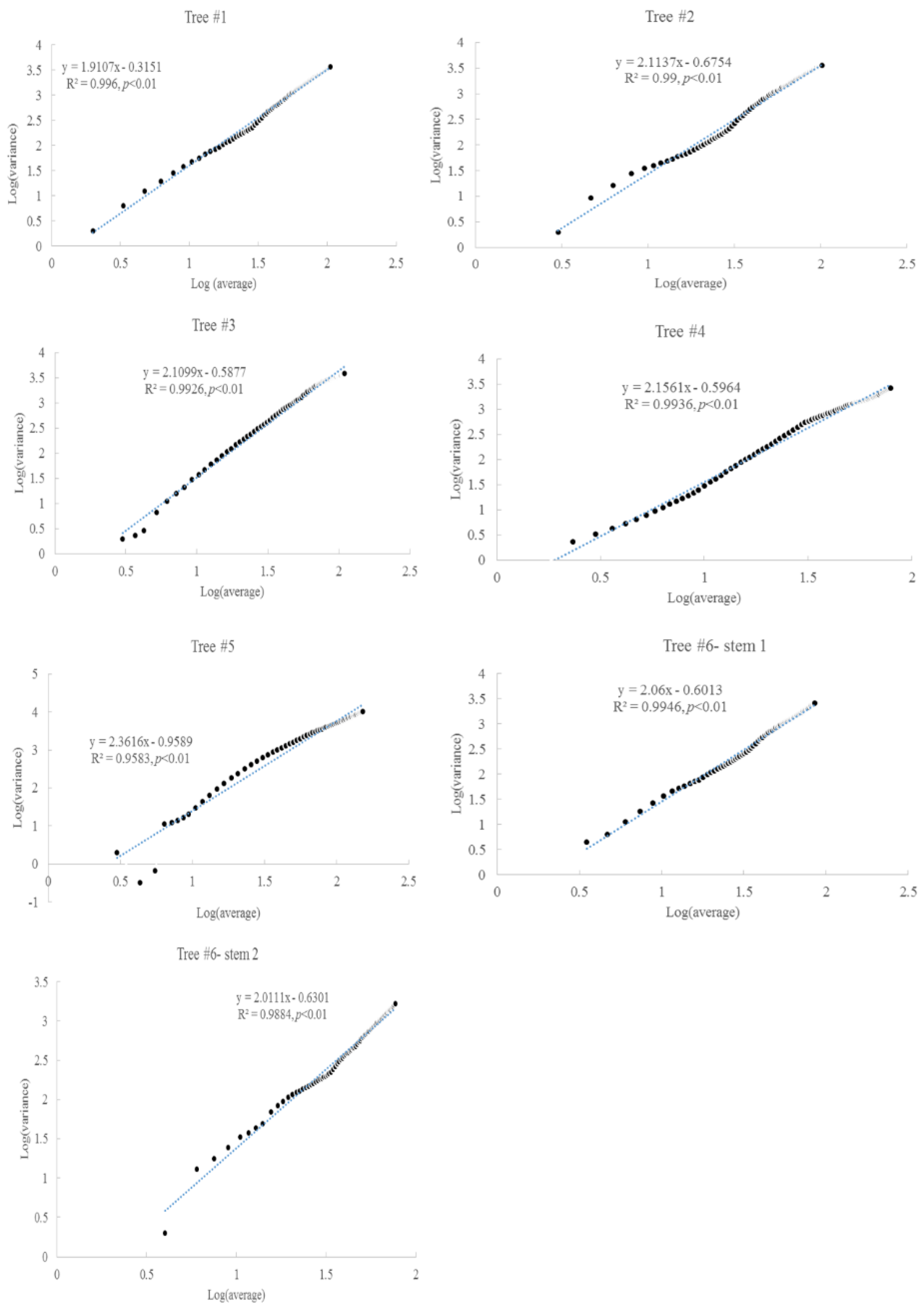
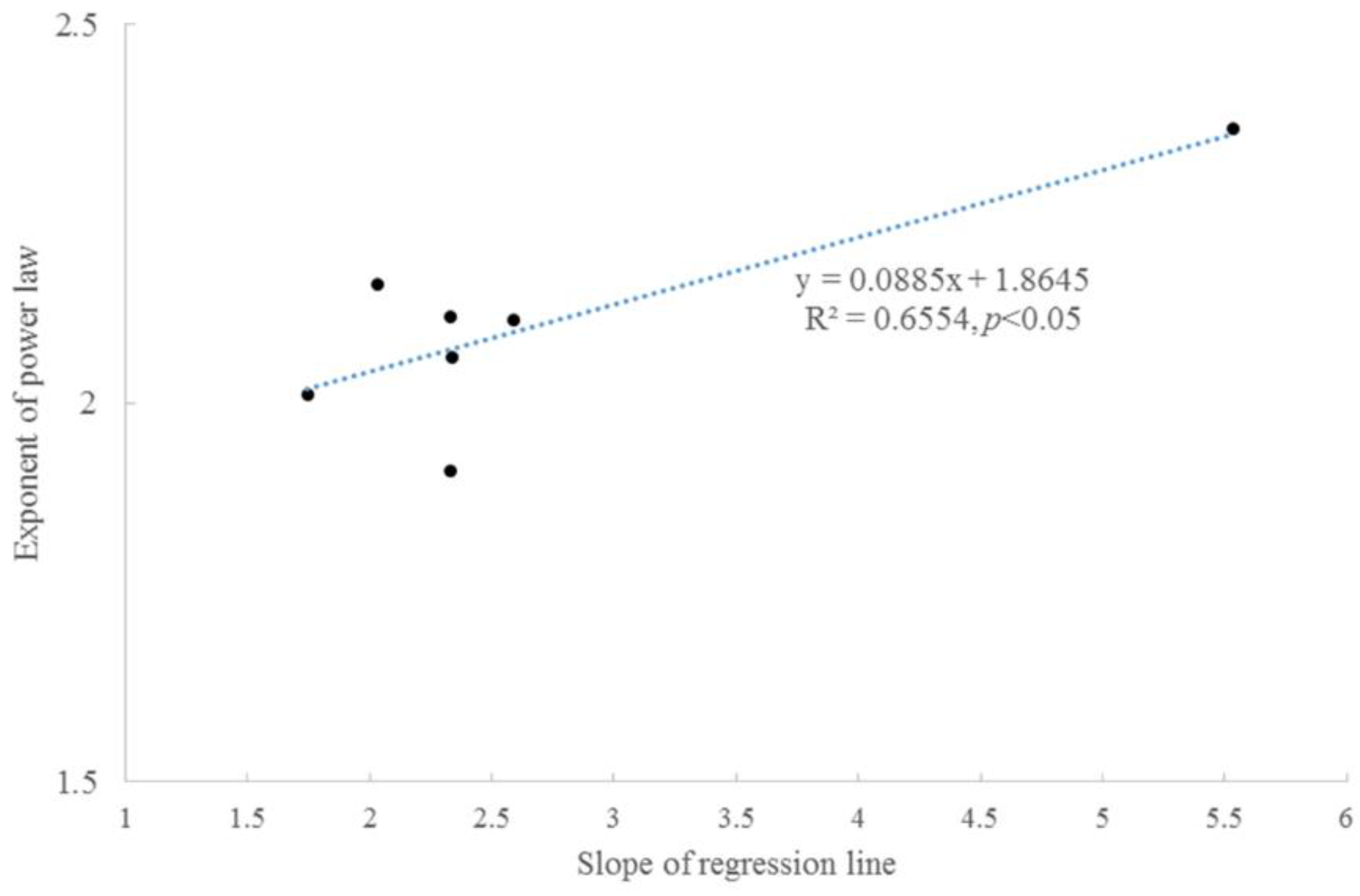
| Item | Tree #1 | Tree #2 | Tree #3 | Tree #4 | Tree #5 | Tree #6 | |
|---|---|---|---|---|---|---|---|
| Stem 1 | Stem 2 | ||||||
| Age (year) | 90 | 89 | 81 | 86 | 63 | 75 | 80 |
| Diameter (mm) | 426 | 408 | 382 | 340 | 638 | 368 | 308 |
| Average Ring width (mm) | 2.4 | 2.3 | 2.4 | 2.0 | 5.1 | 2.5 | 1.9 |
| Maximum Ring width (mm) | 5 | 5 | 9 | 7 | 8 | 8 | 7 |
| Minimum ring width (mm) | 0.5 | 1 | 0.5 | 0.3 | 1 | 1 | 1 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X. Historical Radial Growth of Chinese Torreya Trees and Adaptation to Climate Change. Atmosphere 2020, 11, 691. https://doi.org/10.3390/atmos11070691
Chen X. Historical Radial Growth of Chinese Torreya Trees and Adaptation to Climate Change. Atmosphere. 2020; 11(7):691. https://doi.org/10.3390/atmos11070691
Chicago/Turabian StyleChen, Xiongwen. 2020. "Historical Radial Growth of Chinese Torreya Trees and Adaptation to Climate Change" Atmosphere 11, no. 7: 691. https://doi.org/10.3390/atmos11070691
APA StyleChen, X. (2020). Historical Radial Growth of Chinese Torreya Trees and Adaptation to Climate Change. Atmosphere, 11(7), 691. https://doi.org/10.3390/atmos11070691





