Secondary Organic Aerosol Formation from Nitrophenols Photolysis under Atmospheric Conditions
Abstract
1. Introduction
2. Experiments
2.1. Chamber Description and Equipments
2.2. Experimental Methods and Procedures
2.3. Chemicals
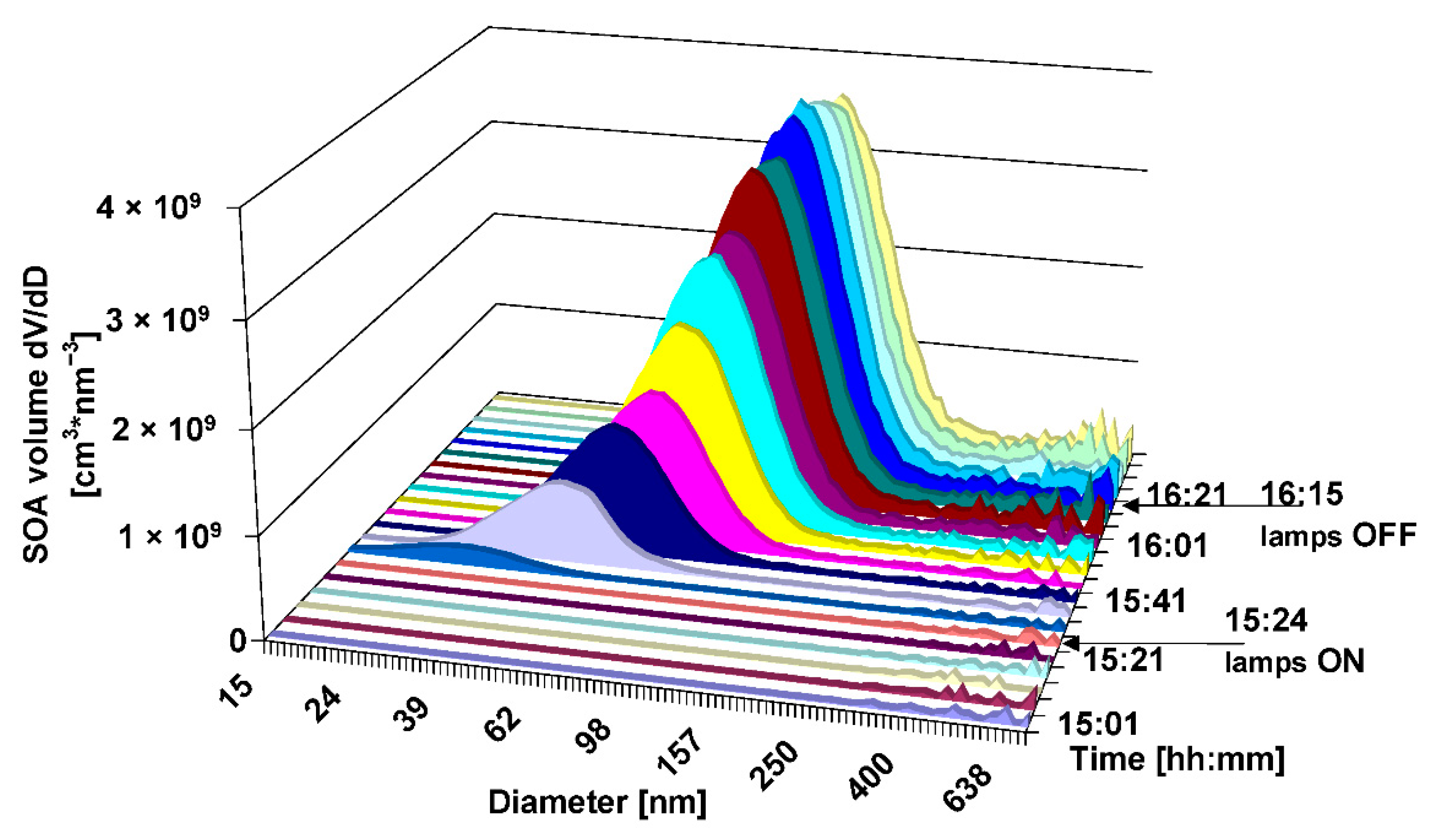
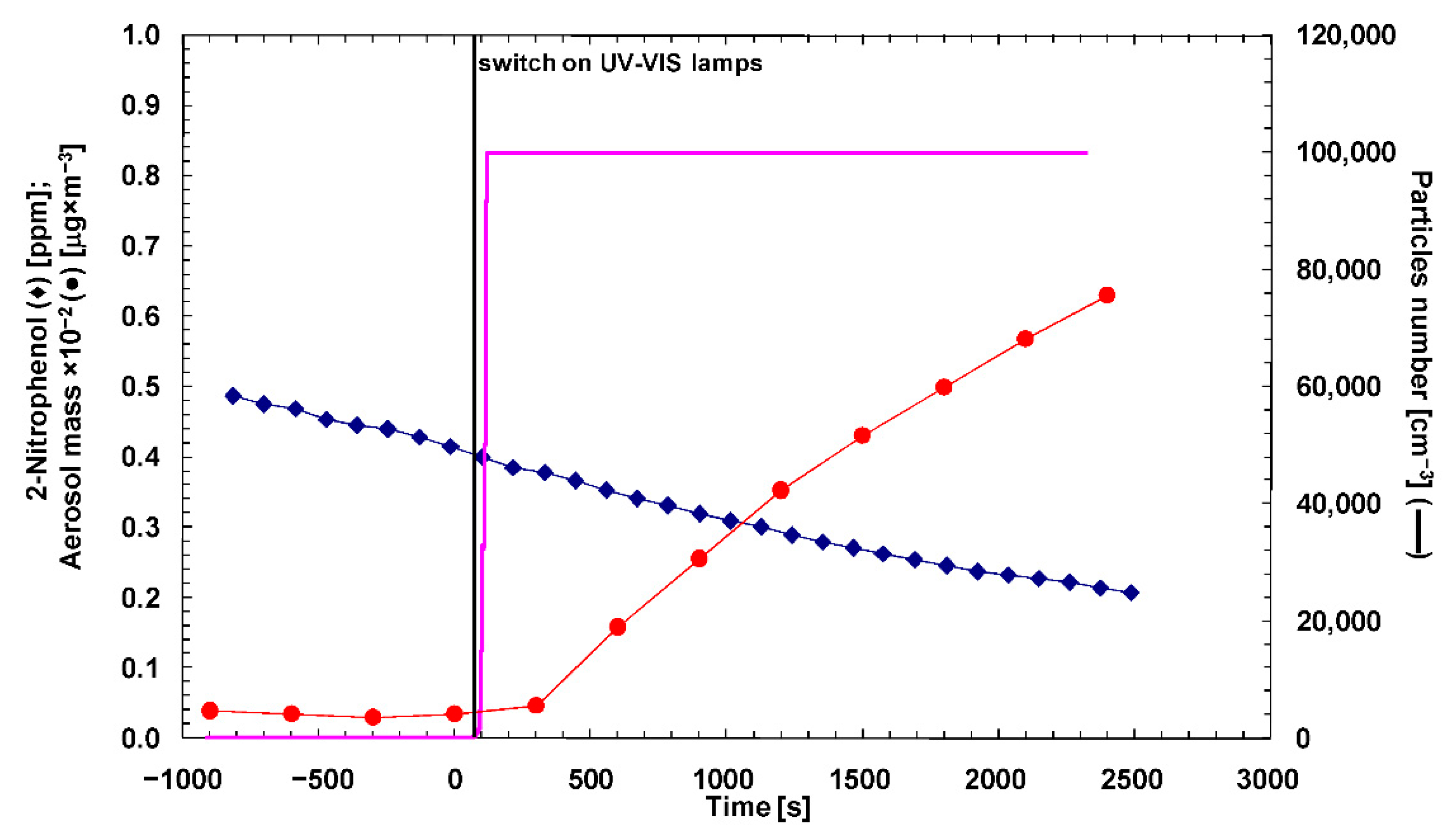
3. Results
4. Discussion
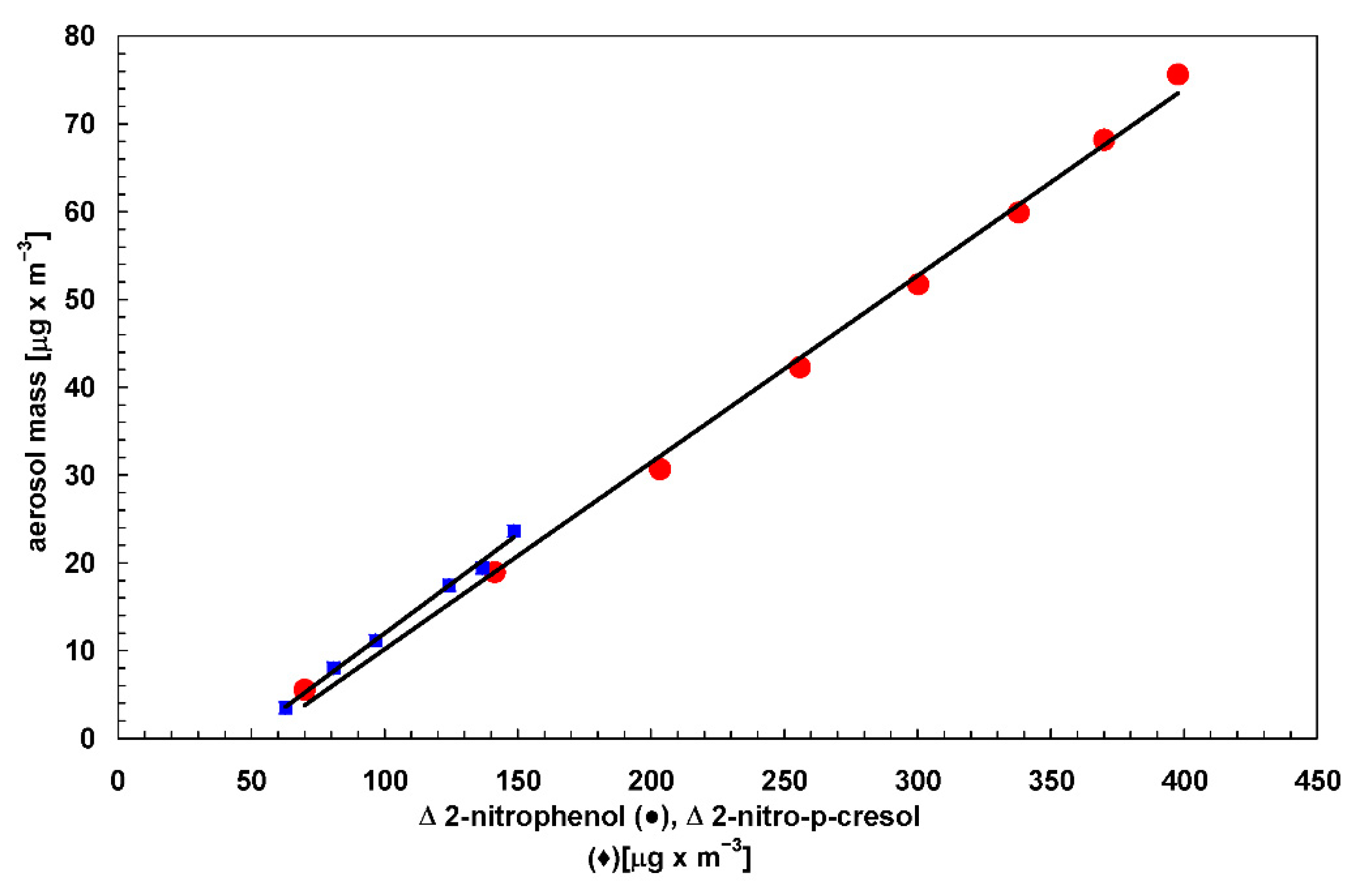
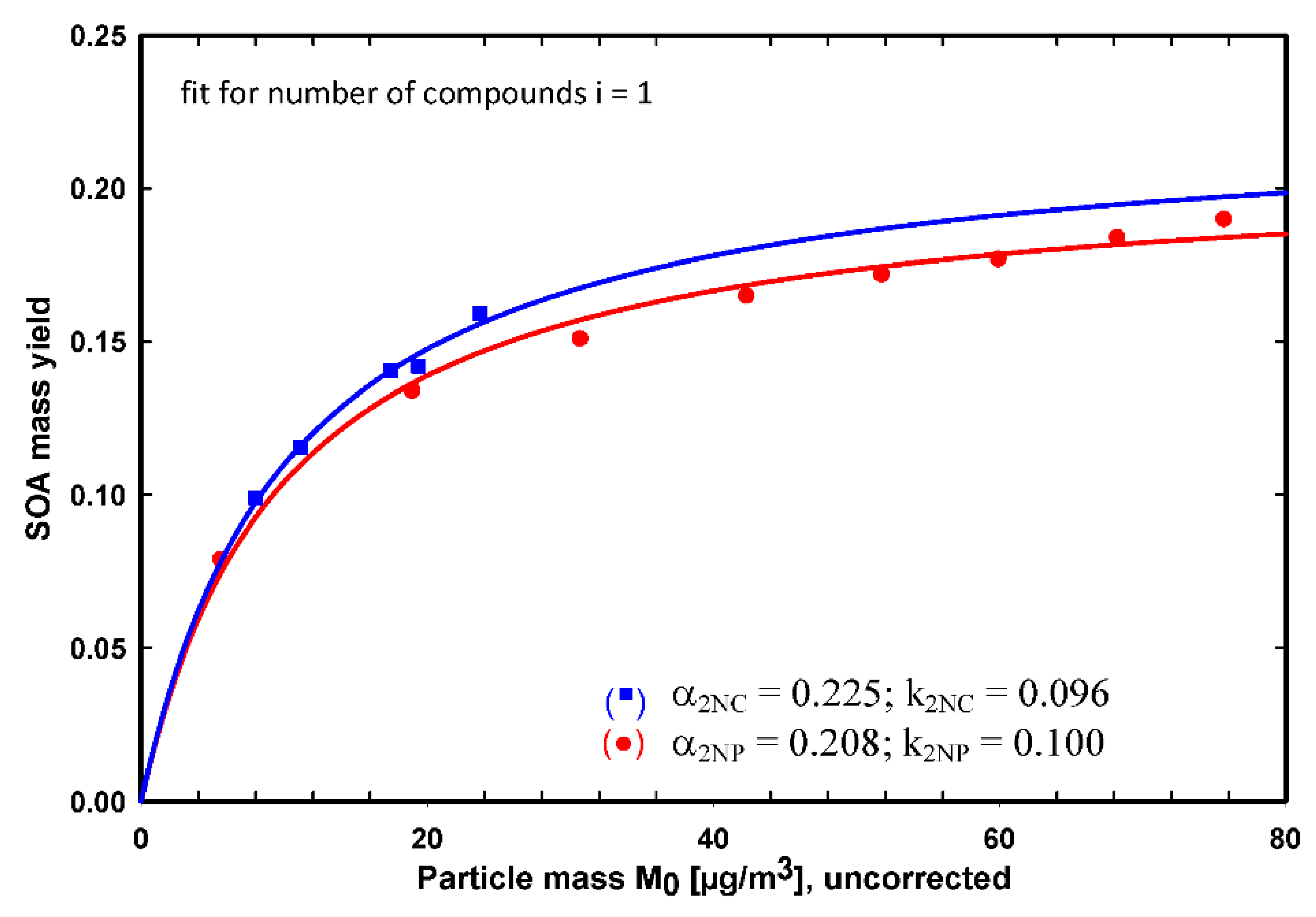
4.1. Expression for SOA Yield
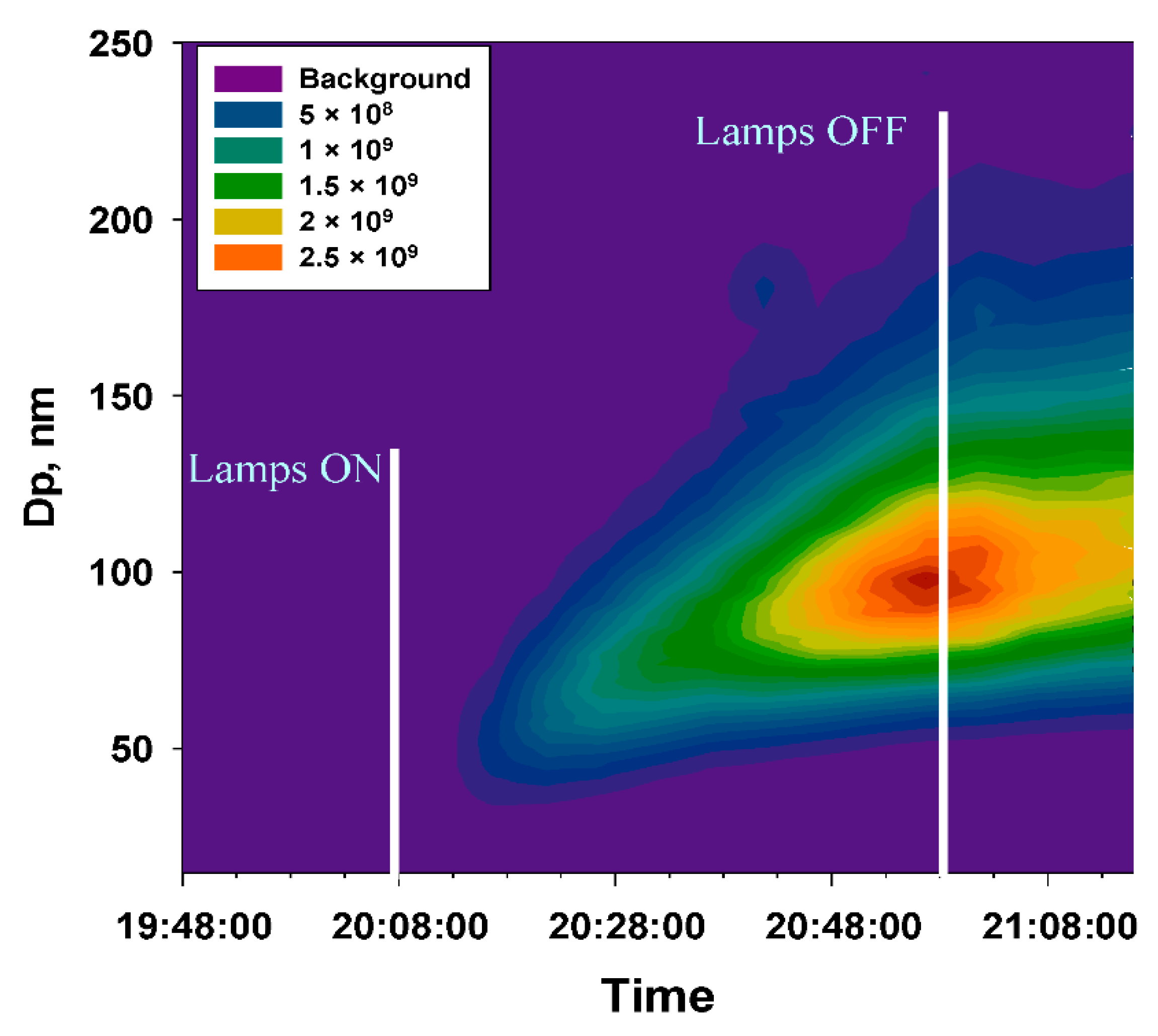
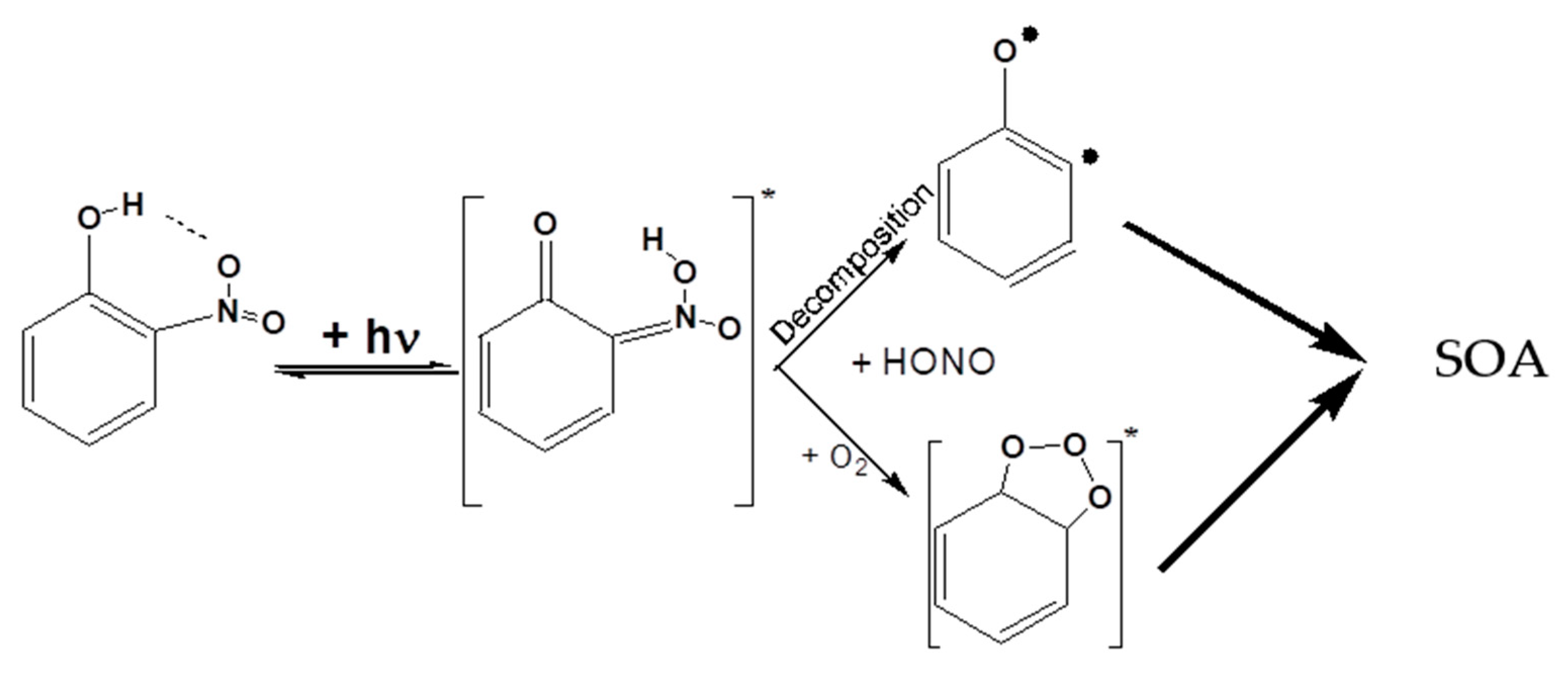
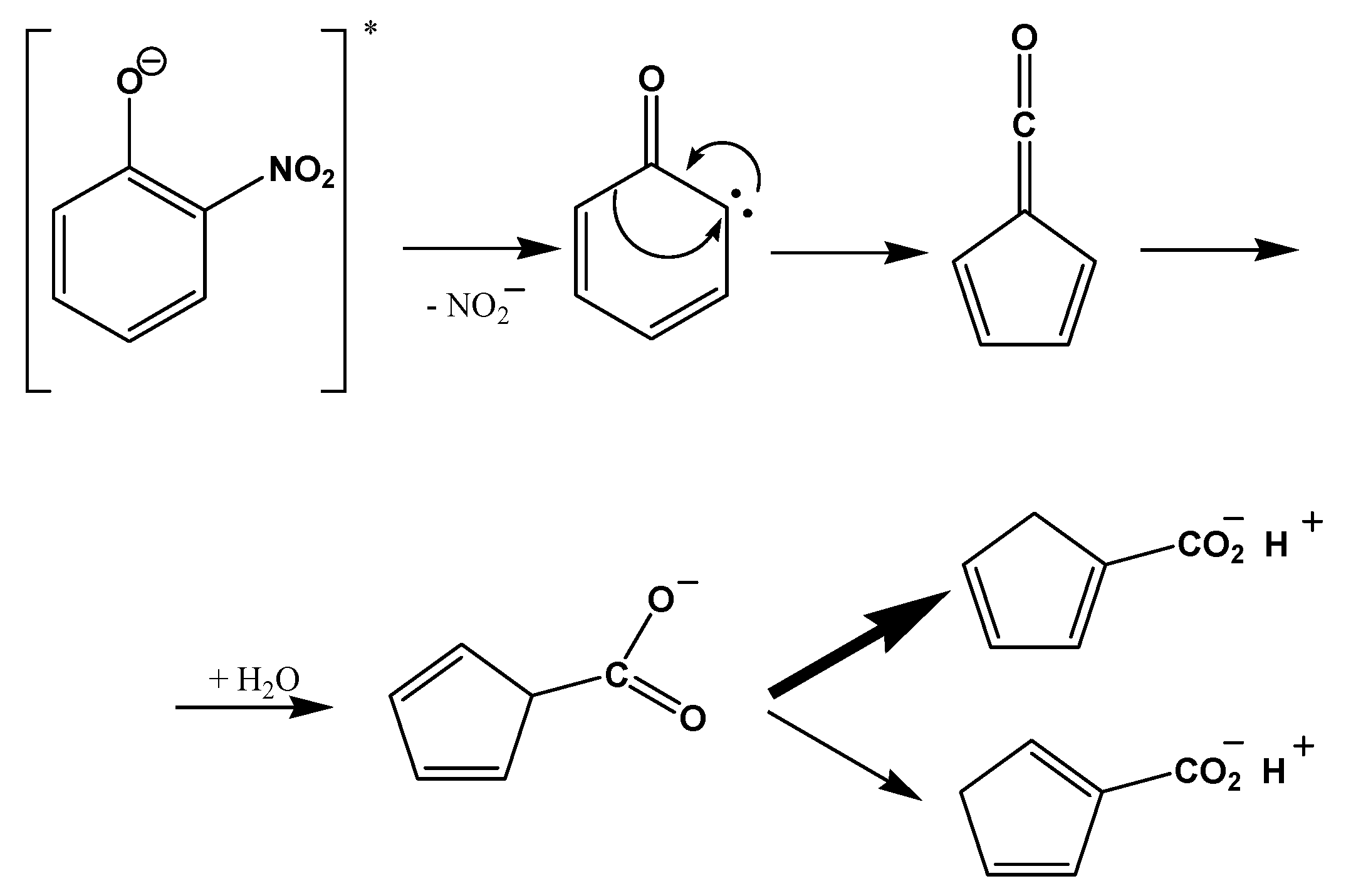
4.2. Proposed Pathways to SOA Formation
4.3. OH Radical Influence on Aerosol Formation
4.4. NOx Effect on Aerosol Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments and Applications; Academic Press: San Diego, CA, USA; London, UK, 2000; pp. 1–969. [Google Scholar]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics, from Air Pollution to Climate Change, 3rd ed.; John and Wiley Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Stockwell, W.R.; Lawson, C.V.; Saunders, E.; Goliff, W.S. A Review of Tropospheric Atmospheric Chemistry and Gas-Phase Chemical Mechanisms for Air Quality Modeling. Atmosphere 2012, 3, 1–32. [Google Scholar] [CrossRef]
- Calvert, J.; Atkinson, R.; Becker, K.H.; Kamens, R.; Seinfeld, J.; Wallington, T.; Yarwood, G. The Mechanisms of the Atmospheric Oxidation of Aromatic Hydrocarbons; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Derwent, R.G.; Jenkin, M.E.; Saunders, S.M.; Pilling, M.J. Photochemical Ozone Creation Potentials for Organic Compounds in Northwest Europe Calculated with a Master Chemical Mechanism. Atmos. Environ. 1998, 32, 2429–2441. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Derwent, R.G.; Wallington, T.J. Photochemical ozone creation potentials for volatile organic compounds: Rationalization and estimation. Atmos. Environ. 2017, 163, 128–137. [Google Scholar] [CrossRef]
- Wang, S.; Newland, M.J.; Deng, W.; Rickard, A.R.; Hamilton, J.F.; Muñoz, A.; Ródenas, M.; Vázquez, M.M.; Wang, L.; Wang, X. Aromatic Photo-oxidation, A New Source of Atmospheric Acidity. Environ. Sci. Technol. 2020, 54, 7798–7806. [Google Scholar] [CrossRef] [PubMed]
- Olariu, R.I.; Bejan, I.; Barnes, I.; Klotz, B.; Becker, K.H.; Wirtz, K. Rate Coefficients for the Gas-Phase Reaction of NO3 Radicals with Selected Dihydroxybenzenes. Int. J. Chem. Kin. 2004, 36, 577–583. [Google Scholar] [CrossRef]
- Pereira, K.L.; Hamilton, J.F.; Rickard, A.R.; Bloss, W.J.; Alam, M.S.; Camredon, M.; Ward, M.W.; Wyche, K.P.; Muñoz, A.; Vera, T.; et al. Insights into the Formation and Evolution of Individual Compounds in the Particulate Phase during Aromatic Photo-Oxidation. Environ. Sci. Technol. 2015, 49, 13168–13178. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, D.; Xiao, M.; Ye, Q.; Stolzenburg, D.; Hofbauer, V.; Ye, P.; Vogel, A.L.; Mauldin, R.L.; Amorim, A.; et al. Photo-oxidation of aromatic hydrocarbons produces low-volatility organic compounds. Environ. Sci. Technol. 2020, 54, 13, 7911–7921. [Google Scholar] [CrossRef]
- Bejan, I.; Abd El Aal, Y.; Barnes, I.; Benter, T.; Bohn, B.; Wiesen, P.; Kleffmann, J. The Photolysis of ortho-Nitrophenols: A new Gas Phase Source of HONO. Phys. Chem. Chem. Phys. 2006, 8, 2028–2035. [Google Scholar] [CrossRef]
- Bejan, I.; Barnes, I.; Olariu, R.; Zhou, S.; Wiesen, P.; Benter, T. Investigations on the gas-phase photolysis and OH radical kinetics of methyl-2-nitrophenols. Phys. Chem. Chem. Phys. 2007, 9, 5686–5692. [Google Scholar] [CrossRef]
- Samir, B.; Kalalian, C.; Roth, E.; Salghi, R.; Chakir, A. Gas-phase UV absorption spectra of pyrazine, pyrimidine and pyridazine. Chem. Phys. Lett. 2020, 751, 137469. [Google Scholar] [CrossRef]
- Yan, J.; Wang, X.; Gong, P.; Wang, C. Nitrated polycyclic aromatic compounds in the atmospheric environment: A review. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ravishankara, A.R. Heterogeneous and Multiphase Chemistry in the Troposphere. Science 1997, 276, 1058–1065. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic Aerosol and Global Climate Modelling: A Review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Martin-Reviejo, M.; Wirtz, K. Is Benzene a Precursor for Secondary Organic Aerosol? Environ. Sci. Technol. 2005, 39, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Nojima, K.; Fukaya, K.; Fukui, S.; Kanno, S. Studies on Photochemistry of Aromatic Hydrocarbons. II—The Formation of Nitrophenols and Nitrobenzene by the Photochemical Reaction of Benzene in the Presence of Nitrogen Monoxide. Chemosphere 1975, 2, 77–82. [Google Scholar] [CrossRef]
- Rippen, G.; Zietz, E.; Frank, R.; Knacker, T.; Klöpffer, W. Do Airborne Nitrophenols Contribute to Forest Decline? Environ. Technol. Lett. 1987, 8, 475–482. [Google Scholar] [CrossRef]
- Isayev, O.; Rasulev, B.; Gorb, L.; Leszczynski, J. Structure-toxicity Relationships of Nitroaromatic Compounds. Mol. Divers. 2006, 10, 233–245. [Google Scholar] [CrossRef]
- Grosjean, D. Atmospheric Fate of Toxic Aromatic Compounds. Sci. Total Environ. 1991, 100, 367–414. [Google Scholar] [CrossRef]
- Harrison, M.A.J.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated Phenols in the Atmosphere: A Review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Geissler, A.; Schöler, H.F. Gas Chromatographic Determination of Phenol, Methylphenols, Chlorophenols, Nitrophenols and Nitroquinones in Water. Wat. Res. 1994, 28, 2047–2053. [Google Scholar]
- Herterich, R. Gas Chromatographic Determination of Nitrophenols in Atmospheric Liquid Water and Airborne Particulates. J. Chromat. 1991, 549, 313–324. [Google Scholar] [CrossRef]
- Schüssler, W.; Nitschke, L. Nitrophenols in Precipitation. Chemosphere 2001, 42, 277–283. [Google Scholar] [CrossRef]
- Lüttke, J.; Levsen, K. Phase Partitioning of Phenol and Nitrophenols in Clouds. Atmos. Environ. 1997, 31, 2649–2655. [Google Scholar] [CrossRef]
- Voznakova, Z.; Podehradska, J.; Kohlickova, M. Determination of Nitrophenol in Soil. Chemosphere 1996, 33, 285–291. [Google Scholar] [CrossRef]
- Kawamura, K.; Kaplan, I.R. Biogenic and Anthropogenic Organic Compounds in Rain and Snow Samples Collected in Southern California. Atmos. Environ. 1986, 20, 115–124. [Google Scholar] [CrossRef]
- Tremp, J.; Mattrel, P.; Fingler, S.; Giger, W. Phenol and Nitrophenols as Tropospheric Pollutants: Emissions from Automobile Exhausts and Phase Transfer in the Atmosphere. Wat. Air Soil Pollut. 1993, 68, 113–123. [Google Scholar] [CrossRef]
- Grosjean, D. Reactions of o-Cresol and Nitrocresol with NOx in Sunlight and with Ozone Nitrogen Dioxide Mixtures in the Dark. Environ. Sci. Technol. 1985, 19, 968–974. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Arey, J. Reactions of OH and NO3 Radicals with Phenol, Cresols, and 2-Nitrophenol at 296 ± 2 K. Environ. Sci. Technol. 1992, 26, 1397–1403. [Google Scholar] [CrossRef]
- Bolzacchini, E.; Bruschi, M.; Hjorth, J.; Meinardi, S.; Orlandi, M.; Rindone, B.; Rosenbohm, E. Gas-Phase Reaction of Phenol with NO3. Environ. Sci. Technol. 2001, 35, 1791–1797. [Google Scholar] [CrossRef]
- Olariu, R.I.; Klotz, B.; Barnes, I.; Becker, K.H.; Mocanu, R. FT-IR Study of the Ring-Retaining Products from the Reaction of OH Radicals with Phenol, o-, m- and p-Cresol. Atmos. Environ. 2002, 36, 3685–3697. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 136760. [Google Scholar] [CrossRef] [PubMed]
- Hidy, G.M. Atmospheric Chemistry in a Box or a Bag. Atmosphere 2019, 10, 401. [Google Scholar] [CrossRef]
- Olariu, R.I.; Barnes, I.; Bejan, I.; Arsene, C.; Vione, D.; Klotz, B.; Becker, K.H. FT-IR product study of the reactions of NO3 radicals with ortho-, meta- and para-cresol. Environ. Sci. Technol. 2013, 47, 7729–7738. [Google Scholar] [CrossRef] [PubMed]
- Alif, A.; Pilichowski, J.-F.; Boule, P. Photochemistry and Environment XIII: Phototransformation of 2-Nitrophenol in Aqueous Solution. J. Photochem. Photobiol. A Chem. 1991, 59, 209–219. [Google Scholar] [CrossRef]
- Chen, B.; Chun, Y.; Khang, G.N. Direct Photolysis of Nitroaromatic Compounds in Aqueous Solutions. J. Environ. Sci.—China 2005, 17, 598–604. [Google Scholar]
- Bardini, P. Atmospheric Oxidation of Dimethylphenols and Nitrophenols. Ph.D. Thesis, University of Cork, Cork, Ireland, 2005. [Google Scholar]
- Zádor, J.; Turányi, T.; Wirtz, K.; Pilling, M.J. Measurement and investigation of chamber radical sources in the European Photoreactor (EUPHORE). J. Atmos. Chem. 2006, 55, 147–166. [Google Scholar] [CrossRef]
- Barnes, I.; Becker, K.H.; Mihalopoulos, N. An FT-IR Product Study of the Photo-Oxidation of Dimethyl Disulfide. J. Atmos. Chem. 1994, 18, 267–289. [Google Scholar] [CrossRef]
- Atkinson, R. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Odum, J.R.; Hoffmann, T.; Bowman, F.; Collins, D.; Flagan, R.C.; Seinfeld, J.F. Gas/Particle Partitioning and Secondary Organic Aerosol Yields. Environ. Sci. Technol. 1996, 30, 2580–2585. [Google Scholar] [CrossRef]
- Nagaya, M.; Kudoh, S.; Nakata, M. Infrared Spectrum and Structure of aci-nitro Form of 2-Nitrophenol in a Low-temperature Argon Matrix. Chem. Phys. Lett. 2006, 427, 67–71. [Google Scholar] [CrossRef]
- Healy, R.M.; Chen, Y.; Kourtchev, I.; Kalberer, K.; O’Shea, D.; Wenger, J.C. Rapid Formation of Secondary Organic Aerosol from the Photolysis of 1‑Nitronaphthalene: Role of Naphthoxy Radical Self-reaction. Environ. Sci. Technol. 2012, 46, 11813–11820. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary Organic Aerosol Formation from Isoprene Photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Murphy, S.M.; Kroll, J.H.; Ng, N.L.; Hildebrandt, L.; Sorooshian, A.; Szmigielski, R.; Vermeylen, R.; Maenhaut, W.; Claeys, M.; et al. Chemical Composition of Secondary Organic Aerosol Formed from the Photooxidation of Isoprene. J. Phys. Chem. A 2006, 110, 9665–9690. [Google Scholar] [CrossRef] [PubMed]
- Bejan, I.; Schürmann, A.; Barnes, I.; Benter, T. Kinetics of the gas-phase reactions of OH radicals with a series of trimethylphenols. Int. J. Chem. Kinet. 2012, 44, 117–124. [Google Scholar] [CrossRef]
- Docherty, K.S.; Ziemann, P.J. Effects of Stabilized Criegee Intermediate and OH Radical Scavengers on Aerosol Formation from Reactions of beta-Pinene with O3. Aerosol Sci. Technol. 2003, 37, 877–891. [Google Scholar] [CrossRef]
- Keywood, M.D.; Kroll, J.H.; Varutbangkul, V.; Bahreini, R.; Flagan, R.C.; Seinfeld, J.H. Secondary Organic Aerosol Formation from Cyclohexene Ozonolysis: Effect of OH Scavenger and the Role of Radical Chemistry. Environ. Sci. Technol. 2004, 38, 3343–3350. [Google Scholar] [CrossRef]
- Volkamer, R.; Klotz, B.; Barnes, I.; Imamura, T.; Wirtz, K.; Washida, N.; Becker, K.H.; Platt, U. OH-Initiated Oxidation of Benzene. Part I. Phenol Formation under Atmospheric Conditions. Phys. Chem. Chem. Phys. 2002, 4, 1598–1610. [Google Scholar] [CrossRef]
- Hurley, M.D.; Sokolov, O.; Wallington, T.J.; Takekawa, H.; Karasawa, M.; Klotz, B.; Barnes, I.; Becker, K.H. Organic aerosol formation during the atmospheric degradation of toluene. Environ. Sci. Technol. 2001, 35, 1358–1366. [Google Scholar] [CrossRef]
- Song, C.; Na, K.; Cocker, D.R. Impact of the Hydrocarbon to NOx Ratio on Secondary Organic Aerosol Formation. Environ. Sci. Technol. 2005, 39, 3143–3149. [Google Scholar] [CrossRef]
- Tsiligiannis, E.; Hammes, J.; Salvador, C.M.; Mentel, T.F.; Hallquist, M. Effect of NOx on 1,3,5-trimethylbenzene (TMB) oxidation product distribution and particle formation. Atmos. Chem. Phys. 2019, 19, 15073–15086. [Google Scholar] [CrossRef]
- Derpmann, V.; Mueller, D.; Bejan, I.; Sonderfeld, H.; Wilberscheid, S.; Koppmann, R.; Brockmann, K.J.; Benter, T. Capillary atmospheric pressure electron capture ionization (cAPECI): A highly efficient ionization method for nitroaromatic compounds. J. Am. Soc. Mass Spectrom. 2014, 25, 329–342. [Google Scholar] [CrossRef] [PubMed]
| Experiments Name | 2-Nitrophenol [ppmv] | Aerosol Yield [%] | Wall Loss Rate [×105 s−1] | Photolysis Rate [×105 s−1] | Exp. Conditions |
|---|---|---|---|---|---|
| 100305 | 0.80 | 22.2 ± 1.15 | 7.3 ± 0.22 | 10.3 ± 0.49 | |
| 111005 | 0.49 | 21.3 ± 0.98 | 18.4 ± 1.53 | 9.3 ± 0.44 | |
| 061105 | 1.23 | 19.6 ± 1.64 | 9.8 ± 0.91 | 8.4 ± 0.60 | |
| 110305 | 1.12 | 6.9 ± 1.00 | 7.3 ± 0.45 | 5.8 ± 0.29 | scav. 1 |
| 120305 | 0.66 | 2.9 ± 0.44 | 7.4 ± 2.60 | 7.0 ± 0.30 | scav. 1 |
| 180305 | 1.14 | 7.5 ± 2.10 | 8.8 ± 0.72 | 4.6 ± 0.10 | scav. 1 |
| 060705_1 | 0.70 | - 2 | 8.2 ± 0.43 | 7.3 ± 0.78 | NOx |
| 060705_2 | 0.92 | - 2 | 9.0 ± 0.68 | 6.5 ± 0.24 | NOx |
| 070705 | 0.61 | - 2 | 11.0 ± 1.44 | 9.7 ± 0.58 | NOx |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejan, I.G.; Olariu, R.-I.; Wiesen, P. Secondary Organic Aerosol Formation from Nitrophenols Photolysis under Atmospheric Conditions. Atmosphere 2020, 11, 1346. https://doi.org/10.3390/atmos11121346
Bejan IG, Olariu R-I, Wiesen P. Secondary Organic Aerosol Formation from Nitrophenols Photolysis under Atmospheric Conditions. Atmosphere. 2020; 11(12):1346. https://doi.org/10.3390/atmos11121346
Chicago/Turabian StyleBejan, Iustinian Gabriel, Romeo-Iulian Olariu, and Peter Wiesen. 2020. "Secondary Organic Aerosol Formation from Nitrophenols Photolysis under Atmospheric Conditions" Atmosphere 11, no. 12: 1346. https://doi.org/10.3390/atmos11121346
APA StyleBejan, I. G., Olariu, R.-I., & Wiesen, P. (2020). Secondary Organic Aerosol Formation from Nitrophenols Photolysis under Atmospheric Conditions. Atmosphere, 11(12), 1346. https://doi.org/10.3390/atmos11121346







