3.1. Gas-Phase Products
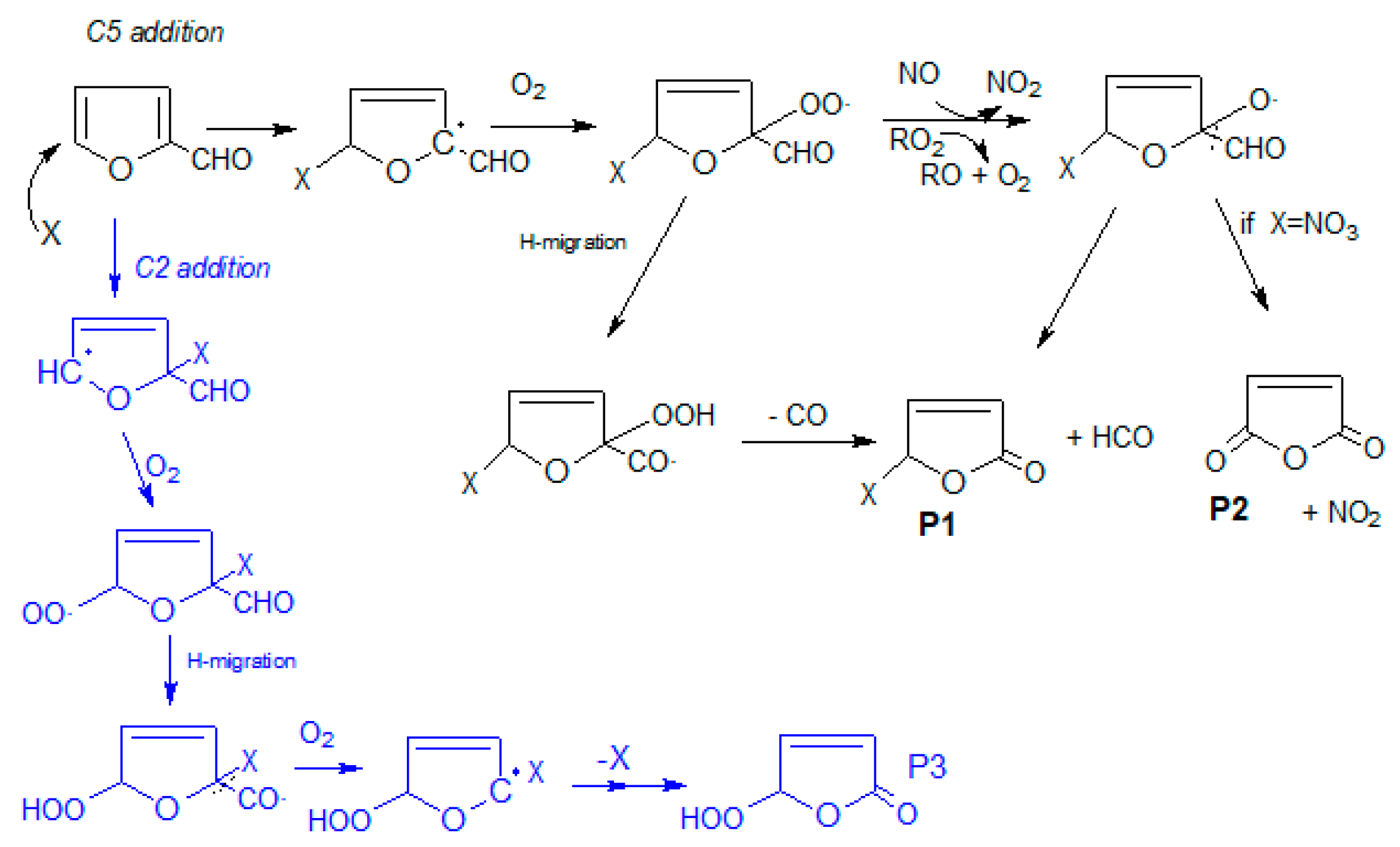
In order to facilitate the identification of the generated products, a general mechanism has been proposed for hydrogen abstraction and double bond addition. The presence of the –CHO group in C2 leads to the deactivation of the C3 and C5 positions of the aromatic ring due to its negative inductive effect. However, according to the number of resonance forms generated by the attack of the oxidant X (Cl, OH or NO
3;
Scheme S1, Supplementary Materials), the most favored positions seem to be C2 and C5. This assumption was confirmed by theoretical calculations [
9,
10]. Of these two positions, C2 and C5, the first is hindered due to the presence of the carbonyl group in the same position. As a consequence, it is expected that the C5 attack will be preferred. Similar double bond addition mechanisms have been proposed for the reactivity of alkylfurans [
1,
17]. Moreover, the furfural can react by aldehydic hydrogen abstraction but, according to the bibliographic data, the abstraction of hydrogen from the aromatic ring is less likely [
18]. This behavior has also been described in previous theoretical works regarding the reactivity of furfural with OH [
10] and NO
3 [
9]. According to these works, a similar reaction mechanism for the reactions with chlorine atoms has been proposed (see
Scheme 1).
The formation of maleic anhydride (P2) and 5-chloro-2-(5H)-furanone (P1) as reaction products was evidenced by GC–TOFMS. The presence of maleic anhydride was confirmed by comparing the retention time and mass spectrum with those of the commercial compound. Furthermore, 5-chloro-2-(5H)-furanone is not commercially available so its formation was confirmed by comparison with the EI mass spectrum obtained in previous works [
17]. The formation of this compound was also observed in the experiments performed by FTIR spectroscopy. An example of the IR bands due to the reaction products generated in the reaction of furfural with Cl atoms is shown in
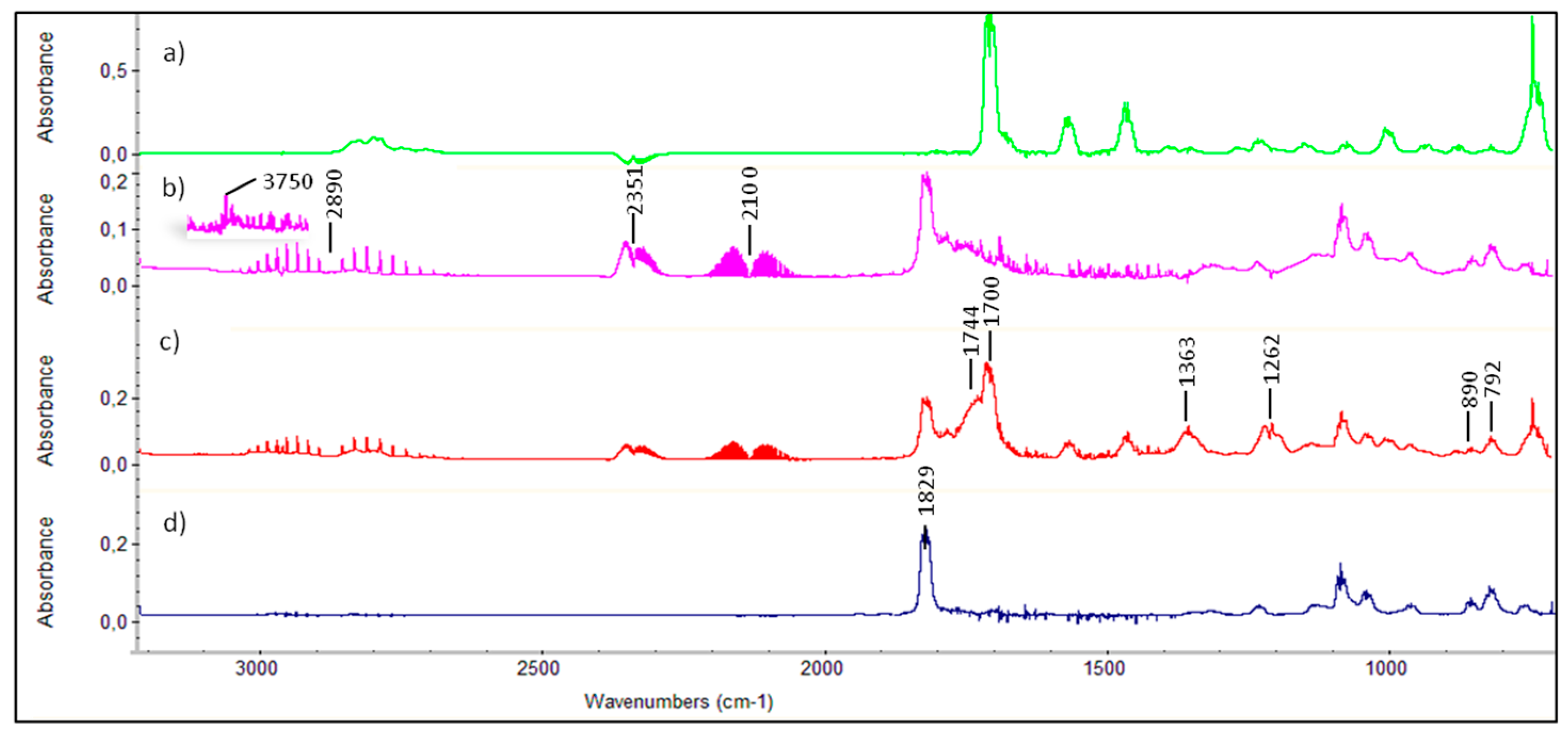
Figure 1.
The bands centered at 2890, 2351 and 2100 cm
−1 (
Figure 1b) are assigned to HCl, CO
2 and CO, respectively. CO may be generated by the decarboxylation of the CHO group and HCl is a coproduct of the hydrogen abstraction process. However, the concentrations of these compounds must be treated with caution since they are also present in the chamber when air and Cl
2 are exposed to visible light. The absorption band at 1829 cm
−1 is assigned to 5-chloro-2-(5
H)-furanone, which has been proposed as a product from the C5 addition. This type of furanone has also been detected in previous works as a product of the reaction of alkylfurans with Cl atoms [
17]. There is a very good agreement between the residual product spectrum and the bibliographic spectra of 5-chloro-2(5
H)-furanone [
17] (
Figure 1d), which strongly supports the finding that the residual FTIR features are mainly attributable to 5-chloro-2(5
H)-furanone.
In the residual product spectrum, the formation of maleic anhydride was not observed, possibly due to overlap with the chlorofuranone bands (
Supplementary Materials Figure S2). However, the formation of a broad band at around 1797–1760 cm
−1 suggests the presence of one or more compounds with a lactone-like structure. This fact, along with the band observed at 3750 cm
−1 (νO–H), could indicate the formation of 5-hydroperoxy-2(5H)-furanone (P3), which could be generated from the chlorine addition in C2—as proposed in the literature for NO
3 [
9] and OH [
10] radicals (blue route in
Scheme 1). In the latter case, ring-opening products have also been theoretically proposed in the literature, although this route is only expected to be minor. Moreover, maleic anhydride, which was detected in our experiments, can also be formed in the H abstraction route (
Scheme 2), albeit to a lesser extent.
Product formation for the reaction of furfural with chlorine atoms in the presence of NO was also studied. Two analogous mechanisms have been proposed by considering the reaction with NOx. The same products were detected as in the absence of NOx. Besides, the presence of NOx leads to the formation of nitrate and peroxyacylnitrate products.
The formation of 5-chloro-2(5
H)-furanone is evidenced in
Figure 1c along with sets of IR bands at 792, 1262 and 1744 cm
−1 and 890, 1163 and 1700 cm
−1, which are absorption patterns attributed to –OONO
2 and –ONO
2 groups, respectively. Moreover, the band at 1798 cm
−1 may be due to the presence of a substituted lactone and the band at 1843 cm
−1 may be due to the presence of a carbonyl group of a nitrate compound.
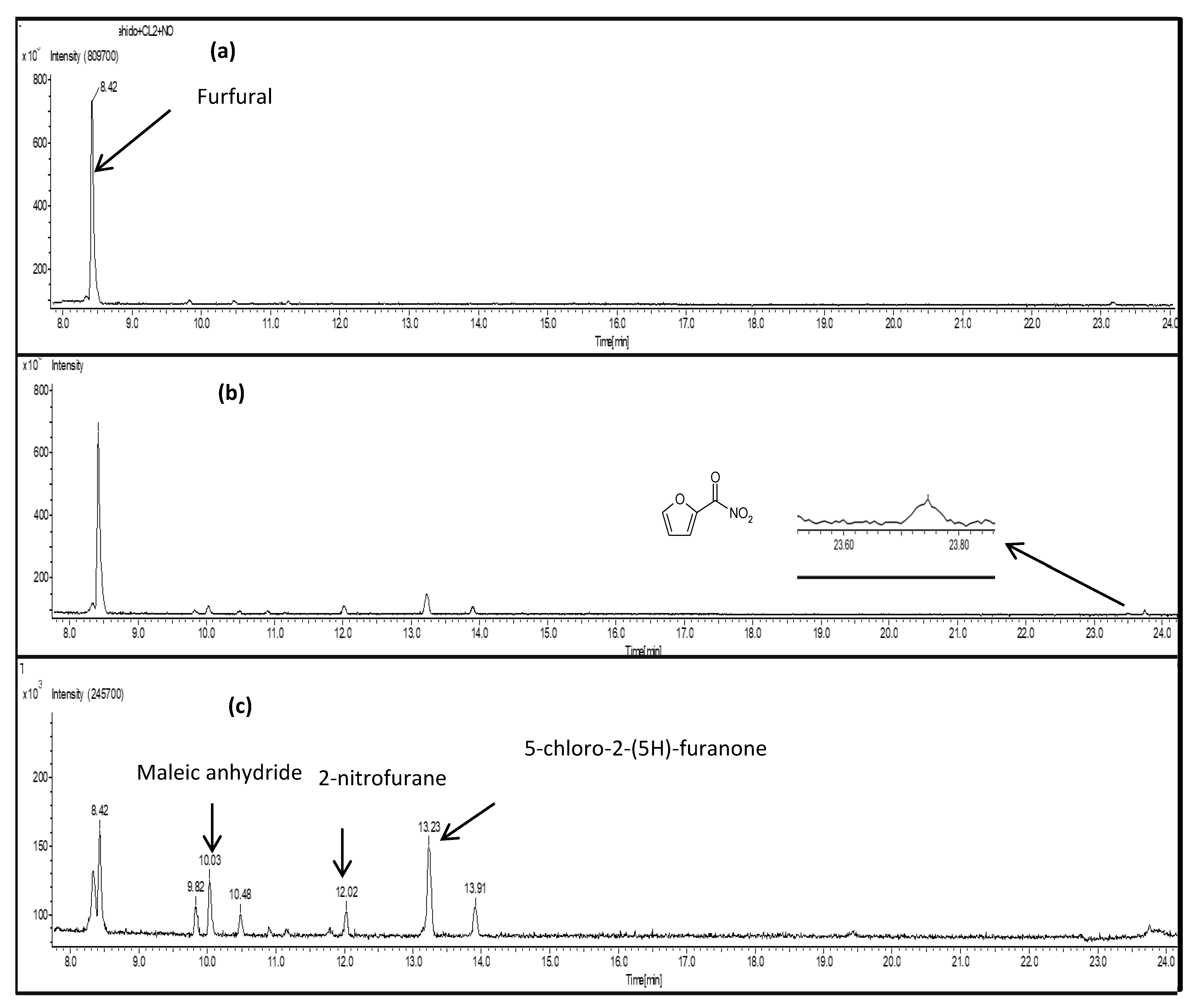
For GC–TOFMS experiments, four product peaks were observed at 10.03, 12.02, 13.23 and 23.73 min in the gas chromatogram (see
Figure 2). Peaks at 10.03 and 13.23 min were assigned to maleic anhydride and 5-chloro-2-(5
H)-furanone (P1), respectively. The peak at 12.02 min was identified as being due to 2-nitrofuran. The assignment was confirmed by comparing the retention times and mass spectra with those of commercial samples: 2-nitrofuran and maleic anhydride. For the identification of 5-chloro-2-(5
H)-furanone, the IR and MS spectra obtained for the synthesized products in our previous work were employed [
17].
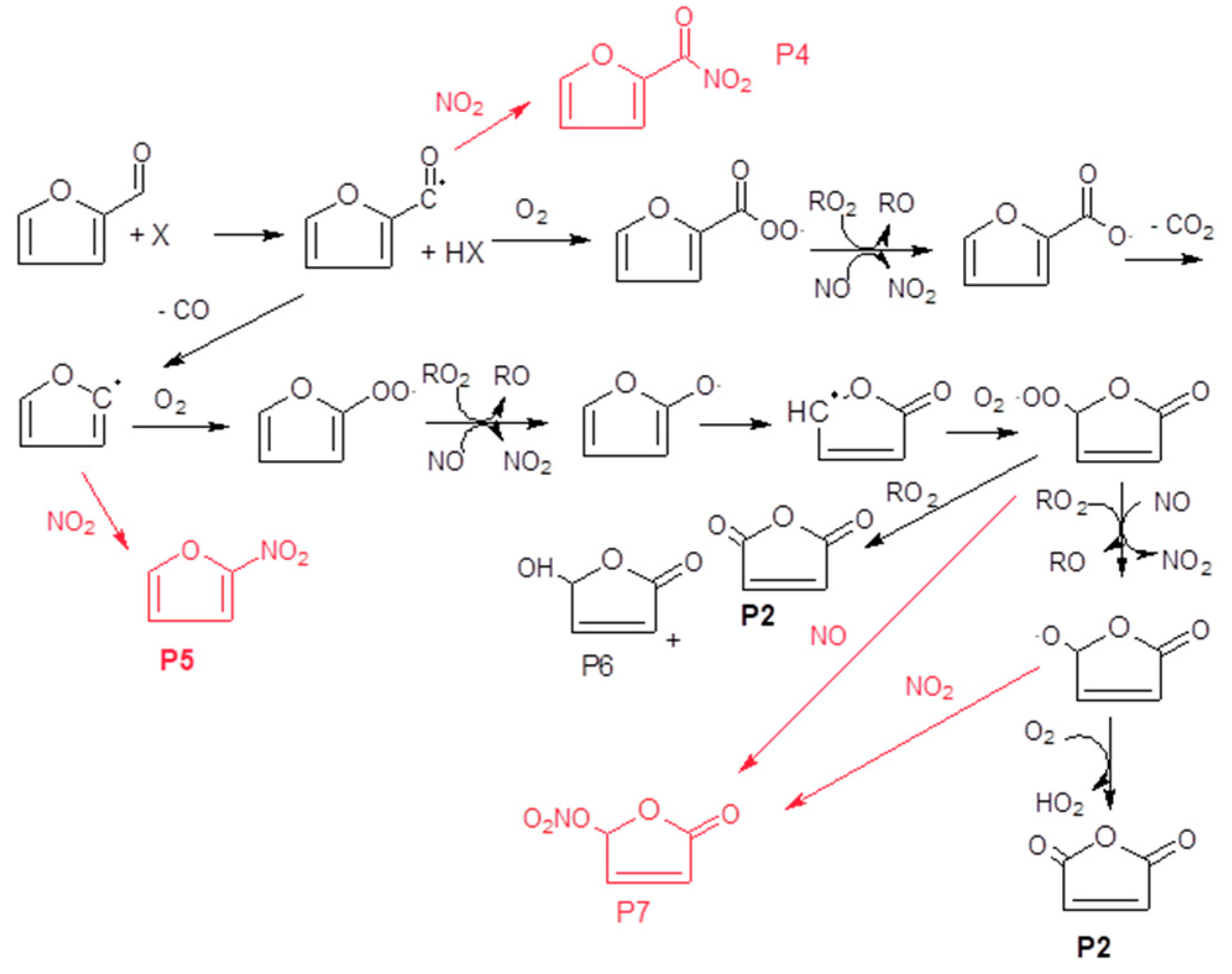
Matches were not found in the database for the peak observed at 23.73 min, but according to the FTIR data, one would expect the formation of a nitrate compound containing a carbonyl group. Therefore, taking in account the FTIR data, the
m/
z value (141) and pattern observed (
Figure S2), this peak is tentatively assigned to 4-nitrooxidanyl-γ-crotonlactone (P4,
Scheme 2). However, the presence of this product was not confirmed because it is not commercially available.
The formation of 2-furylnitroketone and 2-nitrofuran (P5) can be explained by the reaction between the intermediate radicals generated and NO
2, which is present in the NOx mixture (red route in
Scheme 2). These radicals react slowly with O
2 since it is a slightly exothermic and highly reversible reaction, but, alternatively, they would react with other species such as O
3, NO
2 and RO
2 [
10].
Analogous mechanisms were proposed for the reaction of furfural with OH radicals. According to the literature, 2-oxo-3-pentene–1,5-dialdehyde, 5-hydroxy-2-(5
H)-furanone, 4–oxo-2-butenoic acid and maleic anhydride are the main products expected for the OH oxidation of furfural [
10]. From this list of proposed products, only the formation of maleic anhydride was observed in our experiments by GC–TOFMS. The FTIR spectra are in this case more difficult to analyze due to the overlap of the OH precursors employed (CH
3ONO + NO) and the decomposition products (formaldehyde, CO and NO
2). However, the IR bands observed in the product spectra after 30 min of photolysis (see
Figure S3, Supplementary Materials) have been associated with the presence of –ONO
2 and –OONO
2 groups, as described previously. These bands may correspond to the products P4 and P5, which may be formed when certain levels of NOx are present in the gas mixture.
In the case of the reaction of furfural with NO3, the precursor employed (N2O5) decomposes into NO2 and nitric acid (HNO3). Assuming a reaction mechanism similar to that proposed for the reaction with chlorine atoms and OH radicals, one would expect the formation of the addition product 5-nitrate-2-(5H)-furanone (P1), maleic anhydride (P2) and other nitrate products.
In GC–TOFMS experiments, similar results were obtained as in the case of the reaction with chlorine atoms in the presence of NOx. Three products were identified by gas chromatography after 3.5 h of reaction: maleic anhydride, 2-nitrofuran and 2-furylnitroketone (not confirmed).
For FTIR experiments, after 60 min of reaction, the typical IR bands of –ONO
2 (852, 1288 and 1666 cm
−1) and –OONO
2 (792, 1030 and 1726 cm
−1) groups were observed (
Figure S4, Supplementary Materials). Once again, the band at 1805 cm
−1 can be assigned to the presence of a substituted lactone (in this case 5-hydroxy-2(5
H)-furanone (P6), maleic anhydride (P2), or 5-nitrate-2-(5
H)-furanone (P1)). The band at 1840 cm
−1 may be due to the presence of another C=O group of a nitrate compound, which could be assigned to the formation of 2-furylcetone mentioned above.
The formation of 5-nitrate-2-(5
H)-furanone and maleic anhydride have also been suggested in the literature [
9]. The IR absorption bands observed are also consistent with the formation of 5-hydroperoxy-2-(5
H)-furanone and 3-nitrate-2-hydroperoxy–3(2
H)-furanone, which were theoretically proposed in work by Huang et al. [
9]. Once again, the presence of these products was not confirmed because they are not commercially available.
The quantification of the identified products was only possible in certain cases. The product concentrations were estimated using calibration curves obtained previously by introducing into the reactor a set of samples of known concentration.
For some products, such as 5-chloro-2(5
H)-furanone and NO
2, the concentration profiles were obtained using IR absorption cross-sections found in the literature [
19]. In the case of 5-chloro-2-(5
H)-furanone, the concentration was estimated using the IR absorption coefficient of the 2-(5
H)-furanone [
19] because of their structural similarity and since the former is not commercially available. A value for this coefficient of (1.65 ± 0.27) × 10
−3 ppm V
−1 m
−1 for the band of 1811 cm
−1 was used. For NO
2, a value of (1.46 ± 0.04) × 10
−3 ppm V
−1 m
−1 for the band at 1630 cm
−1 was taken for the quantitative analysis. In addition to these two products, the concentrations of maleic anhydride, CO, HCl, HNO
3 and 2-nitrofuran were obtained. An example of the concentration–time profiles for the reaction of Cl atoms and furfural is shown in
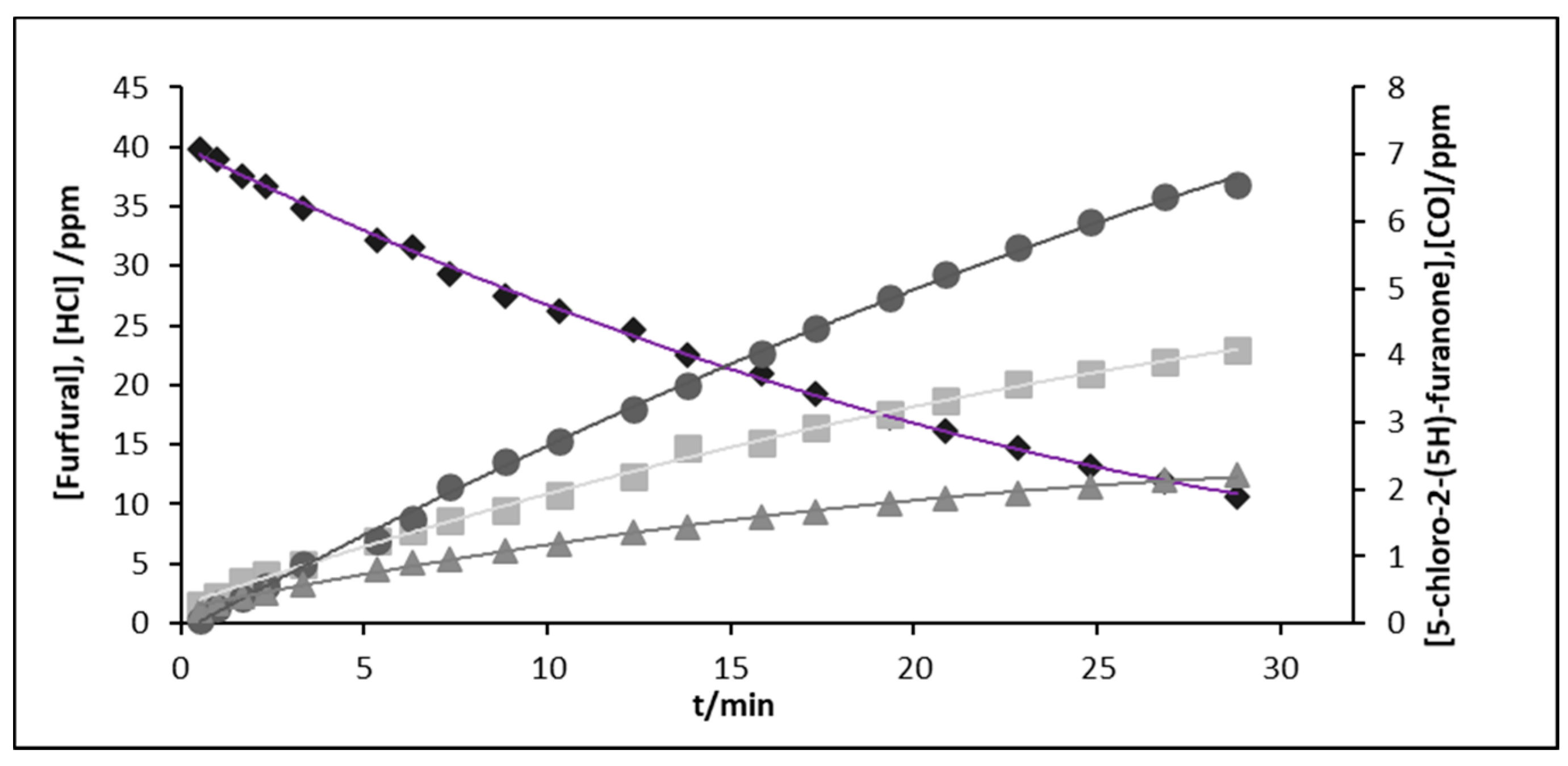
Figure 3.
The profiles are typical of primary products that undergo a progressive increase in the concentration from the early stages of the reaction. Similar time–concentration profiles were obtained for 5-chloro-2-(5
H)-furanone, CO and HCl in the case of the reaction of furfural with chlorine atoms in the presence of NOx. In the cases of maleic anhydride and 2-nitrofuran, the curves obtained after 4–6 min of reaction and using GC–MS/TOF suggest the loss of these products through secondary reactions (
Figure S5, Supplementary Materials). As mentioned before, chlorofuranone has a typical primary product profile while the nitro-2-furylketone (P7) has a secondary product profile (
Figure S6, Supplementary Materials). This may indicate that high levels of NO
2 are required for the generation of this product (P7).
The yields of the primary products were obtained from the slopes of plots of the amounts of reaction product formed versus the amounts of consumed furfural. The product yields obtained for the reaction of furfural with different oxidants are provided in
Table 1.
For the reactions of furfural with chlorine atoms in the absence and presence of NOx, the molar yields obtained for 5-chloro-2-(5H)-furanone were similar for both reactions, although the levels of HCl and CO quantified were lower for the reaction in the presence of NOx. Yields of HCl and CO are overestimated due to wall secondary reactions and traces of humidity.
For the reaction of furfural with the OH radical, only the levels of CO (using FTIR) and maleic anhydride (using GC–MS/TOF) were confirmed. Both products lead to primary concentration–time profiles, as in the case of chlorine reactions. In this case, the yield obtained for the anhydride was slightly higher than that obtained for the reaction with chlorine atoms.
For the reaction with NO
3 radicals, only the levels of NO
2, CO and HNO
3 were confirmed by FTIR analysis. A progressive increase in the concentration of NO
2 during the reaction can be seen (
Figure S7 Supplementary Materials), but this began to decay over time as a result of secondary reactions. The high concentrations detected initially are attributable to the thermal decomposition of the precursor (N
2O
5). The yield was estimated to be (78.3 ± 6.6)%. In this case, the high levels of NO
2 detected could be of great importance since it can react with the reaction intermediates generated. The concentration profiles for CO and HNO
3 are more or less constant, with a slight decay observed for the latter throughout the reaction. This trend can be attributed to the deposition of nitric acid on the reactor walls. However, HNO
3 levels are difficult to evaluate since, as mentioned before, they are strongly influenced by the existence of secondary reactions. Molar yields of (58.6 ± 8.6)% and (20 ± 3)% for 2-nitrofuran and maleic anhydride, respectively, were quantified by GC–TOFMS. As can be seen from the results in
Table 1, this yield is considerably higher than in the case of the other oxidants, which suggests in this case an extra route for the generation of maleic anhydride (
Scheme 1). As mentioned above, the curve contours observed for maleic anhydride after 2 h of reaction suggest loss due to secondary reactions. The molar yield for the 2-furylnitroketone could not be obtained since it is not commercially available.
The studied oxidants led to the formation of the common product maleic anhydride. This compound may be generated by hydrogen abstraction of the –CHO group (via I), since the formation of its coproduct (5-hydroxy-2-(5
H)-furanone) via II has not been confirmed. For reactions with chlorine atoms and the OH radical, the yield for maleic anhydride obtained is between 6 and 9%. However, this yield is underestimated because it is suspected that this compound undergoes a photolysis process [
20]. However, in the case of the nitrate radical, as mentioned above, the anhydride has an extra contribution by the additional pathway. Its yield (~20%) is approximately 2–3 times greater than for other reactions. The results obtained in this study confirm that for reactions with chlorine atoms and the OH and NO
3 radicals, furfural reacts by both mechanisms, namely C5 addition and aldehydic hydrogen abstraction. According to the estimated yield for 2-nitrofuran, it is believed that the abstraction process occurs to a greater extent for nitrate radicals. The reaction with NO
3 radicals led to the formation of various nitrated products such as 2-nitrofuran (P5) and unconfirmed products such nitro-2-furylketone (P4), which are common products to those obtained in the reaction with chlorine atoms in the presence of NOx. This may be due to high levels of NO
2, which make furfural react by following a parallel path (marked in red in the proposed reaction mechanism). This behavior was not observed with the OH radical, where the concentrations of NO
2 due to the decomposition of methylnitrite employed as the precursor is lower.
3.2. SOA Size Distributions
The few products identified in the gas-phase study and their low yields lead us to believe that some of the reaction products generated are not being observed in the gas-phase. This may be because many of the furfural reaction products are in a condensed phase, and therefore, they are not being correctly sampled. For this purpose, a series of experiments was performed under atmospheric pressure and room temperature (similar experimental conditions to those used in the products’ experiments) to study the formation of aerosols in the reaction of furfural with Cl, OH and O3. The same set of experiments was performed to study the reaction with NO3, but particle formation was not observed.
The decay of furfural versus the variation of SOA mass (M
0) for the reaction with chlorine atoms is represented in
Figure 4. The steady state for aerosol production was observed after 30 min, with a maximum mass of around 9 × 10
2 µg m
−3 for the reaction with chlorine atoms. A typical concentration profile, as described in the literature [
21], was observed in this experiment, thus suggesting a strong relationship between the concentration of furfural and aerosol generation.
As SOA wall loss was observed, the aerosol mass concentration (M0) was corrected by using the wall deposition coefficient rate obtained in this work (kw = (2.95 ± 0.46) × 10−5 s−1).
A significant amount of particulate matter, i.e., between 60 and 950 µg m−3, was observed in the oxidation of furfural with chlorine atoms, OH radicals and ozone at the stationary state. This state is rapidly reached after 30 min for the chlorine and ozone reactions and after 5 min for the OH reaction. The furfural concentration was monitored by offline SPME sampling and analyzed by GC–TOFMS. The fiber was exposed in the reactor during 60 s and then thermally desorbed during 15 min at 250 °C in the injection port of the gas chromatograph. The reactor was filled with purified air, a quantity of reactant (2.5–3.5 µg m−3) and oxidant (~2.5 × 1014 molecule cm−3). For the study with chlorine atoms and OH radicals, six visible lamps were employed to start the reaction. Ozone was obtained from an ozonizer as mentioned above. Prior tests were performed to confirm the absence of particle generation from the exposure of furfural to light.
All of the experiments were carried out in the absence of inorganic seed aerosol. The initial concentrations employed for the oxidant ([Ox.]0) and furfural or reactive organic gas ([ROG]0) are listed in
Table 2 together with the reacted furfural (Δ[ROG]) and aerosol yield, Y. The overall organic aerosol yield was determined by employing Equation (1):
where M
0 is the organic aerosol mass concentration and ∆[ROG] is the reacted furfural, both in µg m
−3.
It can be seen from the results that atmospheric degradation of furfural leads to the formation of low vapor pressure products (particulate matter), as previously proposed in the gas-phase study.
The yield of the reaction with chlorine atoms is between 6 to 10 times greater than in the cases of ozone and OH. The particle size obtained is also larger for the reaction with chlorine (diameters 107–523 nm) and this suggests that the reaction of furfural with this oxidant leads to a more effective formation of aerosols.
In the reaction of furfural with the OH radical, particles were formed by irradiation of the precursor mixture (CH3ONO and NO) with visible lamps in air. The concentration and particle size observed under the same experimental conditions were lower than those obtained in the reaction with chlorine atoms. In this reaction, due to the formation of preexisting ultrafine particles from the degradation of the OH precursor, it is believed that these act as condensation nuclei that promote the growth of the particles in this process. However, the existence of a nucleation process cannot be dismissed.
For the study of the SOA formation in the reaction of furfural with ozone, several individual additions of a small quantity of an O
2/O
3 mixture were carried out, but only after the addition of a large excess of O
3, and particle formation was observed. A similar evolution of the size distribution as in the case of chlorine atoms was observed. The SOA distribution for the reaction of furfural with chlorine atoms is shown in
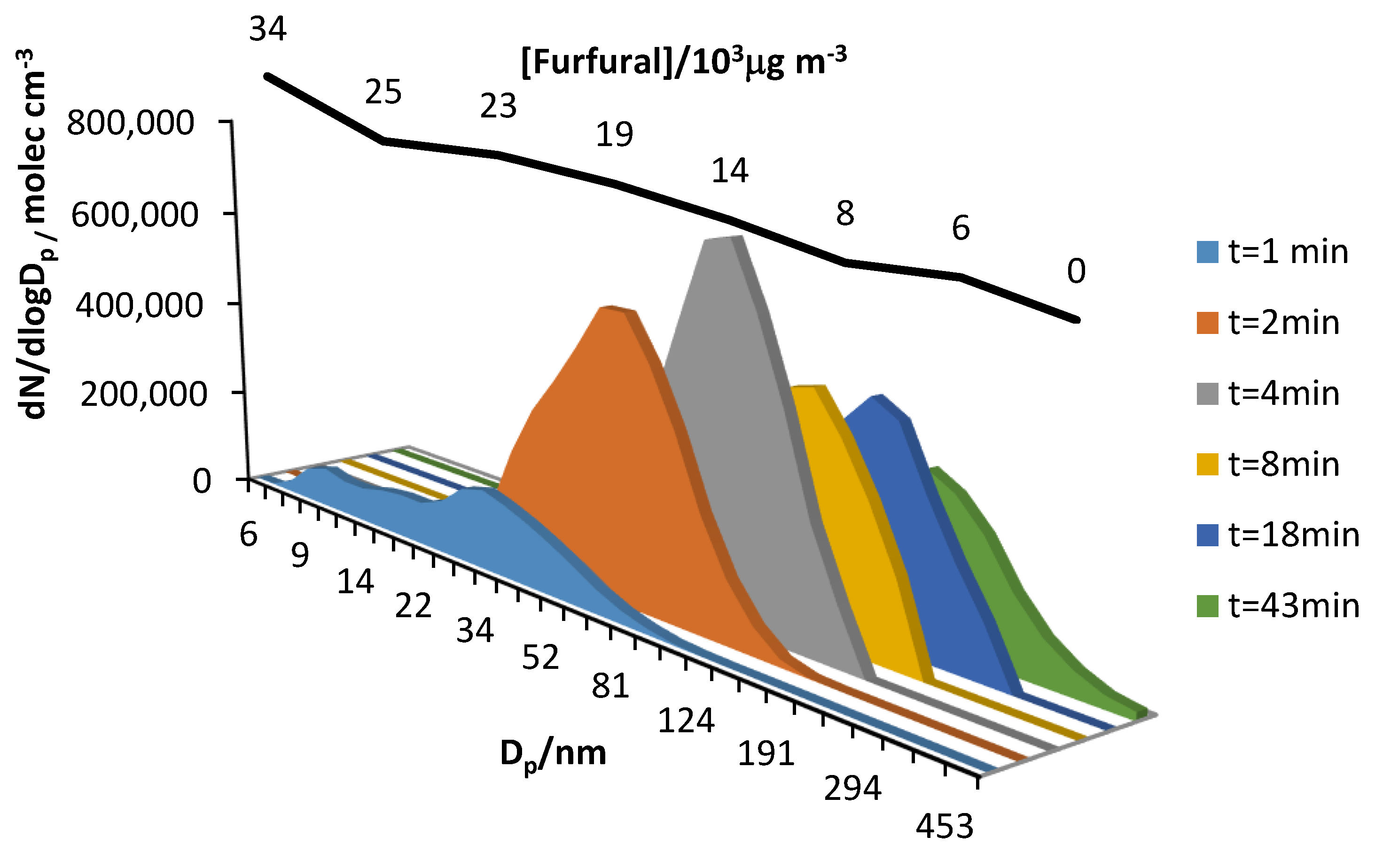
Figure 5.
A set of experiments at different furfural/oxidant concentrations were carried out in order to study its influence of this parameter on the aerosol yield for the reaction with chlorine atoms. The results are summarized in
Table 3.
In most experiments, aerosol yields in the 5–7% range were obtained. The lowest aerosol yield was obtained in Experiment 5, in which a concentration ratio <1 was employed. Reaction 2, with a ratio close to one, also gave a low value of Y.
The aerosol yields obtained in this work for the photoxidation and ozonolysis of furfural are low, but these values are of the same order of magnitude as those reported in the literature for isoprene [
22,
23] and pinene [
24] oxidations. Nevertheless, these values may represent lower limits.

 t = 1 min,
t = 1 min,  t = 2 min,
t = 2 min,  t = 4 min,
t = 4 min,  t = 8 min,
t = 8 min,  t = 18 min,
t = 18 min,  t = 43 min. Variation of furfural concentration during the reaction. Solid line
t = 43 min. Variation of furfural concentration during the reaction. Solid line  .
.
 t = 1 min,
t = 1 min,  t = 2 min,
t = 2 min,  t = 4 min,
t = 4 min,  t = 8 min,
t = 8 min,  t = 18 min,
t = 18 min,  t = 43 min. Variation of furfural concentration during the reaction. Solid line
t = 43 min. Variation of furfural concentration during the reaction. Solid line  .
.