Characteristics and Sources of Water-Soluble Ions in PM2.5 in the Sichuan Basin, China
Abstract
1. Introduction
2. Experiments
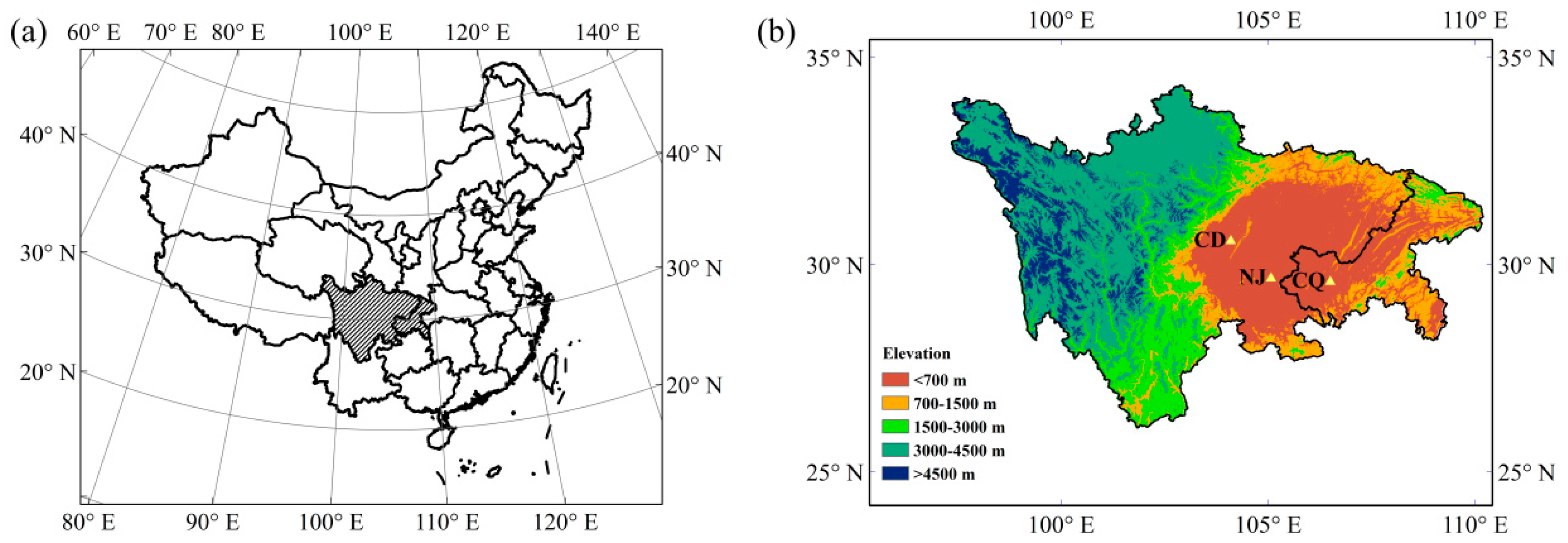
2.1. Site Description and Field Sampling
2.2. Water-Soluble Ion Measurement
3. Results and Discussion
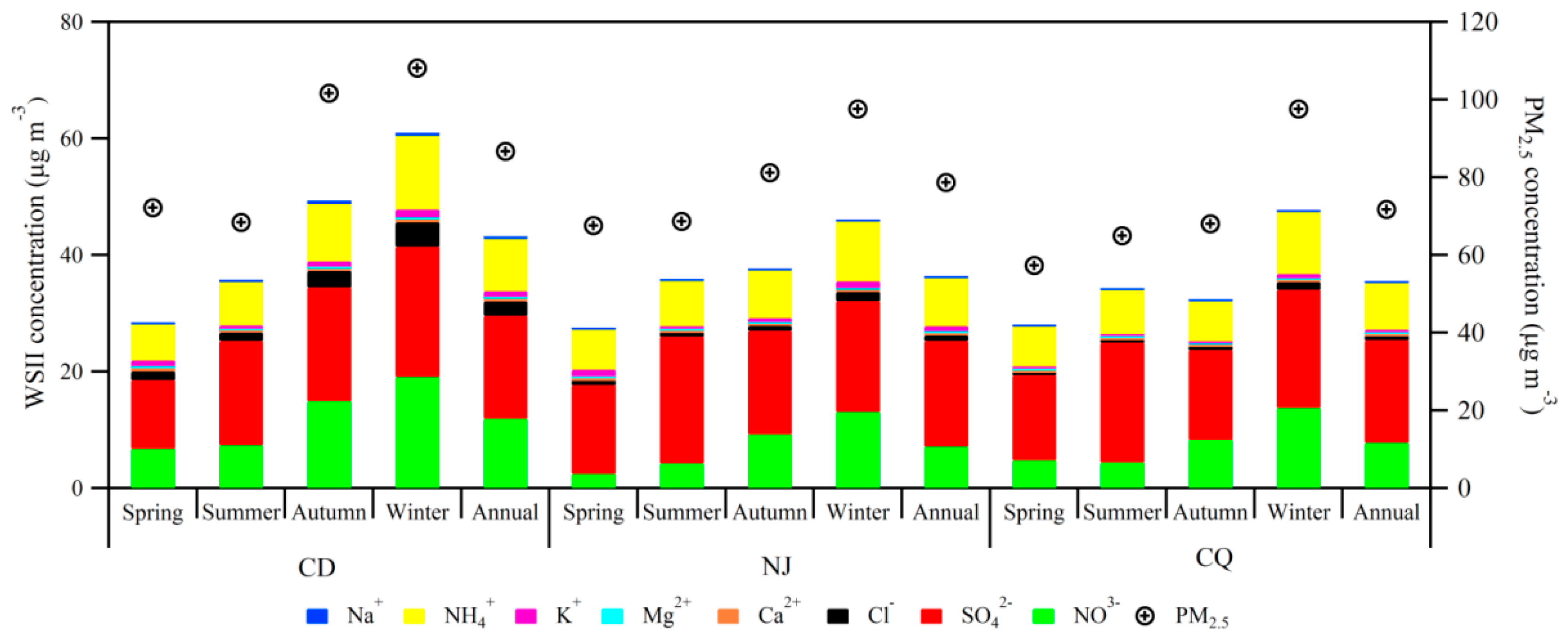
3.1. Concentrations of PM10, PM2.5, and WSIIs
3.2. Seasonal Variations of PM2.5 and WSIIs
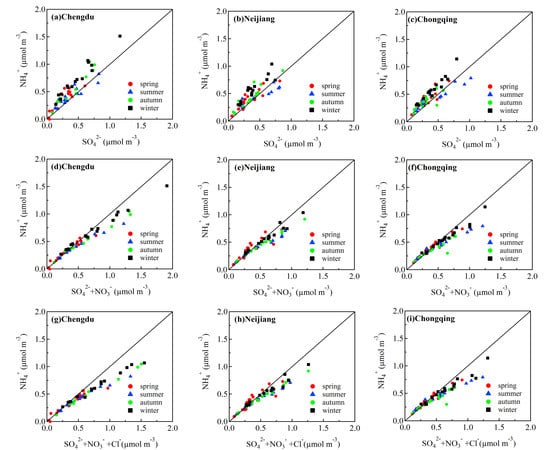
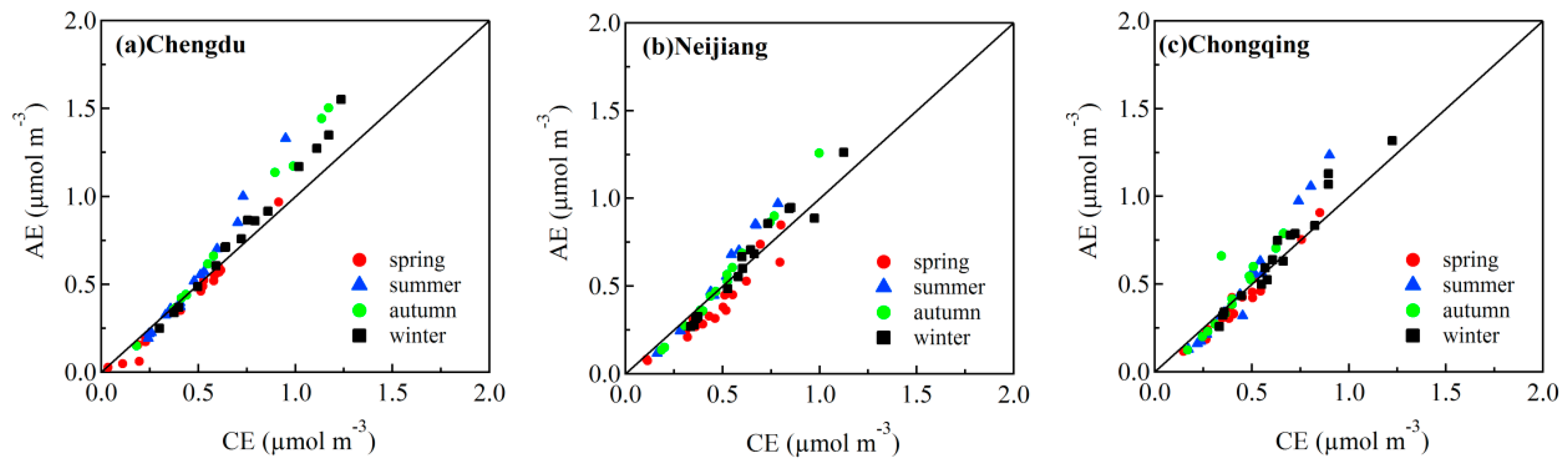
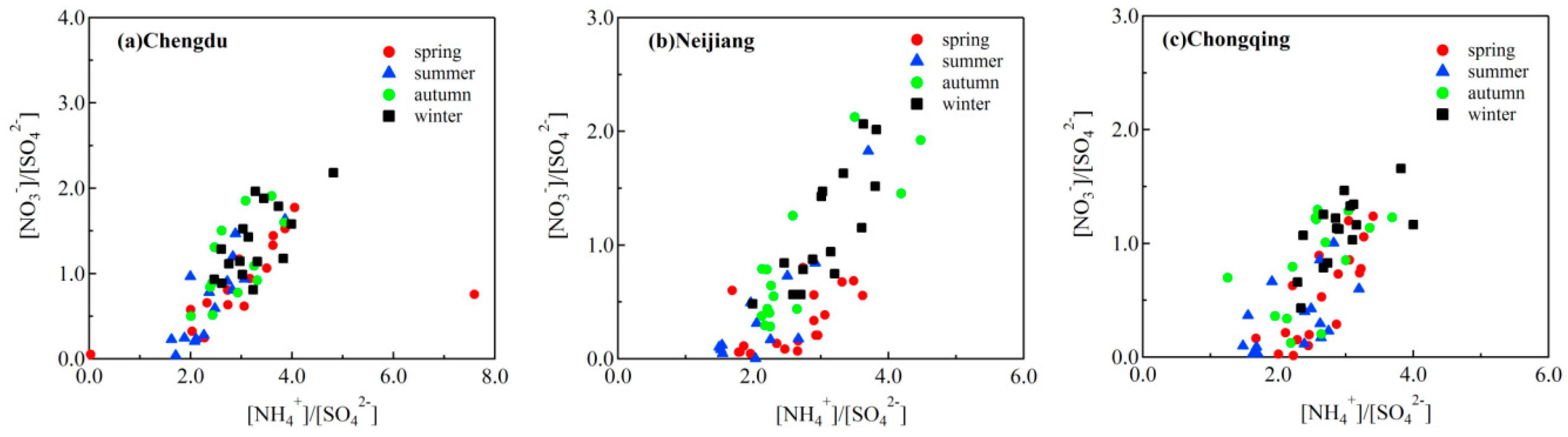
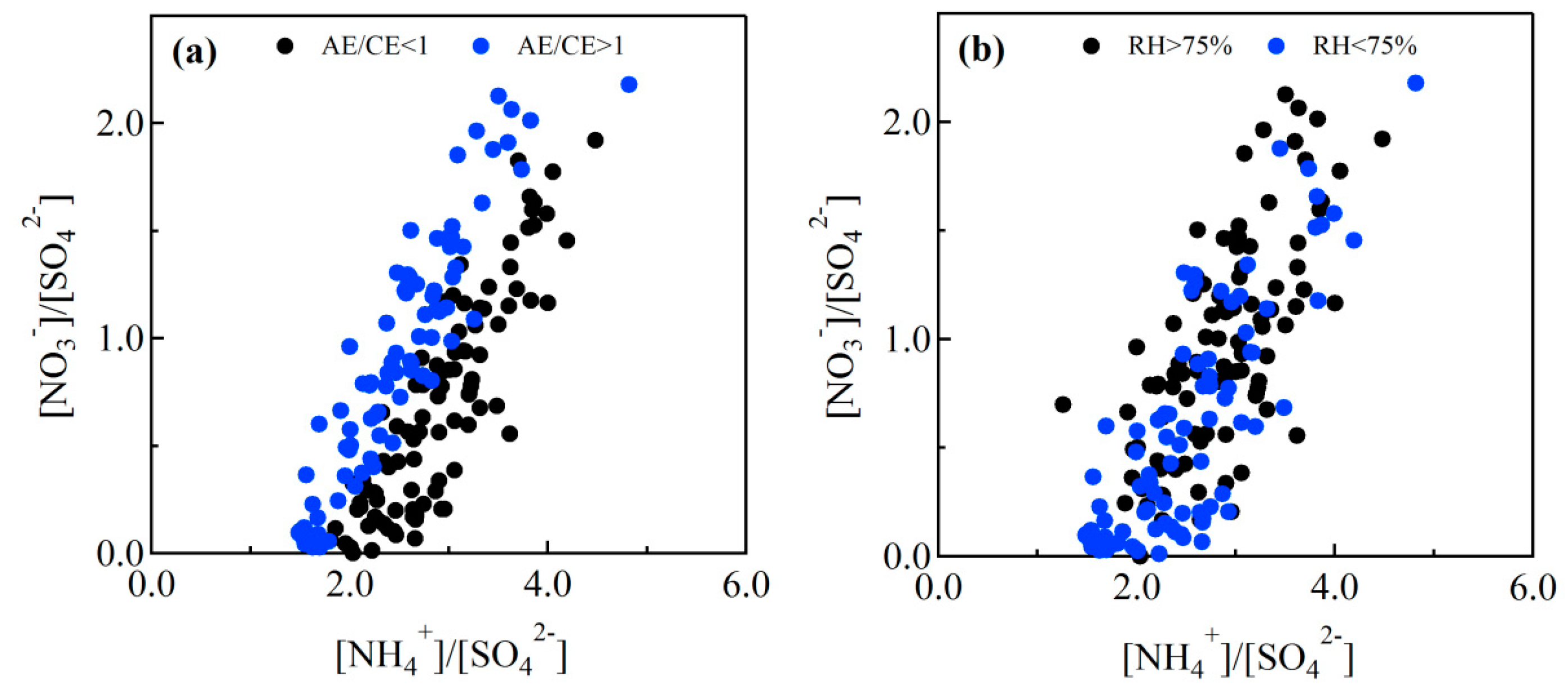
3.3. Stoichiometric Analysis of Cations and Anions
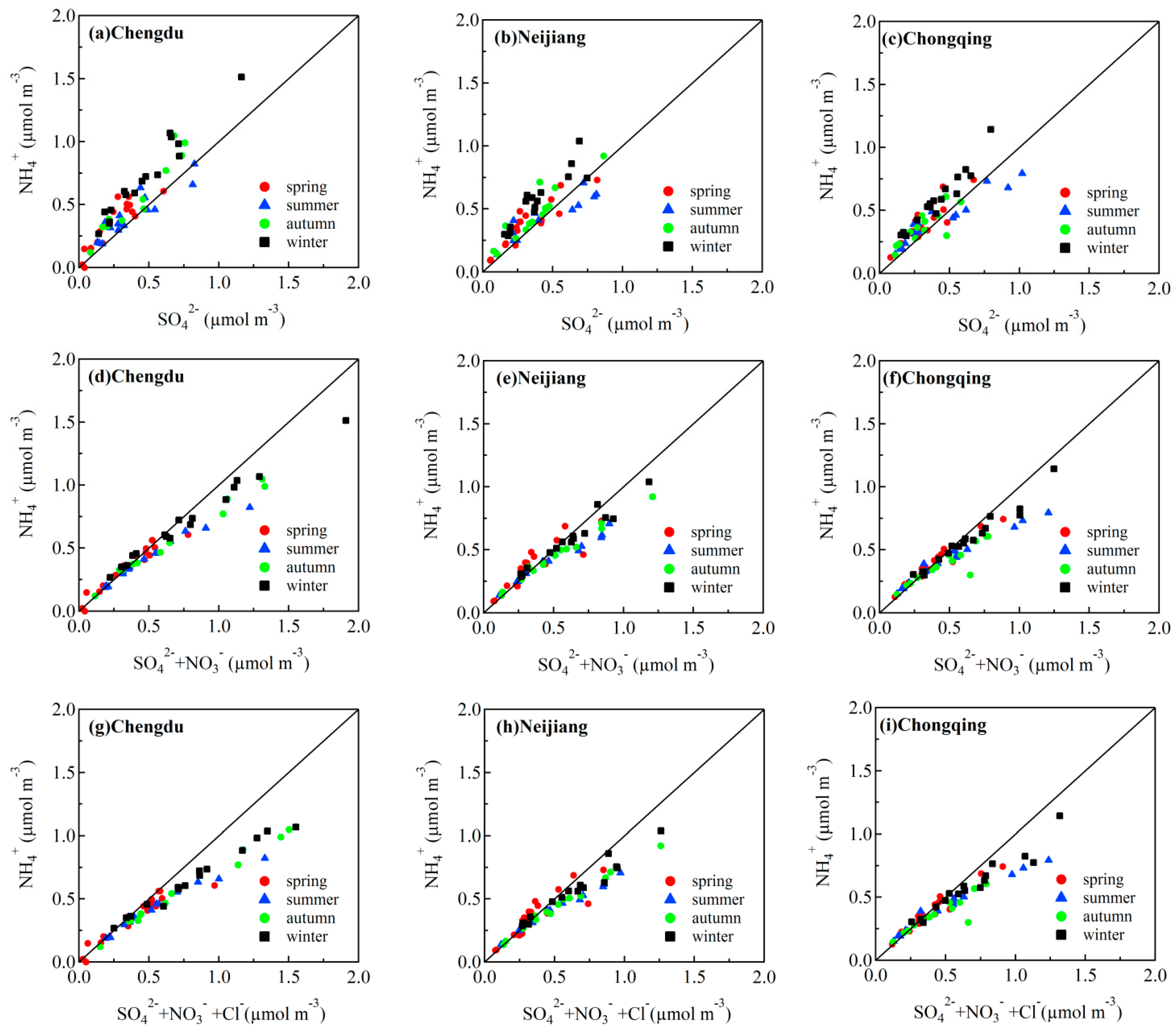
3.4. Chemical Forms of Nitrate and Sulfate
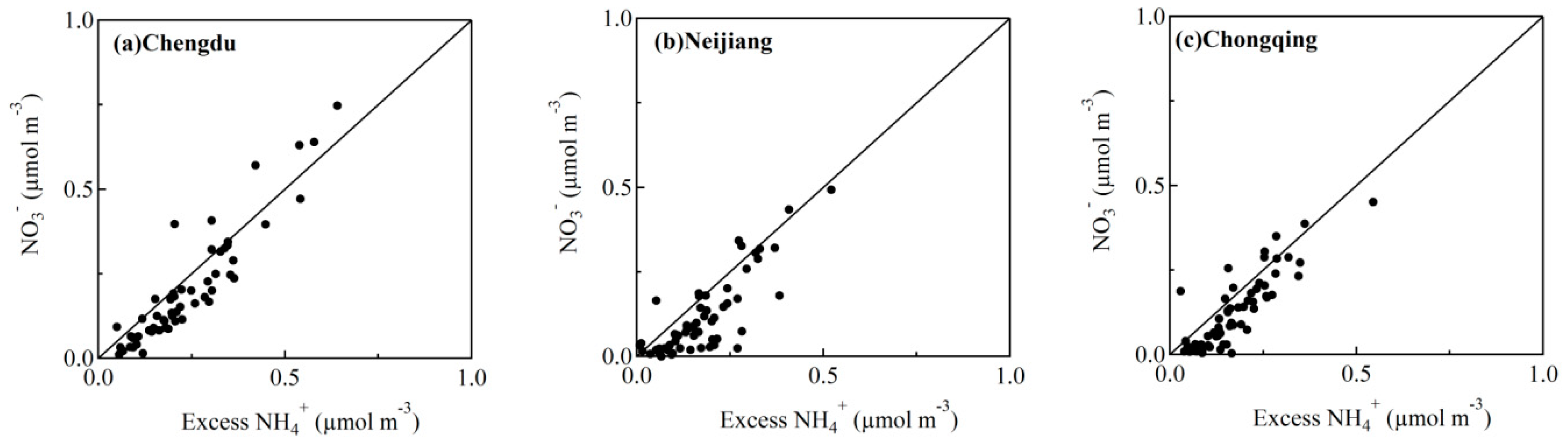
3.5. Formation Mechanism of Nitrate and Sulfate
3.6. Source Analysis of WSIIs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, X.Y.; Wang, Y.Q.; Niu, T.; Zhang, X.C.; Gong, S.L.; Zhang, Y.M.; Sun, J.Y. Atmospheric aerosol compositions in China: Spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos. Chem. Phys. 2012, 12, 779–799. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Cao, J.; Zhang, R. A review of current knowledge concerning PM2. 5 chemical composition, aerosol optical properties and their relationships across China. Atmos. Chem. Phys. 2017, 17, 9485–9518. [Google Scholar] [CrossRef]
- Andreae, M.O.; Schmid, O.; Yang, H.; Chand, D.; Yu, J.Z.; Zeng, L.-M.; Zhang, Y.-H. Optical properties and chemical composition of the atmospheric aerosol in urban Guangzhou, China. Atmos. Environ. 2008, 42, 6335–6350. [Google Scholar] [CrossRef]
- Zheng, S.; Pozzer, A.; Cao, C.X.; Lelieveld, J. Long-term (2001–2012) concentrations of fine particulate matter (PM2.5) and the impact on human health in Beijing, China. Atmos. Chem. Phys. 2015, 15, 5715–5725. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Tang, A.; Yuan, H.; Sun, Y.; Chen, S.; Zheng, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Seinfeld, J.H. Atmospheric Chemistry and Physics of Air Pollution; Wiley: New York, NY, USA, 1986; p. 348. [Google Scholar]
- Wang, Y.; Zhuang, G.; Sun, Y.; An, Z. The variation of characteristics and formation mechanisms of aerosols in dust, haze, and clear days in Beijing. Atmos. Environ. 2006, 40, 6579–6591. [Google Scholar] [CrossRef]
- Khoder, M.I. Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 2002, 49, 675–684. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.D. Temporal and spatial visibility trends in the Sichuan Basin, China, 1973 to 2010. Atmos. Res. 2012, 112, 25–34. [Google Scholar] [CrossRef]
- Tao, J.; Gao, J.; Zhang, L.; Zhang, R.; Che, H.; Zhang, Z.; Lin, Z.; Jing, J.; Cao, J.; Hsu, S.C. PM2.5 pollution in a megacity of southwest China: Source apportionment and implication. Atmos. Chem. Phys. 2014, 14, 8679–8699. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.D.; Luo, B.; Zhai, C.Z. Particulate pollution in urban Chongqing of southwest China: Historical trends of variation, chemical characteristics and source apportionment. Sci. Total. Environ. 2017, 584–585, 523–534. [Google Scholar] [CrossRef]
- He, K.; Zhao, Q.; Ma, Y.; Duan, F.; Yang, F.; Shi, Z.; Chen, G. Spatial and seasonal variability of PM2.5 acidity at two Chinese megacities: Insights into the formation of secondary inorganic aerosols. Atmos. Chem. Phys. 2012, 12, 1377–1395. [Google Scholar] [CrossRef]
- Yang, F.; Tan, J.; Zhao, Q.; Du, Z.; He, K.; Ma, Y.; Duan, F.; Chen, G. Characteristics of PM2.5 speciation in representative megacities and across China. Atmos. Chem. Phys. 2011, 11, 5207–5219. [Google Scholar] [CrossRef]
- Zhao, Q.; He, K.; Rahn, K.A.; Ma, Y.; Jia, Y.; Yang, F.; Duan, F.; Lei, Y.; Chen, G.; Cheng, Y.; et al. Dust storms come to Central and Southwestern China, too: Implications from a major dust event in Chongqing. Atmos. Chem. Phys. 2010, 10, 2615–2630. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total. Environ. 2017, 580, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, H.; Chen, Y.; Yang, F.; Zhang, X.; Zou, Q.; Zhang, R.; Ma, Y.; He, K. Characteristics of aerosol pollution during heavy haze events in Suzhou, China. Atmos. Chem. Phys. 2016, 16, 7357–7371. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.-D. Characteristics and formation mechanism of a heavy air pollution episode caused by biomass burning in Chengdu, Southwest China. Sci. Total. Environ. 2014, 473, 507–517. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. Available online: http://www.stats.gov.cn/ (accessed on 1 February 2019).
- Zhou, J.; Xing, Z.; Deng, J.; Du, K. Characterizing and sourcing ambient PM2.5 over key emission regions in China I: Water-soluble ions and carbonaceous fractions. Atmos. Environ. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Zhao, P.S.; Dong, F.; He, D.; Zhao, X.J.; Zhang, X.L.; Zhang, W.Z.; Yao, Q.; Liu, H.Y. Characteristics of concentrations and chemical compositions for PM2.5 in the region of Beijing, Tianjin, and Hebei, China. Atmos. Chem. Phys. 2013, 13, 4631–4644. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Zhang, R.; Jing, J.; Tao, J.; Hsu, S.C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef]
- Wang, Y.G.; Ying, Q.; Hu, J.L.; Zhang, H.L. Spatial and temporal variations of six criteria air pollutants in 31 provincial capital cities in China during 2013–2014. Environ. Int. 2014, 73, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Louie, P.K.K.; Chan, C.K. Characteristics of aerosol acidity in Hong Kong. Atmos. Environ. 2004, 38, 2965–2974. [Google Scholar] [CrossRef]
- Anttila, T.; Kiendlerscharr, A.; Ralf Tillmann, A.; Mentel, T.F. On the reactive uptake of gaseous compounds by organic-coated aqueous aerosols: Theoretical analysis and application to the heterogeneous hydrolysis of N2O5. J. Phys. Chem. A 2006, 110, 10435. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Wang, S.; Ai, J.; Gui, K.; Duan, B.; Zhao, Q.; Zhang, X.; Jiang, W.; Sun, Y. Heavy pollution episodes, transport pathways and potential sources of PM2.5 during the winter of 2013 in Chengdu (China). Sci. Total. Environ. 2017, 584–585, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Wu, W.S.; Wang, T. Summertime PM2.5 ionic species in four major cities of China: Nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 2009, 9, 1711–1722. [Google Scholar] [CrossRef]
- Ohta, S.; Okita, T. A ChemicaL characterization of atmospheric aerosol in Sapporo. Atmos. Environ. 1990, 24, 815–822. [Google Scholar] [CrossRef]
- Takekawa, H.; Minoura, H.; Yamazaki, S. Temperature dependence of secondary organic aerosol formation by photo-oxidation of hydrocarbons. Atmos. Environ. 2003, 37, 3413–3424. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Fu, P.; Jiang, Q.; Yang, T.; Li, J.; Ge, X. The impact of relative humidity on aerosol composition and evolution processes during wintertime in Beijing, China. Atmos. Environ. 2013, 77, 927–934. [Google Scholar] [CrossRef]
- Svenningsson, B.; Rissler, J.; Swietlicki, E.; Mircea, M.; Bilde, M.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Zhou, J.; Mønster, J.; et al. Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance. Atmos. Chem. Phys. 2006, 6, 1937–1952. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Zhang, J.; Wen, T.; Ji, D.; Wang, Y. Seasonal variation and secondary formation of size-segregated aerosol water-soluble inorganic ions during pollution episodes in Beijing. Atmos. Res. 2016, 168, 70–79. [Google Scholar] [CrossRef]
- Hewitt, C.N. The atmospheric chemistry of sulphur and nitrogen in power station plumes. Atmos. Environ. 2001, 35, 1155–1170. [Google Scholar] [CrossRef]
- Jansen, R.C.; Yang, S.; Chen, J.; Yunjie, H.U.; Chang, X.U.; Hong, S.; Jiao, L.I. Using hourly measurements to explore the role of secondary inorganic aerosol in PM2.5 during Haze and Fog in Hangzhou, China. Adv. Atmos. Sci. 2014, 31, 1427–1434. [Google Scholar] [CrossRef]
- Pathak, R.; Chan, C. Inter-particle and gas-particle interactions in sampling artifacts of PM2.5 in filter-based samplers. Atmos. Environ. 2005, 39, 1597–1607. [Google Scholar] [CrossRef]
| CD | NJ | CQ | |
|---|---|---|---|
| PM10 (μg m−3) | 125.8 ± 74.4 | 116.3 ± 54.7 | 101.0 ± 51.7 |
| PM2.5 (μg m−3) | 86.7 ± 49.7 | 78.6 ± 36.8 | 71.7 ± 36.9 |
| Na+ (μg m−3) | 0.45 ± 0.23 | 0.21 ± 0.09 | 0.25 ± 0.12 |
| NH4+ (μg m−3) | 9.0 ± 5.1 | 8.2 ± 3.6 | 8.0 ± 3.5 |
| K+ (μg m−3) | 1.23 ± 1.22 | 1.17 ± 0.94 | 0.77 ± 0.45 |
| Mg2+ (μg m−3) | 0.07 ± 0.04 | 0.06 ± 0.06 | 0.04 ± 0.03 |
| Ca2+ (μg m−3) | 0.44 ± 0.28 | 0.35 ± 0.21 | 0.32 ± 0.17 |
| SO42− (μg m−3) | 17.7 ± 11.2 | 18.1 ± 10.0 | 17.6 ± 9.6 |
| NO3− (μg m−3) | 11.9 ± 10.3 | 7.1 ± 7.1 | 7.8 ± 6.5 |
| Cl− (μg m−3) | 2.46 ± 2.17 | 0.95 ± 0.88 | 0.69 ± 0.75 |
| WSII (μg m−3) | 43.0 ± 27.9 | 36.2 ± 18.4 | 35.4 ± 18.4 |
| Number of samples | 60 | 59 | 63 |
| SO2 | 33.3 ± 20.6 | 34.7 ± 16.9 | 28.1 ± 17.3 |
| NO2 | 76.6 ± 27.4 | 33.2 ± 15.9 | 39.8 ± 18.9 |
| NO3− | Season | Na+ | NH4+ | K+ | Mg2+ | Ca2+ |
|---|---|---|---|---|---|---|
| CD | Spring | 0.52 | 0.89 | 0.32 | 0.20 | −0.17 |
| Summer | 0.72 | 0.76 | 0.71 | −0.15 | 0.40 | |
| Autumn | 0.75 | 0.95 | 0.80 | 0.40 | 0.78 | |
| Winter | 0.84 | 0.95 | 0.67 | 0.59 | 0.59 | |
| NJ | Spring | 0.12 | 0.19 | 0.62 | 0.00 | 0.06 |
| Summer | 0.59 | 0.38 | 0.73 | 0.62 | −0.08 | |
| Autumn | 0.65 | 0.87 | 0.56 | 0.19 | −0.06 | |
| Winter | 0.55 | 0.76 | 0.37 | 0.08 | 0.06 | |
| CQ | Spring | 0.64 | 0.70 | 0.47 | 0.13 | 0.01 |
| Summer | 0.75 | 0.67 | 0.59 | 0.01 | 0.57 | |
| Autumn | 0.70 | 0.81 | 0.67 | 0.39 | 0.20 | |
| Winter | 0.76 | 0.90 | 0.32 | 0.20 | 0.40 |
| SOR | NOR | |||||
|---|---|---|---|---|---|---|
| CD | NJ | CQ | CD | NJ | CQ | |
| Spring | 0.27 | 0.28 | 0.44 | 0.08 | 0.07 | 0.23 |
| Summer | 0.49 | 0.41 | 0.50 | 0.09 | 0.11 | 0.07 |
| Autumn | 0.33 | 0.43 | 0.34 | 0.14 | 0.25 | 0.17 |
| Winter | 0.33 | 0.29 | 0.41 | 0.18 | 0.26 | 0.28 |
| Annual | 0.35 | 0.34 | 0.41 | 0.12 | 0.17 | 0.19 |
| PM2.5 (μg m−3) | CD | NJ | CQ | |||
|---|---|---|---|---|---|---|
| NO3−/SO42− | NOR/SOR | NO3−/SO42− | NOR/SOR | NO3−/SO42− | NOR/SOR | |
| PM2.5 < 75 | 0.96 | 0.30 | 0.60 | 0.43 | 0.62 | 0.37 |
| 75 ≤ PM2.5 < 115 | 0.89 | 0.34 | 0.72 | 0.51 | 0.99 | 0.75 |
| 115 ≤ PM2.5 < 150 | 1.32 | 0.59 | 0.79 | 0.72 | 0.64 | 0.66 |
| 150 ≤ PM2.5 | 1.34 | 0.57 | 0.93 | 0.86 | 1.15 | 0.84 |
| CD | NJ | CQ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F 1 | F 2 | F 3 | F 1 | F 2 | F 3 | F 1 | F 2 | F 3 | |
| Na+ | 0.74 | 0.45 | 0.22 | 0.70 | 0.34 | 0.45 | 0.53 | 0.61 | 0.39 |
| NH4+ | 0.96 | 0.08 | 0.19 | 0.07 | 0.51 | 0.84 | 0.79 | 0.55 | 0.04 |
| K+ | 0.47 | 0.73 | 0.01 | 0.68 | 0.34 | 0.21 | 0.79 | 0.27 | 0.23 |
| Mg2+ | 0.27 | 0.25 | 0.89 | 0.81 | 0.03 | −0.04 | 0.02 | 0.10 | 0.88 |
| Ca2+ | −0.04 | 0.81 | 0.35 | 0.83 | −0.18 | 0.15 | 0.40 | 0.04 | 0.77 |
| Cl− | 0.77 | 0.48 | 0.03 | 0.20 | 0.88 | 0.10 | 0.19 | 0.92 | 0.16 |
| SO42− | 0.85 | 0.06 | 0.35 | 0.22 | 0.08 | 0.96 | 0.89 | 0.23 | 0.19 |
| NO3− | 0.93 | 0.13 | 0.08 | −0.81 | 0.88 | 0.30 | 0.37 | 0.87 | −0.05 |
| Eigenvalues | 4.90 | 1.19 | 0.68 | 3.71 | 1.80 | 0.93 | 4.62 | 1.32 | 0.77 |
| % of variance | 61.1 | 14.9 | 8.5 | 46.4 | 22.5 | 11.7 | 57.8 | 16.5 | 9.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xie, S.-d.; Luo, B.; Zhai, C. Characteristics and Sources of Water-Soluble Ions in PM2.5 in the Sichuan Basin, China. Atmosphere 2019, 10, 78. https://doi.org/10.3390/atmos10020078
Chen Y, Xie S-d, Luo B, Zhai C. Characteristics and Sources of Water-Soluble Ions in PM2.5 in the Sichuan Basin, China. Atmosphere. 2019; 10(2):78. https://doi.org/10.3390/atmos10020078
Chicago/Turabian StyleChen, Yuan, Shao-dong Xie, Bin Luo, and Chongzhi Zhai. 2019. "Characteristics and Sources of Water-Soluble Ions in PM2.5 in the Sichuan Basin, China" Atmosphere 10, no. 2: 78. https://doi.org/10.3390/atmos10020078
APA StyleChen, Y., Xie, S.-d., Luo, B., & Zhai, C. (2019). Characteristics and Sources of Water-Soluble Ions in PM2.5 in the Sichuan Basin, China. Atmosphere, 10(2), 78. https://doi.org/10.3390/atmos10020078