Abstract
Heterochiasmy, a sex-based difference in recombination rate, has been detected in many species of animals and plants. Several hypotheses about evolutionary causes of heterochiasmy were proposed. However, there is a shortage of empirical data. In this paper, we compared recombination related traits in females and males of the barn swallow Hirundo rustica (Linnaeus, 1758), the species under strong sexual selection, with those in the pale martin Riparia diluta (Sharpe and Wyatt, 1893), a related and ecologically similar species with the same karyotype (2N = 78), but without obvious sexual dimorphism. Recombination traits were examined in pachytene chromosome spreads prepared from spermatocytes and oocytes. Synaptonemal complexes and mature recombination nodules were visualized with antibodies to SYCP3 and MLH1 proteins, correspondingly. Recombination rate was significantly higher (p = 0.0001) in barn swallow females (55.6 ± 6.3 recombination nodules per autosomal genome), caused by the higher number of nodules at the macrochromosomes, than in males (49.0 ± 4.5). They also showed more even distribution of recombination nodules along the macrochromosomes. At the same time, in the pale martin, sexual differences in recombination rate and distributions were rather small. We speculate that an elevated recombination rate in the female barn swallows might have evolved as a compensatory reaction to runaway sexual selection in males.
1. Introduction
Recombination plays an important role in reshuffling alleles from generation to generation. Genetic variation of the number and distribution of recombination events has been detected in many species of plants and animals [1,2,3].
Sex-based differences in recombination (heterochiasmy) have attracted special attention since the early days of genetics. Heterochiasmy is distributed erratically among taxa and varies in magnitude and direction [3,4]. A higher recombination rate is more often observed in females than in males [4,5,6]. Sex-based differences in the distribution of the recombination event along the chromosomes have also been detected. Males usually show more polarized distribution with stronger peaks at telomeres. Females display more even distribution slightly shifted towards centromeres [7,8].
The proximate and ultimate causes of heterochiasmy are not clear. The higher rate and flatter distribution of recombination in females have been ascribed to longer synaptonemal complexes (SC) and weaker chiasma interference [7,9].
Several hypotheses about evolutionary causes of heterochiasmy were proposed. It has been considered as a collateral result of selection against recombination between the sex chromosomes in the heterogametic sex [10]. However, many examples of higher recombination rate in the heterogametic females contradict this generalization [4,8].
Lenormand [11] suggested that sex differences in recombination can result from selection on the haploid phase of the life cycle. This mechanism might operate in plants, but it is hardly applicable to animals [8,12]. Selection against meiotic drive in female meiosis can also contribute to the increase of recombination rate and preferential location of crossovers near centromeres [6,8,13].
Trivers [14] suggested that sexual selection should favor tighter linkage between loci important for male reproductive success. Lenormand [11] carried out a population genetic model analysis of this hypothesis. He concluded that sexual selection might result in decreased recombination in males if the sex difference in the strength of epistasis between alleles of different loci depended on the phase of their linkage (coupling or repulsion). Sardell and Kirkpatrick [8] modified this model, suggesting that sex differences in epistasis between coding regions and their cis regulatory regions.
The abundance of theoretical models of heterochiasmy contrasts with scarcity of data. Genetic linkage studies on non-model species are expensive and time consuming. Cytological analysis of recombination nodules makes it possible to analyze large number of meiosis in both sexes [15,16].
In this study, using immunolocalization of the proteins involved in SC and recombination nodules at the pachytene chromosomes, we compared recombination rate and distribution in females and males of the barn swallow Hirundo rustica (Linnaeus, 1758), the species under strong sexual selection [17,18], with those in the pale martin Riparia diluta (Sharpe and Wyatt, 1893), the related and ecologically similar species showing no sexual dimorphism in morphology [19,20].
2. Materials and Methods
2.1. Specimens
Adult males were captured by bird nets near the nests, at the beginning of breeding season at the end of May. Nestling females on days 3–6 after hatching were collected from the nests. The number of specimens examined and the coordinates of the trapping localities are listed in Supplementary Table S1.
The barn swallows were identified morphologically. The pale martins were identified by DNA barcoding. DNA was isolated from heart and kidney samples by routine phenol-chloroform technique. Primers and PCR conditions for the amplification of a fragment of the mitochondrial COI gene were used according to Hebert et al. [21]. The amplicons were Sanger sequenced. The sequences were processed using MEGA7 (https://megasoftware.net) and then analyzed using the distance-based and tree-based identification tools of the BOLD v.4 database (http://boldsystems.org) [22]. The DNA sequences confirmed correct identification of the individuals as being the pale martin (GenBank accession number MN216343) according to Pavlova et al. [19].
Capture, handling, and euthanasia of the birds followed the protocols approved by the Ethics Committee on Animal Care and Use of the Institute of Cytology and Genetics (approval No. 45/2 of 10 January, 2019). Experiments described in this manuscript were carried out in accordance with the approved national guidelines for the care and use of animals.
2.2. Chromosome Spreading and Staining
Pachytene chromosome spreads were prepared from spermatocytes or juvenile oocytes according to the protocol described by Peters et al. [23]. Immunostaining was performed according to Anderson et al. [24] using the following set of primary antibodies: rabbit polyclonal anti-SYCP3 (1:500; Abcam, Cambridge, UK), mouse monoclonal anti-MLH1 (1:50; Abcam, Cambridge, UK), and human anticentromere (ACA) (1:100; Antibodies Incorporated, Davis, CA, USA). For the secondary antibodies, we used Cy3-conjugated goat anti-rabbit (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA), FITC-conjugated goat anti-mouse (1:50; Jackson ImmunoResearch), and AMCA-conjugated donkey anti-human (1:100; Jackson ImmunoResearch). Antibodies were diluted in PBT (3% bovine serum albumin and 0.05% Tween 20 in phosphate-buffered saline). A solution of 10% PBT was used for blocking. Primary antibodies were incubated overnight at 37 °C; and secondary antibodies for 1 h at 37°C in a humid chamber. Slides were mounted in Vectashield antifade mounting medium (Vector Laboratories, Burlingame, CA, USA) to reduce fluorescence fading.
The preparations were examined with an Axioplan 2 imaging microscope (Carl Zeiss, Oberkochen, Germany) equipped with a CCD camera (CV M300, JAI Corporation, Yokohama, Japan, CHROMA filter sets, and the ISIS4 image-processing package (MetaSystems, Altlußheim, Germany). Brightness and contrast of the images were enhanced using Corel PaintShop Photo Pro X6 (Corel Corporation, Ottawa, ON, Canada).
2.3. Chromosome Measurements and Generation of Recombination Maps
The centromeres were identified by ACA foci. The MLH1 signals were scored if they were localized on SCs. The length of the SC of each chromosome arm was measured in micrometers, and the positions of centromeres and MLH1 foci in relation to the centromere were recorded using MicroMeasure 3.3 [25]. Individual SCs of macrochromosomes were identified by their relative lengths and centromeric indexes. To generate recombination maps of the macrochromosomes, we calculated the absolute position of each MLH1 focus by multiplying the relative position of each focus by the average absolute length of the chromosome arm. These data were pooled for each arm and graphed to represent a recombination map.
The STATISTICA 6.0 software package (StatSoft, Tulsa, OK, USA) was used for descriptive statistics. Values in the text and tables are presented as means ± S.D. Differences between the sexes and species in the average number of MLH1 foci and SC length were estimated by Mann–Whitney non-parametric test. The result p < 0.01 was considered to be statistically significant.
3. Results
3.1. Pachytene Karyotype of the Barn Swallow
The barn swallow karyotype has not been described yet. Its pachytene karyotype contained 38 autosomal SCs and a ZZ/ZW pair (Figure 1). We identified seven largest macroSCs by their relative lengths and centromeric indices. The SC1, SC4, and SCZZ were large metacentrics; they differed from each other in length and centromeric indices (p < 0.001). The SC2 and SC3 were large submetacentrics and differed from each other in centromeric indices. The SC5 and SC6, medium-sized submetacentrics, also differed from each other in centromeric indices. The macroSCs 7, 9, 10, and all microSCs but two, were acrocentric, with gradually decreasing chromosomal sizes (Figure S1). Thus, barn swallow pachytene karyotype was similar to that described in pale martin and sand martin Riparia riparia (Linnaeus, 1758) [26], with their only difference concerning germline restricted chromosome (GRC). The GRC was one of the microchromosomes found in the barn swallow and the largest acrocentric chromosome in pale martin and sand martin [26,27]. In male barn swallows, GRC always appeared as a univalent lacking MLH1 signal and was heavily labelled by centromere antibodies (Figure 1b).
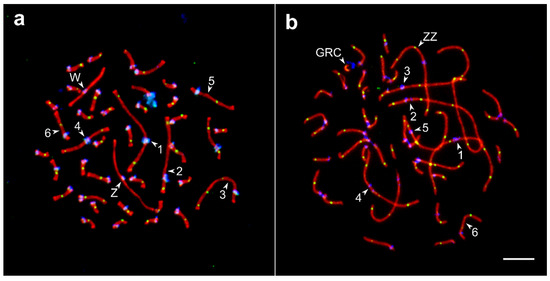
Figure 1.
Synaptonemal complexes (SC) spreads of the oocyte (a) and spermatocyte (b) of the barn swallow after immunolocalization of the lateral elements of the SC (SYCP3—red signal), recombination nodules (MLH1—green), and centromeres (ANA-C—blue). Numbers indicate SCs of the macrochromosomes, letters—ZZ, ZW, and GRC. Bar—5 µm.
3.2. Recombination Rate
Table 1 shows recombination characteristics of studied females and males of barn swallow and pale martin. The barn swallow showed a pronounced sexual dimorphism in the total number of MLH1 foci at autosomes: in the oocytes, it was 13.5% higher than in spermatocytes (Mann–Whitney test z = 12.1; p < 0.00001). The pale martin demonstrated less pronounced (4.5%), although significant, sex difference in this trait (z = 6.9, p < 0.00001). In this species, spermatocytes contained more MLH1 foci at autosomes than oocytes. The pale martin males were sampled from two different locations: three from Novosibirsk and three from Tomsk (Table S1). The difference between them was not significant (z = 0.4; p = 0.7).

Table 1.
Number of MLH1 foci at autosomes and total length of autosomal SC (m ± S.D.) in female and male barn swallows and pale martins.
In both species, we detected significant sex differences in the total SC length: in males, it was longer than in females (z = 10.6 in barn swallow and 10.9 in pale martin; p < 0.00001).
The sex differences of the barn swallow in the total number of autosomal MLH1 foci was mainly due to the macrochromosomes. Almost all microchromosomes in the barn swallow and pale martin, as well as in all bird species examined, contained single recombination nodules. The microchromosomes with two MLH1 foci were rare.
In the female barn swallows, five out of six macroSCs had significantly more MLH1 foci than in the males (p < 0.00001). The SC 6 was an exception (z = 0.8, p = 0.4). None of the pale martin macroSCs showed significant sex differences in the number of MLH1 foci (p > 0.01) (Table 2 and Table S2).

Table 2.
Number of MLH1 foci and SC length (m ± S.D.) at the macroSCs in females and males of barn swallow and pale martin (number of SCs examined is shown in Table S1).
The sex difference in the SC length in the barn swallow was also significant, but with opposite signs: the males had longer macroSCs than females. Thus, the high recombination rate of the macroSCs in the female barn swallows was achieved by a decrease of the distance between the recombination nodules, which in turn indicate a decrease in crossover interference.
Besides the autosomes, we also examined recombination characteristics of the sex chromosomes. In both species, the sex bivalent was similar to that of most songbird species studied. The SC lateral element of Z chromosome was substantially longer than that of W at zygotene at the beginning of pachytene, when they were paired by their distal ends. As the synapsis progressed, it involved equalization of the elements (contraction of Z and elongation of W chromosomes). At the mid-pachytene, ZW bivalent was completely paired (Figure 1), and usually contained a single MLH1 focus at its distal end. The ZZ bivalent usually had two (rarely three) MLH1 foci, similar to the autosomal bivalents of a comparable length (Table 2). The difference in the number of MLH1 foci in ZZ of barn swallow and pale martin was not significant (p = 0.02).
3.3. Recombination Distribution along the Macrochromosomes
The pattern of MLH1 foci distribution in the spermatocytes of both species, and in the oocytes of the pale martin, was rather similar. The peaks of the MLH1 foci were observed near the SC ends, while the rest of the chromosome arms contained a relatively low number of them (Figure 2).
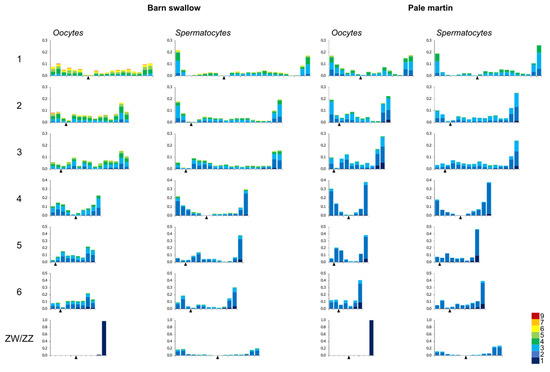
Figure 2.
Distribution of MLH1 foci along individual SCs in pachytene oocytes and spermatocytes of barn swallow and pale martin. On the x-axis: the relative position of MLH1 foci at the six largest macroSCs and ZW/ZZ bivalents in relation to the centromere (black triangle). The width of the interval is approximately 1 μm. On the y-axis: the proportion of MLH1 focus number in each interval. Colors indicate bivalents with 1–5 MLH1 foci per bivalent. The scale shows the color codes. The numbers to the left of the y-axis stand for chromosome numbers.
The recombination landscape of the macrochromosomes of the barn swallow oocytes was quite different. The telomeric peaks were less pronounced and the distribution of the MLH1 foci was more even. Thus, the recombination in the macrochromosomes of the barn swallow oocytes was not only higher than spermatocytes of the same species and in the oocytes of the pale martin, but also more evenly distributed.
4. Discussion
We found that the barn swallow, the species with sex differences in morphology, demonstrated a substantial heterochiasmy, with sex differences in recombination rate and distribution along the macrochromosomes. Oocytes showed 13.5% higher recombination rate and more even distribution of recombination nodules along the macrochromosomes than spermatocytes. These two factors should lead to a higher outcome of crossovers and a faster break up of linkage disequilibrium in female barn swallow meiosis.
The related hirundine species, the pale martin, did not show obvious sexual dimorphism in morphology and difference in recombination characteristics.
Sexual dimorphism is usually considered as a result of sexual selection [28]. Several lines of evidence indicate that male barn swallows are under strong selection for display [17,29]. Therefore, their morphological traits might be considered as derived, and female traits as ancestral.
What about recombination characteristics? Should we consider the lower recombination rate and polarized distribution of crossovers typical to the barn swallow males as derived traits, and higher recombination rate and even distribution typical to the barn swallow females as ancestral? If the answer is yes, then our results match the prediction of the sexual selection hypothesis of heterochiasmy.
However, there are facts that cast doubts on this explanation. The barn swallow males are similar in their recombination pattern to sexually monomorphic pale martins (Table 1). Recombination rate of the barn swallow females is highest among the female songbird studied (Table 3). To save the standard explanation, we should suppose that the ancestors of the barn swallow had evolved for an increase of recombination rate in both sexes, and then sexual selection decreased it in males.

Table 3.
Heterochiasmy and sexual dimorphism in birds.
Alternatively, we may suggest that the low recombination rate is an ancestral trait, which facilitated an establishment and maintenance of linkage disequilibrium between the alleles, controlling male display and female preference to the display. Because the sex difference in recombination in the barn swallow is most pronounced in macrochromosomes, one might suppose that the genes conserved by sexual selection are located there. The strong selection for linkage disequilibrium would lead to accumulation of deleterious mutations in these regions. An increase of recombination in macrochromosomes in female meiosis might be aimed to purge them.
Of course, this hypothesis is highly speculative. We need more data on the occurrence, magnitude, and direction of heterochiasmy among the birds with and without sexual dimorphism in morphology.
Besides the two species described here, recombination rate has been studied so far in both sexes in only a few species (Table 3). Some studies used a cytological approach, while others used a linkage analysis with different density of markers. To make these data comparable, we transformed the number of recombination nodules into genetic map distances (one recombination nodule = 50 cM) and calculated heterochiasmy index as (female genetic map length – male genetic map length)/sex average genetic map length.
Table 3 shows no obvious phylogenetic clustering for magnitude and sign of the heterochiasmy index. There is no obvious correlation between the heterochiasmy and sexual dimorphism. All four species of domesticated Galloanserae show sexual dimorphism in morphology and differ from each other in heterochiasmy: it is female-biased in the domestic goose, male-biased in turkey, and negligible in Japanese quail and domestic chicken. Four of the seven songbird species studied showed female-biased heterochiasmy, one species showed male-biased and two species exhibited no heterochiasmy. In this group of species, we also did not see a coincidence between sexual dimorphism in morphology and heterochiasmy. Two species with a highly positive heterochiasmy index, the Siberian jay and great reed warbler, show no sexual dimorphism, while the obviously dimorphic zebra finch shows no heterochiasmy.
Apparently, the macro-phylogenetic comparison fails to reveal the coevolution of sexual dimorphism in morphology and recombination rate. To shed a light on the evolution of heterochiasmy in birds, we need to compare the related and ecologically similar species. This comparison must take into account not only overall recombination, but also the pattern of its distribution between and along the particular chromosomes (even vs. polarized, centromere vs. telomere biased). We also need an insight into genetic control of sex-restricted traits and its chromosomal localization. Swallows (17 species in genus Hirundo and seven subspecies in H. rustica), martins (six species in genus Riparia and four subspecies in R. diluta), and the genera between them (such as Delichon, for example), with their large and permanent colonies and strong monogamy, provide a good model for such studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/10/1119/s1. Figure S1: Idiograms of pachytene karyotypes of barn swallow (a) and pale martin (b), Table S1: Number of specimens examined and the coordinates of the trapping localities, Table S2: Mann–Whitney test for sex difference in the same species and species difference in the same sex, Supplementary file 1: raw data.
Author Contributions
Conceptualization, P.M.B., E.P.S., A.A.T.; methodology, K.T.; software, A.A.T.; formal analysis, P.M.B.; resources, E.P.S.; investigation, L.P.M., K.T.; data curation, P.M.B.; writing—original draft preparation, P.M.B.; writing—review and editing, P.M.B., A.A.T.; visualization, L.P.M.; supervision, P.M.B.; project administration, A.A.T.; funding acquisition, A.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
Data collection and analysis was funded by Russian Science Foundation, grant number 20-64-46021. Microscopy was carried out at the Microscopy Center of the Siberian Branch of the Russian Academy of Sciences and funded by the Ministry of Science and Higher Education of the Russian Federation, grant numbers 0324-2019-0042 and #2019-0546 (FSUS-2020-0040).
Acknowledgments
We thank Alexei Maslov for trapping birds and Olga Shishkina for the help in image collection and processing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ritz, K.R.; Noor, M.A.F.; Singh, N.D. Variation in recombination rate: Adaptive or not? Trends Genet. 2017, 33, 364–374. [Google Scholar] [CrossRef]
- Stapley, J.; Feulner, P.G.D.; Johnston, S.E.; Santure, A.W.; Smadja, C.M. Variation in recombination frequency and distribution across eukaryotes: Patterns and processes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160455. [Google Scholar] [CrossRef]
- Lenormand, T.; Engelstädter, J.; Johnston, S.E.; Wijnker, E.; Haag, C.R. Evolutionary mysteries in meiosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Burt, A.; Bell, G.; Harvey, P.H. Sex-differences in recombination. J. Evol. Biol. 1991, 4, 259–277. [Google Scholar] [CrossRef]
- Lenormand, T.; Dutheil, J. Recombination difference between sexes: A role for haploid selection. PLoS Biol. 2005, 3, e63. [Google Scholar] [CrossRef]
- Brandvain, Y.; Coop, G. Scrambling eggs: Meiotic drive and the evolution of female recombination rates. Genetics 2012, 190, 709–723. [Google Scholar] [CrossRef]
- Gruhn, J.R.; Rubio, C.; Broman, K.W.; Hunt, P.A.; Hassold, T. Cytological studies of human meiosis: Sex-specific differences in recombination originate at, or prior to, establishment of double-strand breaks. PLoS ONE 2013, 8, e85075. [Google Scholar] [CrossRef]
- Sardell, J.M.; Kirkpatrick, M. Sex differences in the recombination landscape. Am. Nat. 2020, 195, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Petkov, P.M.; Broman, K.W.; Szatkiewicz, J.P.; Paigen, K. Crossover interference underlies sex differences in recombination rates. Trends Genet. 2007, 23, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. Sex-ratio and unisexual sterility in hybrid animals. J. Genet. 1922, 12, 101. [Google Scholar] [CrossRef]
- Lenormand, T. The evolution of sex dimorphism in recombination. Genetics 2003, 163, 811. [Google Scholar] [PubMed]
- Mank, J.E. The evolution of heterochiasmy: The role of sexual selection and sperm competition in determining sex-specific recombination rates in eutherian mammals. Genet. Res. 2009, 91, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Haig, D. Games in tetrads: Segregation, recombination, and meiotic drive. Am. Nat. 2010, 176, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Trivers, R. Sex differences in rates of recombination and sexual selection. In The Evolution of Sex: An Examination of Current Ideas; Michod, R.E., Levin, B.R., Eds.; Sinauer Press: Sunderland, MA, USA, 1988; pp. 270–286. [Google Scholar]
- Hassold, T.; Sherman, S.; Hunt, P. Counting cross-overs: Characterizing meiotic recombination in mammals. Hum. Mol. Genet. 2000, 9, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Torgasheva, A.A.; Borodin, P.M. Immunocytological analysis of meiotic recombination in the gray goose (Anser anser). Cytogenet. Genome Res. 2017, 151, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Sexual Selection and the Barn Swallow. Oxford Series in Ecology and Evolution; Oxford University Press: Oxford, UK, 1994; ISBN 9780198540281. [Google Scholar]
- Romano, A.; Costanzo, A.; Rubolini, D.; Saino, N.; Møller, A.P. Geographical and seasonal variation in the intensity of sexual selection in the barn swallow Hirundo rustica: A meta-analysis. Biol. Rev. 2017, 92, 1582–1600. [Google Scholar] [CrossRef]
- Pavlova, A.; Zink, R.M.; Drovetski, S.V.; Rohwer, S. Pleistocene evolution of closely related sand martins Riparia riparia and R. diluta. Mol. Phylogenet. Evol. 2008, 48, 61–73. [Google Scholar] [CrossRef]
- Sheldon, F.H.; Whittingham, L.A.; Moyle, R.G.; Slikas, B.; Winkler, D.W. Phylogeny of swallows (Aves: Hirundinidae) estimated from nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2005, 35, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of birds through DNA barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. Available online: http://www.barcodinglife.org (accessed on 4 September 2020). [CrossRef]
- Peters, A.H.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosom. Res. 1997, 5, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.K.; Reeves, A.; Webb, L.M.; Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 1999, 151, 1569–1579. [Google Scholar] [PubMed]
- Reeves, A. MicroMeasure: A new computer program for the collection and analysis of cytogenetic data. Genome 2001, 44, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Akberdina, E.A.; Kizilova, E.A.; Romanenko, M.V.; Shnaider, E.P.; Scherbakova, M.M.; Korobitsyn, I.G.; Rubtsov, N.B.; et al. Germline-restricted chromosome (GRC) in the sand martin and the pale martin (Hirundinidae, Aves): Synapsis, recombination and copy number variation. Sci. Rep. 2020, 10, 1058. [Google Scholar] [CrossRef]
- Torgasheva, A.A.; Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Kizilova, E.A.; Akberdina, E.A.; Pristyazhnyuk, I.E.; Shnaider, E.P.; Volodkina, V.A.; Saifitdinova, A.F.; et al. Germline-restricted chromosome (GRC) is widespread among songbirds. Proc. Natl. Acad. Sci. USA 2019, 116, 11650–11845. [Google Scholar] [CrossRef]
- Owens, I.P.F.; Hartley, I.R. Sexual dimorphism in birds: Why are there so many different forms of dimorphism? Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 397–407. [Google Scholar] [CrossRef]
- Romano, A.; Saino, N.; Møller, A.P. Viability and expression of sexual ornaments in the barn swallow Hirundo rustica: A meta-analysis. J. Evol. Biol. 2017, 30, 1929–1935. [Google Scholar] [CrossRef]
- Parés-Casanova, P.M. An analysis of sexual size dimorphism in goose. Br. Poult. Sci. 2014, 55, 143–147. [Google Scholar] [CrossRef]
- Aslam, M.L.; Bastiaansen, J.W.M.; Crooijmans, R.P.M.A.; Vereijken, A.; Megens, H.J.; Groenen, M.A.M. A SNP based linkage map of the turkey genome reveals multiple intrachromosomal rearrangements between the Turkey and Chicken genomes. BMC Genom. 2010, 11, 647. [Google Scholar] [CrossRef]
- Hammond, J.C.; Marsden, S.J. Sexing Turkeys from Hatching to Maturity. Poult. Sci. 1937, 16, 287–288. [Google Scholar] [CrossRef]
- Pigozzi, M.I. Distribution of MLH1 foci on the synaptonemal complexes of chicken oocytes. Cytogenet. Cell Genet. 2001, 95, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Remes, V.; Szekely, T. Domestic chickens defy Rensch’s rule: Sexual size dimorphism in chicken breeds. J. Evol. Biol. 2010, 23, 2754–2759. [Google Scholar] [CrossRef]
- Groenen, M.A.M.; Wahlberg, P.; Foglio, M.; Cheng, H.H.; Megens, H.J.; Crooijmans, R.P.M.A.R.P.; Besnier, F.; Lathrop, M.; Muir, W.M.; Wong, G.K.S.; et al. A high-density SNP-based linkage map of the chicken genome reveals sequence features correlated with recombination rate. Genome Res. 2009, 19, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Calderon, P.L.; Pigozzi, M.I.; Calderón, P.L.; Pigozzi, M.I. MLH1-focus mapping in birds shows equal recombination between sexes and diversity of crossover patterns. Chromosom. Res. 2006, 14, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Huss, D.; Poynter, G.; Lansford, R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Anim. 2008, 37, 513–519. [Google Scholar] [CrossRef]
- Pigozzi, M.I.; Solari, A.J. Equal frequencies of recombination nodules in both sexes of the pigeon suggest a basic difference with eutherian mammals. Genome 1999, 42, 315–321. [Google Scholar] [CrossRef]
- Horng, Y.-M.; Wu, C.-P.; Wang, Y.-C.; Huang, M.-C. A novel molecular genetic marker for gender determination of pigeons. Theriogenology 2006, 65, 1759–1768. [Google Scholar] [CrossRef]
- Jaari, S.; Li, M.-H.; Merilä, J. A first-generation microsatellite-based genetic linkage map of the Siberian jay (Perisoreus infaustus): Insights into avian genome evolution. BMC Genom. 2009, 10, 1. [Google Scholar] [CrossRef]
- Ekman, J.; Bylin, A.; TegelstrÎm, H. Increased lifetime reproductive success for Siberian jay (Perisoreus infaustus) males with delayed dispersal. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999, 266, 911–915. [Google Scholar] [CrossRef]
- Møller, A.P. Sexual selection in the barn swallow (Hirundo rustica). IV. Patterns of fluctuating asymmetry and selection against asymmetry. Evolution 1994, 48, 658. [Google Scholar] [CrossRef]
- Åkesson, M.; Hansson, B.; Hasselquist, D.; Bensch, S. Linkage mapping of AFLP markers in a wild population of great reed warblers: Importance of heterozygosity and number of genotyped individuals. Mol. Ecol. 2007, 16, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Forstmeier, W. Repertoire size, sexual selection, and offspring viability in the great reed warbler: Changing patterns in space and time. Behav. Ecol. 2004, 15, 555–563. [Google Scholar] [CrossRef]
- Hansson, B.; Ljungqvist, M.; Dawson, D.; Mueller, J.C.; Olano-Marin, J.; Ellegren, H.; Nilsson, J. Avian genome evolution: Insights from a linkage map of the blue tit (Cyanistes caeruleus). Heredity 2010, 104, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Örnborg, J.; Andersson, M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 445–450. [Google Scholar] [CrossRef]
- Backström, N.; Karaiskou, N.; Leder, E.H.; Gustafsson, L.; Primmer, C.R.; Qvarnström, A.; Ellegren, H. A gene-based genetic linkage map of the collared flycatcher (Ficedula albicollis) reveals extensive synteny and gene-order conservation during 100 million years of avian evolution. Genetics 2008, 179, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Siitari, H. Individual color variation and male quality in pied flycatchers (Ficedula hypoleuca): A role of ultraviolet reflectance. Behav. Ecol. 2002, 13, 737–741. [Google Scholar] [CrossRef]
- Mak, S.-S.; Wrabel, A.; Nagai, H.; Ladher, R.K.; Sheng, G. Zebra finch as a developmental model. Genesis 2015, 53, 669–677. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).