Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Origin
2.3. Chromosome Preparation, Staining, and Painting
3. Results
3.1. Nomenclature of A. baerii Chromosomes
3.2. Conventional Cytogenetics of the Siberian Sturgeon Karyotype: GTG- and CBG-Banding
3.2.1. GTG-Banding of A. baerii Chromosomes
3.2.2. C-Banding of A. baerii Chromosomes
3.3. Chromosomal Mapping of Microsatellite DNAs
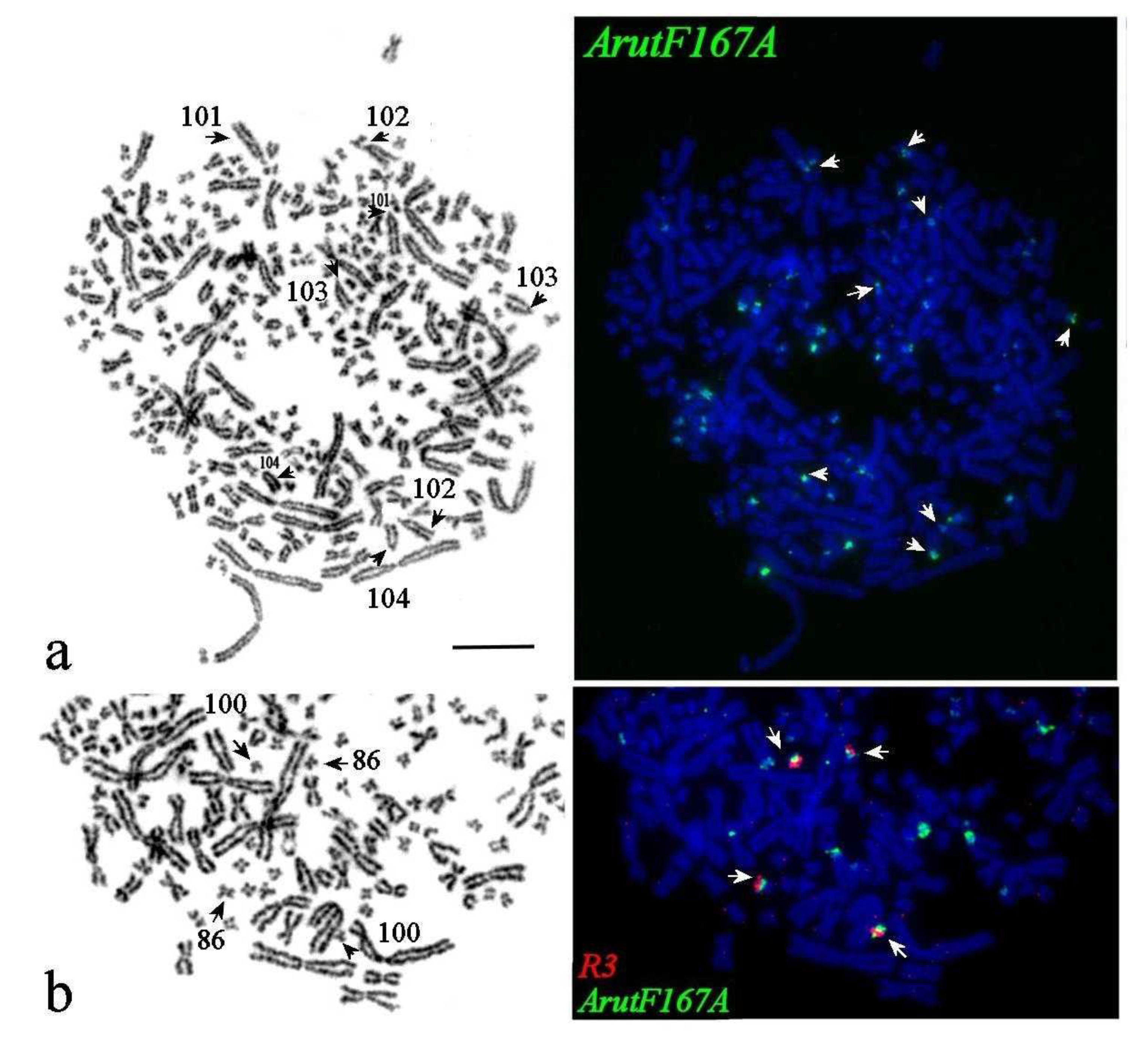
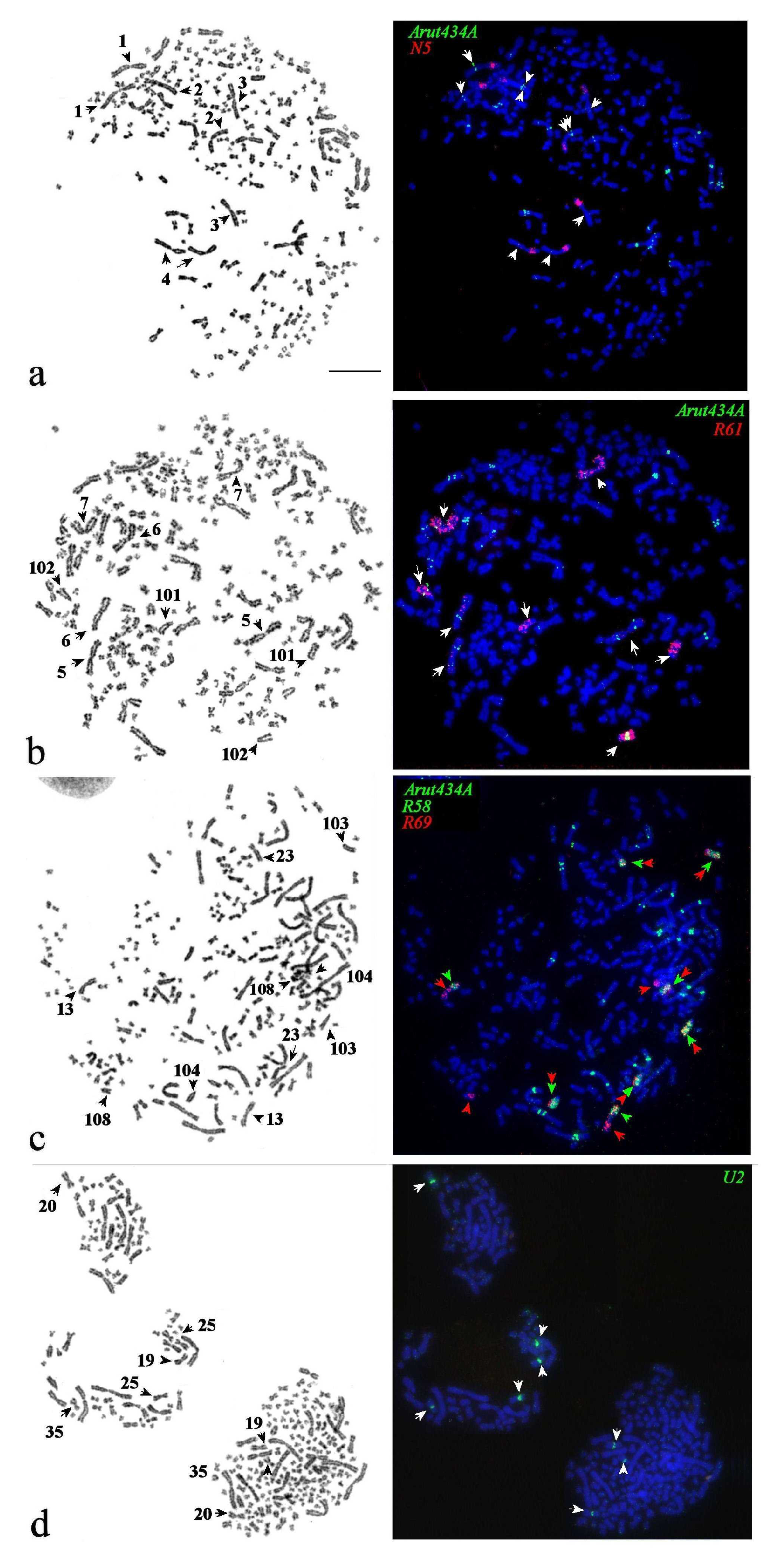
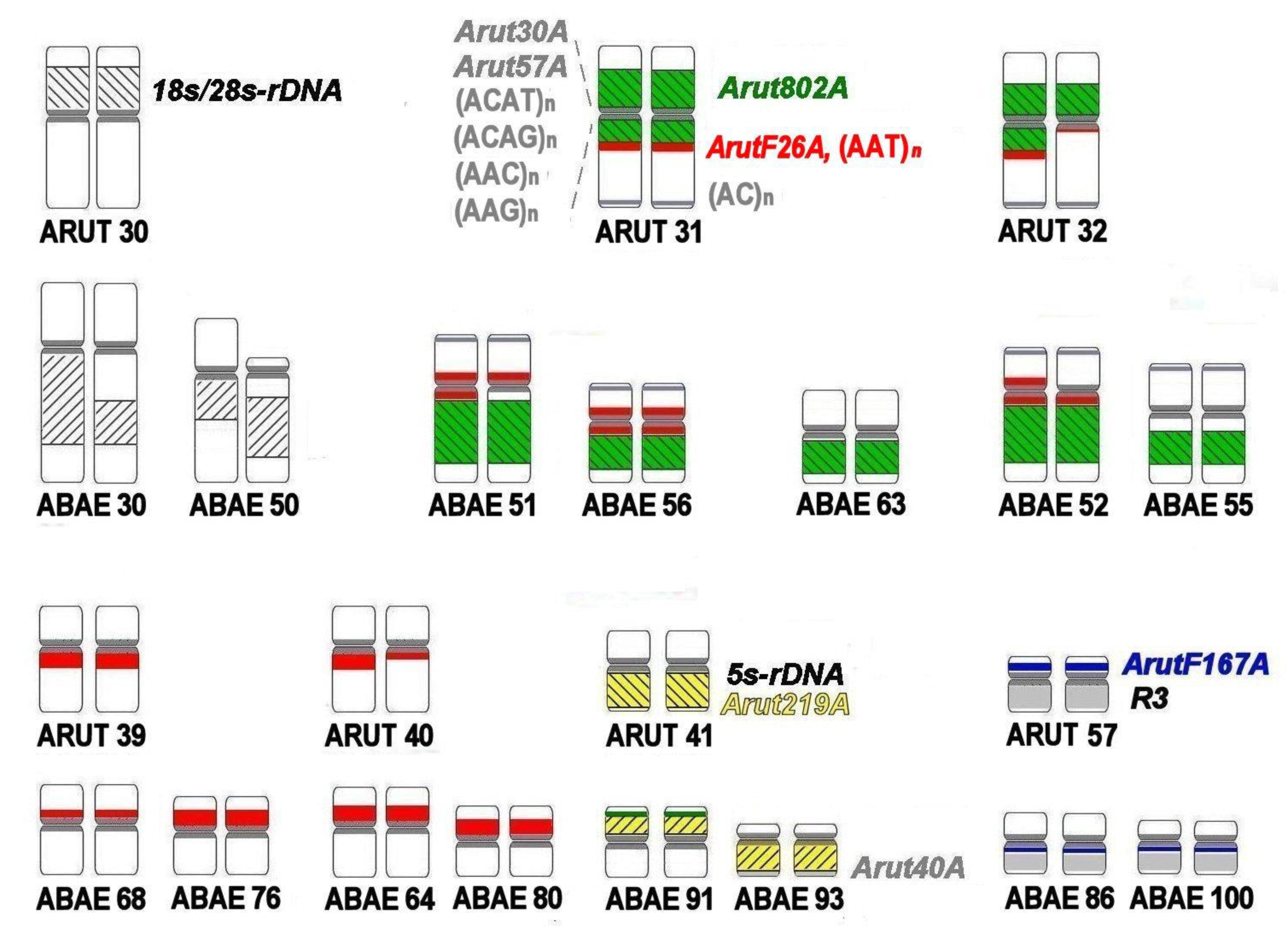
3.4. Localization of Arut19A, Arut30A, Arut40A, and Arut57A Satellite DNA Probes on Chromosomes of A. baerii
3.5. Satellites with Chromosome-Specific Location
3.6. NOR-Bearing Chromosome-Specific Repeats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ludwig, A.; Belfiore, N.M.; Pitra, C.; Svirsky, V.; Jenneckens, I. Genome Duplication Events and Functional Reduction of Ploidy Levels in Sturgeon (Acipenser, Huso and Scaphirhynchus). Genetics 2001, 158, 1203–1215. [Google Scholar] [PubMed]
- Krieger, J.; Hett, A.K.; Fuerst, P.A.; Artyukhin, E.; Ludwig, A. The Molecular Phylogeny of the Order Acipenseriformes Revisited. J. Appl. Ichthyol. 2008, 24, 36–45. [Google Scholar] [CrossRef]
- Crow, K.D.; Smith, C.D.; Cheng, J.-F.; Wagner, G.P.; Amemiya, C.T. An Independent Genome Duplication Inferred from Hox Paralogs in the American Paddlefish—A Representative Basal Ray-Finned Fish and Important Comparative Reference. Genome Biol. Evol. 2012, 4, 937–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, F.; Tagliavini, J.; Congiu, L. Sturgeon Genetics and Cytogenetics: Recent Advancements and Perspectives. Genetics 2001, 111, 359–373. [Google Scholar] [CrossRef]
- Zhou, H.; Fujimoto, T.; Adachi, S.; Yamaha, E.; Arai, K. Genome Size Variation Estimated by Flow Cytometry in Acipenser Mikadoi, Huso Dauricus in Relation to Other Species of Acipenseriformes. J. Appl. Ichthyol. 2011, 27, 484–491. [Google Scholar] [CrossRef]
- Du, K.; Stöck, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.I.; Kichigin, I.; et al. The Sterlet Sturgeon Genome Sequence and the Mechanisms of Segmental Rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Trifonov, V.A.; Romanenko, S.S.; Beklemisheva, V.R.; Biltueva, L.S.; Makunin, A.I.; Lemskaya, N.A.; Kulemzina, A.I.; Stanyon, R.; Graphodatsky, A.S. Evolutionary Plasticity of Acipenseriform Genomes. Chromosoma 2016, 125, 661–668. [Google Scholar] [CrossRef]
- Andreyushkova, D.A.; Makunin, A.I.; Beklemisheva, V.R.; Romanenko, S.A.; Druzhkova, A.S.; Biltueva, L.B.; Serdyukova, N.A.; Graphodatsky, A.S.; Trifonov, V.A. Next Generation Sequencing of Chromosome-Specific Libraries Sheds Light on Genome Evolution in Paleotetraploid Sterlet (Acipenser ruthenus). Genes 2017, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.; Muramoto, J.; Stenius, C.; Christian, L.; Kittrell, W.A.; Atkin, N.B. Microchromosomes in Holocephalian, Chondrostean and Holostean Fishes. Chromosoma 1969, 26, 35–40. [Google Scholar] [CrossRef]
- Kim, D.S.; Nam, Y.K.; Noh, J.K.; Park, C.H.; Chapman, F.A. Karyotype of North American Shortnose Sturgeon Acipenser Brevirostrum With the Highest Chromosome Number in the Acipenseriformes. Ichthyol. Res. 2005, 52, 94–97. [Google Scholar] [CrossRef]
- Dingerkus, G.; Howell, W. Karyotypic Analysis and Evidence of Tetraploidy in the North American Paddlefish, Polyodon Spathula. Science 1976, 194, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Lanfredi, M.; Rossi, R.; Bronzi, P.; Arlati, G. Karyotypic Characterization of Acipenser Gueldenstaedti with C-, AgNO3, and Fluorescence Banding Techniques. Ital. J. Zool. 1996, 63, 113–118. [Google Scholar] [CrossRef]
- Eenennaam, A.; Murray, J.; Medrano, J. Mitotic Analysis of the North American White Sturgeon, Acipenser Transmontanus Richardson (Pisces, Acipenseridae), a Fish with a Very High Chromosome Number. Genome 1998, 41, 266–271. [Google Scholar] [CrossRef]
- Fontana, F.; Lanfredi, M.; Congiu, L.; Leis, M.; Chicca, M.; Rossi, R. Chromosomal Mapping of 18S–28S and 5S rRNA Genes by Two-Colour Fluorescent in Situ Hybridization in Six Sturgeon Species. Genome 2003, 46, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Drouin, G.; De Sá, M.M. The Concerted Evolution of 5s Ribosomal Genes Linked to the Repeat Units of Other Multigene Families. Mol. Biol. Evol. 1995, 12, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, W.F.; Amorim, K.D.J.; Cioffi, M.B.; Bertollo, L.A.C.; Soares, R.X.; De Souza, A.S.; Da Costa, G.W.W.F. Co-located 18S/5S rDNA Arrays: An Ancient and Unusual Chromosomal Trait in Julidini Species (Labridae, Perciformes). Comp. Cytogenet. 2016, 10, 555–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barros, L.C.; Junior, P.M.G.; Feldberg, E. Mapping 45S and 5S Ribosomal Genes in Chromosomes of Anostomidae Fish Species (Ostariophysi, Characiformes) From Different Amazonian Water Types. Hydrobiologia 2016, 789, 77–89. [Google Scholar] [CrossRef]
- Pisano, E.; Ghigliotti, L. Ribosomal Genes in Notothenioid Fishes: Focus on the Chromosomal Organisation. Mar. Genom. 2009, 2, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Hett, A.K.; Fuerst, P.A.; Birstein, V.J.; Ludwig, A. Unusual Intraindividual Variation of the Nuclear 18S rRNA Gene is Widespread Within the Acipenseridae. J. Hered. 2006, 97, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnason, U.; Grétarsdóttir, S.; Widegren, B. Mysticete (Baleen Whale) Relationships Based Upon the Sequence of the Common Cetacean DNA Satellite. Mol. Biol. Evol. 1992, 9, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- De La Herrán, R.; Fontana, F.; Lanfredi, M.; Congiu, L.; Leis, M.; Rossi, R.; Rejón, C.R.; Rejón, M.R.; Garrido-Ramos, M.A. Slow Rates of Evolution and Sequence Homogenization in an Ancient Satellite DNA Family of Sturgeons. Mol. Biol. Evol. 2001, 18, 432–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, F.; De La Herrán, R.; Ludwig, A.; Rejón, C.R.; Rejón, M.R.; Garrido-Ramos, M.A. Evolution of Ancient Satellite DNAs in Sturgeon Genomes. Gene 2004, 338, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lanfredi, M.; Congiu, L.; Garrido-Ramos, M.A.; De La Herrán, R.; Leis, M.; Chicca, M.; Rossi, R.; Tagliavini, J.; Rejón, C.R.; Rejón, M.R.; et al. Chromosomal Location and Evolution of a Satellite DNA Family in Seven Sturgeon Species. Chromosom. Res. 2001, 9, 47–52. [Google Scholar] [CrossRef]
- Fontana, F.; Lanfredi, M.; Kirschbaum, F.; Garrido-Ramos, M.A.; Robles, F.; Forlani, A.; Congiu, L. Comparison of Karyotypes of Acipenser Oxyrinchus and a. Sturio by Chromosome Banding and Fluorescent in Situ Hybridization. Genetica 2007, 132, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Koop, B.F.; Sandve, S.R.; Miller, J.R.; Kent, M.P.; Nome, T.; Hvidsten, T.R.; Leong, J.S.; Minkley, D.R.; Zimin, A.; et al. The Atlantic Salmon Genome Provides Insights Into Rediploidization. Nature 2016, 533, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Chalopin, D.; Volff, J.-N. Analysis of the Spotted Gar Genome Suggests Absence of Causative Link Between Ancestral Genome Duplication and Transposable Element Diversification in Teleost Fish. J. Exp. Zool. Part B Mol. Dev. Evol. 2017, 328, 629–637. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Biltueva, L.B.; Serdyukova, N.A.; Kulemzina, A.; Beklemisheva, V.R.; Gladkikh, O.L.; Lemskaya, N.A.; Interesova, E.A.; Korentovich, M.A.; Vorobieva, N.V.; et al. Segmental Paleotetraploidy Revealed in Sterlet (Acipenser Ruthenus) Genome by Chromosome Painting. Mol. Cytogenet. 2015, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Biltueva, L.S.; Prokopov, D.Y.; Makunin, A.I.; Komissarov, A.S.; Kudryavtseva, A.V.; Lemskaya, N.A.; Vorobieva, N.V.; Serdyukova, N.A.; Romanenko, S.A.; Gladkikh, O.L.; et al. Genomic Organization and Physical Mapping of Tandemly Arranged Repetitive DNAs in Sterlet (Acipenser ruthenus). Cytogenet. Genome Res. 2017, 152, 148–157. [Google Scholar] [CrossRef]
- Bishani, A.; Prokopov, D.Y.; Romanenko, S.A.; Molodtseva, A.S.; Perelman, P.L.; Interesova, E.A.; Beklemisheva, V.R.; Graphodatsky, A.S.; Trifonov, V.A. Evolution of Tandemly Arranged Repetitive DNAs in Three Species of Cyprinoidei With Different Ploidy Levels. Cytogenet. Genome Res. 2020, in press. [Google Scholar]
- Seabright, M. A Rapid Banding Technique for Human Chromosomes. Lancet 1971, 298, 971–972. [Google Scholar] [CrossRef]
- Sumner, A. A Simple Technique for Demonstrating Centromeric Heterochromatin. Exp. Cell Res. 1972, 75, 304–306. [Google Scholar] [CrossRef]
- Vasilyev, V.P.; Sokolov, L.I.; Serebryakova, Y. Karyotypes of the Siberian Sturgeon, Acipenser Baeri of the Lena River and Some Aspects of Karyotype Evolution in Acipenseriformes. Available online: http://sveb.unife.it/it/ricerca-1/laboratori/geneweb/testi/karyotypes-of-the-siberian-sturgeon-acipenser-baeri-of-the-lena-river-and-some-aspects-of-karyotype-evolution-in-acipenseriformes (accessed on 11 September 2020).
- Fontana, F. Chromosomal Nucleolar Organizer Regions in Four Sturgeon Species as Markers of Karyotype Evolution in Acipenseriformes (Pisces). Genome 1994, 37, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, Y.; Zhao, Q.; Zhao, L.; Ludwig, A.; Peng, Z. Highly Resolved Phylogenetic Relationships within Order Acipenseriformes According to Novel Nuclear Markers. Genes 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, F.; Colombo, G. The Chromosomes of Italian Sturgeons. Experientia 1974, 30, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Birstein, V.J.; Vasiliev, V.P. Tetraploid-Octoploid Relationships and Karyological Evolution in the Order Acipenseriformes (Pisces) Karyotypes, Nucleoli, and Nucleolus-Organizer Regions in Four Acipenserid Species. Genetica 1987, 72, 3–12. [Google Scholar] [CrossRef]
- Zhou, G.; Gui, L.; Li, Z.Q.; Yuan, X.P.; Zhang, Q.-Y. Establishment of a Chinese Sturgeon Acipenser Sinensistail-Fin Cell Line and Its Susceptibility to Frog Iridovirus. J. Fish Biol. 2008, 73, 2058–2067. [Google Scholar] [CrossRef]
- Peng, Z.; Ludwig, A.; Wang, D.; Diogo, R.; Wei, Q.; He, S. Age and Biogeography of Major Clades in Sturgeons and Paddlefishes (Pisces: Acipenseriformes). Mol. Phylogenet. Evol. 2007, 42, 854–862. [Google Scholar] [CrossRef]


| Species | Ploidy Level | Chromosome Number | Number of Chromosomes Carrying Molecular Markers, or Their Presence (+)/Absence (−) in the Genome | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Ag-NOR | 18S/28S rDNA | 5S rDNA | HindIII satDNA | PstI satDNA | ||||
| Polyodon spathula, | 2n | 120 | 4 | 6 | 2 | - | - | [11,22] |
| Scaphirhynchus Platorynchus | 2n | 112 | [1] | |||||
| A. sturio1 | 2n | 121 ± 3 | 6 | 8 | 2 | - | + | [22,23,24] |
| A. oxyrinchus1 | 2n | 121 ± 3 | - | + | [22,24] | |||
| Huso huso2 | 2n | 116 ± 4 | 4 | 6 | 2 | 8 | + | [1,22,23] |
| A. stellatus2 | 2n | 118 ± 2 | 6 | 6-8 | 10 | 4 | [1,22] | |
| A. ruthenus2 | 2n | 118 ± 2 | 4 | 6 | 2 | 2(8) | + | [1,8,22,23,35] |
| A. fulvescens2 | 4n | 262 ± 6 | + | [22,33] | ||||
| A. brevirostrum2 | 6n | 372 | + | [10,22] | ||||
| A. baerii2 | 4n | 249 ± 5 | 10–12 | 4 | 38 ± 3 | + | [22,23], this study | |
| A. naccarii2 | 4n | 239 ± 7 | 10–12 | 4 | 50 ± 4 | + | [22,23,36] | |
| A. gueldenstaedtii2 | 4n | 250 ± 8 | 4 | 80 ± 4 | 12 | [22,23,36] | ||
| A. sinensis3 | 4n | 264 ± 4 | + | [22,37] | ||||
| A. transmontanus3 | 4n | 248 ± 8 | 10–12 | 60 | + | [13,22,23] | ||
| NN | Chromosome Numbers | Chromosome Rearrangements | ||
|---|---|---|---|---|
| AcA, 2n ~ 60 | ARUT, 2n ~ 120 | ABAE, 2n ~ 250 | ||
| 1 | 1 | 1 | 1; 2 | |
| 2 | 3; 4 | |||
| 2 | 2 | 3 | 5; 6 | centromeric fission of one ortholog of ARUT 4 resulted in two acrocentrics: ABAE 101 and 102 |
| 4 | 7; 101;102 | |||
| 3 | 3 | 5 | 8; 9 | |
| 6 | 10; 11 | |||
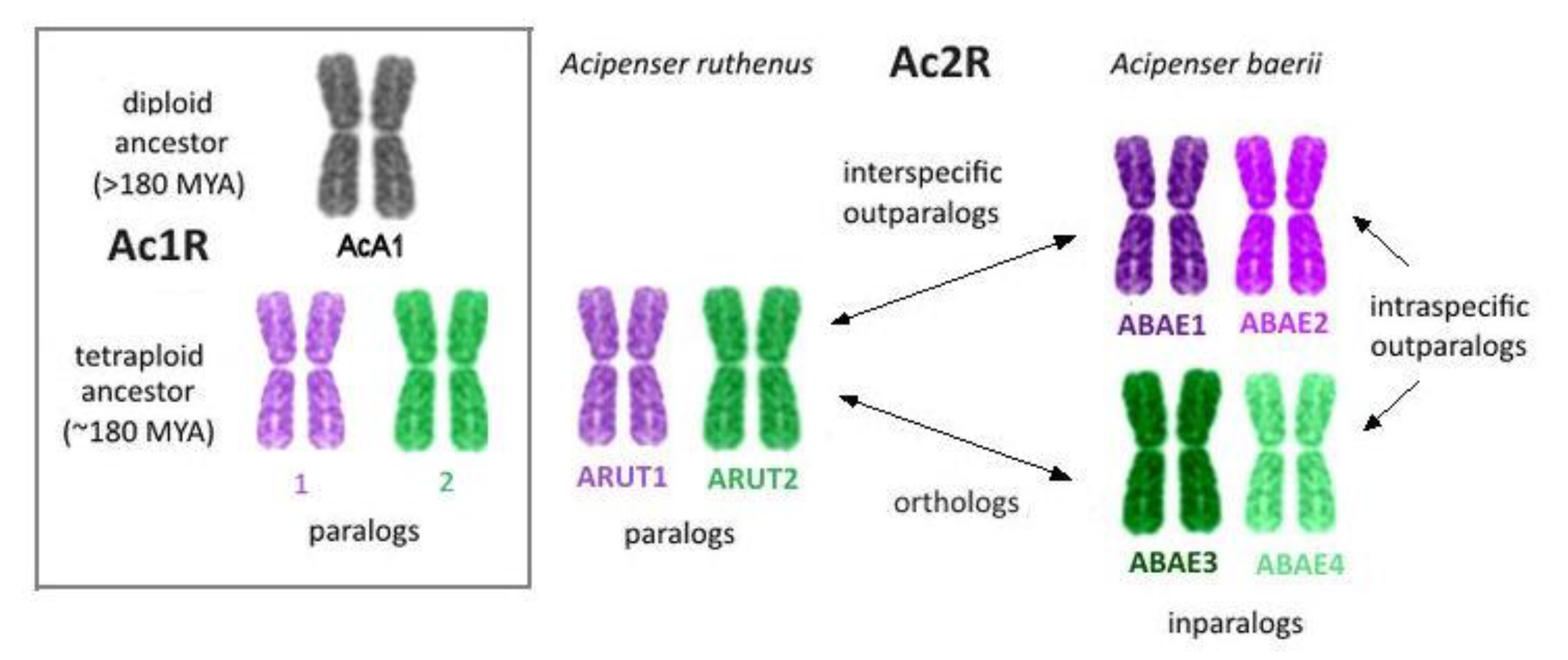
| 4 | 4 | 7 | 13; 23;108 | centromeric fission of one ortholog of the putative ancestral chromosome 4 formed two acrocentrics: ARUT 7q and 14; fusion of ARUT 7q and 7p; centromeric fission of one ortholog of ARUT 7 with formation of 2 acrocentrics: ABAE 23q and 108; fusion of ABAE 23q and 23p |
| 14 | 103; 104 | |||
| 5 | 5 | 8 | 12; 14 | |
| 9 | 15; 16 | |||
| 6 | 6 | 10cent | 19p cent | |
| 20p cent | ||||
| 12cent | 25pq cent | |||
| 35pq cent | ||||
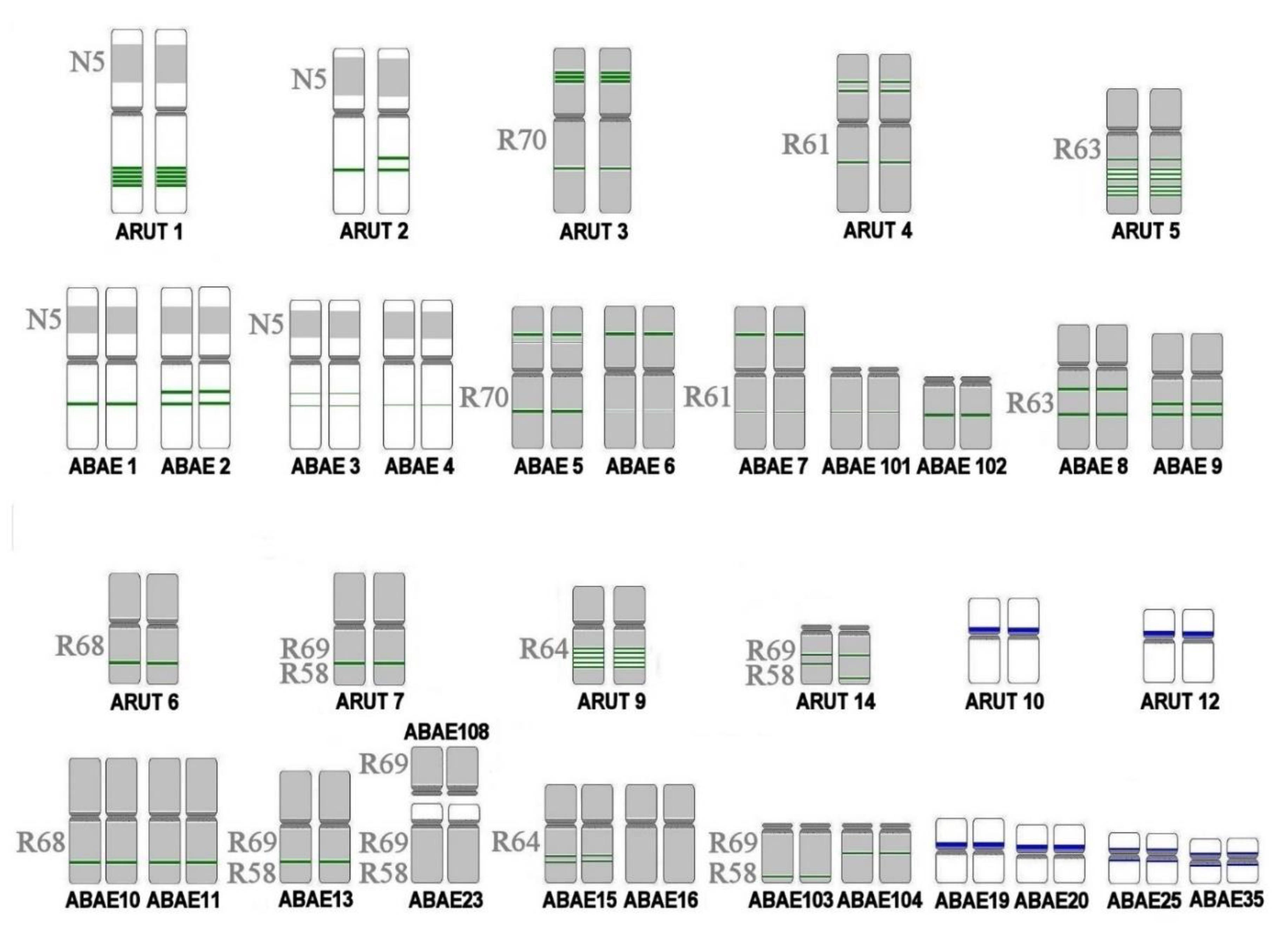
| 7 | 15 | 30 | 30; 50 | fusion of ABAE 30q and 30p; loss of ABAE 50p in one of the homologues |
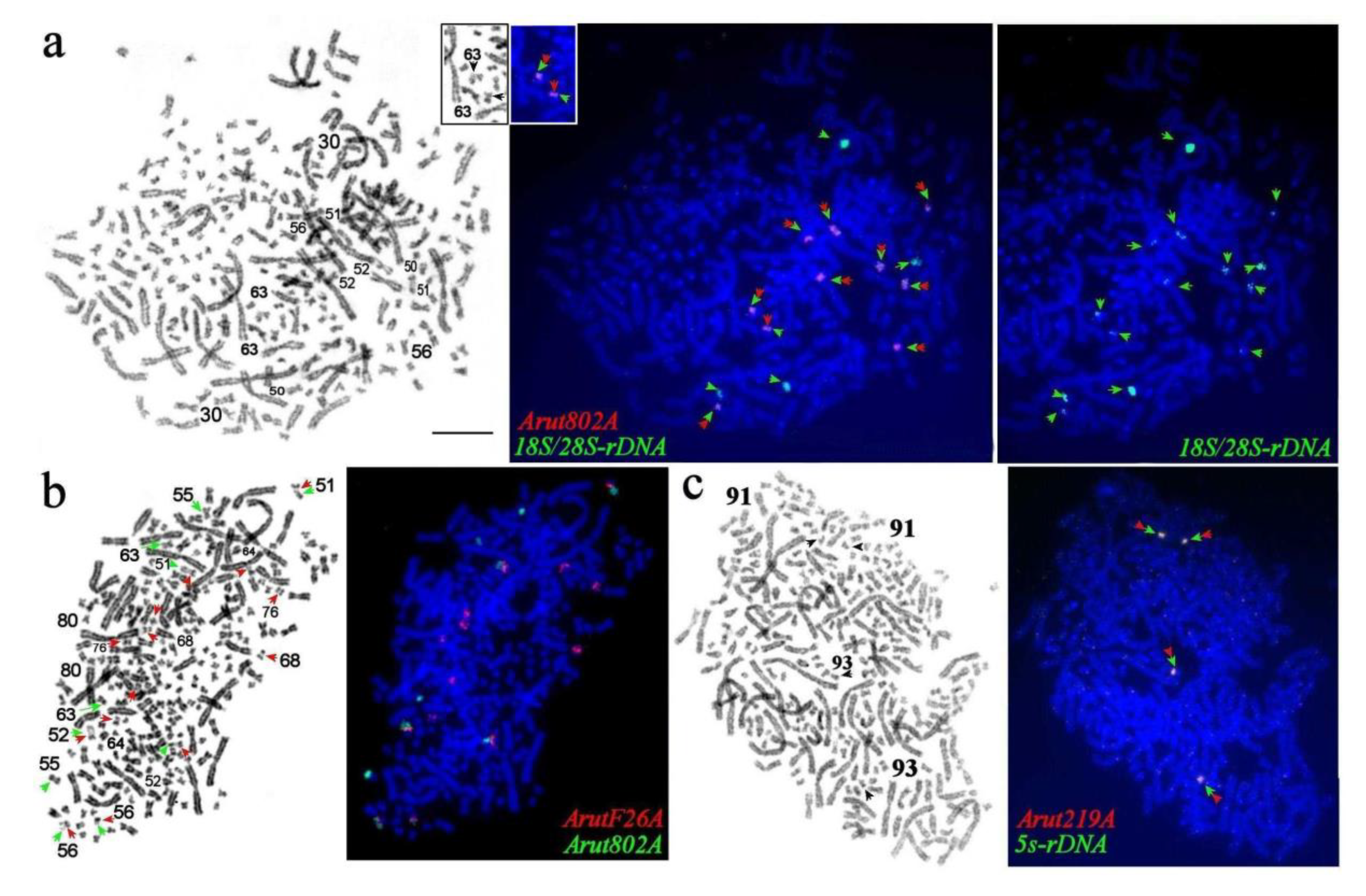
| 8 | 16 | 31 | 51; 56 | fissions of ABAE 55 and 56; formation of a new site of 18S/28S rDNA on ABAE 63 |
| 63 | ||||
| 32 | ||||
| 52; 55 | ||||
| 9 | 20 | 39 | 68; 76 | fusion in orthologs ARUT 39 and 40, resulting in the appearance of ABAE 64 and 68 which are bigger than their inparalogs ABAE 80 and 76, respectively |
| 40 | 64; 80 | |||
| 10 | 21 | 41 | 91; 93 | fission of one of ARUT 41 ortholog resulted in the appearance of small submetacentric ABAE 93, which is almost twice smaller than its inparalog ABAE 91 |
| 11 | 29 | 57 | 86q; 100 | fusion of ABAE 86q and 86p |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biltueva, L.S.; Prokopov, D.Y.; Romanenko, S.A.; Interesova, E.A.; Schartl, M.; Trifonov, V.A. Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii). Genes 2020, 11, 1375. https://doi.org/10.3390/genes11111375
Biltueva LS, Prokopov DY, Romanenko SA, Interesova EA, Schartl M, Trifonov VA. Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii). Genes. 2020; 11(11):1375. https://doi.org/10.3390/genes11111375
Chicago/Turabian StyleBiltueva, Larisa S., Dmitry Yu. Prokopov, Svetlana A. Romanenko, Elena A. Interesova, Manfred Schartl, and Vladimir A. Trifonov. 2020. "Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii)" Genes 11, no. 11: 1375. https://doi.org/10.3390/genes11111375
APA StyleBiltueva, L. S., Prokopov, D. Y., Romanenko, S. A., Interesova, E. A., Schartl, M., & Trifonov, V. A. (2020). Chromosome Distribution of Highly Conserved Tandemly Arranged Repetitive DNAs in the Siberian Sturgeon (Acipenser baerii). Genes, 11(11), 1375. https://doi.org/10.3390/genes11111375






