Intracellular Trafficking of HBV Particles
Abstract
:1. Introduction
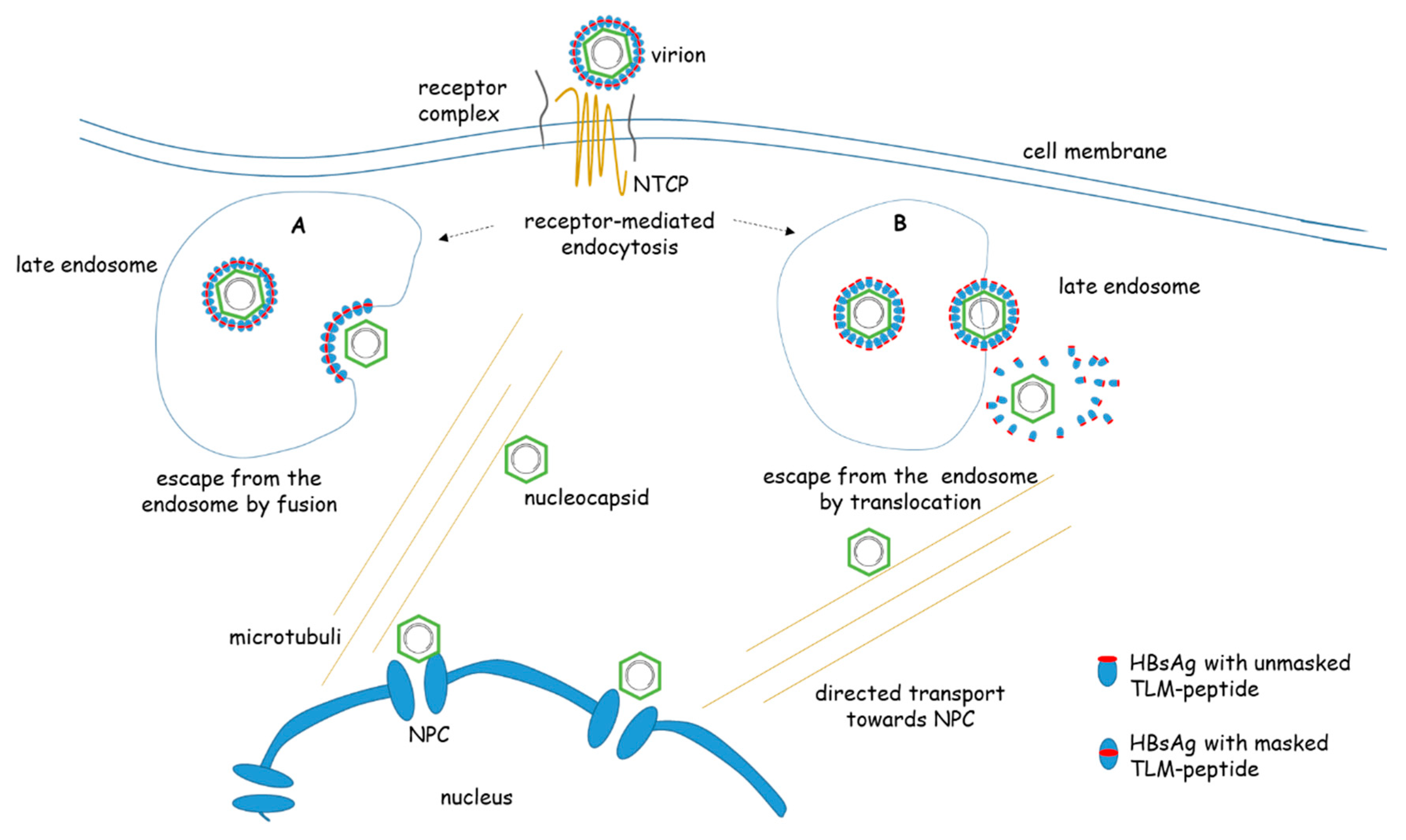
2. Intracellular Cytoplasmic Transport after Viral Uptake
3. Nuclear Transport
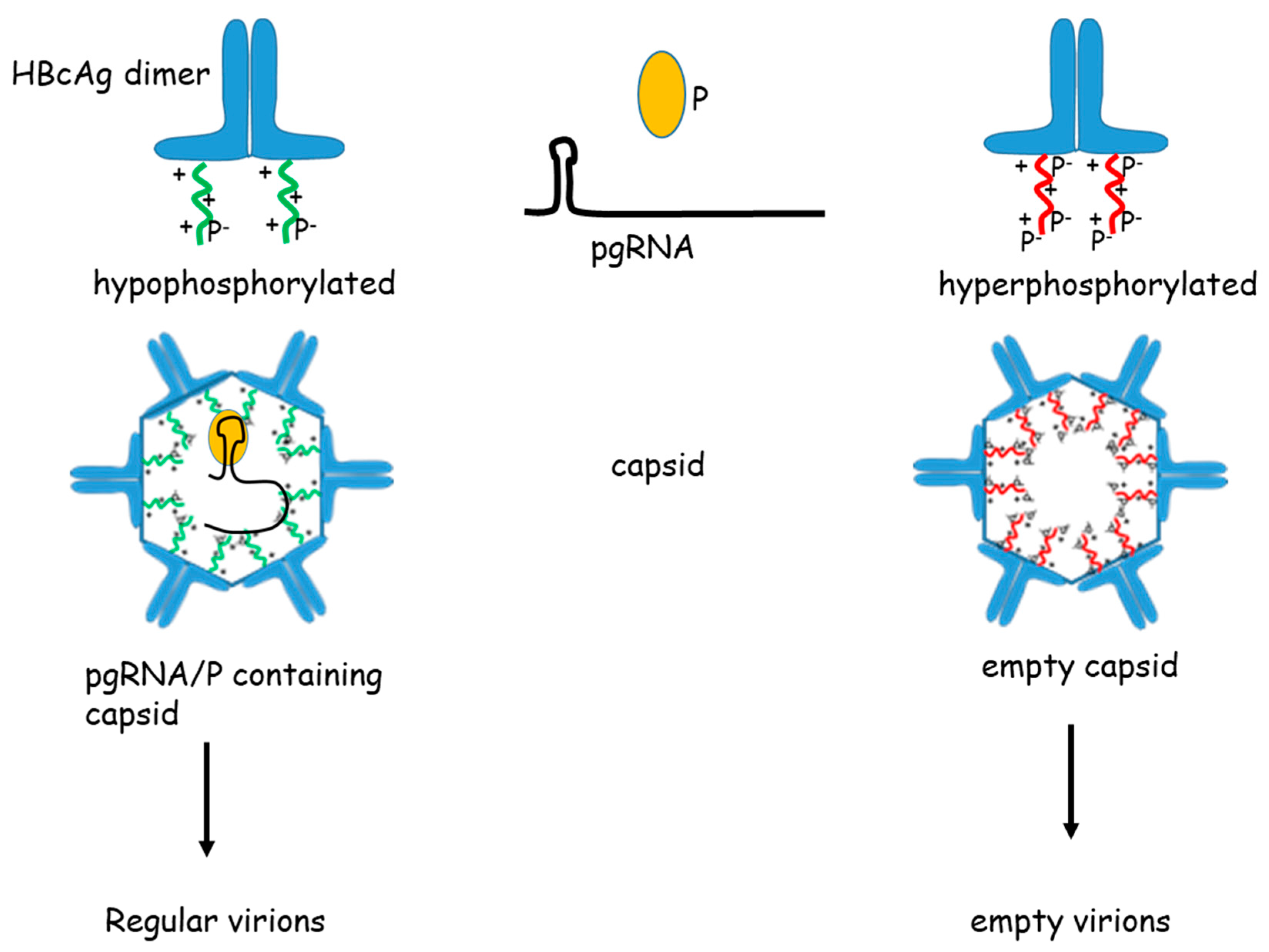
4. Capsid Maturation
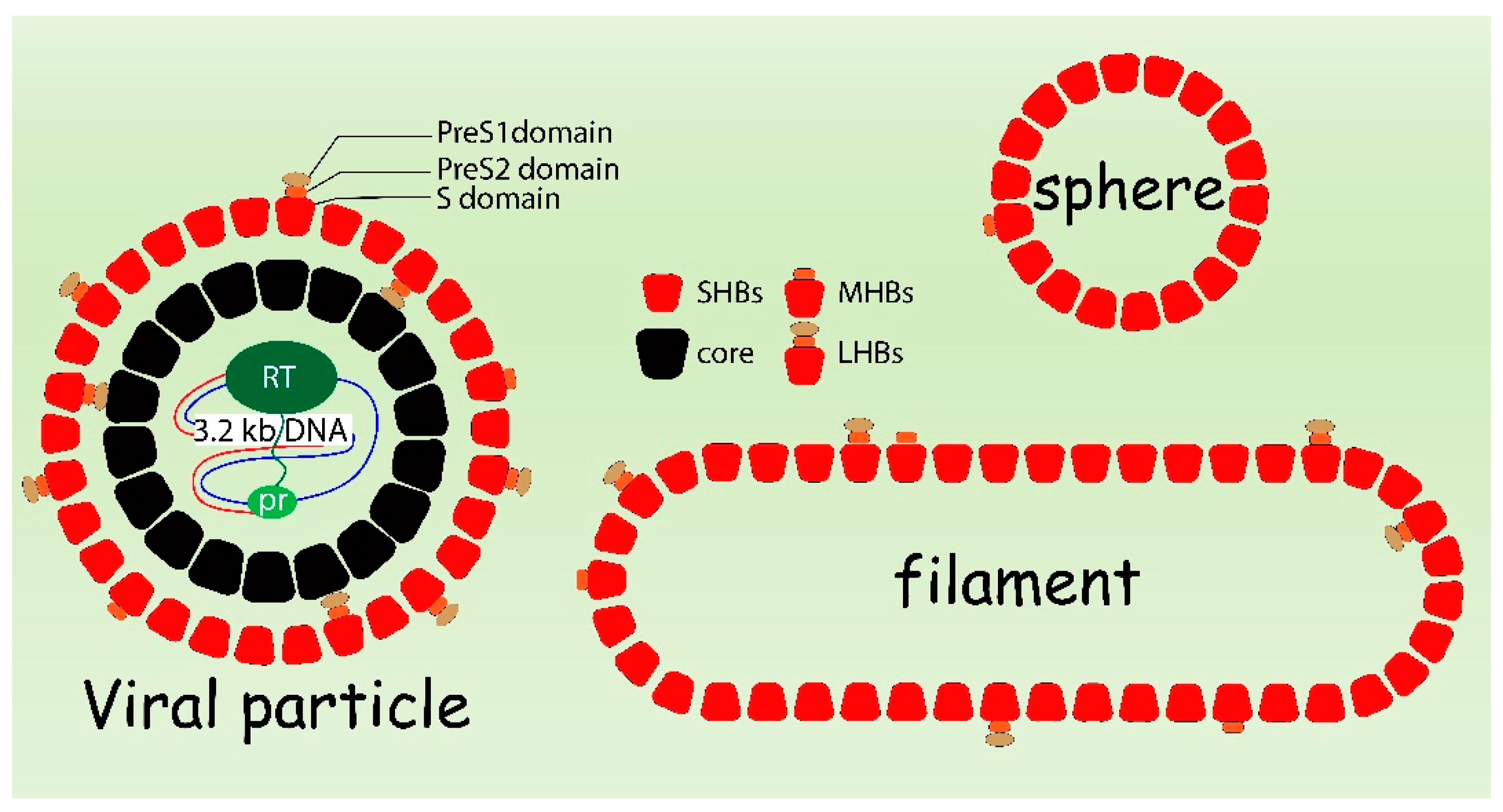
5. Envelopment
6. Release of HBV Subviral Spheres
7. Release of HBV Viral Particles and Filaments
8. Release of Naked Capsids
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gough, N.M. Core and E antigen synthesis in rodent cells transformed with hepatitis B virus DNA is associated with greater than genome length viral messenger RNAs. J. Mol. Biol. 1983, 165, 683–699. [Google Scholar] [CrossRef]
- Treinin, M.; Laub, O. Identification of a promoter element located upstream from the hepatitis B virus X gene. Mol. Cell. Biol. 1987, 7, 545–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, R.; Will, H.; Schaller, H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984, 3, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Eble, B.E.; Lingappa, V.R.; Ganem, D. Hepatitis B surface antigen: An unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol. Cell. Biol. 1986, 6, 1454–1463. [Google Scholar] [CrossRef]
- Eble, B.E.; MacRae, D.R.; Lingappa, V.R.; Ganem, D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol. Cell. Biol. 1987, 7, 3591–3601. [Google Scholar] [CrossRef]
- Short, J.M.; Chen, S.; Roseman, A.M.; Butler, P.J.G.; Crowther, R.A. Structure of hepatitis B surface antigen from subviral tubes determined by electron cryomicroscopy. J. Mol. Biol. 2009, 390, 135–141. [Google Scholar] [CrossRef]
- Eble, B.E.; Lingappa, V.R.; Ganem, D. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J. Virol. 1990, 64, 1414–1419. [Google Scholar] [CrossRef] [Green Version]
- Bruss, V.; Hagelstein, J.; Gerhardt, E.; Galle, P.R. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 1996, 218, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Gripon, P.; Le Seyec, J.; Rumin, S.; Guguen-Guillouzo, C. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 1995, 213, 292–299. [Google Scholar] [CrossRef]
- Ostapchuk, P.; Hearing, P.; Ganem, D. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 1994, 13, 1048–1057. [Google Scholar] [CrossRef]
- Bruss, V.; Lu, X.; Thomssen, R.; Gerlich, W.H. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 1994, 13, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Prange, R.; Streeck, R.E. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 1995, 14, 247–256. [Google Scholar] [CrossRef]
- Le Seyec, J.; Chouteau, P.; Cannie, I.; Guguen-Guillouzo, C.; Gripon, P. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 1999, 73, 2052–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruss, V. A short linear sequence in the pre-S domain of the large hepatitis B virus envelope protein required for virion formation. J. Virol. 1997, 71, 9350–9357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildt, E.; Munz, B.; Saher, G.; Reifenberg, K.; Hofschneider, P.H. The PreS2 activator MHBst of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002, 21, 525–535. [Google Scholar] [CrossRef]
- Hildt, E.; Saher, G.; Bruss, V.; Hofschneider, P.H. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology 1996, 225, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponsel, D.; Bruss, V. Mapping of Amino Acid Side Chains on the Surface of Hepatitis B Virus Capsids Required for Envelopment and Virion Formation. J. Virol. 2003, 77, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koschel, M.; Thomssen, R.; Bruss, V. Extensive Mutagenesis of the Hepatitis B Virus Core Gene and Mapping of Mutations That Allow Capsid Formation. J. Virol. 1999, 73, 2153–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selzer, L.; Katen, S.P.; Zlotnick, A. The Hepatitis B Virus Core Protein Intradimer Interface Modulates Capsid Assembly and Stability. Biochemistry 2014, 53, 5496–5504. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.F.; Cheng, N.; Zlotnick, A.; Wingfield, P.T.; Stahl, S.J.; Steven, A.C. Visualization of a 4-helix bundle in the hepatitis B virus capsid by cryo-electron microscopy. Nature 1997, 386, 91–94. [Google Scholar] [CrossRef]
- Böttcher, B.; Wynne, S.A.; Crowther, R.A. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 1997, 386, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Standring, D.N. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc. Natl. Acad. Sci. USA 1992, 89, 10046–10050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassal, M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J. Virol. 1992, 66, 4107–4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlotnick, A.; Cheng, N.; Conway, J.F.; Booy, F.P.; Steven, A.C.; Stahl, S.J.; Wingfield, P.T. Dimorphism of hepatitis B virus capsids is strongly influenced by the C-terminus of the capsid protein. Biochemistry 1996, 35, 7412–7421. [Google Scholar] [CrossRef] [PubMed]
- Crowther, R. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 1994, 77, 943–950. [Google Scholar] [CrossRef]
- Roseman, A.M.; Berriman, J.A.; Wynne, S.A.; Butler, P.J.G.; Crowther, R.A. A structural model for maturation of the hepatitis B virus core. Proc. Natl. Acad. Sci. USA 2005, 102, 15821–15826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dryden, K.A.; Wieland, S.F.; Whitten-Bauer, C.; Gerin, J.L.; Chisari, F.V.; Yeager, M. Native hepatitis B virions and capsids visualized by electron cryomicroscopy. Mol. Cell 2006, 22, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Lewellyn, E.B.; Loeb, D.D. The arginine clusters of the carboxy-terminal domain of the core protein of hepatitis B virus make pleiotropic contributions to genome replication. J. Virol. 2011, 85, 1298–1309. [Google Scholar] [CrossRef] [Green Version]
- Rat, V.; Pinson, X.; Seigneuret, F.; Durand, S.; Herrscher, C.; Lemoine, R.; Burlaud-Gaillard, J.; Raynal, P.-Y.; Hourioux, C.; Roingeard, P.; et al. Hepatitis B Virus Core Protein Domains Essential for Viral Capsid Assembly in a Cellular Context. J. Mol. Biol. 2020, 432, 3802–3819. [Google Scholar] [CrossRef]
- Ludgate, L.; Liu, K.; Luckenbaugh, L.; Streck, N.; Eng, S.; Voitenleitner, C.; Delaney, W.E.; Hu, J. Cell-Free Hepatitis B Virus Capsid Assembly Dependent on the Core Protein C-Terminal Domain and Regulated by Phosphorylation. J. Virol. 2016, 90, 5830–5844. [Google Scholar] [CrossRef] [Green Version]
- Gallina, A.; Bonelli, F.; Zentilin, L.; Rindi, G.; Muttini, M.; Milanesi, G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J. Virol. 1989, 63, 4645–4652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlotnick, A.; Cheng, N.; Stahl, S.J.; Conway, J.F.; Steven, A.C.; Wingfield, P.T. Localization of the C terminus of the assembly domain of hepatitis B virus capsid protein: Implications for morphogenesis and organization of encapsidated RNA. Proc. Natl. Acad. Sci. USA 1997, 94, 9556–9561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Ludgate, L.; Yuan, Z.; Hu, J. Regulation of multiple stages of hepadnavirus replication by the carboxyl-terminal domain of viral core protein in trans. J. Virol. 2015, 89, 2918–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, X.; Basagoudanavar, S.H.; Liu, K.; Luckenbaugh, L.; Wei, D.; Wang, C.; Wei, B.; Zhao, Y.; Yan, T.; Delaney, W.; et al. Capsid Phosphorylation State and Hepadnavirus Virion Secretion. J. Virol. 2017, 91, e00092-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-C.; Huang, E.-Y.; Su, P.-Y.; Wu, S.-Y.; Yang, C.-C.; Lin, Y.-S.; Chang, W.-C.; Shih, C. Nuclear export and import of human hepatitis B virus capsid protein and particles. PLoS Pathog. 2010, 6, e1001162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckhardt, S.G.; Milich, D.R.; McLachlan, A. Hepatitis B virus core antigen has two nuclear localization sequences in the arginine-rich carboxyl terminus. J. Virol. 1991, 65, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlotnick, A.; Venkatakrishnan, B.; Tan, Z.; Lewellyn, E.; Turner, W.; Francis, S. Core protein: A pleiotropic keystone in the HBV lifecycle. Antivir. Res. 2015, 121, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Watts, N.R.; Conway, J.F.; Cheng, N.; Stahl, S.J.; Belnap, D.M.; Steven, A.C.; Wingfield, P.T. The morphogenic linker peptide of HBV capsid protein forms a mobile array on the interior surface. EMBO J. 2002, 21, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, F.; Nassal, M. Hepatitis B virus nucleocapsid assembly: Primary structure requirements in the core protein. J. Virol. 1990, 64, 3319–3330. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Luckenbaugh, L.; Ning, X.; Xi, J.; Hu, J. Multiple roles of core protein linker in hepatitis B virus replication. PLoS Pathog. 2018, 14, e1007085. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Watts, N.R.; Cheng, N.; Huang, R.; Steven, A.C.; Wingfield, P.T. Expression of quasi-equivalence and capsid dimorphism in the Hepadnaviridae. PLoS Comput. Biol. 2020, 16, e1007782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; White, S.J.; Thompson, R.F.; Bingham, R.; Weiß, E.U.; Maskell, D.P.; Zlotnick, A.; Dykeman, E.; Tuma, R.; Twarock, R.; et al. HBV RNA pre-genome encodes specific motifs that mediate interactions with the viral core protein that promote nucleocapsid assembly. Nat. Microbiol. 2017, 2, 17098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef] [PubMed]
- Gripon, P.; Cannie, I.; Urban, S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 2005, 79, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Falco, S.; Ruvo, M.; Verdoliva, A.; Scarallo, A.; Raimondo, D.; Raucci, A.; Fassina, G. N-terminal myristylation of HBV preS1 domain enhances receptor recognition. J. Pept. Res. 2001, 57, 390–400. [Google Scholar] [CrossRef]
- Offensperger, W.-B.; Offensperger, S.; Walter, E.; Blum, H.E.; Gerok, W. Inhibition of duck hepatitis B virus infection by lysosomotropic agents. Virology 1991, 183, 415–418. [Google Scholar] [CrossRef]
- Macovei, A.; Radulescu, C.; Lazar, C.; Petrescu, S.; Durantel, D.; Dwek, R.A.; Zitzmann, N.; Nichita, N.B. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J. Virol. 2010, 84, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrscher, C.; Pastor, F.; Burlaud-Gaillard, J.; Dumans, A.; Seigneuret, F.; Moreau, A.; Patient, R.; Eymieux, S.; de Rocquigny, H.; Hourioux, C.; et al. Hepatitis B virus entry into HepG2-NTCP cells requires clathrin-mediated endocytosis. Cell. Microbiol. 2020, 22, e13205. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chen, C.-C.; Chang, W.-C.; Tao, M.-H.; Huang, C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J. Virol. 2012, 86, 9443–9453. [Google Scholar] [CrossRef] [Green Version]
- Macovei, A.; Petrareanu, C.; Lazar, C.; Florian, P.; Branza-Nichita, N. Regulation of hepatitis B virus infection by Rab5, Rab7, and the endolysosomal compartment. J. Virol. 2013, 87, 6415–6427. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.-H.; et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, M.; Saso, W.; Nishioka, K.; Ohashi, H.; Sugiyama, R.; Ryo, A.; Ohki, M.; Yun, J.-H.; Park, S.-Y.; Ohshima, T.; et al. The machinery for endocytosis of epidermal growth factor receptor coordinates the transport of incoming hepatitis B virus to the endosomal network. J. Biol. Chem. 2020, 295, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.L.; Núñez, E.; Yélamos, B.; Gómez-Gutiérrez, J.; Peterson, D.L.; Gavilanes, F. Study of the putative fusion regions of the preS domain of hepatitis B virus. Biochim. Biophys. Acta 2015, 1848, 895–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez, E.; Yélamos, B.; Delgado, C.; Gómez-Gutiérrez, J.; Peterson, D.L.; Gavilanes, F. Interaction of preS domains of hepatitis B virus with phospholipid vesicles. Biochim. Biophys. Acta 2009, 1788, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Somiya, M.; Shimada, N.; Sakamoto, W.; Yoshimoto, N.; Iijima, M.; Tatematsu, K.; Nakai, T.; Okajima, T.; Maruyama, A.; et al. Mutational analysis of hepatitis B virus pre-S1 (9-24) fusogenic peptide. Biochem. Biophys. Res. Commun. 2016, 474, 406–412. [Google Scholar] [CrossRef]
- Somiya, M.; Sasaki, Y.; Matsuzaki, T.; Liu, Q.; Iijima, M.; Yoshimoto, N.; Niimi, T.; Maturana, A.D.; Kuroda, S.I. Intracellular trafficking of bio-nanocapsule-liposome complex: Identification of fusogenic activity in the pre-S1 region of hepatitis B virus surface antigen L protein. J. Control. Release 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Berting, A.; Fischer, C.; Schaefer, S.; Garten, W.; Klenk, H.-D.; Gerlich, W.H. Hemifusion activity of a chimeric influenza virus hemagglutinin with a putative fusion peptide from hepatitis B virus. Virus Res. 2000, 68, 35–49. [Google Scholar] [CrossRef]
- Chojnacki, J.; Anderson, D.A.; Grgacic, E.V.L. A hydrophobic domain in the large envelope protein is essential for fusion of duck hepatitis B virus at the late endosome. J. Virol. 2005, 79, 14945–14955. [Google Scholar] [CrossRef] [Green Version]
- Mariyanna, L.; Priyadarshini, P.; Hofmann-Sieber, H.; Krepstakies, M.; Walz, N.; Grundhoff, A.; Buchholz, F.; Hildt, E.; Hauber, J. Excision of HIV-1 proviral DNA by recombinant cell permeable tre-recombinase. PLoS ONE 2012, 7, e31576. [Google Scholar] [CrossRef]
- Zahn, T.; Akhras, S.; Spengler, C.; Murra, R.O.; Holzhauser, T.; Hildt, E. A new approach for therapeutic vaccination against chronic HBV infections. Vaccine 2020, 38, 3105–3120. [Google Scholar] [CrossRef]
- Akhras, S.; Toda, M.; Boller, K.; Himmelsbach, K.; Elgner, F.; Biehl, M.; Scheurer, S.; Gratz, M.; Vieths, S.; Hildt, E. Cell-permeable capsids as universal antigen carrier for the induction of an antigen-specific CD8+ T-cell response. Sci. Rep. 2017, 7, 9630. [Google Scholar] [CrossRef] [PubMed]
- Bleifuss, E.; Kammertoens, T.; Hutloff, A.; Quarcoo, D.; Dorner, M.; Straub, P.; Uckert, W.; Hildt, E. The translocation motif of hepatitis B virus improves protein vaccination. Cell. Mol. Life Sci. 2006, 63, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Brandenburg, B.; Hildt, E. Reconstitution of gene expression from a regulatory-protein-deficient hepatitis B virus genome by cell-permeable HBx protein. EMBO Rep. 2003, 4, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oess, S.; Hildt, E. Novel cell permeable motif derived from the PreS2-domain of hepatitis-B virus surface antigens. Gene Ther. 2000, 7, 750–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, B.; Stockl, L.; Gutzeit, C.; Roos, M.; Lupberger, J.; Schwartlander, R.; Gelderblom, H.; Sauer, I.M.; Hofschneider, P.H.; Hildt, E. A novel system for efficient gene transfer into primary human hepatocytes via cell-permeable hepatitis B virus-like particle. Hepatology 2005, 42, 1300–1309. [Google Scholar] [CrossRef]
- Hildt, E.; Urban, S.; Hofschneider, P.H. Characterization of essential domains for the functionality of the MHBst transcriptional activator and identification of a minimal MHBst activator. Oncogene 1995, 11, 2055–2066. [Google Scholar]
- Stoeckl, L.; Funk, A.; Kopitzki, A.; Brandenburg, B.; Oess, S.; Will, H.; Sirma, H.; Hildt, E. Identification of a structural motif crucial for infectivity of hepatitis B viruses. Proc. Natl. Acad. Sci. USA 2006, 103, 6730–6734. [Google Scholar] [CrossRef] [Green Version]
- Gudima, S.; Meier, A.; Dunbrack, R.; Taylor, J.; Bruss, V. Two potentially important elements of the hepatitis B virus large envelope protein are dispensable for the infectivity of hepatitis delta virus. J. Virol. 2007, 81, 4343–4347. [Google Scholar] [CrossRef] [Green Version]
- Lepère, C.; Régeard, M.; Le Seyec, J.; Gripon, P. The translocation motif of hepatitis B virus envelope proteins is dispensable for infectivity. J. Virol. 2007, 81, 7816–7818. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, M.; Sureau, C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J. Virol. 2007, 81, 5841–5849. [Google Scholar] [CrossRef] [Green Version]
- Rabe, B.; Glebe, D.; Kann, M. Lipid-mediated introduction of hepatitis B virus capsids into nonsusceptible cells allows highly efficient replication and facilitates the study of early infection events. J. Virol. 2006, 80, 5465–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döhner, K.; Sodeik, B. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 2005, 285, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Döhner, K.; Nagel, C.-H.; Sodeik, B. Viral stop-and-go along microtubules: Taking a ride with dynein and kinesins. Trends Microbiol. 2005, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Osseman, Q.; Gallucci, L.; Au, S.; Cazenave, C.; Berdance, E.; Blondot, M.-L.; Cassany, A.; Bégu, D.; Ragues, J.; Aknin, C.; et al. The chaperone dynein LL1 mediates cytoplasmic transport of empty and mature hepatitis B virus capsids. J. Hepatol. 2018, 68, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Kann, M.; Sodeik, B.; Vlachou, A.; Gerlich, W.H.; Helenius, A. Phosphorylation-dependent binding of hepatitis B virus core particles to the nuclear pore complex. J. Cell Biol. 1999, 145, 45–55. [Google Scholar] [CrossRef]
- Rabe, B.; Vlachou, A.; Panté, N.; Helenius, A.; Kann, M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc. Natl. Acad. Sci. USA 2003, 100, 9849–9854. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.T.; Liaw, Y.F.; Ou, J.H. The arginine-rich domain of hepatitis B virus precore and core proteins contains a signal for nuclear transport. J. Virol. 1990, 64, 6141–6147. [Google Scholar] [CrossRef] [Green Version]
- Heger-Stevic, J.; Zimmermann, P.; Lecoq, L.; Böttcher, B.; Nassal, M. Hepatitis B virus core protein phosphorylation: Identification of the SRPK1 target sites and impact of their occupancy on RNA binding and capsid structure. PLoS Pathog. 2018, 14, e1007488. [Google Scholar] [CrossRef] [Green Version]
- Lill, Y.; Lill, M.A.; Fahrenkrog, B.; Schwarz-Herion, K.; Paulillo, S.; Aebi, U.; Hecht, B. Single hepatitis-B virus core capsid binding to individual nuclear pore complexes in Hela cells. Biophys. J. 2006, 91, 3123–3130. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Mao, R.; Block, T.M.; Guo, J.-T. Production and function of the cytoplasmic deproteinized relaxed circular DNA of hepadnaviruses. J. Virol. 2010, 84, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, L.; Kann, M. Nuclear Import of Hepatitis B Virus Capsids and Genome. Viruses 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, W.H.; Goldmann, U.; Müller, R.; Stibbe, W.; Wolff, W. Specificity and localization of the hepatitis B virus-associated protein kinase. J. Virol. 1982, 42, 761–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Xi, J.; Gao, L.; Hu, J. Role of Hepatitis B virus capsid phosphorylation in nucleocapsid disassembly and covalently closed circular DNA formation. PLoS Pathog. 2020, 16, e1008459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupberger, J.; Schaedler, S.; Peiran, A.; Hildt, E. Identification and characterization of a novel bipartite nuclear localization signal in the hepatitis B virus polymerase. World J. Gastroenterol. 2013, 19, 8000–8010. [Google Scholar] [CrossRef] [PubMed]
- Junker-Niepmann, M.; Bartenschlager, R.; Schaller, H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990, 9, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.R.; Ganem, D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J. Virol. 1993, 67, 3254–3263. [Google Scholar] [CrossRef] [Green Version]
- Knaus, T.; Nassal, M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 1993, 21, 3967–3975. [Google Scholar] [CrossRef]
- Bartenschlager, R.; Junker-Niepmann, M.; Schaller, H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J. Virol. 1990, 64, 5324–5332. [Google Scholar] [CrossRef] [Green Version]
- Gerelsaikhan, T.; Tavis, J.E.; Bruss, V. Hepatitis B virus nucleocapsid envelopment does not occur without genomic DNA synthesis. J. Virol. 1996, 70, 4269–4274. [Google Scholar] [CrossRef] [Green Version]
- Summers, J.; Mason, W.S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 1982, 29, 403–415. [Google Scholar] [CrossRef]
- Perlman, D.H.; Berg, E.A.; O’connor, P.B.; Costello, C.E.; Hu, J. Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc. Natl. Acad. Sci. USA 2005, 102, 9020–9025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.T.; Li, J.; Liao, W.; Ou, J. Roles of the three major phosphorylation sites of hepatitis B virus core protein in viral replication. Virology 1999, 259, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazina, E.V.; Fielding, J.E.; Lin, B.; Anderson, D.A. Core protein phosphorylation modulates pregenomic RNA encapsidation to different extents in human and duck hepatitis B viruses. J. Virol. 2000, 74, 4721–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, B.; Nassal, M. Structure of Mutant Hepatitis B Core Protein Capsids with Premature Secretion Phenotype. J. Mol. Biol. 2018, 430, 4941–4954. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Nguyen, D.; Mentzer, L.; Adams, C.; Lee, H.; Ashley, R.; Hafenstein, S.; Hu, J. Secretion of genome-free hepatitis B virus--single strand blocking model for virion morphogenesis of para-retrovirus. PLoS Pathog. 2011, 7, e1002255. [Google Scholar] [CrossRef] [PubMed]
- Bruss, V. Envelopment of the hepatitis B virus nucleocapsid. Virus Res. 2004, 106, 199–209. [Google Scholar] [CrossRef]
- Melegari, M.; Wolf, S.K.; Schneider, R.J. Hepatitis B virus DNA replication is coordinated by core protein serine phosphorylation and HBx expression. J. Virol. 2005, 79, 9810–9820. [Google Scholar] [CrossRef] [Green Version]
- Daub, H.; Blencke, S.; Habenberger, P.; Kurtenbach, A.; Dennenmoser, J.; Wissing, J.; Ullrich, A.; Cotten, M. Identification of SRPK1 and SRPK2 as the Major Cellular Protein Kinases Phosphorylating Hepatitis B Virus Core Protein. J. Virol. 2002, 76, 8124–8137. [Google Scholar] [CrossRef] [Green Version]
- Ludgate, L.; Ning, X.; Nguyen, D.H.; Adams, C.; Mentzer, L.; Hu, J. Cyclin-dependent kinase 2 phosphorylates s/t-p sites in the hepadnavirus core protein C-terminal domain and is incorporated into viral capsids. J. Virol. 2012, 86, 12237–12250. [Google Scholar] [CrossRef] [Green Version]
- Kann, M.; Gerlich, W.H. Effect of core protein phosphorylation by protein kinase C on encapsidation of RNA within core particles of hepatitis B virus. J. Virol. 1994, 68, 7993–8000. [Google Scholar] [CrossRef] [Green Version]
- Diab, A.; Foca, A.; Fusil, F.; Lahlali, T.; Jalaguier, P.; Amirache, F.; N’Guyen, L.; Isorce, N.; Cosset, F.-L.; Zoulim, F.; et al. Polo-like-kinase 1 is a proviral host factor for hepatitis B virus replication. Hepatology 2017, 66, 1750–1765. [Google Scholar] [CrossRef] [PubMed]
- Bartusch, C.; Döring, T.; Prange, R. Rab33B Controls Hepatitis B Virus Assembly by Regulating Core Membrane Association and Nucleocapsid Processing. Viruses 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernholz, D.; Stemler, M.; Brunetto, M.; Bonino, F.; Will, H. Replicating and virion secreting hepatitis B mutant virus unable to produce preS2 protein. J. Hepatol. 1991, 13, S102–S104. [Google Scholar] [CrossRef]
- Bruss, V.; Ganem, D. The role of envelope proteins in hepatitis B virus assembly. Proc. Natl. Acad. Sci. USA 1991, 88, 1059–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruss, V.; Vieluf, K. Functions of the internal pre-S domain of the large surface protein in hepatitis B virus particle morphogenesis. J. Virol. 1995, 69, 6652–6657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruss, V.; Thomssen, R. Mapping a region of the large envelope protein required for hepatitis B virion maturation. J. Virol. 1994, 68, 1643–1650. [Google Scholar] [CrossRef] [Green Version]
- Dyson, M.R.; Murray, K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: Association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. USA 1995, 92, 2194–2198. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wu, Q.; Kuhnhenn, L.; Akhras, S.; Spengler, C.; Boller, K.; Peiffer, K.-H.; Hildt, E. Formation of semi-enveloped particles as a unique feature of a hepatitis B virus PreS1 deletion mutant. Aliment. Pharmacol. Ther. 2019, 50, 940–954. [Google Scholar] [CrossRef]
- Poisson, F.; Severac, A.; Hourioux, C.; Goudeau, A.; Roingeard, P. Both pre-S1 and S domains of hepatitis B virus envelope proteins interact with the core particle. Virology 1997, 228, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Löffler-Mary, H.; Dumortier, J.; Klentsch-Zimmer, C.; Prange, R. Hepatitis B virus assembly is sensitive to changes in the cytosolic S loop of the envelope proteins. Virology 2000, 270, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, B.; Tsuji, N.; Takahashi, H.; Dyson, M.R.; Zhao, S.; Crowther, R.A.; Murray, K. Peptides that block hepatitis B virus assembly: Analysis by cryomicroscopy, mutagenesis and transfection. EMBO J. 1998, 17, 6839–6845. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Urban, S.; Antoni, C.; Böttcher, B. Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions. EMBO J. 2007, 26, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Pastor, F.; Herrscher, C.; Patient, R.; Eymieux, S.; Moreau, A.; Burlaud-Gaillard, J.; Seigneuret, F.; de Rocquigny, H.; Roingeard, P.; Hourioux, C. Direct interaction between the hepatitis B virus core and envelope proteins analyzed in a cellular context. Sci. Rep. 2019, 9, 16178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laub, O.; Rall, L.B.; Truett, M.; Shaul, Y.; Standring, D.N.; Valenzuela, P.; Rutter, W.J. Synthesis of hepatitis B surface antigen in mammalian cells: Expression of the entire gene and the coding region. J. Virol. 1983, 48, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Diminsky, D.; Schirmbeck, R.; Reimann, J.; Barenholz, Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): Composition, structure and immunogenicity. Vaccine 1997, 15, 637–647. [Google Scholar] [CrossRef]
- Gilbert, R.J.C.; Beales, L.; Blond, D.; Simon, M.N.; Lin, B.Y.; Chisari, F.V.; Stuart, D.I.; Rowlands, D.J. Hepatitis B small surface antigen particles are octahedral. Proc. Natl. Acad. Sci. USA 2005, 102, 14783–14788. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhang, J.; Lu, Y.; Luo, S.; Zhang, J.; Zhu, P. Cryo-EM structure of native spherical subviral particles isolated from HBV carriers. Virus Res. 2019, 259, 90–96. [Google Scholar] [CrossRef]
- Patzer, E.J.; Nakamura, G.R.; Simonsen, C.C.; Levinson, A.D.; Brands, R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J. Virol. 1986, 58, 884–892. [Google Scholar] [CrossRef] [Green Version]
- Huovila, A.P.; Eder, A.M.; Fuller, S.D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 1992, 118, 1305–1320. [Google Scholar] [CrossRef]
- Patient, R.; Hourioux, C.; Roingeard, P. Morphogenesis of hepatitis B virus and its subviral envelope particles. Cell. Microbiol. 2009, 11, 1561–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patient, R.; Hourioux, C.; Sizaret, P.-Y.; Trassard, S.; Sureau, C.; Roingeard, P. Hepatitis B virus subviral envelope particle morphogenesis and intracellular trafficking. J. Virol. 2007, 81, 3842–3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prange, R.; Nagel, R.; Streeck, R.E. Deletions in the hepatitis B virus small envelope protein: Effect on assembly and secretion of surface antigen particles. J. Virol. 1992, 66, 5832–5841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurtley, S.M.; Helenius, A. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 1989, 5, 277–307. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Thomé, N.; Kluck, C.J.; Prange, R. Functional incorporation of green fluorescent protein into hepatitis B virus envelope particles. Virology 2004, 330, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Schädler, S.; Hildt, E. HBV life cycle: Entry and morphogenesis. Viruses 2009, 1, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Bruss, V. Hepatitis B virus morphogenesis. World J. Gastroenterol. 2007, 13, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, J.; Boehm, C.; Himmelsbach, K.; Donnerhak, C.; Roettger, H.; Weiss, T.S.; Ploen, D.; Hildt, E. Identification of α-taxilin as an essential factor for the life cycle of hepatitis B virus. J. Hepatol. 2013, 59, 934–941. [Google Scholar] [CrossRef]
- Jiang, B.; Himmelsbach, K.; Ren, H.; Boller, K.; Hildt, E. Subviral Hepatitis B Virus Filaments, like Infectious Viral Particles, Are Released via Multivesicular Bodies. J. Virol. 2015, 90, 3330–3341. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.-F.; Tsai, M.-L.; Huang, J.-Y.; Chang, Y.-S.; Shih, C. The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion. PLoS Pathog. 2015, 11, e1005123. [Google Scholar] [CrossRef] [Green Version]
- Stieler, J.T.; Prange, R. Involvement of ESCRT-II in hepatitis B virus morphogenesis. PLoS ONE 2014, 9, e91279. [Google Scholar] [CrossRef] [PubMed]
- Kian Chua, P.; Lin, M.-H.; Shih, C. Potent inhibition of human Hepatitis B virus replication by a host factor Vps4. Virology 2006, 354, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Sorensen, E.M.; Naito, A.; Schott, M.; Kim, S.; Ahlquist, P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc. Natl. Acad. Sci. USA 2007, 104, 10205–10210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, C.; Döring, T.; Prange, R. Hepatitis B virus maturation is sensitive to functional inhibition of ESCRT-III, Vps4, and gamma 2-adaptin. J. Virol. 2007, 81, 9050–9060. [Google Scholar] [CrossRef] [Green Version]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, P.I.; Cashikar, A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.L.; Urbé, S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007, 8, 355–368. [Google Scholar] [CrossRef]
- Martin-Serrano, J.; Neil, S.J.D. Host factors involved in retroviral budding and release. Nat. Rev. Microbiol. 2011, 9, 519–531. [Google Scholar] [CrossRef]
- Raiborg, C.; Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joshi, A.; Nagashima, K.; Freed, E.O.; Hurley, J.H. Structural basis for viral late-domain binding to Alix. Nat. Struct. Mol. Biol. 2007, 14, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.D.; Chung, H.-Y.; Zhai, Q.; Robinson, H.; Sundquist, W.I.; Hill, C.P. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 2007, 128, 841–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirov, D.G.; Ono, A.; Orenstein, J.M.; Freed, E.O. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 2002, 99, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göttlinger, H.G.; Dorfman, T.; Sodroski, J.G.; Haseltine, W.A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 1991, 88, 3195–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parent, L.J.; Bennett, R.P.; Craven, R.C.; Nelle, T.D.; Krishna, N.K.; Bowzard, J.B.; Wilson, C.B.; Puffer, B.A.; Montelaro, R.C.; Wills, J.W. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 1995, 69, 5455–5460. [Google Scholar] [CrossRef] [Green Version]
- Garnier, L.; Wills, J.W.; Verderame, M.F.; Sudol, M. WW domains and retrovirus budding. Nature 1996, 381, 744–745. [Google Scholar] [CrossRef]
- Hartmann-Stühler, C.; Prange, R. Hepatitis B virus large envelope protein interacts with gamma2-adaptin, a clathrin adaptor-related protein. J. Virol. 2001, 75, 5343–5351. [Google Scholar] [CrossRef] [Green Version]
- Rost, M.; Döring, T.; Prange, R. gamma2-Adaptin, a ubiquitin-interacting adaptor, is a substrate to coupled ubiquitination by the ubiquitin ligase Nedd4 and functions in the endosomal pathway. J. Biol. Chem. 2008, 283, 32119–32130. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.L.; Byfield, R.; Robek, M.D. Hepatitis B virus replication and release are independent of core lysine ubiquitination. J. Virol. 2009, 83, 4923–4933. [Google Scholar] [CrossRef] [Green Version]
- Rost, M.; Mann, S.; Lambert, C.; Döring, T.; Thomé, N.; Prange, R. Gamma-adaptin, a novel ubiquitin-interacting adaptor, and Nedd4 ubiquitin ligase control hepatitis B virus maturation. J. Biol. Chem. 2006, 281, 29297–29308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Yamada, G.; Mizuno, M.; Nishihara, T.; Kinoyama, S.; Kobayashi, T.; Takahashi, T.; Nagashima, H. Full and empty particles of hepatitis B virus in hepatocytes from patients with HBsAg-positive chronic active hepatitis. Lab. Investig. 1983, 48, 678–682. [Google Scholar] [PubMed]
- Kaplan, P.M.; Ford, E.C.; Purcell, R.H.; Gerin, J.L. Demonstration of subpopulations of Dane particles. J. Virol. 1976, 17, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerin, J.L.; Ford, E.C.; Purcell, R.H. Biochemical characterization of Australia antigen. Evidence for defective particles of hepatitis B virus. Am. J. Pathol. 1975, 81, 651–668. [Google Scholar] [PubMed]
- Hu, J.; Liu, K. Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application. Viruses 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heermann, K.H.; Goldmann, U.; Schwartz, W.; Seyffarth, T.; Baumgarten, H.; Gerlich, W.H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 1984, 52, 396–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar-Kimber, K.L.; Jarocki-Witek, V.; Dheer, S.K.; Vernon, S.K.; Conley, A.J.; Davis, A.R.; Hung, P.P. Distinctive properties of the hepatitis B virus envelope proteins. J. Virol. 1988, 62, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Standring, D.N.; Ou, J.H.; Rutter, W.J. Assembly of viral particles in Xenopus oocytes: Pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc. Natl. Acad. Sci. USA 1986, 83, 9338–9342. [Google Scholar] [CrossRef] [Green Version]
- Prange, R.; Clemen, A.; Streeck, R.E. Myristylation is involved in intracellular retention of hepatitis B virus envelope proteins. J. Virol. 1991, 65, 3919–3923. [Google Scholar] [CrossRef] [Green Version]
- Roingeard, P.; Sureau, C. Ultrastructural analysis of hepatitis B virus in HepG2-transfected cells with special emphasis on subviral filament morphogenesis. Hepatology 1998, 28, 1128–1133. [Google Scholar] [CrossRef]
- Chisari, F.V.; Filippi, P.; Buras, J.; McLachlan, A.; Popper, H.; Pinkert, C.A.; Palmiter, R.D.; Brinster, R.L. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. USA 1987, 84, 6909–6913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisari, F.V.; Klopchin, K.; Moriyama, T.; Pasquinelli, C.; Dunsford, H.A.; Sell, S.; Pinkert, C.A.; Brinster, R.L.; Palmiter, R.D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989, 59, 1145–1156. [Google Scholar] [CrossRef]
- Sells, M.A.; Chen, M.L.; Acs, G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 1987, 84, 1005–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureau, C.; Romet-Lemonne, J.-L.; Mullins, J.I.; Essex, M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell 1986, 47, 37–47. [Google Scholar] [CrossRef]
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down’s Syndrome and hepatitis. Nature 1968, 218, 1057–1059. [Google Scholar] [CrossRef]
- Possehl, C.; Repp, R.; Heermann, K.H.; Korec, E.; Uy, A.; Gerlich, W.H. Absence of free core antigen in anti-HBc negative viremic hepatitis B carriers. Arch. Virol. Suppl. 1992, 4, 39–41. [Google Scholar] [CrossRef]
- Bardens, A.; Döring, T.; Stieler, J.; Prange, R. Alix regulates egress of hepatitis B virus naked capsid particles in an ESCRT-independent manner. Cell. Microbiol. 2011, 13, 602–619. [Google Scholar] [CrossRef]
- Sun, D.; Nassal, M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J. Hepatol. 2006, 45, 636–645. [Google Scholar] [CrossRef]
- Milich, D.R.; Chen, M.; Schödel, F.; Peterson, D.L.; Jones, J.E.; Hughes, J.L. Role of B cells in antigen presentation of the hepatitis B core. Proc. Natl. Acad. Sci. USA 1997, 94, 14648–14653. [Google Scholar] [CrossRef] [Green Version]
- Milich, D.R.; McLachlan, A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 1986, 234, 1398–1401. [Google Scholar] [CrossRef]
- Döring, T.; Prange, R. Rab33B and its autophagic Atg5/12/16L1 effector assist in hepatitis B virus naked capsid formation and release. Cell. Microbiol. 2015, 17, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, X.; Kozlowski, M.; Li, W.; Wu, M.; Liu, J.; Chen, L.; Zhang, J.; Huang, Y.; Yuan, Z. Extracellular Hepatitis B Virus RNAs Are Heterogeneous in Length and Circulate as Capsid-Antibody Complexes in Addition to Virions in Chronic Hepatitis B Patients. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Hildt, E. Intracellular Trafficking of HBV Particles. Cells 2020, 9, 2023. https://doi.org/10.3390/cells9092023
Jiang B, Hildt E. Intracellular Trafficking of HBV Particles. Cells. 2020; 9(9):2023. https://doi.org/10.3390/cells9092023
Chicago/Turabian StyleJiang, Bingfu, and Eberhard Hildt. 2020. "Intracellular Trafficking of HBV Particles" Cells 9, no. 9: 2023. https://doi.org/10.3390/cells9092023
APA StyleJiang, B., & Hildt, E. (2020). Intracellular Trafficking of HBV Particles. Cells, 9(9), 2023. https://doi.org/10.3390/cells9092023





