Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Biological Reagents
2.2. Antibodies
2.3. Cell Culture and Treatment
2.4. Water-Soluble Tetrazolium (WST)-1 Assay
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Scratch Assay
2.7. Growth Curves
2.8. Transwell Migration Assay
2.9. Spheroid Sprouting Assay
2.10. Tube Formation Assay
2.11. NG2 Promotor Analyses
2.12. Reporter Gene Assay
2.13. Cell Transfection
2.14. Western Blot Analysis
2.15. Flow Cytometry
2.16. Quantitative Real-Time PCR (qRT-PCR)
2.17. Animals
2.18. Genotyping
2.19. Aortic Ring Assay
2.20. Matrigel Plug Assay
2.21. Immunohistochemistry
2.22. Statistical Analysis
3. Results
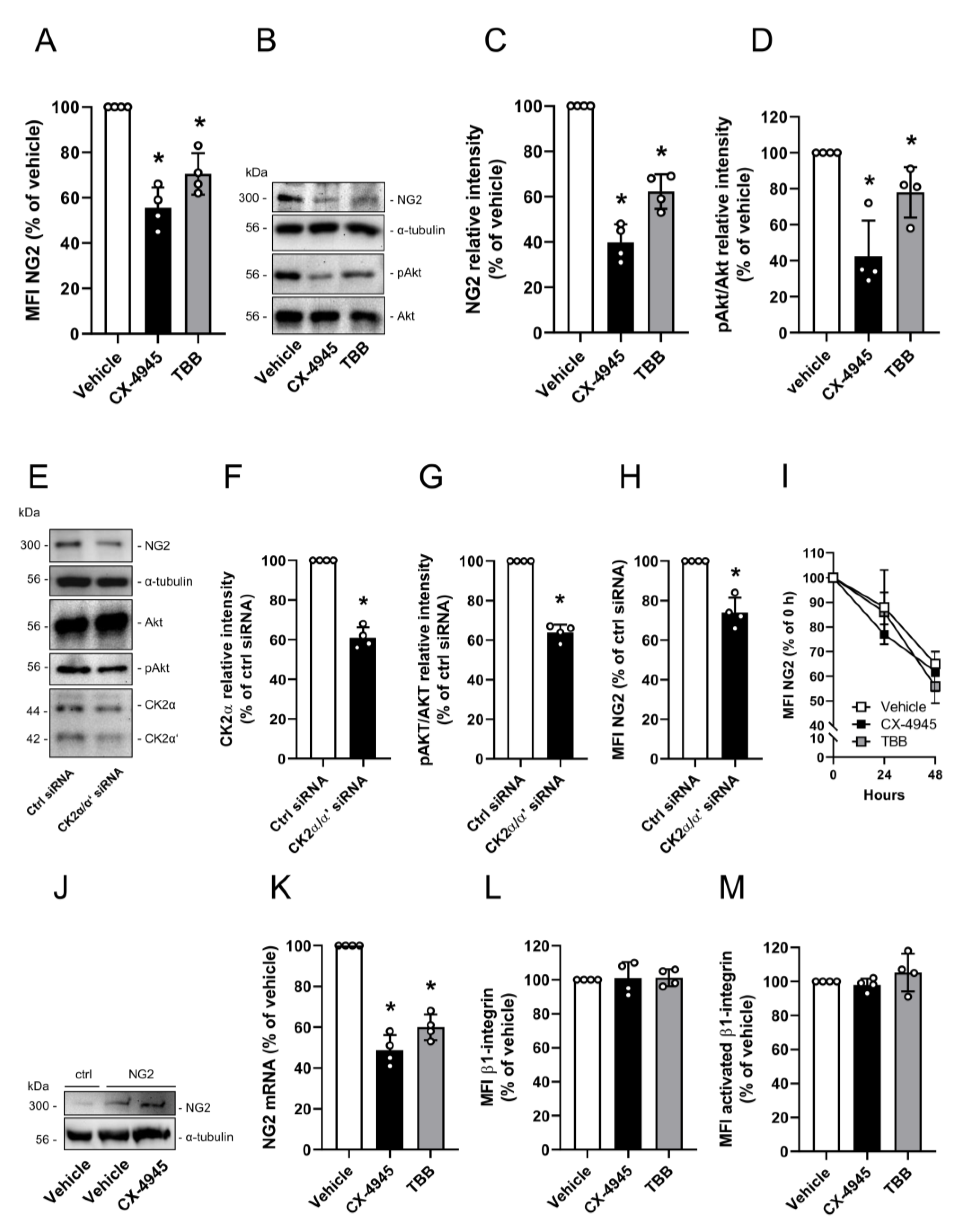
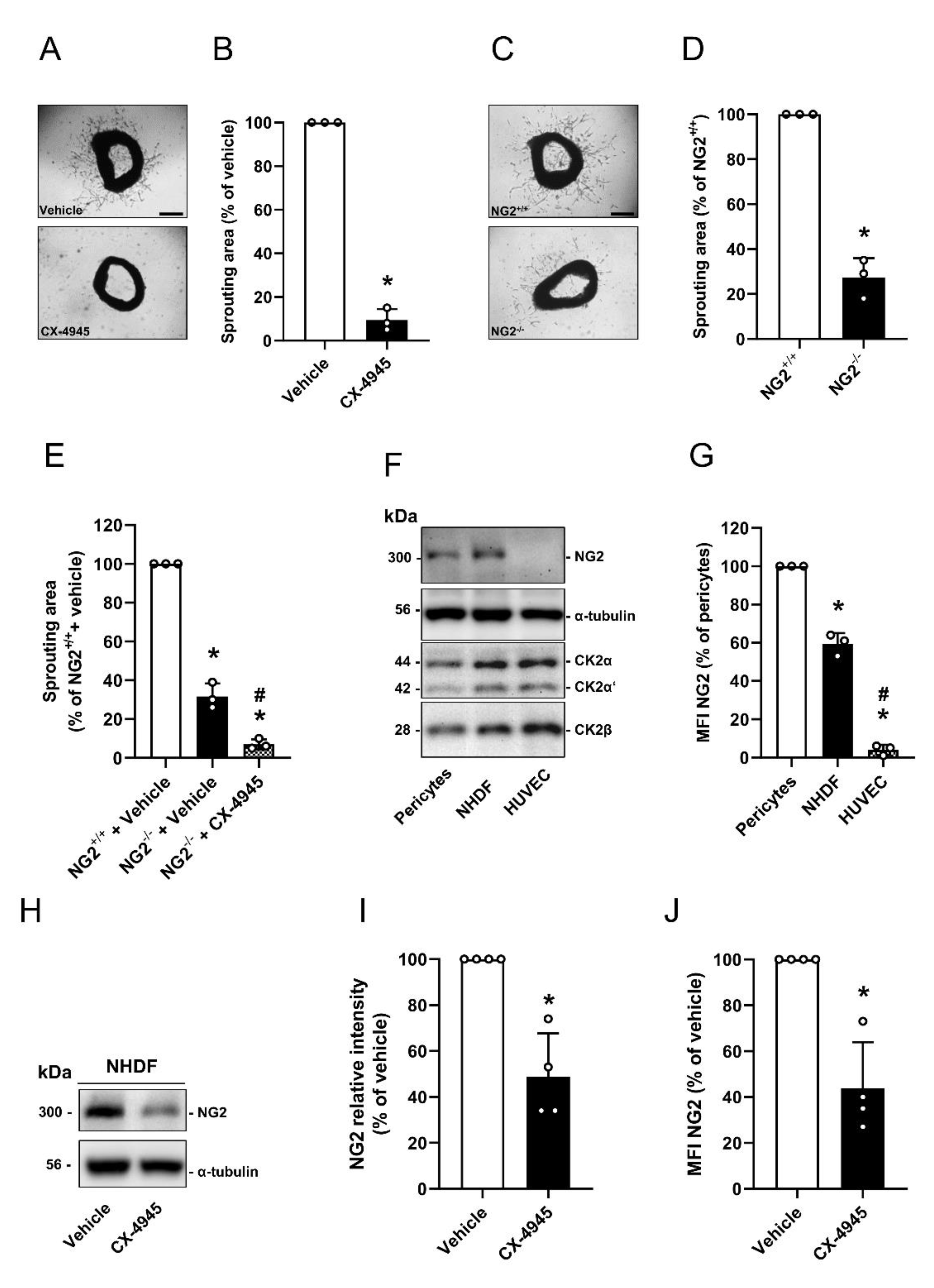
3.1. Effect of CK2 Inhibition on NG2 Expression
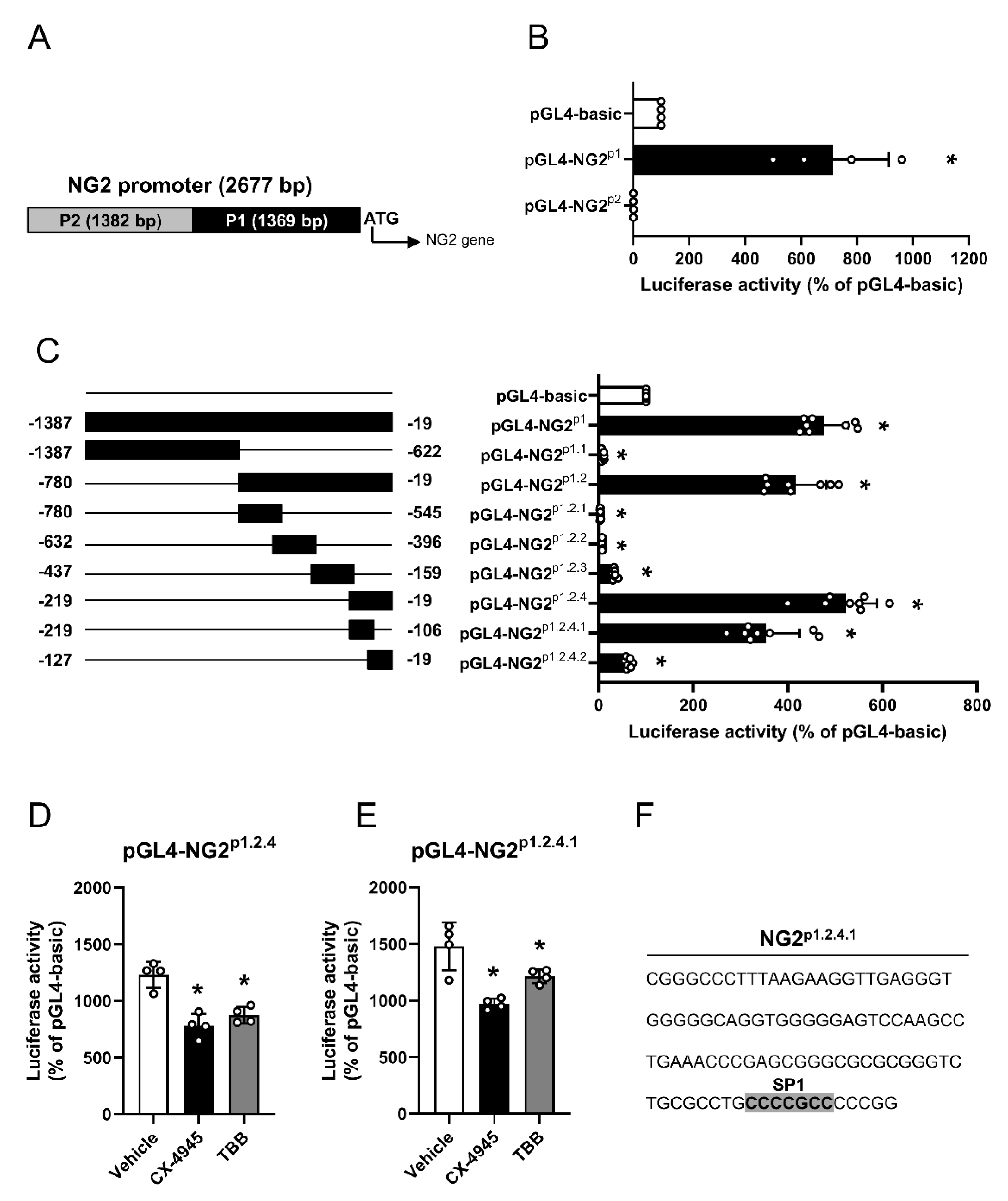
3.2. Effect of CK2 Inhibition on NG2 Promoter Activity
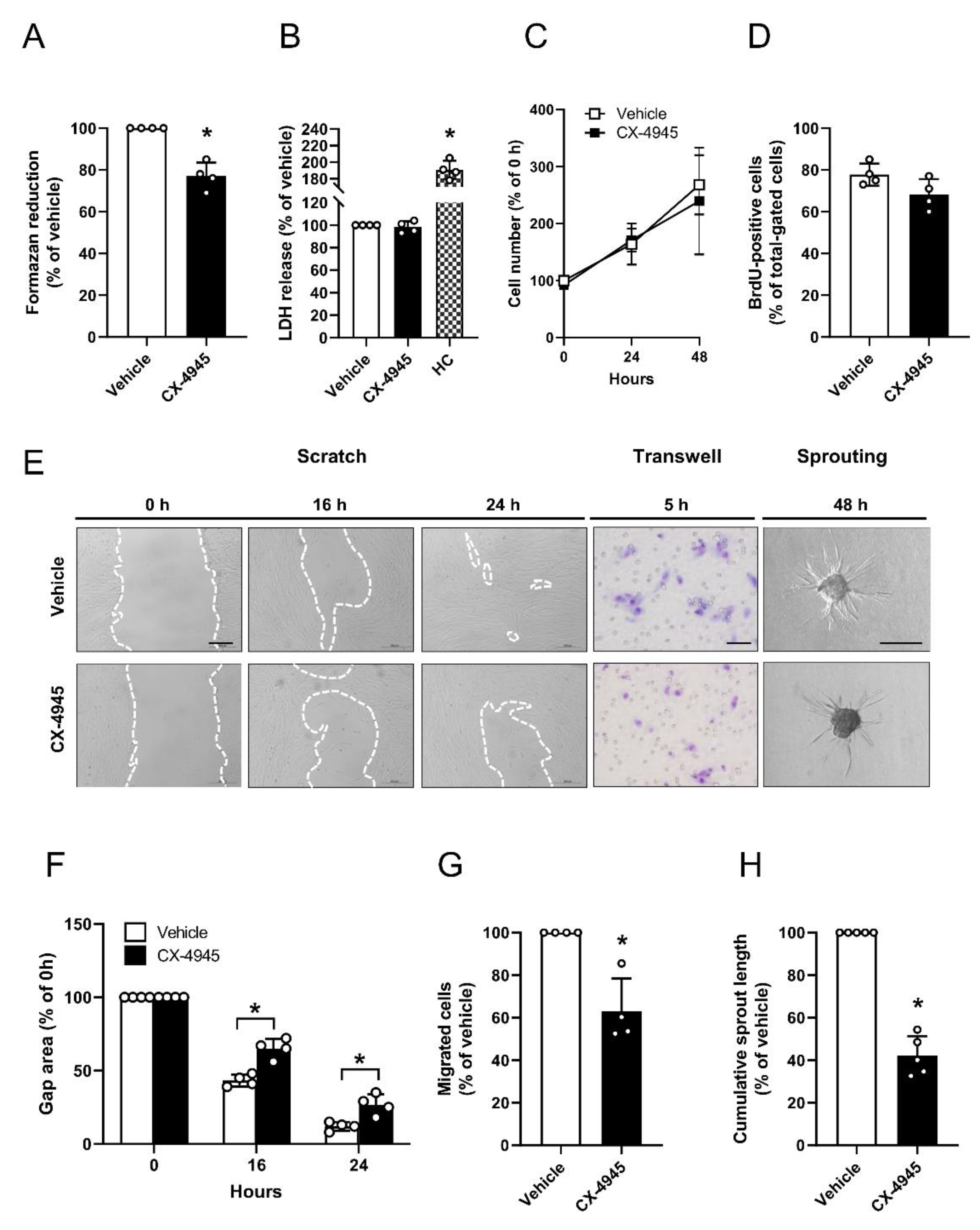
3.3. Effect of CK2 Inhibition on Proliferation, Migration and Sprouting of Pericytes
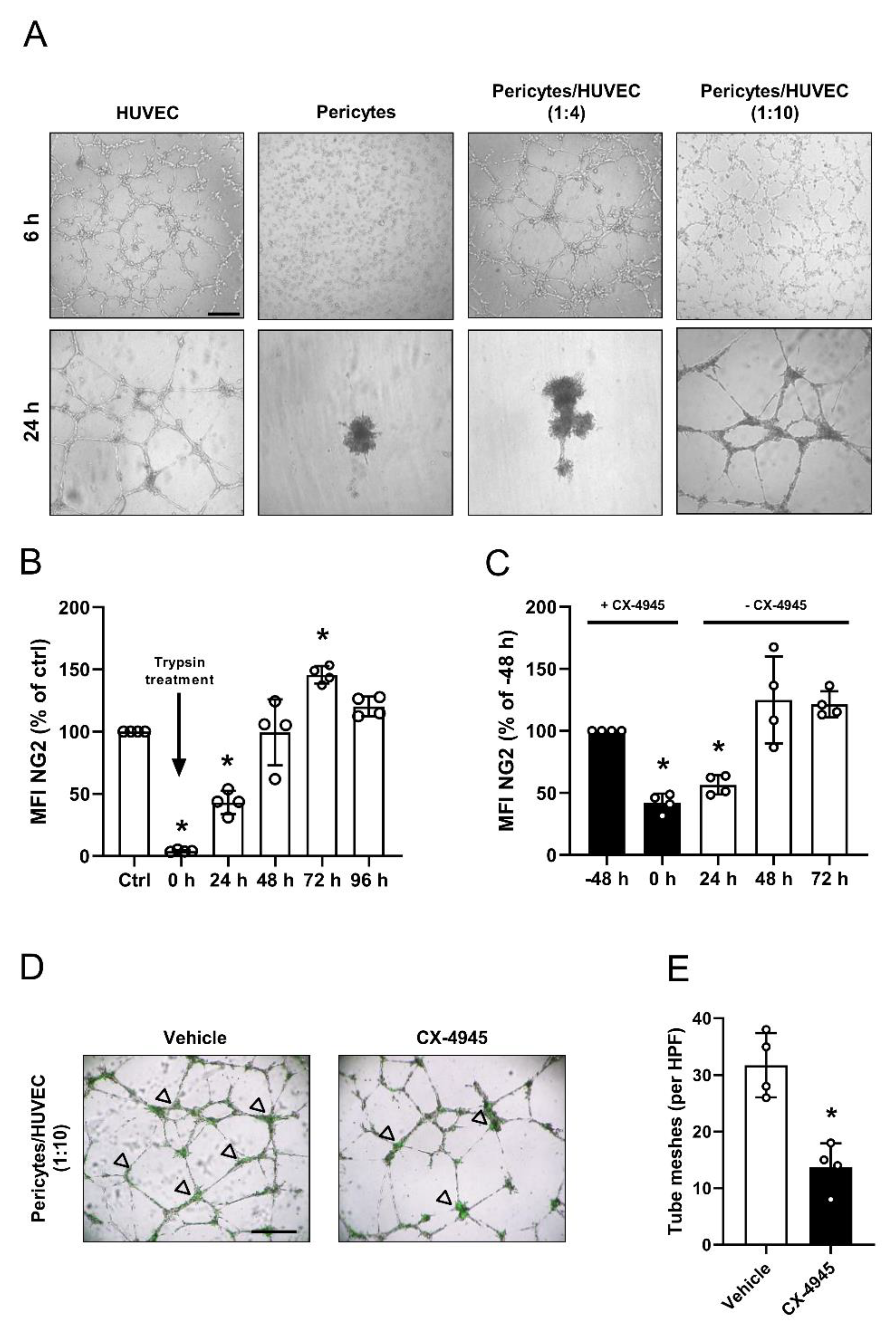
3.4. Effect of CK2 Inhibition in Pericytes on Endothelial Tube Stabilization
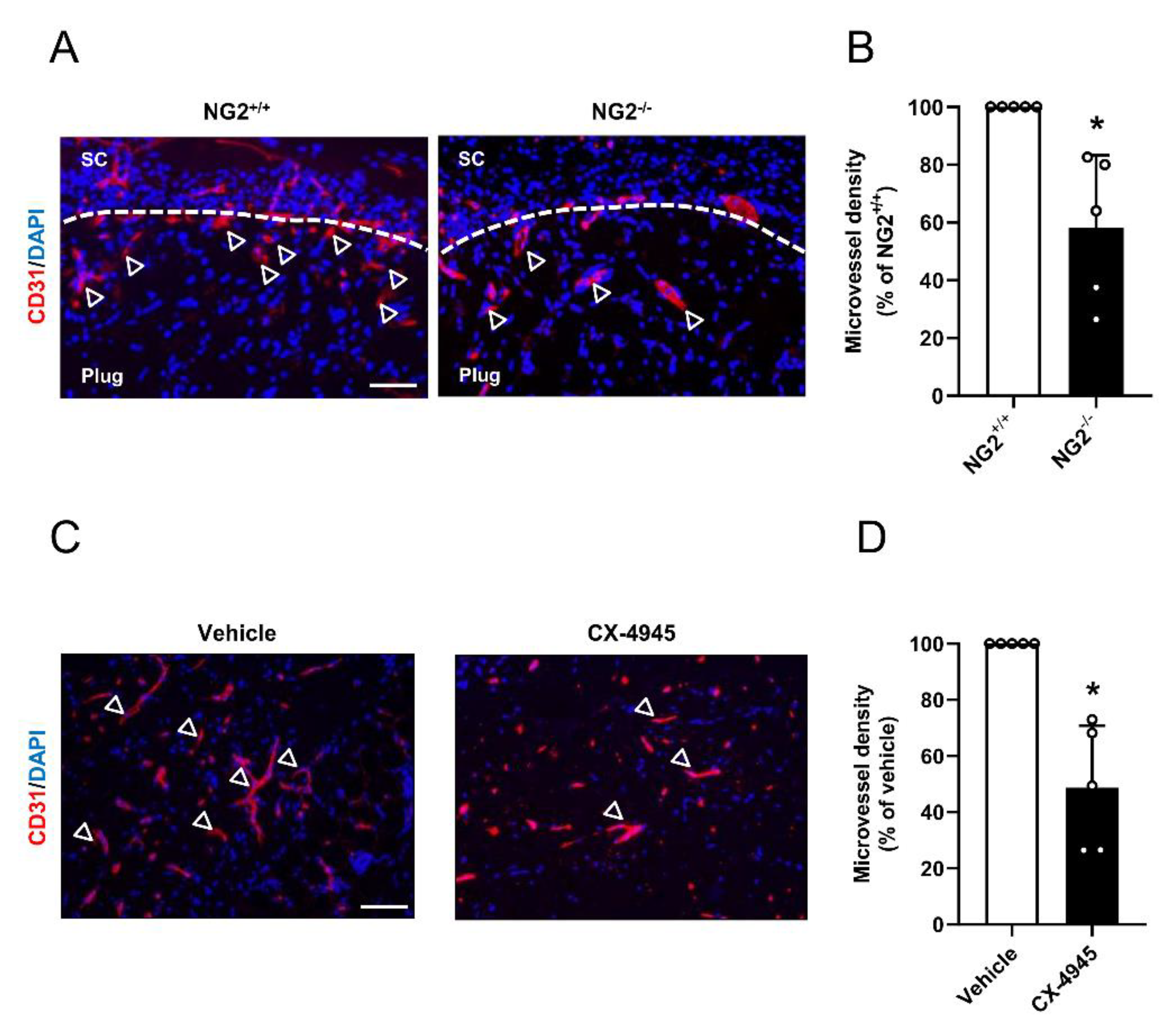
3.5. Effect of CK2 Inhibition in Pericytes on the Angiogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2. FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Laschke, M.W.; Menger, M.D. Function of protein kinase CK2 in thrombus formation. Platelets 2018, 30, 421–427. [Google Scholar] [CrossRef]
- Al Quobaili, F.; Montenarh, M. CK2 and the regulation of the carbohydrate metabolism. Metabolism 2012, 61, 1512–1517. [Google Scholar] [CrossRef]
- Feng, D.; Welker, S.; Körbel, C.; Rudzitis-Auth, J.; Menger, M.D.; Montenarh, M.; Laschke, M.W. Protein kinase CK2 is a regulator of angiogenesis in endometriotic lesions. Angiogenesis 2012, 15, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, M. Protein Kinase CK2 and Angiogenesis. Adv. Clin. Exp. Med. 2014, 23, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozza, G.; Pinna, L.A.; Moro, S. Kinase CK2 inhibition: An update. Curr. Med. Chem. 2013, 20, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Meggio, F.; Ruzzene, M.; Andrzejewska, M.; Kazimierczuk, Z.; Pinna, L.A. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: A novel powerful and selective inhibitor of protein kinase CK2. Biochem. Biophys. Res. Commun. 2004, 321, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Ruzzene, M.; Penzo, D.; Pinna, L.A. Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem. J. 2002, 364, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chon, H.J.; Bae, K.J.; Lee, Y.; Kim, J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front. Pharmacol. 2015, 6, 70. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Boil. 2011, 55, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Armulik, A.; Genové, G.; Betsholtz, C.; Keller, A. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Stapor, P.C.; Sweat, R.S.; Dashti, D.C.; Betancourt, A.M.; Murfee, W.L. Pericyte dynamics during angiogenesis: New insights from new identities. J. Vasc. Res. 2014, 51, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, U.; Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [Green Version]
- Pankova, D.; Jobe, N.; Kratochvílová, M.; Buccione, R.; Brábek, J.; Rösel, D. NG2-mediated Rho activation promotes amoeboid invasiveness of cancer cells. Eur. J. Cell Boil. 2012, 91, 969–977. [Google Scholar] [CrossRef]
- Wilson, B.; Ruberto, G.; Ferrone, S. Immunochemical characterization of a human high molecular weight? melanoma associated antigen identified with monoclonal antibodies. Cancer Immunol. Immunother. 1983, 14, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Stallcup, W.B. The NG2 proteoglycan: Past insights and future prospects. J. Neurocytol. 2003, 31, 423–435. [Google Scholar] [CrossRef]
- Stallcup, W.B. NG2 Proteoglycan Enhances Brain Tumor Progression by Promoting Beta-1 Integrin Activation in both Cis and Trans Orientations. Cancers 2017, 9, 31. [Google Scholar] [CrossRef]
- Schiffer, D.; Mellai, M.; Boldorini, R.; Bisogno, I.; Grifoni, S.; Corona, C.; Bertero, L.; Cassoni, P.; Casalone, C.; Annovazzi, L. The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. Int. J. Mol. Sci. 2018, 19, 2724. [Google Scholar] [CrossRef] [Green Version]
- Burg, M.A.; Tillet, E.; Timpl, R.; Stallcup, W.B. Binding of the NG2 Proteoglycan to Type VI Collagen and Other Extracellular Matrix Molecules. J. Boil. Chem. 1996, 271, 26110–26116. [Google Scholar] [CrossRef] [Green Version]
- Stallcup, W.B.; Dahlin, K.; Healy, P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J. Cell Boil. 1990, 111, 3177–3188. [Google Scholar] [CrossRef] [Green Version]
- Tillet, E.; Gential, B.; Garrone, R.; Stallcup, W.B. NG2 proteoglycan mediates beta1 integrin-independent cell adhesion and spreading on collagen VI. J. Cell Biochem. 2002, 86, 726–736. [Google Scholar] [CrossRef]
- Stallcup, W.B.; You, W.; Kucharova, K.; Cejudo-Martín, P.; Yotsumoto, F. NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Ozerdem, U.; Stallcup, W.B. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis 2004, 7, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Faust, M.; Schuster, N.; Montenarh, M. Specific binding of protein kinase CK2 catalytic subunits to tubulin. FEBS Lett. 1999, 462, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Ampofo, E.; Rudzitis-Auth, J.; Dahmke, I.N.; Rössler, O.G.; Thiel, G.; Montenarh, M.; Menger, M.D.; Laschke, M.W. Inhibition of protein kinase CK2 suppresses tumor necrosis factor (TNF)-α-induced leukocyte–endothelial cell interaction. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 2123–2136. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Ampofo, E.; Menger, M.D.; Laschke, M.W. miR-191 suppresses angiogenesis by activation of NF-kappaB signaling. FASEB J. 2017, 31, 3321–3333. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhao, N.; Bai, X.; Karram, K.; Trotter, J.; Goebbels, S.; Scheller, A.; Kirchhoff, F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 2014, 62, 896–913. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2009, 13, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekenya, M.; Krakstad, C.; Svendsen, A.; Netland, I.A.; Staalesen, V.; Tysnes, B.B.; Selheim, F.; Wang, J.; Sakariassen, P.O.; Sandal, T.; et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene 2008, 27, 5182–5194. [Google Scholar] [CrossRef] [Green Version]
- Sellers, D.L.; Maris, D.O.; Horner, P.J. Post-injury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J. Neurosci. 2009, 29, 6722–6733. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.C.; Nakatsu, M.; Chou, W.; Gershon, P.D.; Hughes, C.C. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Boil. Cell 2011, 22, 3791–3800. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sneddon, J.B.; Diehn, M.; Sood, R.; West, R.B.; Montgomery, K.; Chi, J.-T.; Van De Rijn, M.; Botstein, D.; O Brown, P. Gene Expression Signature of Fibroblast Serum Response Predicts Human Cancer Progression: Similarities between Tumors and Wounds. PLoS Boil. 2004, 2, e7. [Google Scholar] [CrossRef] [Green Version]
- Karen, J.; Rodriguez, A.; Friman, T.; Dencker, L.; Sundberg, C.; Scholz, B. Effects of the Histone Deacetylase Inhibitor Valproic Acid on Human Pericytes In Vitro. PLoS ONE 2011, 6, e24954. [Google Scholar] [CrossRef] [Green Version]
- Tsioumpekou, M.; Cunha, S.I.; Ma, H.; Ahgren, A.; Cedervall, J.; Olsson, A.K.; Heldin, C.H.; Lennartsson, J. Specific targeting of PDGFRbeta in the stroma inhibits growth and angiogenesis in tumors with high PDGF-BB expression. Theranostics 2020, 10, 1122–1135. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. Regulatory mechanisms of NG2/CSPG4 expression. Cell Mol. Biol. Lett. 2017, 22, 4. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, L.; Gilmour, J.; Bonifer, C. The Role of the Ubiquitously Expressed Transcription Factor Sp1 in Tissue-specific Transcriptional Regulation and in Disease. Yale J. Boil. Med. 2016, 89, 513–525. [Google Scholar]
- Armstrong, S.A.; Barry, D.A.; Leggett, R.W.; Mueller, C.R. Casein Kinase II-mediated Phosphorylation of the C Terminus of Sp1 Decreases Its DNA Binding Activity. J. Boil. Chem. 1997, 272, 13489–13495. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Kim, K.-H. Protein Kinase CK2 Down-Regulates Glucose-Activated Expression of the Acetyl-CoA Carboxylase Gene. Arch. Biochem. Biophys. 1997, 338, 227–232. [Google Scholar] [CrossRef]
- Levine, M.; Tjian, R. Transcription regulation and animal diversity. Nature 2003, 424, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Bara, J.J.; Sprecher, C.M.; Menzel, U.; Jalowiec, J.M.; Osinga, R.; Scherberich, A.; Alini, M.; Verrier, S. Pericyte plasticity—comparative investigation of the angiogenic and multilineage potential of pericytes from different human tissues. Eur Cell Mater. 2016, 31, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Bagley, R.G.; Weber, W.; Rouleau, C.; Teicher, B.A. Pericytes and Endothelial Precursor Cells: Cellular Interactions and Contributions to Malignancy. Cancer Res. 2005, 65, 9741–9750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-S.; Zhou, H.-N.; He, S.-S.; Xue, M.-Y.; Li, T.; Liu, L. Research advances in pericyte function and their roles in diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef]
- You, W.-K.; Yotsumoto, F.; Sakimura, K.; Adams, R.H.; Stallcup, W.B. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis 2013, 17, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.-J.; You, W.-K.; Bonaldo, P.; Seyfried, T.N.; Pasquale, E.B.; Stallcup, W.B. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev. Boil. 2010, 344, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Di Maira, G.; Salvi, M.; Arrigoni, G.; Marin, O.; Sarno, S.; Brustolon, F.; A Pinna, L.; Ruzzene, M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005, 12, 668–677. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chatterjee, U.; Ghosh, M.K. Activation of protein kinase CK2 attenuates FOXO3a functioning in a PML-dependent manner: Implications in human prostate cancer. Cell Death Dis. 2013, 4, e543. [Google Scholar] [CrossRef] [Green Version]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef]
- Wietecha, M.S.; Cerny, W.L.; DiPietro, L.A. Mechanisms of Vessel Regression: Toward an Understanding of the Resolution of Angiogenesis. Curr. Top. Microbiol. Immunol. 2012, 367, 3–32. [Google Scholar] [CrossRef]
- Kucharova, K.; Stallcup, W.B. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience 2009, 166, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolosi, P.A.; Dallatomasina, A.; Perris, R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 2015, 5, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, B.M.; Boewe, A.S.; Becker, V.; Nalbach, L.; Gu, Y.; Götz, C.; Menger, M.D.; Laschke, M.W.; Ampofo, E. Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes. Cells 2020, 9, 1546. https://doi.org/10.3390/cells9061546
Schmitt BM, Boewe AS, Becker V, Nalbach L, Gu Y, Götz C, Menger MD, Laschke MW, Ampofo E. Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes. Cells. 2020; 9(6):1546. https://doi.org/10.3390/cells9061546
Chicago/Turabian StyleSchmitt, Beate M., Anne S. Boewe, Vivien Becker, Lisa Nalbach, Yuan Gu, Claudia Götz, Michael D. Menger, Matthias W. Laschke, and Emmanuel Ampofo. 2020. "Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes" Cells 9, no. 6: 1546. https://doi.org/10.3390/cells9061546
APA StyleSchmitt, B. M., Boewe, A. S., Becker, V., Nalbach, L., Gu, Y., Götz, C., Menger, M. D., Laschke, M. W., & Ampofo, E. (2020). Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes. Cells, 9(6), 1546. https://doi.org/10.3390/cells9061546




