A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease
Abstract
1. Introduction
2. Pathology of Cerebral Small Vessel Disease
3. Brain Endothelial Cells
4. Oligodendrocytes
5. WNT Signaling in Endothelial Cells and Oligodendrocytes
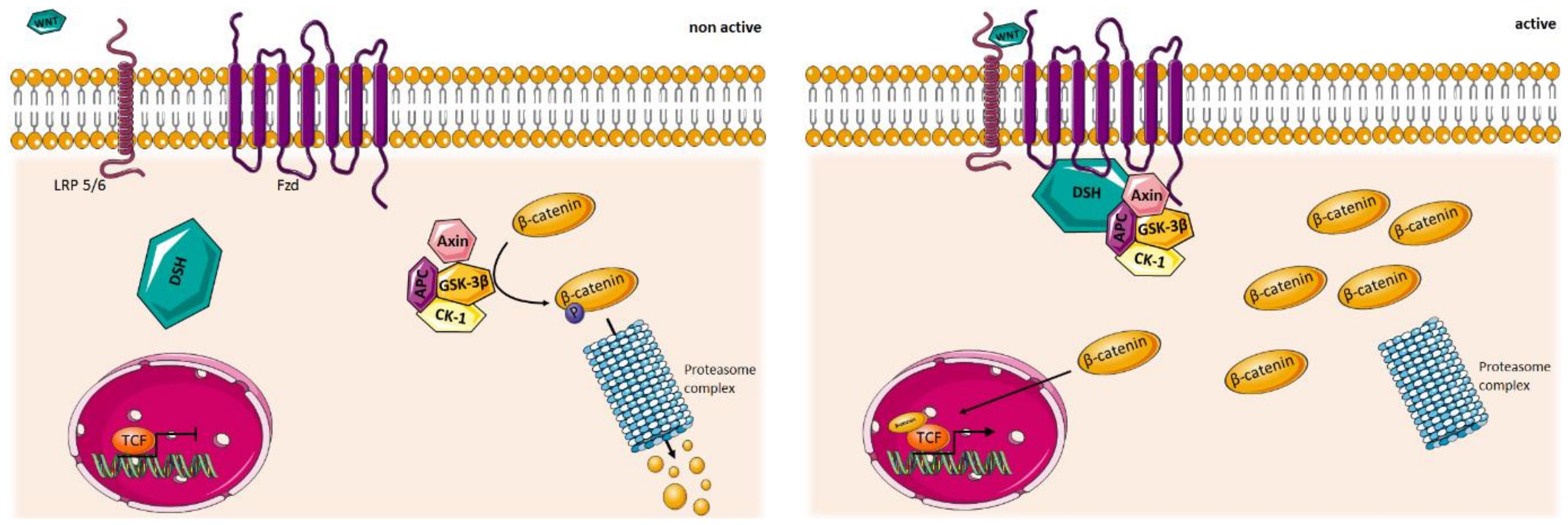
5.1. WNT Signaling
5.2. WNT Signaling in Brain Endothelial Cells
5.3. WNT Signaling in Oligodendrocytes
6. Oligodendrocyte–Endothelial Cell Crosstalk
7. Literature Search Method
8. WNT Signaling in Endothelial Cell–Oligodendrocyte Crosstalk
9. Discussion
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pinter, D.; Ritchie, S.J.; Doubal, F.; Gattringer, T.; Morris, Z.; Bastin, M.E.; Hernández, M.D.C.V.; Royle, N.A.; Corley, J.; Muñoz Maniega, S.; et al. Impact of small vessel disease in the brain on gait and balance. Sci. Rep. 2017, 7, 41637. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood-brain barrier: Ageing and microvascular disease-systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.H.; Lyu, P.Y.; Chen, W.H.; Li, R. Hypertension-Induced Cerebral Small Vessel Disease Leading to Cognitive Impairment. Chin. Med. J. (Engl.) 2018, 131, 615–619. [Google Scholar] [CrossRef]
- Zhang, C.E.; Wong, S.M.; Van De Haar, H.J.; Staals, J.; Jansen, J.F.A.; Jeukens, C.R.L.P.N.; Hofman, P.A.M.; Van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 2017, 88, 426–432. [Google Scholar] [CrossRef]
- Foulquier, S.; Namsolleck, P.; Van Hagen, B.T.; Milanova, I.; Post, M.J.; Blankesteijn, W.M.; Rutten, B.P.; Prickaerts, J.; Van Oostenbrugge, R.J.; Unger, T. Hypertension-induced cognitive impairment: Insights from prolonged angiotensin II infusion in mice. Hypertens. Res. 2018, 41, 817–827. [Google Scholar] [CrossRef]
- Wong, S.M.; Jansen, J.F.A.; Zhang, C.E.; Hoff, E.I.; Staals, J.; van Oostenbrugge, R.J.; Backes, W.H. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 2019, 92, 1669–1677. [Google Scholar] [CrossRef]
- Hawkins, B.T. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Ois Santiago, A.; Roquer Gonzalez, J.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef]
- Kimura, I.; Dohgu, S.; Takata, F.; Matsumoto, J.; Watanabe, T.; Iwao, T.; Yamauchi, A.; Kataoka, Y. Oligodendrocytes upregulate blood-brain barrier function through mechanisms other than the PDGF-BB/PDGFRα pathway in the barrier-tightening effect of oligodendrocyte progenitor cells. Neurosci. Lett. 2020, 715, 134594. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, L.; Tang, X.; Zhu, W.; Zhang, G.; Qin, Y.; Zhu, W. Changes of white matter integrity and structural network connectivity in nondemented cerebral small-vessel disease. J. Magn. Reson. Imaging 2019, 51, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, G.; Ohtomo, R.; Takase, H.; Lok, J.; Arai, K. Role of oligodendrocyte-neurovascular unit in white matter repair. Neurosci. Lett. 2018, 684, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lang, J.; Sohn, J.; Hammond, E.; Chang, M.; Pleasure, D. Canonical Wnt signaling in the oligodendroglial lineage-puzzles remain. Glia 2015, 63, 1671–1693. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral Small Vessel Disease (CSVD)–Lessons from the Animal Models. Front Physiol. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef]
- Mok, V.; Kim, J.S. Prevention and Management of Cerebral Small Vessel Disease. J. Stroke 2015, 17, 111–122. [Google Scholar] [CrossRef]
- Pantoni, L.; Garcia, J.H. The significance of cerebral white matter abnormalities 100 years after binswanger’s report: A review. Stroke 1995, 26, 1293–1301. [Google Scholar] [CrossRef]
- Faraco, G.; Iadecola, C. Hypertension: A harbinger of stroke and dementia. Hypertension 2013, 62, 810–817. [Google Scholar] [CrossRef]
- Hooghiemstra, A.M.; Bertens, A.S.; Leeuwis, A.E.; Bron, E.E.; Bots, M.L.; Brunner-La Rocca, H.P.; De Craen, A.J.M.; Van Der Geest, R.J.; Greving, J.P.; Kappelle, L.J.; et al. The Missing Link in the Pathophysiology of Vascular Cognitive Impairment: Design of the Heart-Brain Study. Cerebrovasc. Dis. Extra 2017, 7, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Liu, Q.; Agca, C.; Agca, Y.; Noble, E.G.; Whitehead, S.N.; Cechetto, D.F. White matter inflammation and cognitive function in a co-morbid metabolic syndrome and prodromal Alzheimer’s disease rat model. J. Neuroinflamm. 2020, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.M.; Williams, A. Endothelial cell–oligodendrocyte interactions in small vessel disease and aging. Clin. Sci. 2017, 131, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Ransom, B.; Goldman, S.A. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003, 26, 523–530. [Google Scholar] [CrossRef]
- Dermietzel, R.; Spray, D.C.; Nedergaard, M. Blood-Brain Barriers: From Ontogeny to Artificial Interfaces; Wiley-Blackwell: Hoboken, NJ, USA, 2007; ISBN 3527310886. [Google Scholar]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Rouhl, R.P.W.; Damoiseaux, J.G.M.C.; Lodder, J.; Theunissen, R.O.M.F.I.H.; Knottnerus, I.L.H.; Staals, J.; Henskens, L.H.G.; Kroon, A.A.; de Leeuw, P.W.; Tervaert, J.W.C.; et al. Vascular inflammation in cerebral small vessel disease. Neurobiol. Aging 2012, 33, 1800–1806. [Google Scholar] [CrossRef]
- Wilkins, A.; Chandran, S.; Compston, A. A role for oligodendrocyte-derived IGF-1 in trophic support of cortical neurons. Glia 2001, 36, 48–57. [Google Scholar] [CrossRef]
- Nave, K.A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Steadman, P.E.; Xia, F.; Ahmed, M.; Mocle, A.J.; Penning, A.R.A.; Geraghty, A.C.; Steenland, H.W.; Monje, M.; Josselyn, S.A.; Frankland, P.W. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020, 105, 150–164. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar]
- Ceprian, M.; Fulton, D. Glial cell AMPA Receptors in nervous system health, injury and disease. Int. J. Mol. Sci. 2019, 20, 2450. [Google Scholar] [CrossRef]
- Fannon, J.; Tarmier, W.; Fulton, D. Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 2015, 63, 1021–1035. [Google Scholar] [CrossRef]
- Butt, A.M.; Hamilton, N.; Hubbard, P.; Pugh, M.; Ibrahim, M. Synantocytes: The fifth element. J. Anat. 2005, 207, 695–706. [Google Scholar] [CrossRef]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Psachoulia, K.; Jamen, F.; Young, K.M.; Richardson, W.D. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009, 5, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.Y.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of Oligodendrocyte Generation and Myelination in the Human Brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.H.; Demir, S.; Wiese, S.; Kruse, N.; Siglienti, I.; Gerhardt, E.; Neumann, H.; Sendtner, M.; Lühder, F.; et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: Therapeutic implications in a model of multiple sclerosis. Brain 2010, 133, 2248–2263. [Google Scholar] [CrossRef]
- Choi, B.R.; Kim, D.H.; Back, D.B.; Kang, C.H.; Moon, W.J.; Han, J.S.; Choi, D.H.; Kwon, K.J.; Shin, C.Y.; Kim, B.R.; et al. Characterization of White Matter Injury in a Rat Model of Chronic Cerebral Hypoperfusion. Stroke 2016, 47, 542–547. [Google Scholar] [CrossRef]
- Miyamoto, N.; Tanaka, R.; Shimura, H.; Watanabe, T.; Mori, H.; Onodera, M.; Mochizuki, H.; Hattori, N.; Urabe, T. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J. Cereb. Blood Flow Metab. 2010, 30, 299–310. [Google Scholar] [CrossRef]
- Jalal, F.Y.; Yang, Y.; Thompson, J.; Lopez, A.C.; Rosenberg, G.A. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 2012, 43, 1115–1122. [Google Scholar] [CrossRef]
- Yuen, T.J.; Silbereis, J.C.; Griveau, A.; Chang, S.M.; Daneman, R.; Fancy, S.P.J.; Zahed, H.; Maltepe, E.; Rowitch, D.H. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 2014, 158, 383–396. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Cattelino, A.; Liebner, S.; Gallini, R.; Zanetti, A.; Balconi, G.; Corsi, A.; Blanco, P.; Wolburg, H.; Moore, R.; Oreda, B.; et al. The conditional inactivation of the β-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 2003, 162, 1111–1122. [Google Scholar] [CrossRef]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef]
- Franco, C.A.; Liebner, S.; Gerhardt, H. Vascular morphogenesis: A Wnt for every vessel? Curr. Opin. Genet. Dev. 2009, 19, 476–483. [Google Scholar] [CrossRef]
- Shivanna, S.; Harrold, I.; Shashar, M.; Meyer, R.; Kiang, C.; Francis, J.; Zhao, Q.; Feng, H.; Edelman, E.R.; Rahimi, N.; et al. The C-Cbl ubiquitin ligase regulates nuclear β-catenin and angiogenesis by its tyrosine phosphorylation mediated through the Wnt signaling pathway. J. Biol. Chem. 2015, 290, 12537–12546. [Google Scholar] [CrossRef]
- Cho, C.; Smallwood, P.M.; Nathans, J. Reck and Gpr124 Are Essential Receptor Cofactors for Wnt7a/Wnt7b-Specific Signaling in Mammalian CNS Angiogenesis and Blood-Brain Barrier Regulation. Neuron 2017, 95, 1056–1073.e5. [Google Scholar] [CrossRef]
- Vanhollebeke, B.; Stone, O.A.; Bostaille, N.; Cho, C.; Zhou, Y.; Maquet, E.; Gauquier, A.; Cabochette, P.; Fukuhara, S.; Mochizuki, N.; et al. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 2015, 8, e06489. [Google Scholar] [CrossRef]
- Benz, F.; Wichitnaowarat, V.; Lehmann, M.; Germano, R.F.; Mihova, D.; Macas, J.; Adams, R.H.; Mark Taketo, M.; Plate, K.H.; Guérit, S.; et al. Low wnt/β-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. eLife 2019, 8, e43818. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kagawa, T.; Wada, T.; Muroyama, Y.; Takada, S.; Ikenaka, K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 2005, 282, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Soomro, S.H.; Jie, J.; Fu, H. Oligodendrocytes Development and Wnt Signaling Pathway. Int. J. Hum. Anat. 2018, 1, 17–35. [Google Scholar] [CrossRef]
- Langseth, A.J.; Munji, R.N.; Choe, Y.; Huynh, T.; Pozniak, C.D.; Pleasure, S.J. Wnts Influence the Timing and Efficiency of Oligodendrocyte Precursor Cell Generation in the Telencephalon. J. Neurosci. 2010, 30, 13367–13372. [Google Scholar] [CrossRef]
- Fancy, S.P.J.; Baranzini, S.E.; Zhao, C.; Yuk, D.I.; Irvine, K.A.; Kaing, S.; Sanai, N.; Franklin, R.J.M.; Rowitch, D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009, 23, 1571–1585. [Google Scholar] [CrossRef]
- Tawk, M.; Makoukji, J.; Belle, M.; Fonte, C.; Trousson, A.; Hawkins, T.; Li, H.; Ghandour, S.; Schumacher, M.; Massaad, C. Wnt/β-Catenin Signaling Is an Essential and Direct Driver of Myelin Gene Expression and Myelinogenesis. J. Neurosci. 2011, 31, 3729–3742. [Google Scholar] [CrossRef]
- Huang, N.; Chen, D.; Wu, X.; Chen, X.; Zhang, X.; Niu, J.; Shen, H.Y.; Xiao, L. Aspirin Promotes Oligodendroglial Differentiation Through Inhibition of Wnt Signaling Pathway. Mol. Neurobiol. 2016, 53, 3258–3266. [Google Scholar] [CrossRef]
- Lang, J.; Maeda, Y.; Bannerman, P.; Xu, J.; Horiuchi, M.; Pleasure, D.; Guo, F. Adenomatous Polyposis Coli Regulates Oligodendroglial Development. J. Neurosci. 2013, 33, 3113–3130. [Google Scholar] [CrossRef]
- Fu, H.; Kesari, S.; Cai, J. Tcf7l2 is tightly controlled during myelin formation. Cell. Mol. Neurobiol. 2012, 32, 345–352. [Google Scholar] [CrossRef]
- Lürbke, A.; Hagemeier, K.; Cui, Q.L.; Metz, I.; Brück, W.; Antel, J.; Kuhlmann, T. Limited TCF7L2 Expression in MS Lesions. PLoS ONE 2013, 8, e72822. [Google Scholar] [CrossRef]
- Ulanska-Poutanen, J.; Mieczkowski, J.; Zhao, C.; Konarzewska, K.; Kaza, B.; Pohl, H.B.F.; Bugajski, L.; Kaminska, B.; Franklin, R.J.M.; Zawadzka, M. Injury-induced perivascular niche supports alternative differentiation of adult rodent CNS progenitor cells. eLife 2018, 7, e30325. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Horie, R. Developmental course of hypertension and regional cerebral blood flow in stroke-prone spontaneously hypertensive rats. Stroke 1977, 8, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Tomimoto, H.; Akiguchi, I.; Wakita, H.; Shibasaki, H.; Horie, R. White matter lesions and alteration of vascular cell composition in the brain of spontaneously hypertensive rats. Neuroreport 2001, 12, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.L.; Smith, C.; Sudlow, C.L.M.; Wardlaw, J.M. Is the spontaneously hypertensive stroke prone rat a pertinent model of sub cortical ischemic stroke? A systematic review. Int. J. Stroke 2011, 6, 434–444. [Google Scholar] [CrossRef]
- Mayes, D.A.; Rizvi, T.A.; Titus-Mitchell, H.; Oberst, R.; Ciraolo, G.M.; Vorhees, C.V.; Robinson, A.P.; Miller, S.D.; Cancelas, J.A.; Stemmer-Rachamimov, A.O.; et al. Nf1 Loss and Ras Hyperactivation in Oligodendrocytes Induce NOS-Driven Defects in Myelin and Vasculature. Cell Rep. 2013, 4, 1197–1212. [Google Scholar] [CrossRef]
- Tong, X.K.; Trigiani, L.J.; Hamel, E. High cholesterol triggers white matter alterations and cognitive deficits in a mouse model of cerebrovascular disease: Benefits of simvastatin. Cell Death Dis. 2019, 10, 89. [Google Scholar] [CrossRef]
- Rajani, R.M.; Quick, S.; Ruigrok, S.R.; Graham, D.; Harris, S.E.; Verhaaren, B.F.J.; Fornage, M.; Seshadri, S.; Atanur, S.S.; Dominiczak, A.F.; et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci. Transl. Med. 2018, 10, eaam9507. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. An Oligovascular Niche: Cerebral Endothelial Cells Promote the Survival and Proliferation of Oligodendrocyte Precursor Cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef]
- Seo, J.H.; Miyamoto, N.; Hayakawa, K.; Pham, L.D.D.; Maki, T.; Ayata, C.; Kim, K.W.; Lo, E.H.; Arai, K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J. Clin. Investig. 2013, 123, 782–786. [Google Scholar] [CrossRef]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef]
- Wang, L.; Geng, J.; Qu, M.; Yuan, F.; Wang, Y.; Pan, J.; Li, Y.; Ma, Y.; Zhou, P.; Zhang, Z.; et al. Oligodendrocyte precursor cells transplantation protects blood–brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Pham, L.D.D.; Seo, J.H.; Kim, K.W.; Lo, E.H.; Arai, K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell. Mol. Life Sci. 2014, 71, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Niu, J.; Munji, R.; Davalos, D.; Chang, J.; Zhang, H.; Tien, A.C.; Kuo, C.J.; Chan, J.R.; Daneman, R.; et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 2016, 351, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Tsai, H.-H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial–vascular interactions disrupt the blood–brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef]
- Iijima, K.; Kurachi, M.; Shibasaki, K.; Naruse, M.; Puentes, S.; Imai, H.; Yoshimoto, Y.; Mikuni, M.; Ishizaki, Y. Transplanted microvascular endothelial cells promote oligodendrocyte precursor cell survival in ischemic demyelinating lesions. J. Neurochem. 2015, 135, 539–550. [Google Scholar] [CrossRef]
- Altun, I.; Oz, F.; Arkaya, S.C.; Altun, I.; Bilge, A.K.; Umman, B.; Turkoglu, U.M. Effect of Statins on Endothelial Function in Patients with Acute Coronary Syndrome: A Prospective Study Using Adhesion Molecules and Flow-Mediated Dilatation. J. Clin. Med. Res. 2014, 6, 354. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014, 358, 633–649. [Google Scholar] [CrossRef]
- Bugiani, M.; Kevelam, S.H.; Bakels, H.S.; Waisfisz, Q.; Ceuterick-De Groote, C.; Niessen, H.W.M.; Abbink, T.E.M.; Lesnik Oberstein, S.A.M.J.; Van Der Knaap, M.S. Cathepsin A-related arteriopathy with strokes and leukoencephalopathy (CARASAL). Neurology 2016, 87, 1777–1786. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Li, L.; Wei, B.; He, A.; Lu, L.; Li, X.; Zhang, L.; Xu, Z.; Sun, M. Methylation-reprogrammed Wnt/β-catenin signalling mediated prenatal hypoxia-induced brain injury in foetal and offspring rats. J. Cell. Mol. Med. 2018, 22, 3866–3874. [Google Scholar] [CrossRef]
- Li, C.; Zheng, X.; Han, Y.; Lv, Y.; Lan, F.; Zhao, J. XAV939 inhibits the proliferation and migration of lung adenocarcinoma A549 cells through the WNT pathway. Oncol. Lett. 2018, 15, 8973–8982. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Yanguas-Casás, N.; Feliú, A.; Mestre, L.; Carrillo-Salinas, F.J.; Riecken, K.; Gomez-Nicola, D.; Guaza, C. Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis. J. Neuroinflamm. 2020, 17, 88. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manukjan, N.; Ahmed, Z.; Fulton, D.; Blankesteijn, W.M.; Foulquier, S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells 2020, 9, 1545. https://doi.org/10.3390/cells9061545
Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells. 2020; 9(6):1545. https://doi.org/10.3390/cells9061545
Chicago/Turabian StyleManukjan, Narek, Zubair Ahmed, Daniel Fulton, W. Matthijs Blankesteijn, and Sébastien Foulquier. 2020. "A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease" Cells 9, no. 6: 1545. https://doi.org/10.3390/cells9061545
APA StyleManukjan, N., Ahmed, Z., Fulton, D., Blankesteijn, W. M., & Foulquier, S. (2020). A Systematic Review of WNT Signaling in Endothelial Cell Oligodendrocyte Interactions: Potential Relevance to Cerebral Small Vessel Disease. Cells, 9(6), 1545. https://doi.org/10.3390/cells9061545







