Insight into Salivary Gland Aquaporins
Abstract
1. Introduction
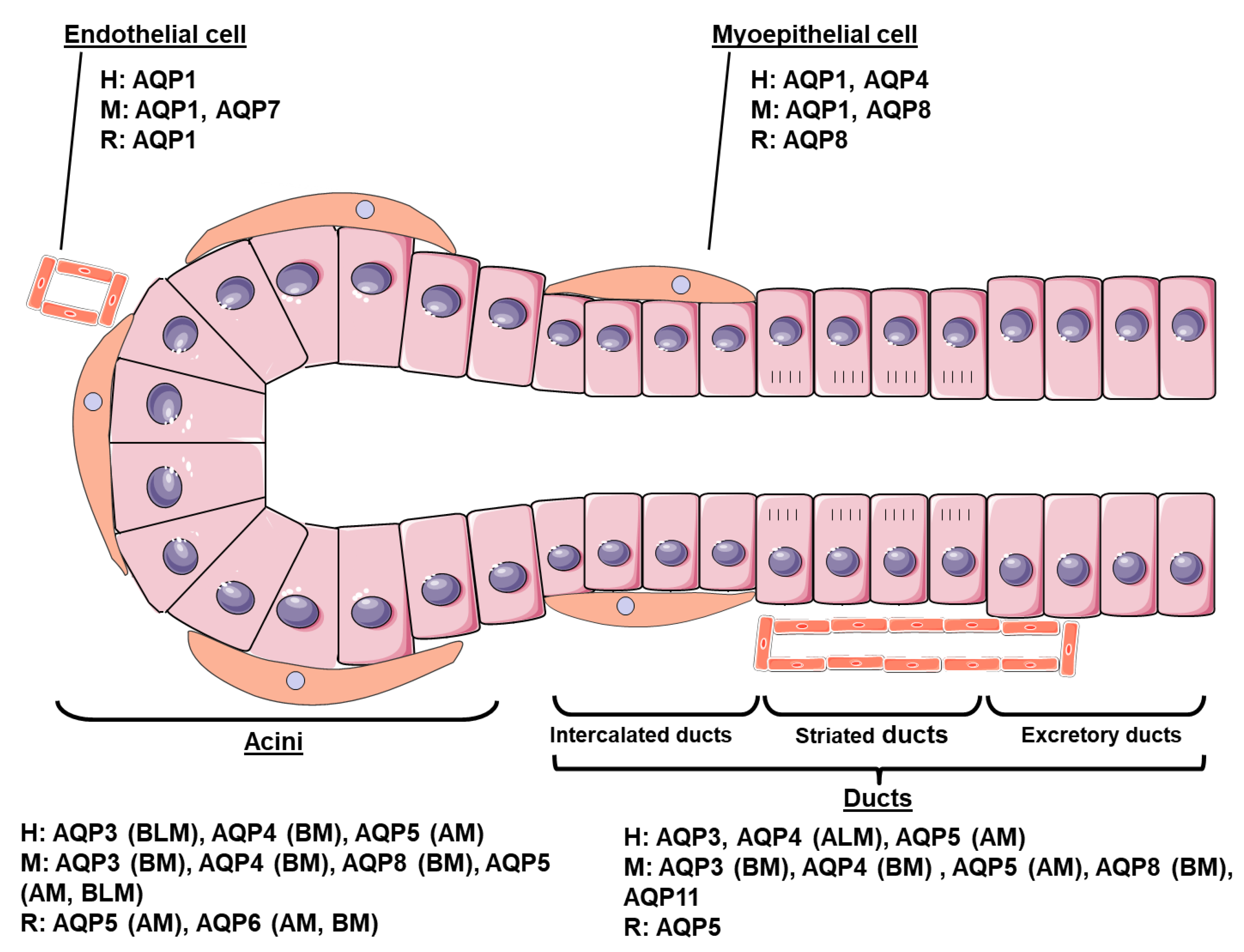
2. Cellular Distribution of AQPs in Adult SG Subsection
2.1. Human
2.2. Mouse
2.3. Rat Subsection
3. Physiological Function of the AQPs in SG
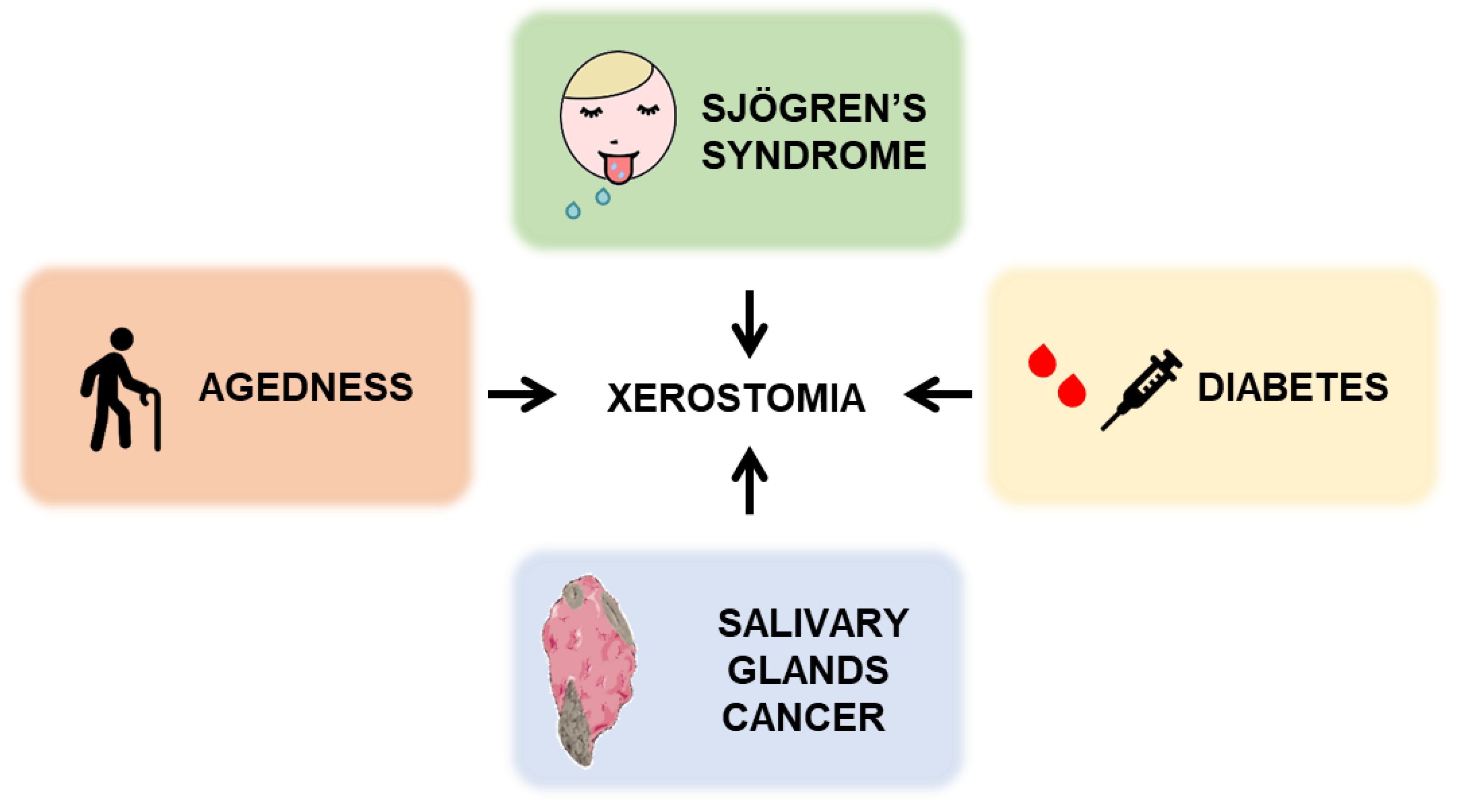
4. Involvement of AQPs in SG Pathologies
4.1. Sjögren’s Syndrome
4.2. Radiotherapy for Head and Neck Cancer
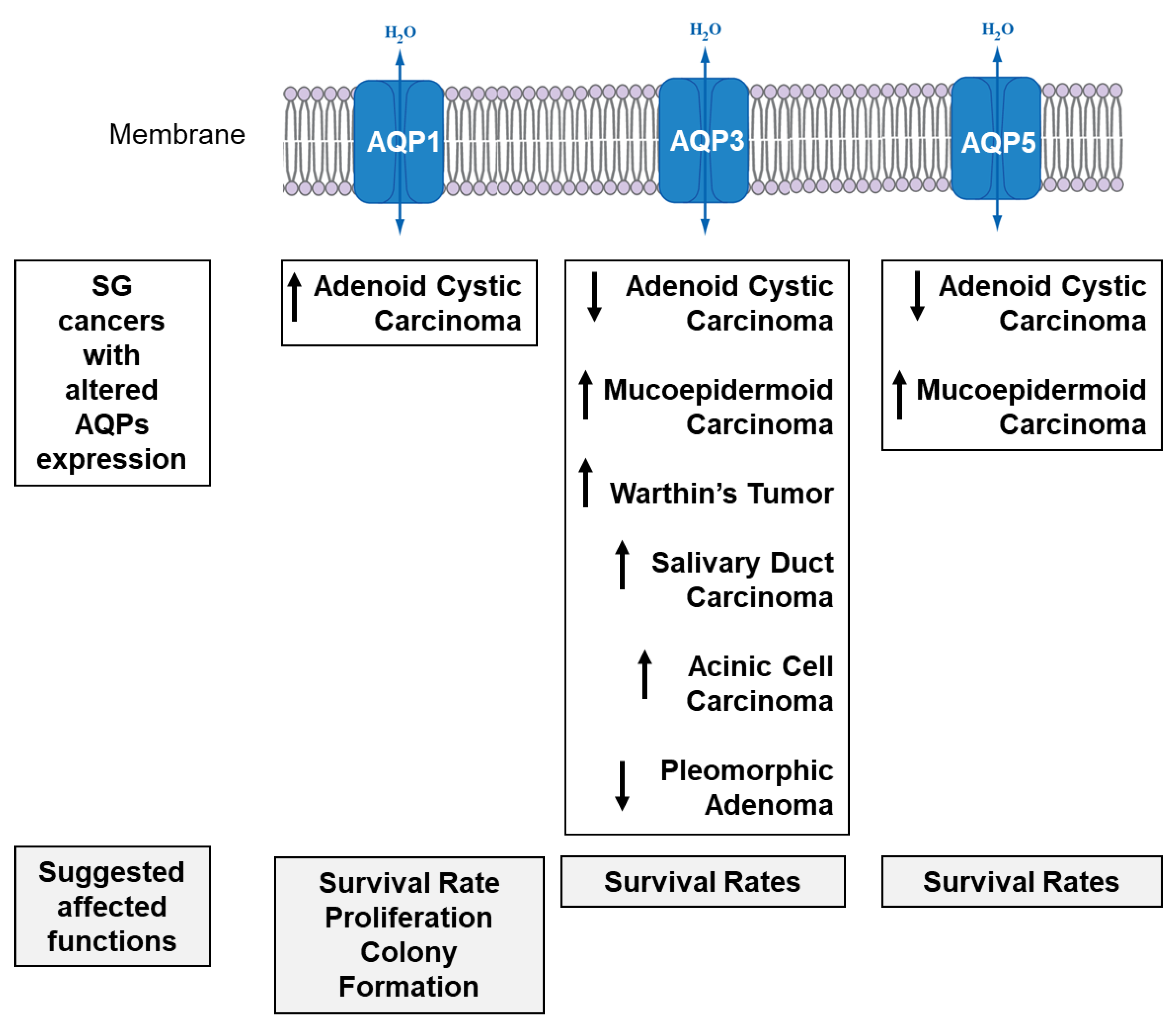
4.3. SG Cancer
4.4. Agedness and Diabetes
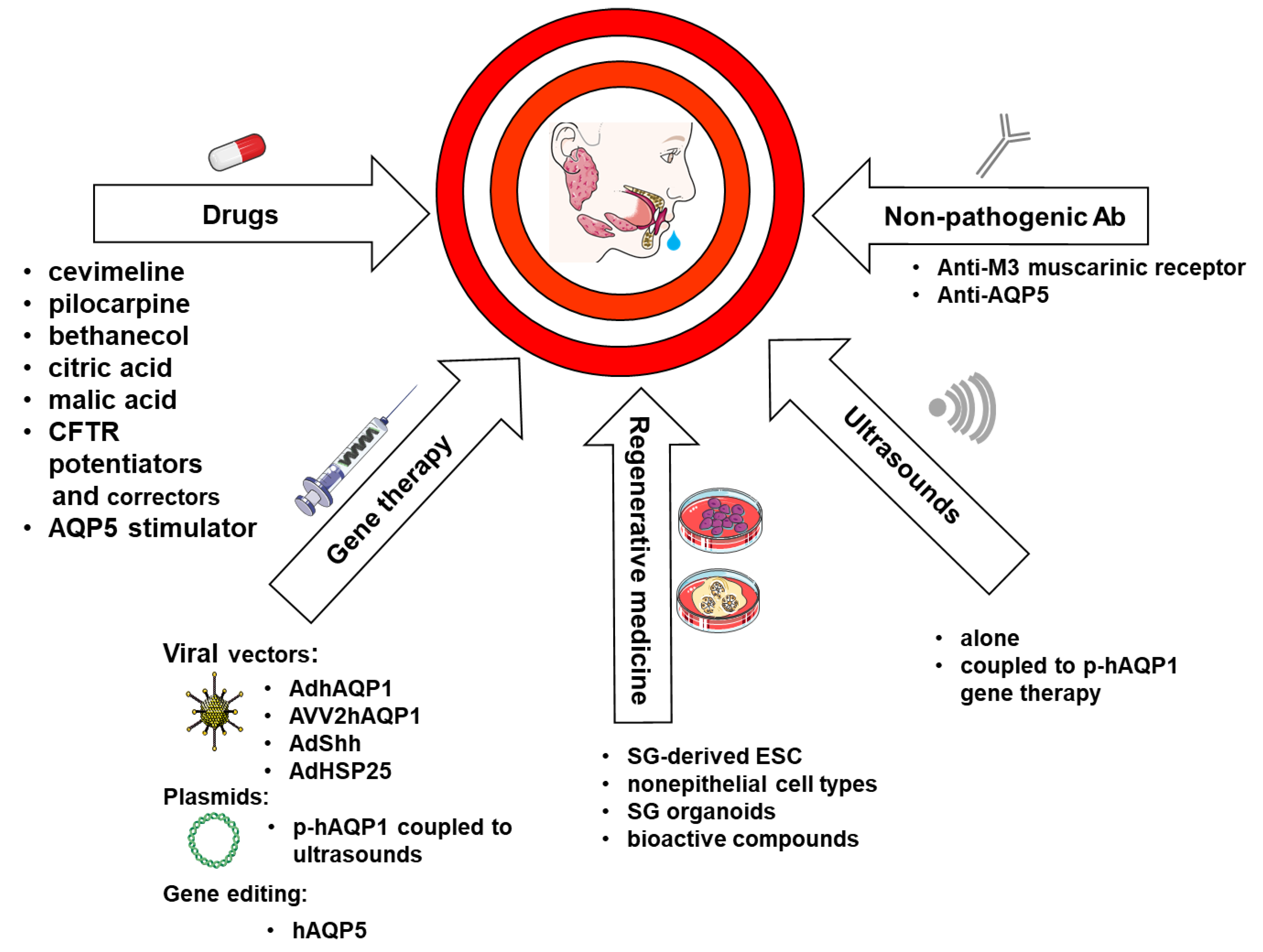
5. Therapeutic Strategies Aiming at AQPs to Treat Xerostomia
5.1. Drugs
5.2. Ultrasounds
5.3. Non-Pathogenic Antibodies
5.4. Gene Therapy
5.5. AQPs and Regenerative Medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melvin, J.E.; Yule, D.; Shuttleworth, T.; Begenisich, T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005, 67, 445–469. [Google Scholar] [CrossRef] [PubMed]
- Amerongen, A.V.N.; Veerman, E.C.I. Saliva--the defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. 1993, 265, F463–476. [Google Scholar] [CrossRef]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels--from atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Conner, M.; Bill, R.M.; Conner, A.C. Structural determinants of oligomerization of the aquaporin-4 channel. J. Biol. Chem. 2016, 291, 6858–6871. [Google Scholar] [CrossRef]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, J.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The Evolutionary Aspects of Aquaporin Family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [Google Scholar] [CrossRef]
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef]
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and obesity. Cell. Mol. Life Sci. Cmls 2015, 72, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Chivasso, C.; Perret, J.; Delporte, C. Involvement of Aquaporins in the Pathogenesis, Diagnosis and Treatment of Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3392. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective Secretion of Saliva in Transgenic Mice Lacking Aquaporin-5 Water Channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef]
- Verkman, A.S.; Yang, B.; Song, Y.; Manley, G.T.; Ma, T. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 2000, 85, 233s–241s. [Google Scholar] [CrossRef]
- Delporte, C. Aquaporins in salivary glands and pancreas. Biochim. Biophys. Acta Bba-Gen. Subj. 2014, 1840, 1524–1532. [Google Scholar] [CrossRef]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Coutinho-Camillo, C.M.; Nico, M.M.S.; Lourenco, S.V. The expression of water channel proteins during human salivary gland development: A topographic study of aquaporins 1, 3 and 5. J. Mol. Histol. 2017, 48, 329–336. [Google Scholar] [CrossRef]
- Stamboni, M.B.; Gomes, Á.N.; de, M.; de Souza, M.M.; Oliveira, K.K.; Arruda, C.F.J.; de Paula, F.; Bettim, B.B.; Marques, M.M.; Kowalski, L.P.; et al. Aquaporin 1, 3, and 5 Patterns in Salivary Gland Mucoepidermoid Carcinoma: Expression in Surgical Specimens and an In Vitro Pilot Study. Int. J. Mol. Sci. 2020, 21, 1287. [Google Scholar] [CrossRef]
- Delporte, C. Aquaporins and Gland Secretion. In Aquaporins; Yang, B., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2017; Volume 969, pp. 63–79. ISBN 978-94-024-1055-6. [Google Scholar]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Nico, B.; Ribatti, D.; Ruggieri, S.; Lofrumento, D.D.; Lisi, S. Abnormal distribution of AQP4 in minor salivary glands of primary Sjögren’s syndrome patients. Autoimmunity 2017, 50, 202–210. [Google Scholar] [CrossRef]
- Hosoi, K.; Yao, C.; Hasegawa, T.; Yoshimura, H.; Akamatsu, T. Dynamics of Salivary Gland AQP5 under Normal and Pathologic Conditions. Int. J. Mol. Sci. 2020, 21, 1182. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Ruus, A.-K.; Galtung, H.K. Aquaporins in the adult mouse submandibular and sublingual salivary glands. J. Mol. Histol. 2014, 45, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Saga, T.; Watanabe, K.; Takahashi, N.; Tabira, Y.; Kusukawa, J.; Yamaki, K.-I. An immunohistochemistry-based study on aquaporin (AQP)-1, 3, 4, 5 and 8 in the parotid glands, submandibular glands and sublingual glands of Sjögren’s syndrome mouse models chronically administered cevimeline. Kurume Med. J. 2013, 60, 7–19. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Yasui, M. Aquaporins and disease: Lessons from mice to humans. Trends Endocrinol. Metab. 2002, 13, 355–360. [Google Scholar] [CrossRef]
- Sapmaz, E.; Uysal, M.; Tumer, M.K.; Sapmaz, H.I.; Somuk, B.T.; Arici, A.; Tas, U. Investigation of age-related changes in the expression of aquaporin-1 and aquaporin-5 in the salivary glands of mice. Acta Otolaryngol. (Stockh.) 2016, 136, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.S.; Aure, M.H.; Peters, S.B.; Larsen, M.; Messelt, E.B.; Kanli Galtung, H. Localization of AQP5 during development of the mouse submandibular salivary gland. J. Mol. Histol. 2011, 42, 71–81. [Google Scholar] [CrossRef]
- Larsen, H.S.; Ruus, A.-K.; Schreurs, O.; Galtung, H.K. Aquaporin 11 in the developing mouse submandibular gland. Eur. J. Oral Sci. 2010, 118, 9–13. [Google Scholar] [CrossRef]
- Araujo, M.V.T.; Spadella, M.A.; Chies, A.B.; Arruda, G.V.; de Santos, M.T.; Cavariani, M.M.; Domeniconi, R.F. Effect of low radiation dose on the expression and location of aquaporins in rat submandibular gland. Tissue Cell 2018, 53, 104–110. [Google Scholar] [CrossRef]
- Gresz, V.; Kwon, T.H.; Hurley, P.T.; Varga, G.; Zelles, T.; Nielsen, S.; Case, R.M.; Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281, G247–G254. [Google Scholar] [CrossRef]
- Delporte, C.; Steinfeld, S. Distribution and roles of aquaporins in salivary glands. Biochim. Biophys. Acta Bba-Biomembr. 2006, 1758, 1061–1070. [Google Scholar] [CrossRef]
- Mangos, J.A.; McSherry, N.R. Micropuncture study of urea excretion in parotid saliva of the rat. Am. J. Physiol. 1970, 218, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Matsuki-Fukushima, M.; Fujita-Yoshigaki, J.; Murakami, M.; Katsumata-Kato, O.; Yokoyama, M.; Sugiya, H. Involvement of AQP6 in the Mercury-Sensitive Osmotic Lysis of Rat Parotid Secretory Granules. J. Membr. Biol. 2013, 246, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S.; Hiramatsu, Y.; Lockwich, T.; Baum, B.J. Activation and regulation of calcium entry in rat parotid gland acinar cells. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1993, 4, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Salivary secretion: Mechanism and neural regulation. Monogr. Oral Sci. 2014, 24, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Azlina, A.; Karabasil, M.R.; Purwanti, N.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Degradation of submandibular gland AQP5 by parasympathetic denervation of chorda tympani and its recovery by cevimeline, an M3 muscarinic receptor agonist. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G112–G123. [Google Scholar] [CrossRef]
- Azlina, A.; Javkhlan, P.; Hiroshima, Y.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Roles of lysosomal proteolytic systems in AQP5 degradation in the submandibular gland of rats following chorda tympani parasympathetic denervation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1106–1117. [Google Scholar] [CrossRef]
- Chen, G.; Yao, C.; Hasegawa, T.; Akamatsu, T.; Yoshimura, H.; Hosoi, K. Effects of isoproterenol on aquaporin 5 levels in the parotid gland of mice in vivo. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E100–108. [Google Scholar] [CrossRef]
- Yang, F.; Kawedia, J.D.; Menon, A.G. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J. Biol. Chem. 2003, 278, 32173–32180. [Google Scholar] [CrossRef]
- Kitchen, P.; Öberg, F.; Sjöhamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Törnroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef]
- Ishii, M.; Kurachi, Y. Muscarinic acetylcholine receptors. Curr. Pharm. Des. 2006, 12, 3573–3581. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporin water channels and endothelial cell function. J. Anat. 2002, 200, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Mammalian aquaporins: Diverse physiological roles and potential clinical significance. Expert Rev. Mol. Med. 2008, 10, e13. [Google Scholar] [CrossRef] [PubMed]
- Login, F.H.; Jensen, H.H.; Pedersen, G.A.; Koffman, J.S.; Kwon, T.-H.; Parsons, M.; Nesjum, L.N. Aquaporins differentially regulate cell-cell adhesion in MDCK cells. Faseb J. 2019, 33, 6980–6994. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren syndrome. Nat. Rev. Dis. Primers. 2016, 2, 16047. [Google Scholar] [CrossRef]
- Thorne, I.; Sutcliffe, N. Sjögren’s syndrome. Br. J. Hosp. Med. Lond. Engl. 2005 2017, 78, 438–442. [Google Scholar] [CrossRef]
- Sandhya, P.; Kurien, B.T.; Danda, D.; Scofield, R.H. Update on Pathogenesis of Sjogren’s Syndrome. Curr. Rheumatol. Rev. 2017, 13, 5–22. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M.; Kordossis, T. Sjögren’s syndrome revisited: Autoimmune epithelitis. Br. J. Rheumatol. 1996, 35, 204–206. [Google Scholar] [CrossRef][Green Version]
- Ohlsson, M.; Jonsson, R.; Brokstad, K.A. Subcellular redistribution and surface exposure of the Ro52, Ro60 and La48 autoantigens during apoptosis in human ductal epithelial cells: A possible mechanism in the pathogenesis of Sjögren’s syndrome. Scand. J. Immunol. 2002, 56, 456–469. [Google Scholar] [CrossRef]
- Fayyaz, A.; Kurien, B.T.; Scofield, R.H. Autoantibodies in Sjögren’s Syndrome. Rheum. Dis. Clin. North. Am 2016, 42, 419–434. [Google Scholar] [CrossRef]
- Kovács, L.; Marczinovits, I.; György, A.; Tóth, G.K.; Dorgai, L.; Pál, J.; Molnár, J.; Pokorny, G. Clinical associations of autoantibodies to human muscarinic acetylcholine receptor 3(213-228) in primary Sjogren’s syndrome. Rheumatol. Oxf. Engl. 2005, 44, 1021–1025. [Google Scholar] [CrossRef][Green Version]
- Jeon, S.; Lee, J.; Park, S.-H.; Kim, H.-D.; Choi, Y. Associations of Anti-Aquaporin 5 Autoantibodies with Serologic and Histopathological Features of Sjögren’s Syndrome. J. Clin. Med. 2019, 8, 1863. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jin, Y.; Zhang, X.; Zhou, Y.; Li, R.; Dai, Y.; Sun, X.; Zhao, J.; Guo, J.; Li, Z. Characteristics of germinal center-like structures in patients with Sjögren’s syndrome. Int. J. Rheum. Dis. 2017, 20, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; Konno, A.; Bolaky, N.; Oak, J.S.; Fruman, D.; Nicaise, C.; Takiguchi, M.; Delporte, C. Link between inflammation and aquaporin-5 distribution in submandibular gland in Sjögren’s syndrome? Oral Dis. 2012, 18, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Soyfoo, M.S.; De Vriese, C.; Debaix, H.; Martin-Martinez, M.D.; Mathieu, C.; Devuyst, O.; Steinfeld, S.D.; Delporte, C. Modified aquaporin 5 expression and distribution in submandibular glands from NOD mice displaying autoimmune exocrinopathy. Arthritis Rheum. 2007, 56, 2566–2574. [Google Scholar] [CrossRef]
- Boumba, D.; Skopouli, F.N.; Moutsopoulos, H.M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br. J. Rheumatol. 1995, 34, 326–333. [Google Scholar] [CrossRef]
- Fox, R.I.; Kang, H.I.; Ando, D.; Abrams, J.; Pisa, E. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J. Immunol. Baltim. Md 1950 1994, 152, 5532–5539. [Google Scholar]
- Jin, J.-O.; Yu, Q. T Cell-Associated Cytokines in the Pathogenesis of Sjögren’s Syndrome. J. Clin. Cell. Immunol. 2013, 11742. [Google Scholar] [CrossRef]
- Cha, S.; Brayer, J.; Gao, J.; Brown, V.; Killedar, S.; Yasunari, U.; Peck, A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004, 60, 552–565. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, J.-O.; Kawai, T.; Yu, Q. Endogenous programmed death ligand-1 restrains the development and onset of Sjӧgren’s syndrome in non-obese diabetic mice. Sci. Rep. 2016, 6, 39105. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Jin, Y.; Zhao, R.; Dong, C.; Zheng, W.; Zhang, T.; Li, J.; Gu, Z. B7-H3 participates in human salivary gland epithelial cells apoptosis through NF-κB pathway in primary Sjögren’s syndrome. J. Transl. Med. 2019, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Arce-Franco, M.; Dominguez-Luis, M.; Pec, M.K.; Martínez-Gimeno, C.; Miranda, P.; Alvarez de la Rosa, D.; Giraldez, T.; García-Verdugo, J.M.; Machado, J.D.; Díaz-González, F. Functional effects of proinflammatory factors present in Sjögren’s syndrome salivary microenvironment in an in vitro model of human salivary gland. Sci. Rep. 2017, 7, 11897. [Google Scholar] [CrossRef] [PubMed]
- Limaye, A.; Hall, B.E.; Zhang, L.; Cho, A.; Prochazkova, M.; Zheng, C.; Walker, M.; Adewusi, F.; Burbelo, P.D.; Sun, Z.J.; et al. Targeted TNF-α Overexpression Drives Salivary Gland Inflammation. J. Dent. Res. 2019, 98, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Motegi, K.; Kani, K.; Takano, H.; Momota, Y.; Aota, K.; Yamanoi, T.; Azuma, M. TNF-α inhibits aquaporin 5 expression in human salivary gland acinar cells via suppression of histone H4 acetylation. J. Cell. Mol. Med. 2012, 16, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kawai, T.; Yu, Q. Pathogenic role of endogenous TNF-α in the development of Sjögren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Nandula, S.R.; Amarnath, S.; Molinolo, A.; Bandyopadhyay, B.C.; Hall, B.; Goldsmith, C.M.; Zheng, C.; Larsson, J.; Sreenath, T.; Chen, W.; et al. Female mice are more susceptible to developing inflammatory disorders due to impaired transforming growth factor beta signaling in salivary glands. Arthritis Rheum. 2007, 56, 1798–1805. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Carcamo, W.C.; Chiorini, J.A.; Peck, A.B. Pathogenic effect of interleukin-17A in induction of Sjögren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res. Ther. 2010, 12, R220. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Chiorini, J.A.; Peck, A.B. IL17: Potential therapeutic target in Sjögren’s syndrome using adenovirus-mediated gene transfer. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 54–62. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Ribatti, D.; Lisi, S. TGFβ1-Smad canonical and -Erk noncanonical pathways participate in interleukin-17-induced epithelial-mesenchymal transition in Sjögren’s syndrome. Lab. Investig. J. Tech. Methods Pathol. 2020. [Google Scholar] [CrossRef]
- Li, C.; Zhu, F.; Wu, B.; Wang, Y. Vasoactive Intestinal Peptide Protects Salivary Glands against Structural Injury and Secretory Dysfunction via IL-17A and AQP5 Regulation in a Model of Sjögren Syndrome. Neuroimmunomodulation 2017, 24, 300–309. [Google Scholar] [CrossRef]
- Jin, J.-O.; Shinohara, Y.; Yu, Q. Innate immune signaling induces interleukin-7 production from salivary gland cells and accelerates the development of primary Sjögren’s syndrome in a mouse model. PLoS ONE 2013, 8, e77605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q. Anti-IL-7 receptor-α treatment ameliorates newly established Sjögren’s-like exocrinopathy in non-obese diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Wee, Y.; Yang, C.-H.; Melvin, J.E.; Baker, O.J. ALX/FPR2 Modulates Anti-Inflammatory Responses in Mouse Submandibular Gland. Sci. Rep. 2016, 6, 24244. [Google Scholar] [CrossRef] [PubMed]
- Beroukas, D.; Hiscock, J.; Gannon, B.J.; Jonsson, R.; Gordon, T.P.; Waterman, S.A. Selective down-regulation of aquaporin-1 in salivary glands in primary Sjögren’s syndrome. Lab. Investig. J. Tech. Methods Pathol. 2002, 82, 1547–1552. [Google Scholar] [CrossRef]
- Hua, Y.; Ying, X.; Qian, Y.; Liu, H.; Lan, Y.; Xie, A.; Zhu, X. Physiological and pathological impact of AQP1 knockout in mice. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Ichiyama, T.; Nakatani, E.; Tatsumi, K.; Hideshima, K.; Urano, T.; Nariai, Y.; Sekine, J. Expression of aquaporin 3 and 5 as a potential marker for distinguishing dry mouth from Sjögren’s syndrome. J. Oral Sci. 2018, 60, 212–220. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Tensing, E.-K.; Laine, M.; Porola, P.; Törnwall, J.; Hukkanen, M. Abnormal distribution of aquaporin-5 in salivary glands in the NOD mouse model for Sjögren’s syndrome. J. Rheumatol. 2005, 32, 1071–1075. [Google Scholar]
- Soyfoo, M.S.; Bolaky, N.; Depoortere, I.; Delporte, C. Relationship between aquaporin-5 expression and saliva flow in streptozotocin-induced diabetic mice? Oral Dis. 2012, 18, 501–505. [Google Scholar] [CrossRef]
- Steinfeld, S.; Cogan, E.; King, L.S.; Agre, P.; Kiss, R.; Delporte, C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab. Investig. J. Tech. Methods Pathol. 2001, 81, 143–148. [Google Scholar] [CrossRef]
- Yoshimura, S.; Nakamura, H.; Horai, Y.; Nakajima, H.; Shiraishi, H.; Hayashi, T.; Takahashi, T.; Kawakami, A. Abnormal distribution of AQP5 in labial salivary glands is associated with poor saliva secretion in patients with Sjögren’s syndrome including neuromyelitis optica complicated patients. Mod. Rheumatol. 2016, 26, 384–390. [Google Scholar] [CrossRef]
- Gresz, V.; Horvath, A.; Gera, I.; Nielsen, S.; Zelles, T. Immunolocalization of AQP5 in resting and stimulated normal labial glands and in Sjögren’s syndrome. Oral Dis. 2015, 21, e114–120. [Google Scholar] [CrossRef] [PubMed]
- Teos, L.Y.; Zhang, Y.; Cotrim, A.P.; Swaim, W.; Won, J.H.; Ambrus, J.; Shen, L.; Bebris, L.; Grisius, M.; Jang, S.-I.; et al. IP3R deficit underlies loss of salivary fluid secretion in Sjögren’s Syndrome. Sci. Rep. 2015, 5, 13953. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Shin, Y.; Choi, S.; Namkoong, E.; Kim, M.; Lee, J.; Song, Y.; Park, K. Effect of Antimuscarinic Autoantibodies in Primary Sjögren’s Syndrome. J. Dent. Res. 2015, 94, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Gauna, A.E.; Perez, G.; Park, Y.; Pauley, K.M.; Kawai, T.; Cha, S. Autoantibodies against muscarinic type 3 receptor in Sjögren’s syndrome inhibit aquaporin 5 trafficking. PLoS ONE 2013, 8, e53113. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Stergiou, C.; Daoussis, D.; Zisimopoulou, P.; Andonopoulos, A.P.; Zolota, V.; Tzartos, S.J. Antibodies to aquaporins are frequent in patients with primary Sjögren’s syndrome. Rheumatol. Oxf. Engl. 2017, 56, 2114–2122. [Google Scholar] [CrossRef]
- Alam, J.; Koh, J.H.; Kim, N.; Kwok, S.-K.; Park, S.-H.; Song, Y.W.; Park, K.; Choi, Y. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjögren’s syndrome. Immunol. Res. 2016, 64, 848–856. [Google Scholar] [CrossRef]
- Martín-Nares, E.; Hernández-Molina, G. Novel autoantibodies in Sjögren’s syndrome: A comprehensive review. Autoimmun Rev. 2019, 18, 192–198. [Google Scholar] [CrossRef]
- Zhang, L.W.; Cong, X.; Zhang, Y.; Wei, T.; Su, Y.C.; Serrão, A.C.A.; Brito, A.R.T.; Yu, G.Y.; Hua, H.; Wu, L.L. Interleukin-17 Impairs Salivary Tight Junction Integrity in Sjögren’s Syndrome. J. Dent. Res. 2016, 95, 784–792. [Google Scholar] [CrossRef]
- Mellas, R.E.; Leigh, N.J.; Nelson, J.W.; McCall, A.D.; Baker, O.J. Zonula occludens-1, occludin and E-cadherin expression and organization in salivary glands with Sjögren’s syndrome. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2015, 63, 45–56. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef]
- Cong, X.; Zhang, X.-M.; Zhang, Y.; Wei, T.; He, Q.-H.; Zhang, L.-W.; Hua, H.; Lee, S.-W.; Park, K.; Yu, G.-Y.; et al. Disruption of endothelial barrier function is linked with hyposecretion and lymphocytic infiltration in salivary glands of Sjögren’s syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Ewert, P.; Aguilera, S.; Alliende, C.; Kwon, Y.-J.; Albornoz, A.; Molina, C.; Urzúa, U.; Quest, A.F.G.; Olea, N.; Pérez, P.; et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010, 62, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.T.; Ferretti, G.A.; Nethery, W.J.; Valdez, I.H.; Fox, P.C.; Ng, D.; Muscoplat, C.C.; Gallagher, S.C. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N. Engl. J. Med. 1993, 329, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-A. Radiotherapy for head and neck cancer. Semin. Plast. Surg. 2010, 24, 127–136. [Google Scholar] [CrossRef]
- Choi, J.H.; Wu, H.-G.; Jung, K.C.; Lee, S.H.; Kwon, E.K. Apoptosis and expression of AQP5 and TGF-beta in the irradiated rat submandibular gland. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2009, 41, 145–154. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, B.K.; Jang, S.J.; Yun, J.W.; Jung, M.H.; Kang, K.M.; Kim, T.G.; Woo, S.H. Alpha-Lipoic Acid Ameliorates Radiation-Induced Salivary Gland Injury by Preserving Parasympathetic Innervation in Rats. Int. J. Mol. Sci. 2020, 21, 2260. [Google Scholar] [CrossRef]
- Takagi, K.; Yamaguchi, K.; Sakurai, T.; Asari, T.; Hashimoto, K.; Terakawa, S. Secretion of saliva in X-irradiated rat submandibular glands. Radiat. Res. 2003, 159, 351–360. [Google Scholar] [CrossRef]
- Asari, T.; Maruyama, K.; Kusama, H. Salivation triggered by pilocarpine involves aquaporin-5 in normal rats but not in irradiated rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 531–538. [Google Scholar] [CrossRef]
- Meyer, R.; Wong, W.Y.; Guzman, R.; Burd, R.; Limesand, K. Radiation Treatment of Organotypic Cultures from Submandibular and Parotid Salivary Glands Models Key In Vivo Characteristics. J. Vis. Exp. Jove 2019. [Google Scholar] [CrossRef]
- Vissink, A.; Kalicharan, D.; S-Gravenmade, E.J.; Jongebloed, W.L.; Ligeon, E.E.; Nieuwenhuis, P.; Konings, A.W. Acute irradiation effects on morphology and function of rat submandibular glands. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 1991, 20, 449–456. [Google Scholar] [CrossRef]
- Taniguchi, A.; Susa, T.; Kogo, H.; Iizuka-Kogo, A.; Yokoo, S.; Matsuzaki, T. Long-term Pilocarpine Treatment Improves Salivary Flow in Irradiated Mice. Acta Histochem. Cytochem. 2019, 52, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Sánchez Silva, R.; Gómez Izquierdo, L.; Márquez, E.; Ortega Ruiz, F.; Cejudo, P.; Barrot Cortés, E.; Toledo Aral, J.J.; Echevarría, M. Overexpression of Aquaporin-1 in lung adenocarcinomas and pleural mesotheliomas. Histol. Histopathol. 2011, 26, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef]
- Yin, T.; Yu, S.; Xiao, L.; Zhang, J.; Liu, C.; Lu, Y.; Liu, C. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J. Huazhong Univ. Sci. Technol. Med. Sci. Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban 2008, 28, 346–348. [Google Scholar] [CrossRef]
- Cao, X.-C.; Zhang, W.-R.; Cao, W.-F.; Liu, B.-W.; Zhang, F.; Zhao, H.-M.; Meng, R.; Zhang, L.; Niu, R.-F.; Hao, X.-S.; et al. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLoS ONE 2013, 8, e56735. [Google Scholar] [CrossRef]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell. Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef]
- Song, T.; Yang, H.; Ho, J.C.M.; Tang, S.C.W.; Sze, S.C.W.; Lao, L.; Wang, Y.; Zhang, K.Y. Expression of aquaporin 5 in primary carcinoma and lymph node metastatic carcinoma of non-small cell lung cancer. Oncol. Lett. 2015, 9, 2799–2804. [Google Scholar] [CrossRef]
- Shi, X.; Wu, S.; Yang, Y.; Tang, L.; Wang, Y.; Dong, J.; Lü, B.; Jiang, G.; Zhao, W. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014, 35, 7035–7045. [Google Scholar] [CrossRef]
- Shao, C.; Sun, W.; Tan, M.; Glazer, C.A.; Bhan, S.; Zhong, X.; Fakhry, C.; Sharma, R.; Westra, W.H.; Hoque, M.O.; et al. Integrated, genome-wide screening for hypomethylated oncogenes in salivary gland adenoid cystic carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4320–4330. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Shao, C.; Bishop, J.A.; Feng, Z.; Trock, B.J.; Westra, W.H.; Ha, P.K. Aquaporin-1 promoter hypermethylation is associated with improved prognosis in salivary gland adenoid cystic carcinoma. Otolaryngol.–Head Neck Surg. Off. J. Am. Acad. Otolaryngol.–Head Neck Surg. 2014, 150, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, S.; Wada, K.; Usami, Y.; Tanaka, N.; Aikawa, T.; Okura, M.; Nakajima, A.; Kogo, M.; Kamisaki, Y. Differential expression of aquaporin 5 and aquaporin 3 in squamous cell carcinoma and adenoid cystic carcinoma. Int. J. Oncol. 2012, 41, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Kondo, T.; Nakazawa, T.; Yamane, T.; Mochizuki, K.; Kawasaki, T.; Matsuzaki, T.; Takata, K.; Katoh, R. Expression of aquaporin3 in human neoplastic tissues. Histopathology 2012, 61, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Lee, H.-Y.; Kim, S.-H.; Park, J.-S.; Kim, H.-R.; Chae, H.-J. Potential Application of Ixeris dentata in the Prevention and Treatment of Aging-Induced Dry Mouth. Nutrients 2018, 10, 1989. [Google Scholar] [CrossRef]
- Miyagi, Y.; Kondo, Y.; Kusuda, Y.; Hori, Y.; Yamazaki, S.; Munemasa, T.; Mukaibo, T.; Masaki, C.; Hosokawa, R. Submandibular gland-specific inflammaging-induced hyposalivation in the male senescence-accelerated mouse prone -1 line (SAM-P1). Biogerontology 2019, 20, 421–432. [Google Scholar] [CrossRef]
- Kuraji, M.; Matsuno, T.; Satoh, T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J. Clin. Biochem. Nutr. 2016, 59, 79–85. [Google Scholar] [CrossRef]
- Inoue, N.; Iida, H.; Yuan, Z.; Ishikawa, Y.; Ishida, H. Age-related decreases in the response of aquaporin-5 to acetylcholine in rat parotid glands. J. Dent. Res. 2003, 82, 476–480. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Moore, P.A.; Guggenheimer, J.; Etzel, K.R.; Weyant, R.J.; Orchard, T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, V.; Nix, P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010, 64, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Lee, H.-Y.; Kim, S.-H.; Kim, H.-R.; Chae, H.-J. Ixeris dentata Extract Increases Salivary Secretion through the Regulation of Endoplasmic Reticulum Stress in a Diabetes-Induced Xerostomia Rat Model. Int. J. Mol. Sci. 2018, 19, 1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, Z.; Inoue, N.; Cho, G.; Shono, M.; Ishikawa, Y. Abnormal subcellular localization of AQP5 and downregulated AQP5 protein in parotid glands of streptozotocin-induced diabetic rats. Biochim. Biophys. Acta 2011, 1810, 543–554. [Google Scholar] [CrossRef]
- El Sadik, A.; Mohamed, E.; El Zainy, A. Postnatal changes in the development of rat submandibular glands in offspring of diabetic mothers: Biochemical, histological and ultrastructural study. PLoS ONE 2018, 13, e0205372. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wang, Y.; Zhang, C.-L.; Yang, Z.-M. Decreased basal and stimulated salivary parameters by histopathological lesions and secretory dysfunction of parotid and submandibular glands in rats with type 2 diabetes. Exp. Ther. Med. 2020, 19, 2707–2719. [Google Scholar] [CrossRef]
- Talha, B.; Swarnkar, S.A. Xerostomia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Al Hamad, A.; Lodi, G.; Porter, S.; Fedele, S.; Mercadante, V. Interventions for dry mouth and hyposalivation in Sjögren’s syndrome: A systematic review and meta-analysis. Oral Dis. 2019, 25, 1027–1047. [Google Scholar] [CrossRef]
- Cifuentes, M.; Del Barrio-Díaz, P.; Vera-Kellet, C. Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: A double-blind randomized controlled trial. Br. J. Dermatol. 2018, 179, 1056–1061. [Google Scholar] [CrossRef]
- Mercadante, V.; Al Hamad, A.; Lodi, G.; Porter, S.; Fedele, S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral Oncol. 2017, 66, 64–74. [Google Scholar] [CrossRef]
- Salum, F.G.; Medella-Junior, F.; de, A.C.; Figueiredo, M.A.Z.; Cherubini, K. Salivary hypofunction: An update on therapeutic strategies. Gerodontology 2018, 35, 305–316. [Google Scholar] [CrossRef]
- Jham, B.C.; Teixeira, I.V.; Aboud, C.G.; Carvalho, A.L.; de Coelho, M.M.; da Freire, S.A.R. A randomized phase III prospective trial of bethanechol to prevent radiotherapy-induced salivary gland damage in patients with head and neck cancer. Oral Oncol. 2007, 43, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.; Mubeen, K.; Vijayalakshmi, K.R. A study on Evaluation of efficacy of bethanechol in the management of chemoradiation-induced xerostomia in oral cancer patients. J. Oral Maxillofac. Pathol. Jomfp 2017, 21, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of muscarinic receptors in rat parotid acinar cells induces AQP5 trafficking to nuclei and apical plasma membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Suzuki, T.; Koyama, H.; Tanaka, S.; Takata, K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: Immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999, 295, 513–521. [Google Scholar] [CrossRef]
- Takakura, K.; Takaki, S.; Takeda, I.; Hanaue, N.; Kizu, Y.; Tonogi, M.; Yamane, G. Effect of cevimeline on radiation-induced salivary gland dysfunction and AQP5 in submandibular gland in mice. Bull. Tokyo Dent. Coll. 2007, 48, 47–56. [Google Scholar] [CrossRef]
- Nishimura, H.; Yakeishi, A.; Saga, T.; Yamaki, K.-I. Effects of cevimeline on the immunolocalization of aquaporin-5 and the ultrastructure of salivary glands in Sjögren’s syndrome model mice. Kurume Med. J. 2009, 56, 39–47. [Google Scholar] [CrossRef]
- Zeng, M.; Szymczak, M.; Ahuja, M.; Zheng, C.; Yin, H.; Swaim, W.; Chiorini, J.A.; Bridges, R.J.; Muallem, S. Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice. Gastroenterology 2017, 153, 1148–1159. [Google Scholar] [CrossRef]
- Beumer, W.; Swildens, J.; Leal, T.; Noel, S.; Anthonijsz, H.; van der Horst, G.; Kuiperij-Boersma, H.; Potman, M.; van Putten, C.; Biasutto, P.; et al. Evaluation of eluforsen, a novel RNA oligonucleotide for restoration of CFTR function in in vitro and murine models of p.Phe508del cystic fibrosis. PLoS ONE 2019, 14, e0219182. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Clancy, J.P.; Nichols, D.P.; Nick, J.A.; De Boeck, K.; Solomon, G.M.; Mall, M.A.; Bolognese, J.; Bouisset, F.; den Hollander, W.; et al. Antisense oligonucleotide eluforsen improves CFTR function in F508del cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2019, 18, 536–542. [Google Scholar] [CrossRef]
- Drevinek, P.; Pressler, T.; Cipolli, M.; De Boeck, K.; Schwarz, C.; Bouisset, F.; Boff, M.; Henig, N.; Paquette-Lamontagne, N.; Montgomery, S.; et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Sato, M.; Kuroda, S.; Mansjur, K.Q.; Khaliunaa, G.; Nagata, K.; Horiuchi, S.; Inubushi, T.; Yamamura, Y.; Azuma, M.; Tanaka, E. Low-intensity pulsed ultrasound rescues insufficient salivary secretion in autoimmune sialadenitis. Arthritis Res. Ther. 2015, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Tradtrantip, L.; Phuan, P.-W.; Bennett, J.L.; Verkman, A.S. Affinity-matured “aquaporumab” anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology 2020, 162, 107827. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Zhang, H.; Saadoun, S.; Phuan, P.-W.; Lam, C.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012, 71, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Baum, B.J. Gene delivery in salivary glands: From the bench to the clinic. Biochim. Biophys. Acta 2011, 1812, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef]
- Teos, L.Y.; Zheng, C.-Y.; Liu, X.; Swaim, W.D.; Goldsmith, C.M.; Cotrim, A.P.; Baum, B.J.; Ambudkar, I.S. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 2016, 23, 572–579. [Google Scholar] [CrossRef]
- Shan, Z.; Li, J.; Zheng, C.; Liu, X.; Fan, Z.; Zhang, C.; Goldsmith, C.M.; Wellner, R.B.; Baum, B.J.; Wang, S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 11, 444–451. [Google Scholar] [CrossRef]
- O’Connell, A.C.; Baccaglini, L.; Fox, P.C.; O’Connell, B.C.; Kenshalo, D.; Oweisy, H.; Hoque, A.T.; Sun, D.; Herscher, L.L.; Braddon, V.R.; et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999, 6, 505–513. [Google Scholar] [CrossRef]
- Lai, Z.; Yin, H.; Cabrera-Pérez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C.; et al. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Goldsmith, C.M.; McCullagh, L.; Berkowitz, T.; Strobl, S.L.; Malyguine, A.; Kopp, W.C.; Chiorini, J.A.; et al. Immune reactivity after adenoviral-mediated aquaporin-1 cDNA transfer to human parotid glands. Oral Dis. 2017, 23, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yan, X.; Zheng, C.; Goldsmith, C.M.; Afione, S.; Hai, B.; Xu, J.; Zhou, J.; Zhang, C.; Chiorini, J.A.; et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011, 18, 38–42. [Google Scholar] [CrossRef]
- Momot, D.; Zheng, C.; Yin, H.; Elbekai, R.H.; Vallant, M.; Chiorini, J.A. Toxicity and biodistribution of the serotype 2 recombinant adeno-associated viral vector, encoding Aquaporin-1, after retroductal delivery to a single mouse parotid gland. PLoS ONE 2014, 9, e92832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Hai, B.; Zhao, Q.; Deveau, M.A.; Liu, F. Delivery of Sonic Hedgehog Gene Repressed Irradiation-induced Cellular Senescence in Salivary Glands by Promoting DNA Repair and Reducing Oxidative Stress. Theranostics 2018, 8, 1159–1167. [Google Scholar] [CrossRef]
- Hu, L.; Zhu, Z.; Hai, B.; Chang, S.; Ma, L.; Xu, Y.; Li, X.; Feng, X.; Wu, X.; Zhao, Q.; et al. Intragland Shh gene delivery mitigated irradiation-induced hyposalivation in a miniature pig model. Theranostics 2018, 8, 4321–4331. [Google Scholar] [CrossRef]
- Nair, R.P.; Sunavala-Dossabhoy, G. Promising Gene Therapeutics for Salivary Gland Radiotoxicity. Aims Med. Sci. 2016, 3, 329–344. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, Y.-J.; Kwon, H.-C.; Bae, S.; Kim, S.-H.; Min, J.-J.; Cho, C.-K.; Lee, Y.-S. Radioprotective effect of heat shock protein 25 on submandibular glands of rats. Am. J. Pathol. 2006, 169, 1601–1611. [Google Scholar] [CrossRef]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M.J. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, S.; Zhang, L.-R.; Zhang, N.; Cheng, Z.; Liu, Q.; Shields, K.J.; Hu, B.; Passineau, M.J. CRISPR-Cas9 HDR system enhances AQP1 gene expression. Oncotarget 2017, 8, 111683–111696. [Google Scholar] [CrossRef]
- Ashmore-Harris, C.; Fruhwirth, G.O. The clinical potential of gene editing as a tool to engineer cell-based therapeutics. Clin. Transl. Med. 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene editing and CRISPR in the clinic: Current and future perspectives. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. Jasn 2006, 17, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Verkman, A.S. Aquaporin-1 water permeability as a novel determinant of axonal regeneration in dorsal root ganglion neurons. Exp. Neurol. 2015, 265, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Hsieh, P.-M.; Hsu, C.-Y.; Lin, C.-W.; Feng, G.-M.; Chen, Y.-S.; Hung, C.-H. Expression of aquaporins in rat liver regeneration. Scand. J. Gastroenterol. 2012, 47, 676–685. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakamura, S.; Suzuki, R.; Islam, N.; Domon, T.; Yamamoto, T.; Wakita, M. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell 2000, 32, 457–463. [Google Scholar] [CrossRef]
- Walker, N.I.; Gobé, G.C. Cell death and cell proliferation during atrophy of the rat parotid gland induced by duct obstruction. J. Pathol. 1987, 153, 333–344. [Google Scholar] [CrossRef]
- Larsen, H.S.; Ruus, A.-K.; Galtung, H.K. Aquaporin expression patterns in the developing mouse salivary gland. Eur. J. Oral Sci. 2009, 117, 655–662. [Google Scholar] [CrossRef]
- Ono Minagi, H.; Usami, Y.; Sakai, M.; Sakai, T. Morphological differences between regenerating salivary glands after salivary gland duct ligation and embryonic salivary glands. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2020, 229, 151482. [Google Scholar] [CrossRef]
- Purwanti, N.; Karabasil, M.R.; Matsuo, S.; Chen, G.; Javkhlan, P.; Azlina, A.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Induction of Sca-1 via activation of STAT3 system in the duct cells of the mouse submandibular gland by ligation of the main excretory duct. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G814–824. [Google Scholar] [CrossRef]
- Yasumitsu, T.; Shimizu, O.; Shiratsuchi, H.; Miyake, Y.; Yonehara, Y. Distribution of aquaporin-5, transforming growth factor-β1 and laminin during regeneration of atrophic rat submandibular glands after duct ligation. J. Oral Sci. 2018, 60, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Maria, S.M.; Redman, R.S.; Maria, A.M.; Saad El-Din, T.A.; Soussa, E.F.; Tran, S.D. Effects of double ligation of Stensen’s duct on the rabbit parotid gland. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2014, 89, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Liu, Y.; Kornete, M.; Roescher, N.; Kodama, S.; Peterson, A.; Piccirillo, C.A.; Tran, S.D. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjögren’s-like disease. PLoS ONE 2012, 7, e38615. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Faustman, D.L.; Liu, Y.; Sumita, Y.; Blank, D.; Peterson, A.; Kodama, S.; Tran, S.D. Treatment for salivary gland hypofunction at both initial and advanced stages of Sjögren-like disease: A comparative study of bone marrow therapy versus spleen cell therapy with a 1-year monitoring period. Cytotherapy 2014, 16, 412–423. [Google Scholar] [CrossRef]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef]
- Misuno, K.; Tran, S.D.; Khalili, S.; Huang, J.; Liu, Y.; Hu, S. Quantitative analysis of protein and gene expression in salivary glands of Sjogren’s-like disease NOD mice treated by bone marrow soup. PLoS ONE 2014, 9, e87158. [Google Scholar] [CrossRef][Green Version]
- Kim, J.W.; Kim, J.M.; Choi, M.E.; Kim, S.-K.; Kim, Y.-M.; Choi, J.-S. Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model. Sci. Rep. 2019, 9, 15752. [Google Scholar] [CrossRef]
- Fang, D.; Hu, S.; Liu, Y.; Quan, V.-H.; Seuntjens, J.; Tran, S.D. Identification of the active components in Bone Marrow Soup: A mitigator against irradiation-injury to salivary glands. Sci. Rep. 2015, 5, 16017. [Google Scholar] [CrossRef]
- Fang, D.; Shang, S.; Liu, Y.; Bakkar, M.; Sumita, Y.; Seuntjens, J.; Tran, S.D. Optimal timing and frequency of bone marrow soup therapy for functional restoration of salivary glands injured by single-dose or fractionated irradiation. J. Tissue Eng. Regen. Med. 2018, 12, e1195–e1205. [Google Scholar] [CrossRef]
- Su, X.; Fang, D.; Liu, Y.; Ruan, G.; Seuntjens, J.; Kinsella, J.M.; Tran, S.D. Lyophilized bone marrow cell extract functionally restores irradiation-injured salivary glands. Oral Dis. 2018, 24, 202–206. [Google Scholar] [CrossRef]
- Su, X.; Liu, Y.; Bakkar, M.; ElKashty, O.; El-Hakim, M.; Seuntjens, J.; Tran, S.D. Labial Stem Cell Extract Mitigates Injury to Irradiated Salivary Glands. J. Dent. Res. 2020, 99, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4, 2498. [Google Scholar] [CrossRef]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Tran, S.D. Synergy between genetic and tissue engineering: Creating an artificial salivary gland. Periodontol. 2000 2006, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Fang, D.; Liu, Y.; Ramamoorthi, M.; Zeitouni, A.; Chen, W.; Tran, S.D. Three-dimensional organotypic culture of human salivary glands: The slice culture model. Oral Dis. 2016, 22, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng. Part. A 2011, 17, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Liu, Y.; El-Hakim, M.; Zeitouni, A.; Tran, S.D. The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J. Tissue Eng. Regen. Med. 2017, 11, 2643–2657. [Google Scholar] [CrossRef]
- Maria, O.M.; Tran, S.D. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev. 2011, 20, 959–967. [Google Scholar] [CrossRef]
- Seo, Y.J.; Lilliu, M.A.; Abu Elghanam, G.; Nguyen, T.T.; Liu, Y.; Lee, J.C.; Presley, J.F.; Zeitouni, A.; El-Hakim, M.; Tran, S.D. Cell culture of differentiated human salivary epithelial cells in a serum-free and scalable suspension system: The salivary functional units model. J. Tissue Eng. Regen. Med. 2019, 13, 1559–1570. [Google Scholar] [CrossRef]
- Maria, O.M.; Maria, A.M.; Cai, Y.; Tran, S.D. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012, 18, 162–168. [Google Scholar] [CrossRef]
- Tran, S.D.; Redman, R.S.; Barrett, A.J.; Pavletic, S.Z.; Key, S.; Liu, Y.; Carpenter, A.; Nguyen, H.M.; Sumita, Y.; Baum, B.J.; et al. Microchimerism in salivary glands after blood- and marrow-derived stem cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Miyauchi, T.; Abe, Y.; Kojić, D.; Tanaka, M.; Chikazawa, N.; Nakatake, Y.; Ko, S.B.H.; Kobayashi, D.; Hazama, A.; et al. Unprecedented cell-selection using ultra-quick freezing combined with aquaporin expression. PLoS ONE 2014, 9, e87644. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, C.; Elkashty, O.A.; Chivasso, C.; Perret, J.; Tran, S.D.; Delporte, C. Insight into Salivary Gland Aquaporins. Cells 2020, 9, 1547. https://doi.org/10.3390/cells9061547
D’Agostino C, Elkashty OA, Chivasso C, Perret J, Tran SD, Delporte C. Insight into Salivary Gland Aquaporins. Cells. 2020; 9(6):1547. https://doi.org/10.3390/cells9061547
Chicago/Turabian StyleD’Agostino, Claudia, Osama A. Elkashty, Clara Chivasso, Jason Perret, Simon D. Tran, and Christine Delporte. 2020. "Insight into Salivary Gland Aquaporins" Cells 9, no. 6: 1547. https://doi.org/10.3390/cells9061547
APA StyleD’Agostino, C., Elkashty, O. A., Chivasso, C., Perret, J., Tran, S. D., & Delporte, C. (2020). Insight into Salivary Gland Aquaporins. Cells, 9(6), 1547. https://doi.org/10.3390/cells9061547