Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Cell Treatment with IL-4, SDF-1, and IL-4 and SDF-1
2.3. Migration Assay
2.4. ELISA
2.5. Co-Culture of ADSCs with C2C12 Myoblasts
2.6. Preparation of Cells for Transplantation
2.7. Skeletal Muscle Injury
2.8. Histological Analyzes—Myofiber Number and Connective Tissue Area
2.9. Localization of Transplanted Cells
2.10. qPCR
2.11. Immunolocalization
2.12. Next Generation RNA Sequencing (NGS)
2.13. Statistical Analysis
3. Results
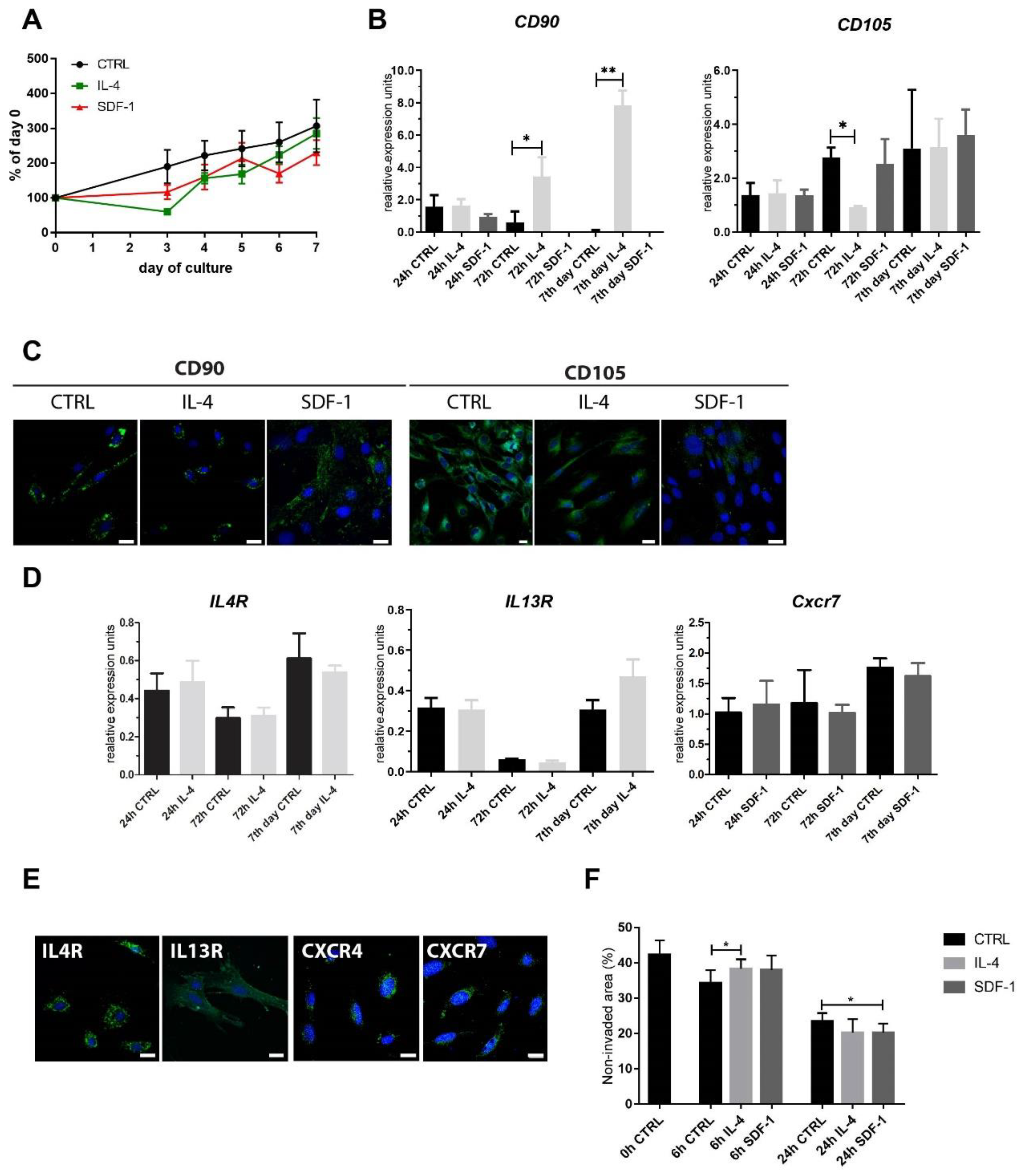
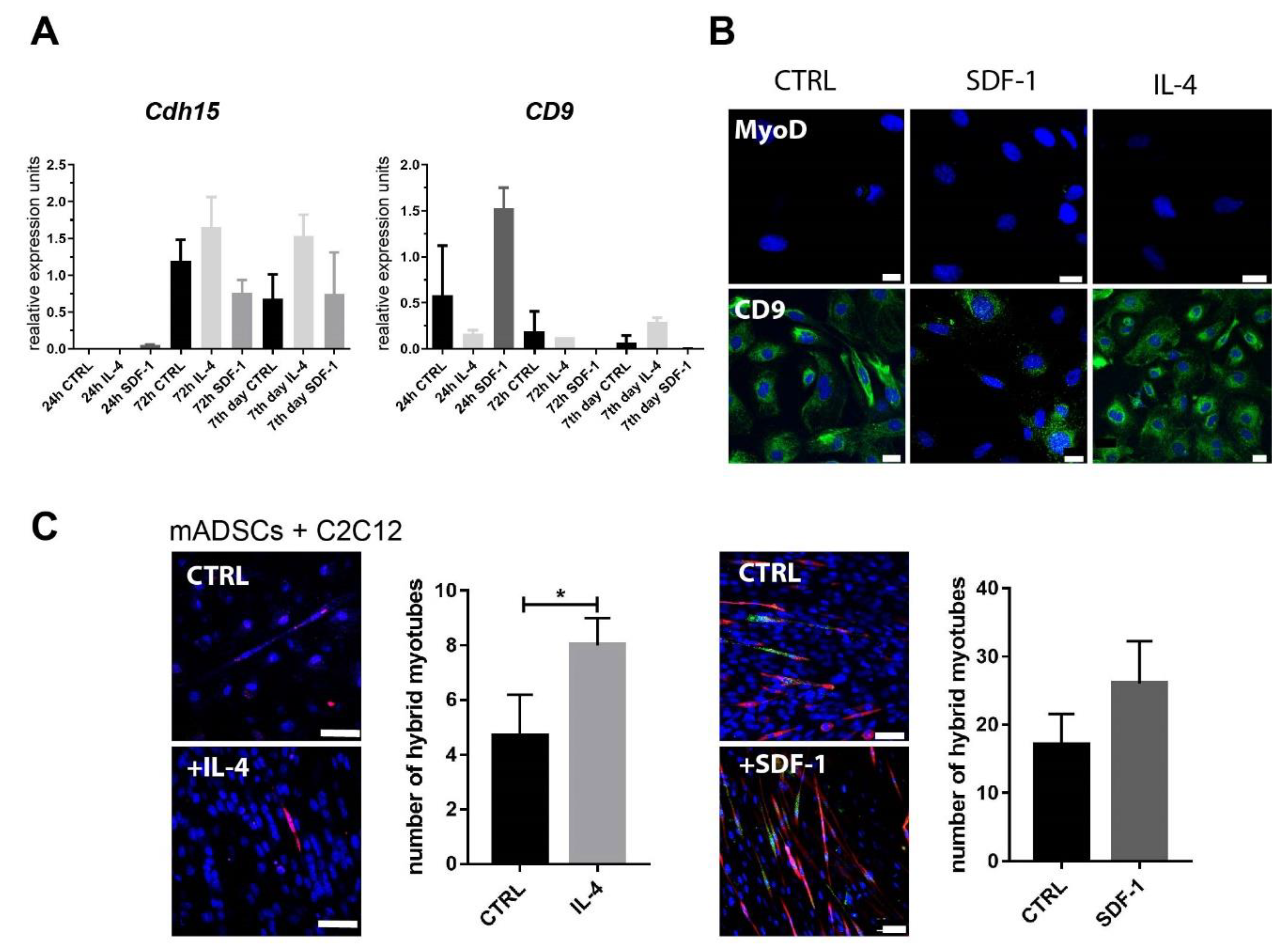
3.1. Mouse ADSC Response to IL-4 or SDF-1 Treatment In Vitro
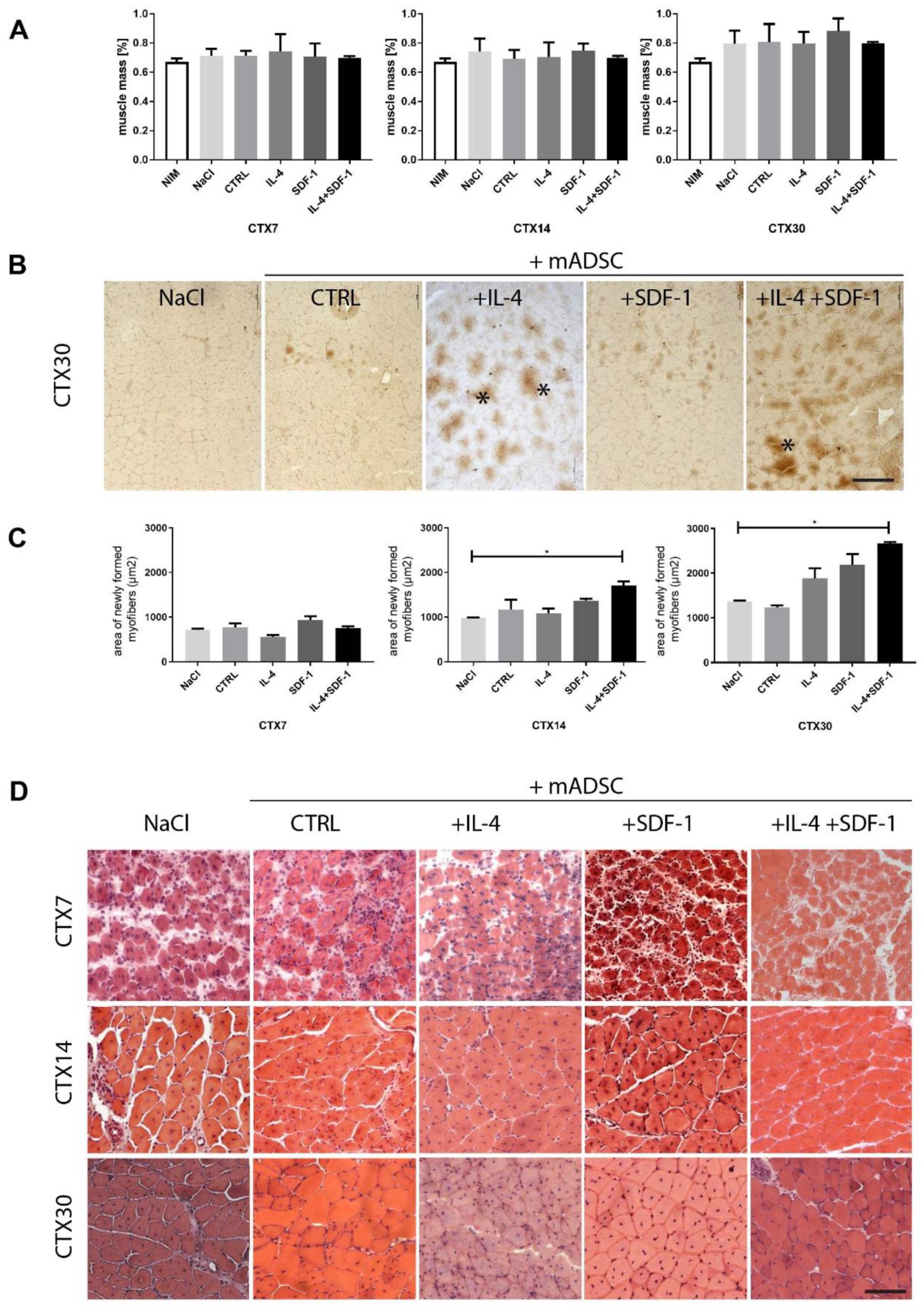
3.2. Transplantation of IL-4- and SDF-1-Treated mADSCs into Regenerating Mouse Muscle
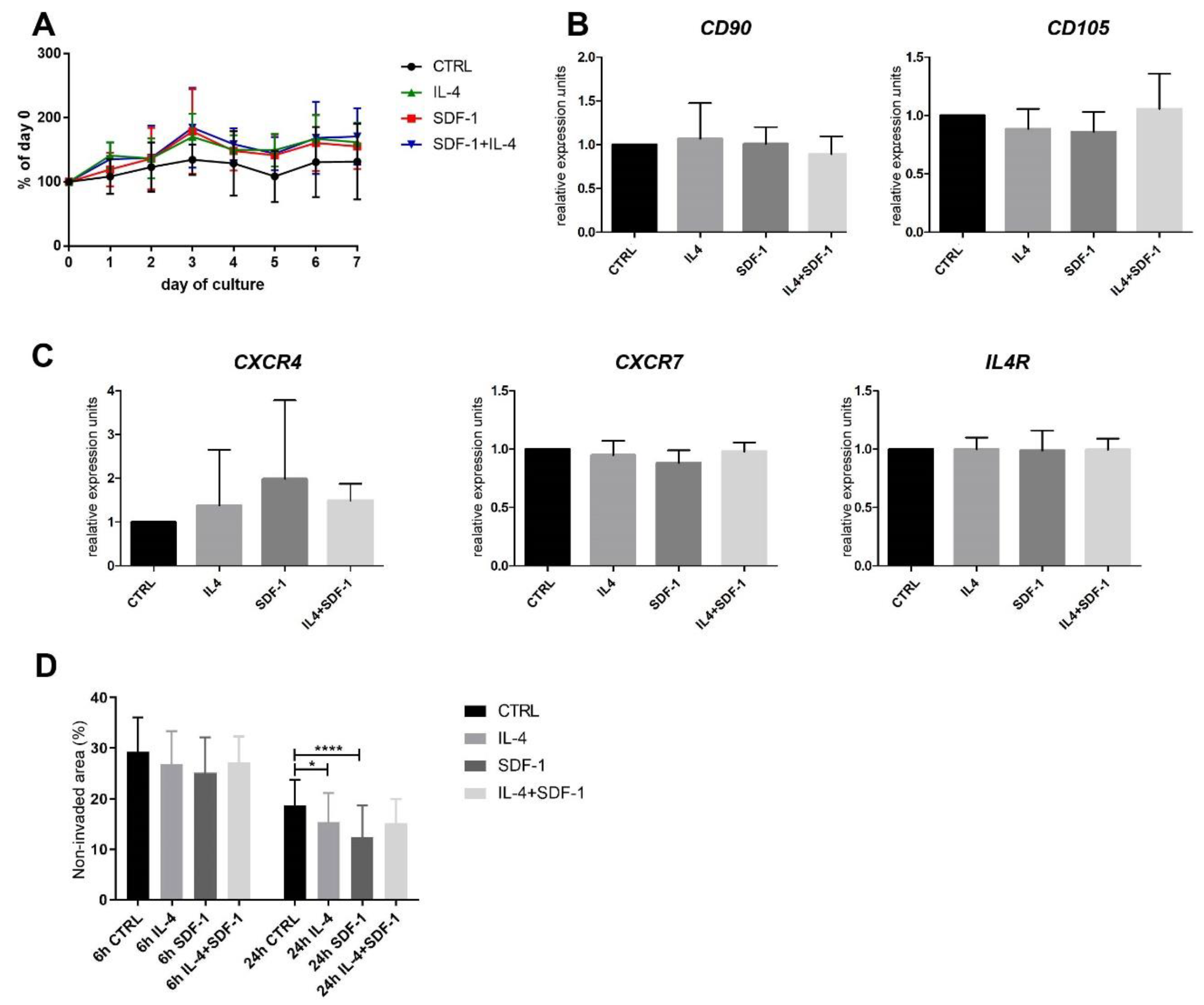
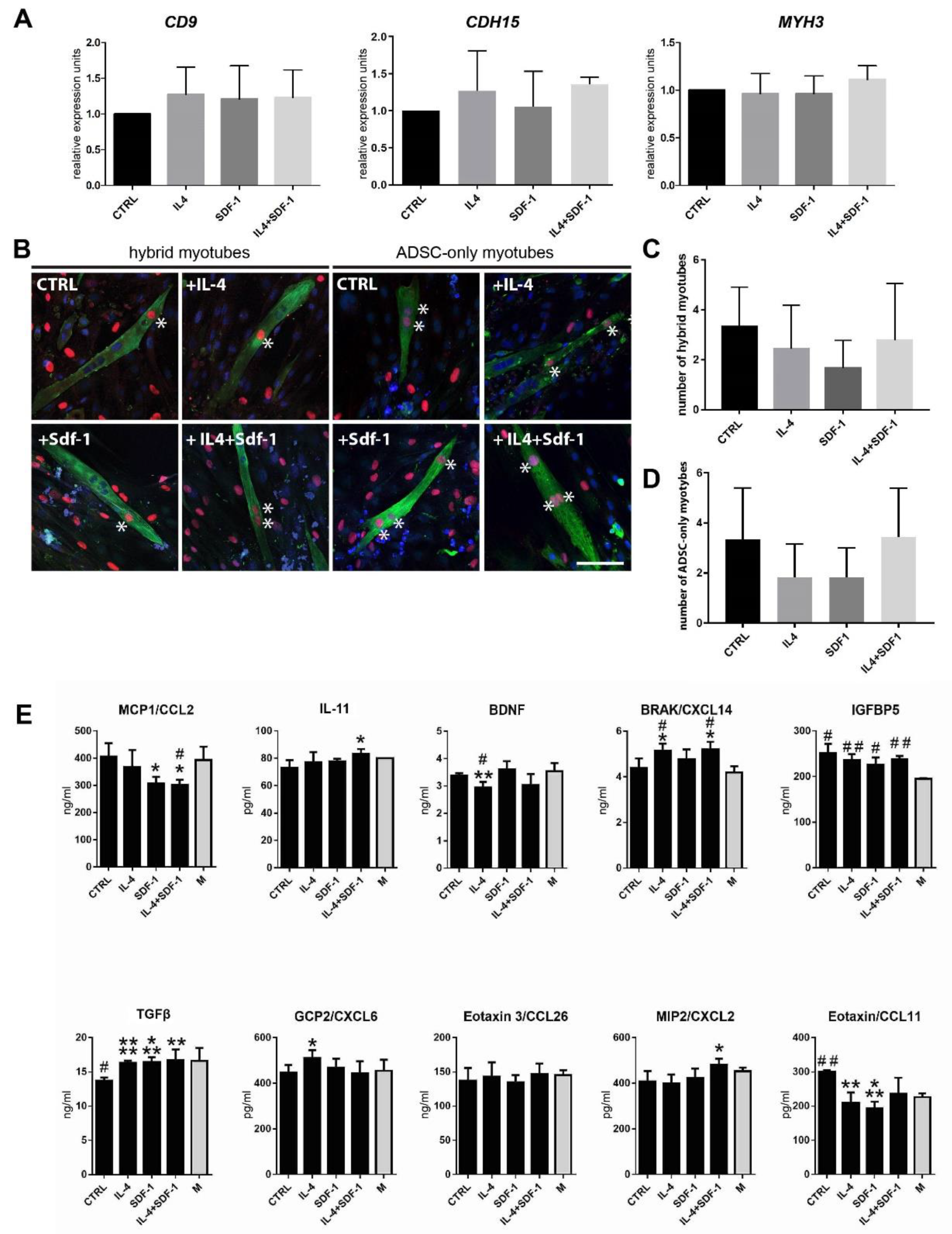
3.3. Human ADSC Response to IL-4 and SDF-1 Treatment In Vitro
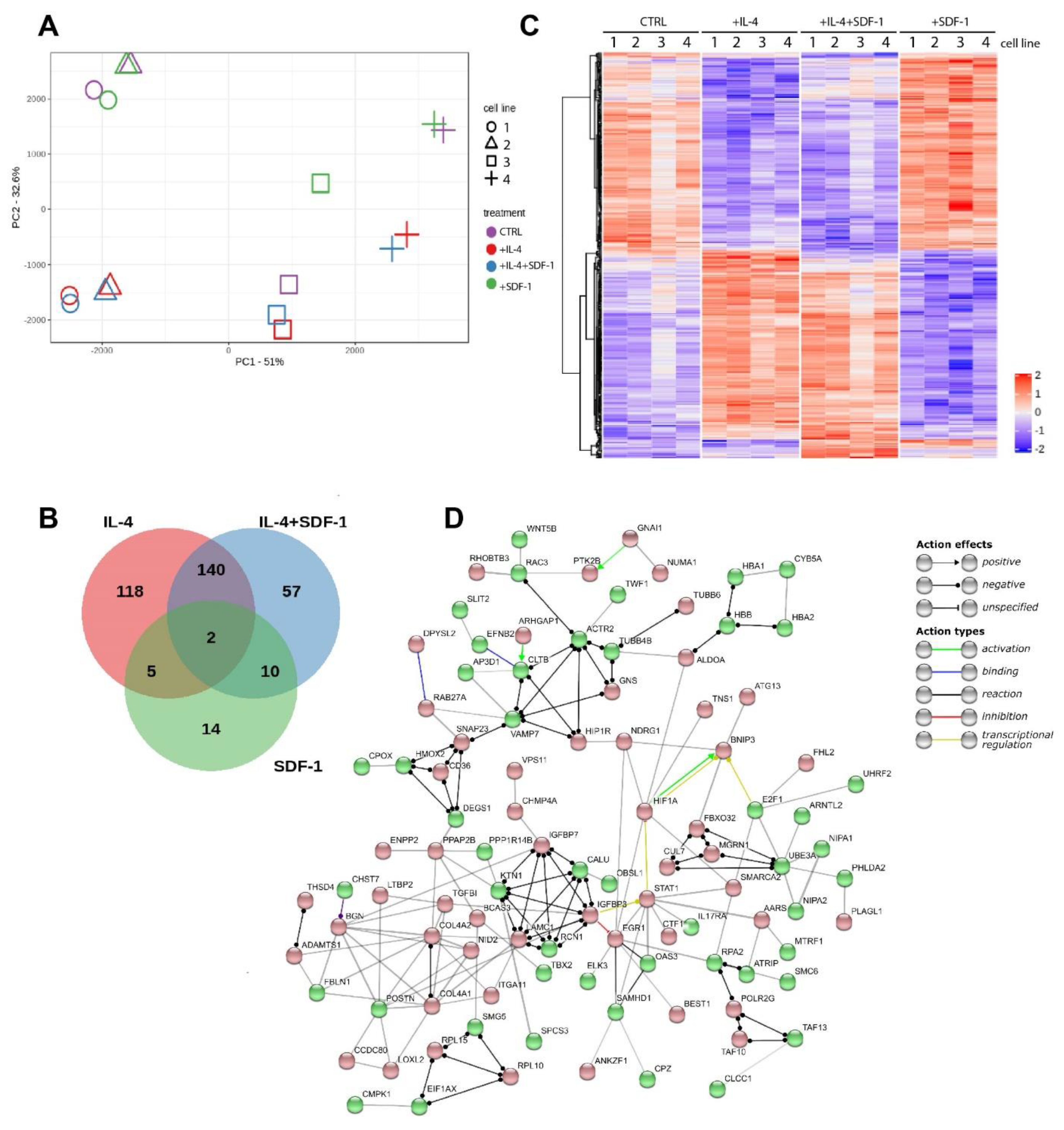
3.4. IL-4, SDF-1 or IL-4 and SDF-1 Impact at hADSC Transctiptome
3.5. Transplantation of IL-4 and SDF-1 Treated hADSCs into Regenerating NOD/SCID Mouse Muscle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grabowska, I.; Archacka, K.; Czerwinska, A.M.; Krupa, M.; Ciemerych, M.A. Mouse and Human Pluripotent Stem Cells and the Means of Their Myogenic Differentiation. Results Probl. Cell Differ. 2012, 55, 321–356. [Google Scholar] [CrossRef] [PubMed]
- Ciemerych, M.A.; Archacka, K.; Grabowska, I.; Przewoźniak, M. Cell Cycle Regulation During Proliferation and Differentiation of Mammalian Muscle Precursor Cells. Results Probl. Cell Differ. 2011, 53, 473–527. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994, 125, 1275–1287. [Google Scholar] [CrossRef]
- Fan, Y.; Maley, M.; Beilharz, M.; Grounds, M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996, 19, 853–860. [Google Scholar] [CrossRef]
- Skuk, D.; Caron, N.J.; Goulet, M.; Roy, B.; Tremblay, J.P. Resetting the problem of cell death following muscle-derived cell transplantation: Detection, dynamics and mechanisms. J. Neuropathol. Exp. Neurol. 2003, 62, 951–967. [Google Scholar] [CrossRef]
- Morosetti, R.; Gidaro, T.; Broccolini, A.; Gliubizzi, C.; Sancricca, C.; Tonali, P.A.; Ricci, E.; Mirabella, M. Mesoangioblasts from facioscapulohumeral muscular dystrophy display in vivo a variable myogenic ability predictable by their in vitro behavior. Cell Transplant. 2011, 20, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Rotini, A.; Martinez-Sarra, E.; Duelen, R.; Costamagna, D.; Di Filippo, E.S.; Giacomazzi, G.; Grosemans, H.; Fulle, S.; Sampaolesi, M. Aging affects the in vivo regenerative potential of human mesoangioblasts. Aging Cell 2018, 17. [Google Scholar] [CrossRef]
- Quattrocelli, M.; Costamagna, D.; Giacomazzi, G.; Camps, J.; Sampaolesi, M. Notch signaling regulates myogenic regenerative capacity of murine and human mesoangioblasts. Cell Death Dis. 2014, 5, e1448. [Google Scholar] [CrossRef]
- Dellavalle, A.; Maroli, G.; Covarello, D.; Azzoni, E.; Innocenzi, A.; Perani, L.; Antonini, S.; Sambasivan, R.; Brunelli, S.; Tajbakhsh, S.; et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2011, 2, 499. [Google Scholar] [CrossRef]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pannerec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.K.; Hall, J.K.; Troy, A.A.; Cornelison, D.D.; Majka, S.M.; Olwin, B.B. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell 2009, 4, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewski, B.; Archacka, K.; Grabowska, I.; Florkowska, A.; Ciemerych, M.A.; Brzoska, E. Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Archacka, K.; Brzoska, E.; Ciemerych, M.A.; Czerwinska, A.M.; Grabowska, I.; Kowalski, K.K.; Zimowska, M. Pluripotent and Mesenchymal Stem Cells—Challenging Sources for Derivation of Myoblast. In Cardiac Cell Culture Technologies; Brzozka, Z., Jastrzebska, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 109–154. [Google Scholar]
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 628767. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef]
- Halvorsen, Y.C.; Wilkison, W.O.; Gimble, J.M. Adipose-derived stromal cells--their utility and potential in bone formation. Int. J. Obes. Relat. Metab. Disord. 2000, 24, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Halvorsen, Y.D.; Bond, A.; Sen, A.; Franklin, D.M.; Lea-Currie, Y.R.; Sujkowski, D.; Ellis, P.N.; Wilkison, W.O.; Gimble, J.M. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: Biochemical, cellular, and molecular analysis. Metabolism 2001, 50, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Lea-Currie, Y.R.; Sujkowska, D.; Franklin, D.M.; Wilkison, W.O.; Halvorsen, Y.D.; Gimble, J.M. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J. Cell Biochem. 2001, 81, 312–319. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Schumacher, A.; Filipowicz, N.; Wardowska, A.; Zielinski, M.; Madanecki, P.; Nowicka, E.; Langa, P.; Deptula, M.; Zielinski, J.; et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci. Rep. 2018, 8, 11339. [Google Scholar] [CrossRef]
- Schipper, B.M.; Marra, K.G.; Zhang, W.; Donnenberg, A.D.; Rubin, J.P. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann. Plast. Surg. 2008, 60, 538–544. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef]
- Efimenko, A.Y.; Kochegura, T.N.; Akopyan, Z.A.; Parfyonova, Y.V. Autologous Stem Cell Therapy: How Aging and Chronic Diseases Affect Stem and Progenitor Cells. Biores. Open Access 2015, 4, 26–38. [Google Scholar] [CrossRef]
- Guilak, F.; Lott, K.E.; Awad, H.A.; Cao, Q.; Hicok, K.C.; Fermor, B.; Gimble, J.M. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J. Cell Physiol. 2006, 206, 229–237. [Google Scholar] [CrossRef]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef]
- Charvet, C.; Houbron, C.; Parlakian, A.; Giordani, J.; Lahoute, C.; Bertrand, A.; Sotiropoulos, A.; Renou, L.; Schmitt, A.; Melki, J.; et al. New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways. Mol. Cell Biol. 2006, 26, 6664–6674. [Google Scholar] [CrossRef] [PubMed]
- Lafreniere, J.F.; Mills, P.; Bouchentouf, M.; Tremblay, J.P. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp. Cell Res. 2006, 312, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hangoc, G.; Bian, H.; Pelus, L.M.; Broxmeyer, H.E. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells 2005, 23, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Cecil, J.; Peng, S.B.; Schrementi, J.; Kovacevic, S.; Paul, D.; Su, E.W.; Wang, J. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene 2006, 374, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, L.; Sanchez-Mateos, P.; Cabanas, C. CXCR7 impact on CXCL12 biology and disease. Trends Mol. Med. 2013, 19, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef]
- Miller, R.J.; Banisadr, G.; Bhattacharyya, B.J. CXCR4 signaling in the regulation of stem cell migration and development. J. Neuroimmunol. 2008, 198, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Decaillot, F.M.; Kazmi, M.A.; Lin, Y.; Ray-Saha, S.; Sakmar, T.P.; Sachdev, P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J. Biol. Chem. 2011, 286, 32188–32197. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Archacki, R.; Archacka, K.; Streminska, W.; Paciorek, A.; Golabek, M.; Ciemerych, M.A.; Brzoska, E. Stromal derived factor-1 and granulocyte-colony stimulating factor treatment improves regeneration of Pax7-/- mice skeletal muscles. J. Cachexia Sarcopenia Muscle 2016, 7, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, E.; Kowalski, K.; Markowska-Zagrajek, A.; Kowalewska, M.; Archacki, R.; Plaskota, I.; Streminska, W.; Janczyk-Ilach, K.; Ciemerych, M.A. Sdf-1 (CXCL12) induces CD9 expression in stem cells engaged in muscle regeneration. Stem Cell Res. Ther. 2015, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Brzoska, E.; Gawrysiak, A.; Streminska, W.; Moraczewski, J.; Polanski, Z.; Hoser, G.; Kawiak, J.; Machaj, E.K.; Pojda, Z.; et al. Restricted Myogenic Potential of Mesenchymal Stromal Cells Isolated From Umbilical Cord. Cell Transplant. 2012, 21, 1711–1726. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Streminska, W.; Janczyk-Ilach, K.; Machaj, E.K.; Pojda, Z.; Hoser, G.; Kawiak, J.; Moraczewski, J.; Ciemerych, M.A.; Brzoska, E. Myogenic potential of mesenchymal stem cells-the case of adhesive fraction of human umbilical cord blood cells. Curr. Stem Cell Res. 2013, 8, 82–90. [Google Scholar] [CrossRef]
- Swierczek-Lasek, B.; Keremidarska-Markova, M.; Hristova-Panusheva, K.; Vladkova, T.; Ciennerych, M.A.; Archacka, K.; Krasteva, N. Polydimethylsiloxane materials with supraphysiological elasticity enable differentiation of myogenic cells. J. Biomed. Mater. Res. Part. A 2019, 107, 2619–2628. [Google Scholar] [CrossRef]
- Siennicka, K.; Zolocinska, A.; Stepien, K.; Lubina-Dabrowska, N.; Maciagowska, M.; Zolocinska, E.; Slysz, A.; Piusinska-Macoch, R.; Mazur, S.; Zdanowicz, U.; et al. Adipose-Derived Cells (Stromal Vascular Fraction) Transplanted for Orthopedical or Neurological Purposes: Are They Safe Enough? Stem Cells Int. 2016, 2016, 5762916. [Google Scholar] [CrossRef]
- Kowalski, K.; Kolodziejczyk, A.; Sikorska, M.H.; Placzkiewicz, J.; Cichosz, P.; Kowalewska, M.; Streminska, W.; Janczyk-Ilach, K.; Koblowska, M.; Fogtman, A.; et al. Stem cells migration during skeletal muscle regenerationthe role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adh. Migr. 2017, 11, 384–398. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Brzoska, E.; Ciemerych, M.A.; Przewozniak, M.; Zimowska, M. Regulation of muscle stem cells activation: The role of growth factors and extracellular matrix. Vitam. Horm. 2011, 87, 239–276. [Google Scholar] [PubMed]
- Juban, G.; Chazaud, B. Metabolic regulation of macrophages during tissue repair: Insights from skeletal muscle regeneration. FEBS Lett. 2017, 591, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B. Macrophages: Supportive cells for tissue repair and regeneration. Immunobiology 2014, 219, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Cohen, T.V.; Many, G.M.; Fleming, B.D.; Gnocchi, V.F.; Ghimbovschi, S.; Mosser, D.M.; Hoffman, E.P.; Partridge, T.A. Upregulated IL-1beta in dysferlin-deficient muscle attenuates regeneration by blunting the response to pro-inflammatory macrophages. Skelet Muscle 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wu, Y.; Wang, L.; Wang, X.; Du, J. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 2013, 288, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Hulderman, T.; Jensen, N.; McKinstry, M.; Mishra, M.; Luster, M.I.; Simeonova, P.P. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002, 16, 1630–1632. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy. Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, M.; Butler, D.M.; Marinova-Mutafchieva, L.; Feldmann, M. An anti-inflammatory role for interleukin-11 in established murine collagen-induced arthritis. Immunology 1998, 95, 31–37. [Google Scholar] [CrossRef]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: A bridge between inflammation and neuroplasticity. Front. Cell Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chatterjee, M.; Schmid, H.; Beck, S.; Gawaz, M. CXCL14 as an emerging immune and inflammatory modulator. J. Inflamm. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Wang, Y.J.; Jia, Z.; Wang, L.P.; Wang, J.S.; Yang, D.M.; Song, J.Q.; Wang, S.L.; Fan, Z.P. Demethylation of IGFBP5 by Histone Demethylase KDM6B Promotes Mesenchymal Stem Cell-Mediated Periodontal Tissue Regeneration by Enhancing Osteogenic Differentiation and Anti-Inflammation Potentials. Stem Cells 2015, 33, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Han, N.A.; Zhang, F.Q.; Li, G.Q.; Zhang, X.L.; Lin, X.; Yang, H.Q.; Wang, L.J.; Cao, Y.Y.; Du, J.; Fan, Z.P. Local application of IGFBP5 protein enhanced periodontal tissue regeneration via increasing the migration, cell proliferation and osteo/dentinogenic differentiation of mesenchymal stem cells in an inflammatory niche. Stem Cell Res. Ther. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.S.; Wang, X.F.; Jiang, G.M.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.Z.; Cai, S.H.; et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Grabowska, I.; Mazur, M.A.; Kowalski, K.; Helinska, A.; Moraczewski, J.; Streminska, W.; Hoser, G.; Kawiak, J.; Ciemerych, M.A.; Brzoska, E. Progression of inflammation during immunodeficient mouse skeletal muscle regeneration. J. Muscle Res. Cell Motil. 2015, 36, 395–404. [Google Scholar] [CrossRef][Green Version]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Wakitani, S.; Saito, T.; Caplan, A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995, 18, 1417–1426. [Google Scholar] [CrossRef]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Buzanska, L.; Jurga, M.; Stachowiak, E.K.; Stachowiak, M.K.; Domanska-Janik, K. Neural stem-like cell line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 2006, 15, 391–406. [Google Scholar] [CrossRef]
- Gussoni, E.; Soneoka, Y.; Strickland, C.D.; Buzney, E.A.; Khan, M.K.; Flint, A.F.; Kunkel, L.M.; Mulligan, R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 401, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Fukada, S.; Miyagoe-Suzuki, Y.; Tsukihara, H.; Yuasa, K.; Higuchi, S.; Ono, S.; Tsujikawa, K.; Takeda, S.; Yamamoto, H. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J. Cell Sci. 2002, 115, 1285–1293. [Google Scholar]
- Brazelton, T.R.; Nystrom, M.; Blau, H.M. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev. Biol. 2003, 262, 64–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, Y.; Cao, J.; Zhang, H.; Chen, M.; Wang, L.; Zhang, C. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum. Mol. Genet. 2015, 24, 6029–6040. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Zimowska, M.; Maciejewska, K.; Jablonska, Z.; Bazga, A.; Ozieblo, M.; Streminska, W.; Bem, J.; Brzoska, E.; Ciemerych, M.A. Adipose Tissue-Derived Stromal Cells in Matrigel Impacts the Regeneration of Severely Damaged Skeletal Muscles. Int. J. Mol. Sci. 2019, 20, 3313. [Google Scholar] [CrossRef] [PubMed]
- Abarbanell, A.M.; Coffey, A.C.; Fehrenbacher, J.W.; Beckman, D.J.; Herrmann, J.L.; Weil, B.; Meldrum, D.R. Proinflammatory cytokine effects on mesenchymal stem cell therapy for the ischemic heart. Ann. Thorac. Surg. 2009, 88, 1036–1043. [Google Scholar] [CrossRef]
- Shohara, R.; Yamamoto, A.; Takikawa, S.; Iwase, A.; Hibi, H.; Kikkawa, F.; Ueda, M. Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy 2012, 14, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.C.; Jeon, E.S.; Lee, I.H.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor necrosis factor-alpha-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J. Investig. Derm. 2011, 131, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.N.; Ahn, J.S.; Lee, S.G.; Lee, H.S.; Choi, A.M.K.; Yoon, J.H. Integrins alphavbeta5 and alphavbeta6 Mediate IL-4-Induced Collective Migration in Human Airway Epithelial Cells. Am. J. Respir Cell Mol. Biol. 2018. [Google Scholar] [CrossRef]
- Puchert, M.; Engele, J. The peculiarities of the SDF-1/CXCL12 system: In some cells, CXCR4 and CXCR7 sing solos, in others, they sing duets. Cell Tissue Res. 2014, 355, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Chen, F.; Liu, J.; Jin, P. Stromal cell-derived factor-1 promotes human adipose tissue-derived stem cell survival and chronic wound healing. Exp. Med. 2016, 12, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Djouad, F.; Bouffi, C.; Ghannam, S.; Noel, D.; Jorgensen, C. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef]
- Zvonic, S.; Lefevre, M.; Kilroy, G.; Floyd, Z.E.; DeLany, J.P.; Kheterpal, I.; Gravois, A.; Dow, R.; White, A.; Wu, X.; et al. Secretome of primary cultures of human adipose-derived stem cells: Modulation of serpins by adipogenesis. Mol. Cell Proteom. 2007, 6, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Carbone, A.; Castellani, S.; Di Gioia, S. Paracrine effects and heterogeneity of marrow-derived stem/progenitor cells: Relevance for the treatment of respiratory diseases. Cells Tissues Organs. 2013, 197, 445–473. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archacka, K.; Bem, J.; Brzoska, E.; Czerwinska, A.M.; Grabowska, I.; Kasprzycka, P.; Hoinkis, D.; Siennicka, K.; Pojda, Z.; Bernas, P.; et al. Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells. Cells 2020, 9, 1479. https://doi.org/10.3390/cells9061479
Archacka K, Bem J, Brzoska E, Czerwinska AM, Grabowska I, Kasprzycka P, Hoinkis D, Siennicka K, Pojda Z, Bernas P, et al. Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells. Cells. 2020; 9(6):1479. https://doi.org/10.3390/cells9061479
Chicago/Turabian StyleArchacka, Karolina, Joanna Bem, Edyta Brzoska, Areta M. Czerwinska, Iwona Grabowska, Paulina Kasprzycka, Dzesika Hoinkis, Katarzyna Siennicka, Zygmunt Pojda, Patrycja Bernas, and et al. 2020. "Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells" Cells 9, no. 6: 1479. https://doi.org/10.3390/cells9061479
APA StyleArchacka, K., Bem, J., Brzoska, E., Czerwinska, A. M., Grabowska, I., Kasprzycka, P., Hoinkis, D., Siennicka, K., Pojda, Z., Bernas, P., Binkowski, R., Jastrzebska, K., Kupiec, A., Malesza, M., Michalczewska, E., Soszynska, M., Ilach, K., Streminska, W., & Ciemerych, M. A. (2020). Beneficial Effect of IL-4 and SDF-1 on Myogenic Potential of Mouse and Human Adipose Tissue-Derived Stromal Cells. Cells, 9(6), 1479. https://doi.org/10.3390/cells9061479




