From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology
Abstract
1. Introduction
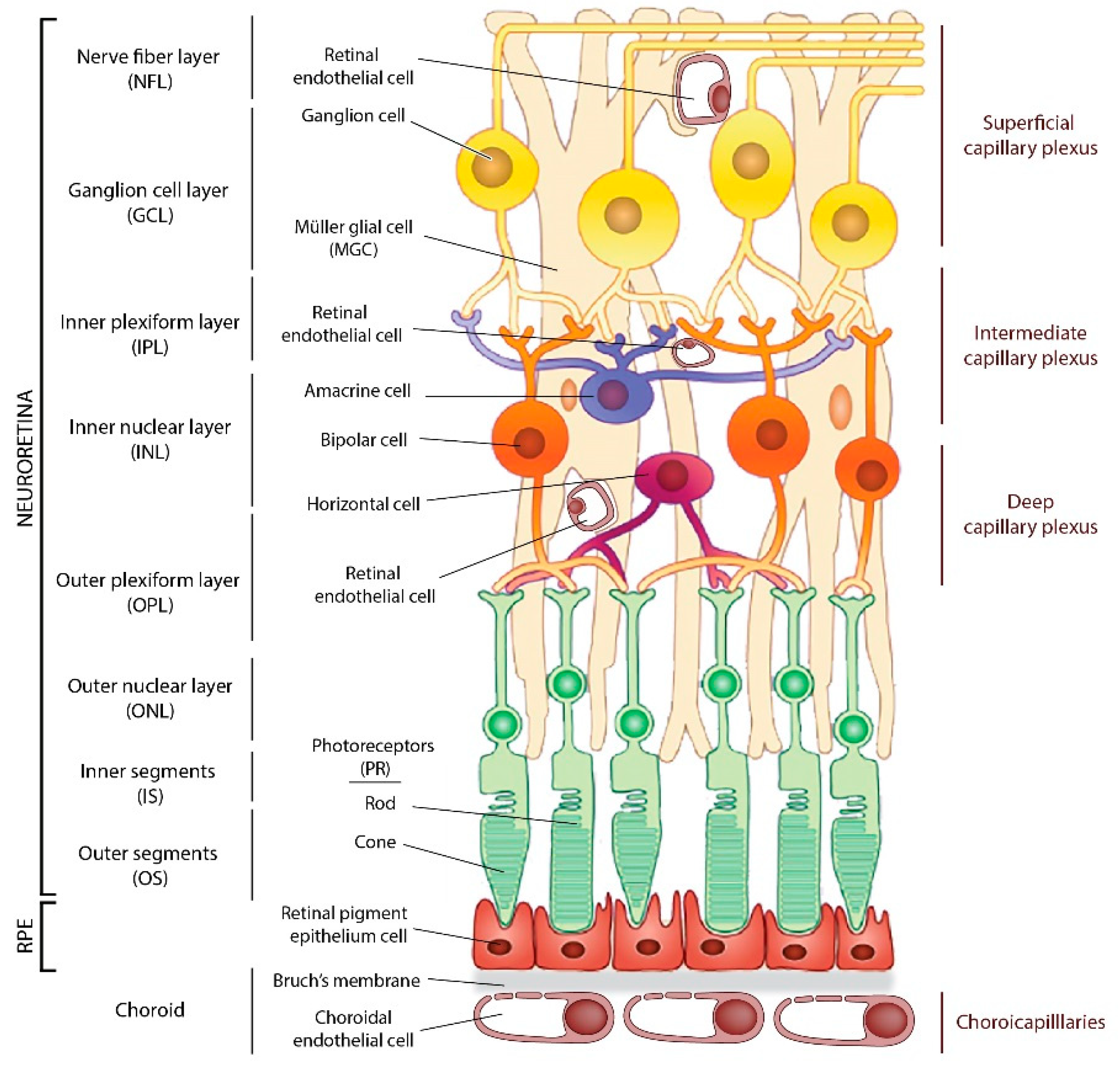
2. The Retina Structure and Retinal Oxygen Supply
3. Iron Homeostasis in the Retina
3.1. Distribution of Iron in the Retina
3.2. Proteins Involved in Retinal Iron Homeostasis
3.2.1. General Iron Homeostasis
3.2.2. Iron Flux in Retina
Transferrin-Bound Iron Transport in the Retina
Non-Transferrin-Bound Iron Transport in the Retina
3.2.3. Iron Regulation in Retina
4. Physiopathological Role of Iron in the Retina
4.1. Iron in Cellular Metabolism/Functions
4.1.1. Iron as a Fe-S Structural Motif Involved in Various Cellular Machinery Proteins
4.1.2. Iron in Nucleic Acids Machinery, Cell Proliferation, and DNA Repair
4.1.3. Iron in Oxygen Transport and Regulation
4.1.4. Iron and Visual Function
4.2. The Dark Side of Iron
4.2.1. The Crucial Role of Iron in Oxidative Stress-Mediated Damages in the Retina
4.2.2. Retinal Cells Death Mechanisms in Iron Overload
4.2.3. Inflammation
4.2.4. Angiogenesis
5. Role of Iron in Retinal Diseases
5.1. Siderosis and Retinal Hemorrhages
5.2. Retinal Manifestations of Inherited Iron Disorders
5.3. Age-Related Macular Degeneration
5.4. Diabetic Retinopathy
5.5. Glaucoma Neuropathy
5.6. Inherited Retinal Dystrophies and Associated Diseases
6. Iron Neutralization as a Therapeutic Strategy for Retinal Diseases
6.1. Chemical Chelators
6.2. Natural Chelators
6.3. Transferrin
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP binding cassette |
| AMD | Age-Related Macular Degeneration |
| APP | amyloid-beta precursor protein |
| BMP | Bone Morphogenetic Protein |
| BRB | Blood Retinal Barrier |
| CEC | Choroidal Endothelial Cell |
| CP | Ceruloplasmin |
| DFO | Deferroxamine |
| DMT1 | divalent metal transporter 1 |
| Fe-S | Cluster Iron-Sulfur |
| FPN | Ferroportin |
| FT | Ferritin |
| HEPC | Hepcidin |
| HEPH | Hephaestin |
| HFE | Hemochromatosis protein |
| HFT | Heavy Ferritin chin |
| HIF | Hypoxia Inducible Factor |
| HJV | Hemojuveline |
| HRE | Hypoxia Responsive Element |
| IL | Interleukin |
| IRE | Iron Responsive Element |
| IRP | Iron Regulatory Protein |
| LCN2 | Lipocalin 2 |
| LF | Lactoferrin |
| LFT | Light Ferritin chain |
| LIP | Labile Iron Pool |
| MacTel 2 | Macular telangiectasia type 2 |
| MGC | Muller Glial cell |
| NTBI | Non-Transferrin Bound Iron |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| PCBP | Poly(rC)-binding proteins |
| PrPC | Prion protein |
| PR | photoreceptor |
| REC | Retinal Endothelial Cell |
| ROS | Reactive Oxygen Species |
| RPE | Retinal Pigment Epithelium |
| SCARA5 | Scavenger receptor class A, member 5 |
| SMAD | Mothers against decapentaplegic homolog 1 |
| TBI | Transferrin Bound Iron |
| TF | Transferrin |
| TFR | Transferrin Receptor |
| VEGF | Vascular Endothelial Growth Factor |
| ZP | Zyloklopen |
| ZIP | ZRT/IRT-like proteins |
References
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Saltzman, H.A. RETINAL OXYGEN UTILIZATION MEASURED BY HYPERBARIC BLACKOUT. Arch. Ophthalmol. 1964, 72, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Linsenmeier, R.A.; Zhang, H.F. Retinal oxygen: From animals to humans. Prog. Retin. Eye Res. 2017, 58, 115–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Törnquist, P.; Bill, A. Glucose metabolism in pig outer retina in light and darkness. Acta Physiol. Scand. 1997, 160, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T. Development and pathological changes of neurovascular unit regulated by hypoxia response in the retina. Prog. Brain Res. 2016, 225, 201–211. [Google Scholar]
- Yang, L.; Wang, D.; Wang, X.-T.; Lu, Y.-P.; Zhu, L. The roles of hypoxia-inducible Factor-1 and iron regulatory protein 1 in iron uptake induced by acute hypoxia. Biochem. Biophys. Res. Commun. 2018, 507, 128–135. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Jeanny, J.C.; Guillonneau, X.; Keller, N.; Nguyen-Legros, J.; Sergeant, C.; Guillou, F.; Courtois, Y. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2343–2351. [Google Scholar]
- Garcia-Castineiras, S. Iron, the retina and the lens: A focused review. Exp. Eye Res. 2010, 90, 664–678. [Google Scholar] [CrossRef]
- Moos, T.; Bernth, N.; Courtois, Y.; Morgan, E.H. Developmental iron uptake and axonal transport in the retina of the rat. Mol. Cell. Neurosci. 2011, 46, 607–613. [Google Scholar] [CrossRef]
- Hahn, P.; Song, Y.; Ying, G.S.; He, X.; Beard, J.; Dunaief, J.L. Age-dependent and gender-specific changes in mouse tissue iron by strain. Exp. Gerontol. 2009, 44, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Ying, G.S.; Beard, J.; Dunaief, J.L. Iron levels in human retina: Sex difference and increase with age. Neuroreport 2006, 17, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, B.; Lukas, T.J.; Suyeoka, G.; Wu, G.; Neufeld, A.H. Changes in iron-regulatory proteins in the aged rodent neural retina. Neurobiol. Aging 2009, 30, 1865–1876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, H.; Lukas, T.J.; Du, N.; Suyeoka, G.; Neufeld, A.H. Dysfunction of the retinal pigment epithelium with age: Increased iron decreases phagocytosis and lysosomal activity. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Merlot, A.M.; Huang, M.L.-H.; Bae, D.-H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 2015, 1853, 1130–1144. [Google Scholar] [CrossRef]
- Lederman, M.; Obolensky, A.; Grunin, M.; Banin, E.; Chowers, I. Retinal Function and Structure in the Hypotransferrinemic Mouse. Investig. Opthalmol. Vis. Sci. 2012, 53, 605. [Google Scholar] [CrossRef][Green Version]
- Rageh, A.A.; Ferrington, D.A.; Roehrich, H.; Yuan, C.; Terluk, M.R.; Nelson, E.F.; Montezuma, S.R. Lactoferrin Expression in Human and Murine Ocular Tissue. Curr. Eye Res. 2016, 41, 883–889. [Google Scholar] [CrossRef]
- Montezuma, S.R.; Dolezal, L.D.; Rageh, A.A.; Mar, K.; Jordan, M.; Ferrington, D.A. Lactoferrin Reduces Chorioretinal Damage in the Murine Laser Model of Choroidal Neovascularization. Curr. Eye Res. 2015, 40, 946–953. [Google Scholar] [CrossRef]
- Parmar, T.; Parmar, V.M.; Arai, E.; Sahu, B.; Perusek, L.; Maeda, A. Acute Stress Responses Are Early Molecular Events of Retinal Degeneration in Abca4−/−Rdh8−/− Mice After Light Exposure. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3257–3267. [Google Scholar] [CrossRef]
- Parmar, T.; Parmar, V.M.; Perusek, L.; Georges, A.; Takahashi, M.; Crabb, J.W.; Maeda, A. Lipocalin 2 Plays an Important Role in Regulating Inflammation in Retinal Degeneration. J. Immunol. 2018, 200, 3128–3141. [Google Scholar] [CrossRef] [PubMed]
- Cases, O.; Obry, A.; Ben-Yacoub, S.; Augustin, S.; Joseph, A.; Toutirais, G.; Simonutti, M.; Christ, A.; Cosette, P.; Kozyraki, R. Impaired vitreous composition and retinal pigment epithelium function in the FoxG1::LRP2 myopic mice. Biochim. Biophys. Acta 2017, 1863, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Dentchev, T.; Qian, Y.; Rouault, T.; Harris, Z.L.; Dunaief, J.L. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol. Vis. 2004, 10, 598–607. [Google Scholar] [PubMed]
- Theurl, M.; Song, D.; Clark, E.; Sterling, J.; Grieco, S.; Altamura, S.; Galy, B.; Hentze, M.; Muckenthaler, M.U.; Dunaief, J.L. Mice with hepcidin-resistant ferroportin accumulate iron in the retina. FASEB J. 2016, 30, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Qian, Y.; Dentchev, T.; Chen, L.; Beard, J.; Harris, Z.L.; Dunaief, J.L. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13850–13855. [Google Scholar] [CrossRef]
- Wolkow, N.; Song, D.; Song, Y.; Chu, S.; Hadziahmetovic, M.; Lee, J.C.; Iacovelli, J.; Grieco, S.; Dunaief, J.L. Ferroxidase hephaestin’s cell-autonomous role in the retinal pigment epithelium. Am. J. Pathol. 2012, 180, 1614–1624. [Google Scholar] [CrossRef][Green Version]
- Wolkow, N.; Song, Y.; Wu, T.-D.; Qian, J.; Guerquin-Kern, J.-L.; Dunaief, J.L. Aceruloplasminemia: Retinal histopathologic manifestations and iron-mediated melanosome degradation. Arch. Ophthalmol. 2011, 129, 1466–1474. [Google Scholar] [CrossRef]
- Dinet, V.; An, N.; Ciccotosto, G.D.; Bruban, J.; Maoui, A.; Bellingham, S.A.; Hill, A.F.; Andersen, O.M.; Nykjaer, A.; Jonet, L.; et al. APP involvement in retinogenesis of mice. Acta Neuropathol. 2011, 121, 351–363. [Google Scholar] [CrossRef]
- Chen, H.; Attieh, Z.K.; Syed, B.A.; Kuo, Y.; Stevens, V.; Fuqua, B.K.; Andersen, H.S.; Naylor, C.E.; Evans, R.W.; Gambling, L.; et al. Identification of Zyklopen, a New Member of the Vertebrate Multicopper Ferroxidase Family, and Characterization in Rodents and Human Cells123. J. Nutr. 2010, 140, 1728–1735. [Google Scholar] [CrossRef]
- He, X.; Hahn, P.; Iacovelli, J.; Wong, R.; King, C.; Bhisitkul, R.; Massaro-Giordano, M.; Dunaief, J.L. Iron homeostasis and toxicity in retinal degeneration. Prog. Retin. Eye Res. 2007, 26, 649–673. [Google Scholar] [CrossRef]
- Sterling, J.; Guttha, S.; Song, Y.; Song, D.; Hadziahmetovic, M.; Dunaief, J.L. Iron importers Zip8 and Zip14 are expressed in retina and regulated by retinal iron levels. Exp. Eye Res. 2017, 155, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Ranchon-Cole, I.; Jonet, L.; Beaumont, C.; Behar-Cohen, F.; Courtois, Y.; Jeanny, J.-C. Light-induced retinal degeneration correlates with changes in iron metabolism gene expression, ferritin level, and aging. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Gnana-Prakasam, J.P.; Roon, P.; Smith, R.G.; Smith, S.B.; Ganapathy, V. Expression and polarized localization of the hemochromatosis gene product HFE in retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4238–4244. [Google Scholar] [CrossRef] [PubMed]
- Gnana-Prakasam, J.P.; Thangaraju, M.; Liu, K.; Ha, Y.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Absence of iron-regulatory protein Hfe results in hyperproliferation of retinal pigment epithelium: Role of cystine/glutamate exchanger. Biochem. J. 2009, 424, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hadziahmetovic, M.; Song, Y.; Wolkow, N.; Iacovelli, J.; Kautz, L.; Roth, M.P.; Dunaief, J.L. Bmp6 regulates retinal iron homeostasis and has altered expression in age-related macular degeneration. Am. J. Pathol. 2011, 179, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Gnana-Prakasam, J.P.; Zhang, M.; Martin, P.M.; Atherton, S.S.; Smith, S.B.; Ganapathy, V. Expression of the iron-regulatory protein haemojuvelin in retina and its regulation during cytomegalovirus infection. Biochem. J. 2009, 419, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Gnana-Prakasam, J.P.; Smith, S.B.; Ganapathy, V. Deletion of hemojuvelin, an iron-regulatory protein, in mice results in abnormal angiogenesis and vasculogenesis in retina along with reactive gliosis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3616–3625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gnana-Prakasam, J.P.; Baldowski, R.B.; Ananth, S.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Retinal expression of the serine protease matriptase-2 (Tmprss6) and its role in retinal iron homeostasis. Mol. Vis. 2014, 20, 561–574. [Google Scholar] [PubMed]
- Hadziahmetovic, M.; Song, Y.; Ponnuru, P.; Iacovelli, J.; Hunter, A.; Haddad, N.; Beard, J.; Connor, J.R.; Vaulont, S.; Dunaief, J.L. Age-Dependent Retinal Iron Accumulation and Degeneration in Hepcidin Knockout Mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kast, B.; Schori, C.; Grimm, C. Hypoxic preconditioning protects photoreceptors against light damage independently of hypoxia inducible transcription factors in rods. Exp. Eye Res. 2016, 146, 60–71. [Google Scholar] [CrossRef]
- Hughes, J.M.; Groot, A.J.; van der Groep, P.; Sersansie, R.; Vooijs, M.; van Diest, P.J.; Van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Active HIF-1 in the normal human retina. J. Histochem. Cytochem. 2010, 58, 247–254. [Google Scholar] [CrossRef]
- Perez Bay, A.E.; Schreiner, R.; Benedicto, I.; Rodriguez-Boulan, E.J. Galectin-4-mediated transcytosis of transferrin receptor. J. Cell. Sci. 2014, 127, 4457–4469. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Le Rouzic, Q.; Oudar, A.; Berdugo, M.; El Sanharawi, M.; Andrieu-Soler, C.; Naud, M.C.; Jonet, L.; Latour, C.; Klein, C.; et al. Targeting iron-mediated retinal degeneration by local delivery of transferrin. Free Radic. Biol. Med. 2015, 89, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Kaczara, P.; Zaręba, M.; Herrnreiter, A.; Skumatz, C.M.B.; Żądło, A.; Sarna, T.; Burke, J.M. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012, 25, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.H.; Shu, W.; Song, Y.; Simpson, E.M.; Lakhal-Littleton, S.; Dunaief, J.L. Ferroportin-mediated iron export from vascular endothelial cells in retina and brain. Exp. Eye Res. 2019, 187, 107728. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Fontaine, I.; Jonet, L.; Guillou, F.; Behar-Cohen, F.; Courtois, Y.; Jeanny, J.C. The protective role of transferrin in Muller glial cells after iron-induced toxicity. Mol. Vis. 2008, 14, 928–941. [Google Scholar] [PubMed]
- Mendes-Jorge, L.; Ramos, D.; Valença, A.; López-Luppo, M.; Pires, V.M.R.; Catita, J.; Nacher, V.; Navarro, M.; Carretero, A.; Rodriguez-Baeza, A.; et al. Correction: L-Ferritin Binding to Scara5: A New Iron Traffic Pathway Potentially Implicated in Retinopathy. PLoS ONE 2017, 12, e0180288. [Google Scholar] [CrossRef]
- Rousseau, E.; Michel, P.P.; Hirsch, E.C. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Homeostasis. Mol. Pharm. 2013, 84, 888–898. [Google Scholar] [CrossRef]
- Valapala, M.; Edwards, M.; Hose, S.; Grebe, R.; Bhutto, I.A.; Cano, M.; Berger, T.; Mak, T.W.; Wawrousek, E.; Handa, J.T.; et al. Increased Lipocalin-2 in the retinal pigment epithelium of Cryba1 cKO mice is associated with a chronic inflammatory response. Aging Cell 2014, 13, 1091–1094. [Google Scholar] [CrossRef]
- Ananth, S.; Gnana-Prakasam, J.P.; Bhutia, Y.D.; Veeranan-Karmegam, R.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2014, 1842, 603–612. [Google Scholar] [CrossRef]
- Anderson, C.P.; Shen, M.; Eisenstein, R.S.; Leibold, E.A. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta 2012, 1823, 1468–1483. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.H.; Shu, W.; Song, Y.; Sterling, J.; Kozmik, Z.; Lakhal-Littleton, S.; Dunaief, J.L. Liver-Specific, but Not Retina-Specific, Hepcidin Knockout Causes Retinal Iron Accumulation and Degeneration. Am. J. Pathol. 2019, 189, 1814–1830. [Google Scholar] [CrossRef] [PubMed]
- Mowat, F.M.; Luhmann, U.F.O.; Smith, A.J.; Lange, C.; Duran, Y.; Harten, S.; Shukla, D.; Maxwell, P.H.; Ali, R.R.; Bainbridge, J.W.B. HIF-1alpha and HIF-2alpha Are Differentially Activated in Distinct Cell Populations in Retinal Ischaemia. PLoS ONE 2010, 5, e11103. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Rouault, T.A. Iron-sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta 2015, 1853, 1493–1512. [Google Scholar] [CrossRef]
- Das, D.; Patra, S.; Bridwell-Rabb, J.; Barondeau, D.P. Mechanism of frataxin “bypass” in human iron–sulfur cluster biosynthesis with implications for Friedreich’s ataxia. J. Biol. Chem. 2019, 294, 9276–9284. [Google Scholar] [CrossRef] [PubMed]
- Efimova, M.G.; Trottier, Y. Distribution of frataxin in eye retina of normal mice and of transgenic R7E mice with retinal degeneration. J. Evol. Biochem. Phys. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Crombie, D.E.; Van Bergen, N.; Davidson, K.C.; Anjomani Virmouni, S.; Mckelvie, P.A.; Chrysostomou, V.; Conquest, A.; Corben, L.A.; Pook, M.A.; Kulkarni, T.; et al. Characterization of the retinal pigment epithelium in Friedreich ataxia. Biochem. Biophys. Rep. 2015, 4, 141–147. [Google Scholar] [CrossRef][Green Version]
- Rouault, T.A.; Maio, N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 2017, 292, 12744–12753. [Google Scholar] [CrossRef]
- Crack, J.C.; Green, J.; Thomson, A.J.; Brun, N.E.L. Iron–Sulfur Clusters as Biological Sensors: The Chemistry of Reactions with Molecular Oxygen and Nitric Oxide. Acc. Chem. Res. 2014, 47, 3196–3205. [Google Scholar] [CrossRef]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Paul, V.D.; Lill, R. Biogenesis of cytosolic and nuclear iron-sulfur proteins and their role in genome stability. Biochim. Biophys. Acta 2015, 1853, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhou, M.; Ji, K.; Zhuang, J.; Dang, W.; Fu, S.; Sun, T.; Zhang, X. Expression of Sirtuins in the Retinal Neurons of Mice, Rats, and Humans. Front. Aging Neurosci. 2017, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The Maintenance of Mitochondrial DNA Integrity--Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, S.W.; Van Houten, B.; Jin, G.F.; Conklin, C.A.; Godley, B.F. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp. Eye Res. 1999, 68, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Barreau, E.; Brossas, J.-Y.; Courtois, Y.; Treton, J.A. Accumulation of Mitochondrial DNA Deletions in Human Retina During Aging. Investig. Ophthalmol. Vis. Sci. 1996, 37, 384–391. [Google Scholar]
- Gkotsi, D.; Begum, R.; Salt, T.; Lascaratos, G.; Hogg, C.; Chau, K.-Y.; Schapira, A.H.V.; Jeffery, G. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp. Eye Res. 2014, 122, 50–53. [Google Scholar] [CrossRef]
- Tezel, T.H.; Geng, L.; Lato, E.B.; Schaal, S.; Liu, Y.; Dean, D.; Klein, J.B.; Kaplan, H.J. Synthesis and Secretion of Hemoglobin by Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2009, 50, 1911. [Google Scholar] [CrossRef]
- Promsote, W.; Makala, L.; Li, B.; Smith, S.B.; Singh, N.; Ganapathy, V.; Pace, B.S.; Martin, P.M. Monomethylfumarate Induces γ-Globin Expression and Fetal Hemoglobin Production in Cultured Human Retinal Pigment Epithelial (RPE) and Erythroid Cells, and in Intact Retina. Investig. Opthalmol. Vis. Sci. 2014, 55, 5382. [Google Scholar] [CrossRef][Green Version]
- Hunt, R.C.; Hunt, D.M.; Gaur, N.; Smith, A. Hemopexin in the human retina: Protection of the retina against heme-mediated toxicity. J. Cell. Physiol. 1996, 168, 71–80. [Google Scholar] [CrossRef]
- Chen, W.; Lu, H.; Dutt, K.; Smith, A.; Hunt, D.M.; Hunt, R.C. Expression of the protective proteins hemopexin and haptoglobin by cells of the neural retina. Exp. Eye Res. 1998, 67, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From structure to function in health and disease. Mol. Asp. Med. 2016, 52, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ma, Z.; Liu, B.; Fang, W.; Qin, L.; Huang, Y.F.; Wang, L.; Gao, Y. Hemin supports the survival of photoreceptors injured by N-Methyl-N-nitrosourea: The contributory role of neuroglobin in photoreceptor degeneration. Brain Res. 2018, 1678, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Qiu, S.; Chen, X.-C.; Dai, Z.-H.; Huang, Y.-C.; Li, Y.-N.; Cai, R.-H.; Lei, H.-T.; Gu, H.-Y. Neuroglobin – A potential biological marker of retinal damage induced by LED light. Neuroscience 2014, 270, 158–167. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.; Xie, L.; Greenberg, D.A. Interactions between Vascular Endothelial Growth Factor and Neuroglobin. Neurosci. Lett. 2012, 519, 47–50. [Google Scholar] [CrossRef]
- Gnana-Prakasam, J.P.; Reddy, S.K.; Veeranan-Karmegam, R.; Smith, S.B.; Martin, P.M.; Ganapathy, V. Polarized distribution of heme transporters in retinal pigment epithelium and their regulation in the iron-overload disease hemochromatosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9279–9286. [Google Scholar] [CrossRef]
- Moiseyev, G.; Takahashi, Y.; Chen, Y.; Gentleman, S.; Redmond, T.M.; Crouch, R.K.; Ma, J.-X. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J. Biol. Chem. 2006, 281, 2835–2840. [Google Scholar] [CrossRef]
- Hamel, C.P.; Tsilou, E.; Pfeffer, B.A.; Hooks, J.J.; Detrick, B.; Redmond, T.M. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J. Biol. Chem. 1993, 268, 15751–15757. [Google Scholar]
- Marlhens, F.; Bareil, C.; Griffoin, J.M.; Zrenner, E.; Amalric, P.; Eliaou, C.; Liu, S.Y.; Harris, E.; Redmond, T.M.; Arnaud, B.; et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat. Genet. 1997, 17, 139–141. [Google Scholar] [CrossRef]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso -zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef]
- Betts-Obregon, B.S.; Gonzalez-Fernandez, F.; Tsin, A.T. Interphotoreceptor retinoid-binding protein (IRBP) promotes retinol uptake and release by rat Müller cells (rMC-1) in vitro: Implications for the cone visual cycle. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6265–6271. [Google Scholar] [CrossRef]
- Unger, E.L.; Earley, C.J.; Beard, J.L. Diurnal cycle influences peripheral and brain iron levels in mice. J. Appl. Physiol. 2009, 106, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.L.; Jones, B.C.; Bianco, L.E.; Allen, R.P.; Earley, C.J. Diurnal variations in brain iron concentrations in BXD RI mice. Neuroscience 2014, 263, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Scholten, A.; Manchala, G.; Cudia, D.; Zlomke-Sell, S.-K.; Koch, K.-W.; Ames, J.B. Structural Characterization of Ferrous Ion Binding to Retinal Guanylate Cyclase Activator Protein 5 from Zebrafish Photoreceptors. Biochemistry 2017, 56, 6652–6661. [Google Scholar] [CrossRef]
- Shichi, H. Microsomal electron transfer system of bovine retinal pigment epithelium. Exp. Eye Res. 1969, 8, 60–68. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Jeanny, J.-C.; Keller, N.; Sergeant, C.; Guillonneau, X.; Beaumont, C.; Courtois, Y. Impaired retinal iron homeostasis associated with defective phagocytosis in Royal College of Surgeons rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 537–545. [Google Scholar]
- McGahan, M.C.; Harned, J.; Mukunnemkeril, M.; Goralska, M.; Fleisher, L.; Ferrell, J.B. Iron alters glutamate secretion by regulating cytosolic aconitase activity. Am. J. Physiol. Cell Physiol. 2005, 288, C1117–C1124. [Google Scholar] [CrossRef]
- Kaushik, P.; Gorin, F.; Vali, S. Dynamics of tyrosine hydroxylase mediated regulation of dopamine synthesis. J. Comput. Neurosci. 2007, 22, 147–160. [Google Scholar] [CrossRef]
- Huang, Q.; Hong, X.; Hao, Q. SNAP-25 is also an iron-sulfur protein. FEBS Lett. 2008, 582, 1431–1436. [Google Scholar] [CrossRef]
- Molday, R.S. Insights into the Molecular Properties of ABCA4 and Its Role in the Visual Cycle and Stargardt Disease. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 415–431. ISBN 978-0-12-801059-4. [Google Scholar]
- Ueda, K.; Kim, H.J.; Zhao, J.; Song, Y.; Dunaief, J.L.; Sparrow, J.R. Iron promotes oxidative cell death caused by bisretinoids of retina. Proc. Natl. Acad. Sci. USA 2018, 115, 4963–4968. [Google Scholar] [CrossRef]
- Lucius, R.; Sievers, J. Postnatal retinal ganglion cells in vitro: Protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996, 743, 56–62. [Google Scholar] [CrossRef]
- Kurz, T.; Karlsson, M.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C. ARPE-19 retinal pigment epithelial cells are highly resistant to oxidative stress and exercise strict control over their lysosomal redox-active iron. Autophagy 2009, 5, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.S.; Symons, R.C.A.; Komeima, K.; Shen, J.; Xiao, W.; Swaim, M.E.; Gong, Y.Y.; Kachi, S.; Campochiaro, P.A. Differential sensitivity of cones to iron-mediated oxidative damage. Investig. Ophthalmol. Vis. Sci. 2007, 48, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Różanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Różanowska, M. Human RPE Melanosomes Protect from Photosensitized and Iron-Mediated Oxidation but Become Pro-oxidant in the Presence of Iron upon Photodegradation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2838–2847. [Google Scholar] [CrossRef]
- Akeo, K.; Hiramitsu, T.; Yorifuji, H.; Okisaka, S. Membranes of retinal pigment epithelial cells in vitro are damaged in the phagocytotic process of the photoreceptor outer segment discs peroxidized by ferrous ions. Pigment Cell Res. 2002, 15, 341–347. [Google Scholar] [CrossRef]
- Harned, J.; Nagar, S.; McGahan, M.C. Hypoxia controls iron metabolism and glutamate secretion in retinal pigmented epithelial cells. Biochim. Biophys. Acta 2014, 1840, 3138–3144. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.E.C.; Del Mar, N.; Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 2018, 64, 96–130. [Google Scholar] [CrossRef]
- Imamura, T.; Hirayama, T.; Tsuruma, K.; Shimazawa, M.; Nagasawa, H.; Hara, H. Hydroxyl radicals cause fluctuation in intracellular ferrous ion levels upon light exposure during photoreceptor cell death. Exp. Eye Res. 2014, 129, 24–30. [Google Scholar] [CrossRef]
- Guajardo, M.H.; Terrasa, A.M.; Catalá, A. Lipid-protein modifications during ascorbate-Fe2+ peroxidation of photoreceptor membranes: Protective effect of melatonin. J. Pineal Res. 2006, 41, 201–210. [Google Scholar] [CrossRef]
- Hunt, R.C.; Handy, I.; Smith, A. Heme-mediated reactive oxygen species toxicity to retinal pigment epithelial cells is reduced by hemopexin. J. Cell. Physiol. 1996, 168, 81–86. [Google Scholar] [CrossRef]
- Tian, Y.; He, Y.; Song, W.; Zhang, E.; Xia, X. Neuroprotective effect of deferoxamine on N-methyl-d-aspartate-induced excitotoxicity in RGC-5 cells. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Thaler, S.; Fiedorowicz, M.; Rejdak, R.; Choragiewicz, T.J.; Sulejczak, D.; Stopa, P.; Zarnowski, T.; Zrenner, E.; Grieb, P.; Schuettauf, F. Neuroprotective effects of tempol on retinal ganglion cells in a partial optic nerve crush rat model with and without iron load. Exp. Eye Res. 2010, 90, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Lam, K.W.; Lam, T.T.; Tso, M.O. Iron-induced apoptosis in the photoreceptor cells of rats. Investig. Ophthalmol. Vis. Sci. 1998, 39, 631–633. [Google Scholar] [PubMed]
- Daruich, A.; Le Rouzic, Q.; Jonet, L.; Naud, M.-C.; Kowalczuk, L.; Pournaras, J.-A.; Boatright, J.H.; Thomas, A.; Turck, N.; Moulin, A.; et al. Iron is neurotoxic in retinal detachment and transferrin confers neuroprotection. Sci. Adv. 2019, 5, eaau9940. [Google Scholar] [CrossRef]
- Chaudhary, K.; Promsote, W.; Ananth, S.; Veeranan-Karmegam, R.; Tawfik, A.; Arjunan, P.; Martin, P.; Smith, S.B.; Thangaraju, M.; Kisselev, O.; et al. Iron Overload Accelerates the Progression of Diabetic Retinopathy in Association with Increased Retinal Renin Expression. Sci. Rep. 2018, 8, 3025. [Google Scholar] [CrossRef]
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Gelfand, B.D.; Wright, C.B.; Kim, Y.; Yasuma, T.; Yasuma, R.; Li, S.; Fowler, B.J.; Bastos-Carvalho, A.; Kerur, N.; Uittenbogaard, A.; et al. Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome. Cell Rep. 2015, 11, 1686–1693. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Song, Y.; Zhao, L.; Wolkow, N.; Tobias, J.W.; Song, W.; Dunaief, J.L. Iron-induced Local Complement Component 3 (C3) Up-regulation via Non-canonical Transforming Growth Factor (TGF)-beta Signaling in the Retinal Pigment Epithelium. J. Biol. Chem 2015, 290, 11918–11934. [Google Scholar] [CrossRef]
- Vogi, W.; Nolte, R.; Brunahl, D. Binding of iron to the 5th component of human complement directs oxygen radical-mediated conversion to specific sites and causes nonenzymic activation. Complement Inflamm 1991, 8, 313–319. [Google Scholar]
- Toomey, C.B.; Johnson, L.V.; Bowes Rickman, C. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog. Retin. Eye Res. 2018, 62, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Asthana, A.; Baksi, S.; Ashok, A.; Karmakar, S.; Mammadova, N.; Kokemuller, R.; Greenlee, M.H.; Kong, Q.; Singh, N. Prion protein facilitates retinal iron uptake and is cleaved at the β-site: Implications for retinal iron homeostasis in prion disorders. Sci. Rep. 2017, 7, 9600. [Google Scholar] [CrossRef] [PubMed]
- You, L.-H.; Yan, C.-Z.; Zheng, B.-J.; Ci, Y.-Z.; Chang, S.-Y.; Yu, P.; Gao, G.-F.; Li, H.-Y.; Dong, T.-Y.; Chang, Y.-Z. Astrocyte hepcidin is a key factor in LPS-induced neuronal apoptosis. Cell Death Dis. 2017, 8, e2676. [Google Scholar] [CrossRef] [PubMed]
- Gnana-Prakasam, J.P.; Martin, P.M.; Mysona, B.A.; Roon, P.; Smith, S.B.; Ganapathy, V. Hepcidin expression in mouse retina and its regulation via lipopolysaccharide/Toll-like receptor-4 pathway independent of Hfe. Biochem. J. 2008, 411, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, P.J.; Hirsch, E.C.; González-Billault, C.; Núñez, M.T. Hepcidin attenuates amyloid beta-induced inflammatory and pro-oxidant responses in astrocytes and microglia. J. Neurochem. 2017, 142, 140–152. [Google Scholar] [CrossRef]
- Ghosh, S.; Shang, P.; Yazdankhah, M.; Bhutto, I.; Hose, S.; Montezuma, S.R.; Luo, T.; Chattopadhyay, S.; Qian, J.; Lutty, G.A.; et al. Activating the AKT2-nuclear factor-κB-lipocalin-2 axis elicits an inflammatory response in age-related macular degeneration: Lipocalin-2 as an indicator of early AMD. J. Pathol. 2017, 241, 583–588. [Google Scholar] [CrossRef]
- Coffman, L.G.; Brown, J.C.; Johnson, D.A.; Parthasarathy, N.; D’Agostino, R.B.; Lively, M.O.; Hua, X.; Tilley, S.L.; Muller-Esterl, W.; Willingham, M.C.; et al. Cleavage of high-molecular-weight kininogen by elastase and tryptase is inhibited by ferritin. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L505–L515. [Google Scholar] [CrossRef]
- Gnana-Prakasam, J.P.; Ananth, S.; Prasad, P.D.; Zhang, M.; Atherton, S.S.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Expression and iron-dependent regulation of succinate receptor GPR91 in retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3751–3758. [Google Scholar] [CrossRef]
- Arjunan, P.; Gnanaprakasam, J.P.; Ananth, S.; Romej, M.A.; Rajalakshmi, V.-K.; Prasad, P.D.; Martin, P.M.; Gurusamy, M.; Thangaraju, M.; Bhutia, Y.D.; et al. Increased Retinal Expression of the Pro-Angiogenic Receptor GPR91 via BMP6 in a Mouse Model of Juvenile Hemochromatosis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1612–1619. [Google Scholar] [CrossRef][Green Version]
- Burke, J.M.; Smith, J.M. Retinal proliferation in response to vitreous hemoglobin or iron. Investig. Ophthalmol. Vis. Sci. 1981, 20, 582–592. [Google Scholar] [PubMed]
- Loporchio, D.; Mukkamala, L.; Gorukanti, K.; Zarbin, M.; Langer, P.; Bhagat, N. Intraocular foreign bodies: A review. Surv. Ophthalmol. 2016, 61, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Konerirajapuram, N.S.; Coral, K.; Punitham, R.; Sharma, T.; Kasinathan, N.; Sivaramakrishnan, R. Trace elements iron, copper and zinc in vitreous of patients with various vitreoretinal diseases. Indian J. Ophthalmol. 2004, 52, 145–148. [Google Scholar] [PubMed]
- Conart, J.-B.; Berrod, J.-P. [Non-traumatic vitreous hemorrhage]. J. Fr. Ophtalmol. 2016, 39, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.V. Retinal hemorrhage in abusive head trauma. Pediatrics 2010, 126, 961–970. [Google Scholar] [CrossRef]
- Casini, G.; Loiudice, P.; Menchini, M.; Sartini, F.; De Cillà, S.; Figus, M.; Nardi, M. Traumatic submacular hemorrhage: Available treatment options and synthesis of the literature. Int. J. Retin. Vitr. 2019, 5, 48. [Google Scholar] [CrossRef]
- Bhisitkul, R.B.; Winn, B.J.; Lee, O.-T.; Wong, J.; de Souza Pereira, D.; Porco, T.C.; He, X.; Hahn, P.; Dunaief, J.L. Neuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhage. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4071–4077. [Google Scholar] [CrossRef]
- Chen-Roetling, J.; Regan, K.A.; Regan, R.F. Protective effect of vitreous against hemoglobin neurotoxicity. Biochem. Biophys. Res. Commun. 2018, 503, 152–156. [Google Scholar] [CrossRef]
- Zerbib, J.; Pierre-Kahn, V.; Sikorav, A.; Oubraham, H.; Sayag, D.; Lobstein, F.; Massonnet-Castel, S.; Haymann-Gawrilow, P.; Souied, E.H. Unusual retinopathy associated with hemochromatosis. Retin Cases Brief Rep. 2015, 9, 190–194. [Google Scholar] [CrossRef]
- Gnana-Prakasam, J.P.; Tawfik, A.; Romej, M.; Ananth, S.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Iron-mediated retinal degeneration in haemojuvelin-knockout mice. Biochem. J. 2012, 441, 599–608. [Google Scholar] [CrossRef]
- Kumar, P.; Nag, T.C.; Jha, K.A.; Dey, S.K.; Kathpalia, P.; Maurya, M.; Gupta, C.L.; Bhatia, J.; Roy, T.S.; Wadhwa, S. Experimental oral iron administration: Histological investigations and expressions of iron handling proteins in rat retina with aging. Toxicology 2017, 392, 22–31. [Google Scholar] [CrossRef]
- Shu, W.; Dunaief, J.L. Potential Treatment of Retinal Diseases with Iron Chelators. Pharmaceuticals (Basel) 2018, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef] [PubMed]
- Biesemeier, A.; Yoeruek, E.; Eibl, O.; Schraermeyer, U. Iron accumulation in Bruch’s membrane and melanosomes of donor eyes with age-related macular degeneration. Exp. Eye Res. 2015, 137, 39–49. [Google Scholar] [CrossRef]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas Affected by Age-Related Macular Degeneration Contain Increased Chelatable Iron in the Retinal Pigment Epithelium and Bruch’s Membrane. Arch. Ophthalmol. 2003, 121, 1099–1105. [Google Scholar] [CrossRef]
- Junemann, A.G.; Stopa, P.; Michalke, B.; Chaudhri, A.; Reulbach, U.; Huchzermeyer, C.; Schlotzer-Schrehardt, U.; Kruse, F.E.; Zrenner, E.; Rejdak, R. Levels of aqueous humor trace elements in patients with non-exsudative age-related macular degeneration: A case-control study. PLoS ONE 2013, 8, e56734. [Google Scholar] [CrossRef]
- Dentchev, T.; Hahn, P.; Dunaief, J.L. Strong labeling for iron and the iron-handling proteins ferritin and ferroportin in the photoreceptor layer in age-related macular degeneration. Arch Ophthalmol. 2005, 123, 1745–1746. [Google Scholar] [CrossRef]
- Chowers, I.; Wong, R.; Dentchev, T.; Farkas, R.H.; Iacovelli, J.; Gunatilaka, T.L.; Medeiros, N.E.; Presley, J.B.; Campochiaro, P.A.; Curcio, C.A.; et al. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2135–2140. [Google Scholar] [CrossRef]
- Čolak, E.; Žorić, L.; Radosavljević, A.; Ignjatović, S. The Association of Serum Iron-Binding Proteins and the Antioxidant Parameter Levels in Age-Related Macular Degeneration. Curr. Eye Res. 2018, 43, 659–665. [Google Scholar] [CrossRef]
- Wysokinski, D.; Danisz, K.; Pawlowska, E.; Dorecka, M.; Romaniuk, D.; Robaszkiewicz, J.; Szaflik, M.; Szaflik, J.; Blasiak, J.; Szaflik, J.P. Transferrin receptor levels and polymorphism of its gene in age-related macular degeneration. Acta Biochim. Pol. 2015, 62, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Pogorzelska, M.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Genetic polymorphism of the iron-regulatory protein-1 and -2 genes in age-related macular degeneration. Mol. Biol. Rep. 2012, 39, 7077–7087. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, E.; Szaflik, J.; Chmielewska, M.; Wozniak, K.; Sklodowska, A.; Waszczyk, M.; Dorecka, M.; Blasiak, J.; Szaflik, J.P. An association between polymorphism of the heme oxygenase-1 and -2 genes and age-related macular degeneration. Mol. Biol. Rep. 2012, 39, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Szemraj, M.; Oszajca, K.; Szemraj, J.; Jurowski, P. MicroRNA Expression Analysis in Serum of Patients with Congenital Hemochromatosis and Age-Related Macular Degeneration (AMD). Med. Sci. Monit. 2017, 23, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wong, T.Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diab. Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Hernández, C.; Simó, R. Iron overload in diabetic retinopathy: A cause or a consequence of impaired mechanisms? Exp. Diabetes Res. 2010, 2010, 714108. [Google Scholar] [CrossRef]
- Baumann, B.; Sterling, J.; Song, Y.; Song, D.; Fruttiger, M.; Gillies, M.; Shen, W.; Dunaief, J.L. Conditional Müller Cell Ablation Leads to Retinal Iron Accumulation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4223–4234. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Wang, H.-W.; Sun, P.; Chen, Y.; Jiang, L.-P.; Wu, H.-P.; Zhang, W.; Gao, F. Research progress on human genes involved in the pathogenesis of glaucoma (Review). Mol. Med. Rep. 2018, 18, 656–674. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Borisuth, N.S.; Tripathi, B.J.; Gotsis, S.S. Quantitative and qualitative analyses of transferrin in aqueous humor from patients with primary and secondary glaucomas. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2866–2873. [Google Scholar]
- Farkas, R.H.; Chowers, I.; Hackam, A.S.; Kageyama, M.; Nickells, R.W.; Otteson, D.C.; Duh, E.J.; Wang, C.; Valenta, D.F.; Gunatilaka, T.L.; et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlötzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Jünemann, A.G.M. Levels of aqueous humor trace elements in patients with open-angle glaucoma. J. Trace Elem. Med. Biol. 2018, 45, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Fick, A.; Jünemann, A.; Michalke, B.; Lucio, M.; Hohberger, B. Levels of serum trace elements in patients with primary open-angle glaucoma. J. Trace Elem. Med. Biol. 2019, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Wang, S.Y.; Yoo, C.; Singh, K.; Lin, S.C. Association between serum ferritin and glaucoma in the South Korean population. JAMA Ophthalmol. 2014, 132, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Sarnat-Kucharczyk, M.; Rokicki, W.; Zalejska-Fiolka, J.; Pojda-Wilczek, D.; Mrukwa-Kominek, E. Determination of Serum Ceruloplasmin Concentration in Patients with Primary Open Angle Glaucoma with Cataract and Patients with Cataract Only: A Pilot Study. Med. Sci. Monit. 2016, 22, 1384–1388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DeToma, A.S.; Dengler-Crish, C.M.; Deb, A.; Braymer, J.J.; Penner-Hahn, J.E.; van der Schyf, C.J.; Lim, M.H.; Crish, S.D. Abnormal metal levels in the primary visual pathway of the DBA/2J mouse model of glaucoma. Biometals 2014, 27, 1291–1301. [Google Scholar] [CrossRef]
- Anders, F.; Teister, J.; Funke, S.; Pfeiffer, N.; Grus, F.; Solon, T.; Prokosch, V. Proteomic profiling reveals crucial retinal protein alterations in the early phase of an experimental glaucoma model. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1395–1407. [Google Scholar] [CrossRef]
- Cheah, J.H.; Kim, S.F.; Hester, L.D.; Clancy, K.W.; Patterson, S.E.; Papadopoulos, V.; Snyder, S.H. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 2006, 51, 431–440. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, M.; Shoeb, M.; Hogan, D.; Tang, L.; Syed, M.F.; Wang, C.Z.; Campbell, G.A.; Ansari, N.H. Metal chelator combined with permeability enhancer ameliorates oxidative stress-associated neurodegeneration in rat eyes with elevated intraocular pressure. Free Radic. Biol. Med. 2014, 69, 289–299. [Google Scholar] [CrossRef]
- Sakamoto, K.; Suzuki, T.; Takahashi, K.; Koguchi, T.; Hirayama, T.; Mori, A.; Nakahara, T.; Nagasawa, H.; Ishii, K. Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina. Exp. Eye Res. 2018, 171, 30–36. [Google Scholar] [CrossRef]
- Sirohi, K.; Chalasani, M.L.S.; Sudhakar, C.; Kumari, A.; Radha, V.; Swarup, G. M98K-OPTN induces transferrin receptor degradation and RAB12-mediated autophagic death in retinal ganglion cells. Autophagy 2013, 9, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Jonet, L.; Sergeant, C.; Vesvres, M.H.; Behar-Cohen, F.; Courtois, Y.; Jeanny, J.C. Overexpressed or intraperitoneally injected human transferrin prevents photoreceptor degeneration in rd10 mice. Mol. Vis. 2010, 16, 2612–2625. [Google Scholar] [PubMed]
- Scerri, T.S.; Quaglieri, A.; Cai, C.; Zernant, J.; Matsunami, N.; Baird, L.; Scheppke, L.; Bonelli, R.; Yannuzzi, L.A.; Friedlander, M.; et al. Genome-wide analyses identify common variants associated with macular telangiectasia type 2. Nat. Genet. 2017, 49, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Obolensky, A.; Berenshtein, E.; Lederman, M.; Bulvik, B.; Alper-Pinus, R.; Yaul, R.; Deleon, E.; Chowers, I.; Chevion, M.; Banin, E. Zinc-desferrioxamine attenuates retinal degeneration in the rd10 mouse model of retinitis pigmentosa. Free Radic. Biol. Med. 2011, 51, 1482–1491. [Google Scholar] [CrossRef]
- Li, Z.L.; Lam, S.; Tso, M.O. Desferrioxamine ameliorates retinal photic injury in albino rats. Curr. Eye Res. 1991, 10, 133–144. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Song, Y.; Wolkow, N.; Iacovelli, J.; Grieco, S.; Lee, J.; Lyubarsky, A.; Pratico, D.; Connelly, J.; Spino, M.; et al. The Oral Iron Chelator Deferiprone Protects against Iron Overload–Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 959–968. [Google Scholar] [CrossRef]
- Song, D.; Zhao, L.; Li, Y.; Hadziahmetovic, M.; Song, Y.; Dunaief, J.L. The oral iron chelator deferiprone protects against iron overload-induced retinal degeneration in Hepcidin knockout mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4525–4532. [Google Scholar] [CrossRef]
- Song, D.; Song, Y.; Hadziahmetovic, M.; Zhong, Y.; Dunaief, J.L. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic. Biol. Med. 2012, 53, 64–71. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Pajic, M.; Grieco, S.; Song, Y.; Song, D.; Li, Y.; Cwanger, A.; Iacovelli, J.; Chu, S.; Ying, G.-S.; et al. The Oral Iron Chelator Deferiprone Protects Against Retinal Degeneration Induced through Diverse Mechanisms. Transl. Vis. Sci. Technol. 2012, 1, 7. [Google Scholar] [CrossRef]
- Arora, A.; Wren, S.; Gregory Evans, K. Desferrioxamine related maculopathy: A case report. Am. J. Hematol. 2004, 76, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, V.; Schocket, S.S.; Jiji, R. Deferoxamine (Desferal)-induced toxic retinal pigmentary degeneration and presumed optic neuropathy. Ophthalmology 1984, 91, 443–451. [Google Scholar] [CrossRef]
- Jauregui, R.; Park, K.S.; Bassuk, A.G.; Mahajan, V.B.; Tsang, S.H. Deferoxamine-induced electronegative ERG responses. Doc. Ophthalmol. 2018, 137, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar] [PubMed]
- Sahlstedt, L.; von Bonsdorff, L.; Ebeling, F.; Ruutu, T.; Parkkinen, J. Effective binding of free iron by a single intravenous dose of human apotransferrin in haematological stem cell transplant patients. Br. J. Haematol. 2002, 119, 547–553. [Google Scholar] [CrossRef]
- Brittenham, G.M. Iron-chelating therapy for transfusional iron overload. N. Engl. J. Med. 2011, 364, 146–156. [Google Scholar] [CrossRef]
- Goya, N.; Miyazaki, S.; Kodate, S.; Ushio, B. A family of congenital atransferrinemia. Blood 1972, 40, 239–245. [Google Scholar] [CrossRef]
- Simon, S.; Athanasiov, P.A.; Jain, R.; Raymond, G.; Gilhotra, J.S. Desferrioxamine-related ocular toxicity: A case report. Indian J. Ophthalmol. 2012, 60, 315–317. [Google Scholar] [CrossRef]
- Di Nicola, M.; Barteselli, G.; Dell’Arti, L.; Ratiglia, R.; Viola, F. Functional and Structural Abnormalities in Deferoxamine Retinopathy: A Review of the Literature. Biomed. Res. Int. 2015, 2015, 249617. [Google Scholar] [CrossRef]
- Beau-Salinas, F.; Guitteny, M.A.; Donadieu, J.; Jonville-Bera, A.P.; Autret-Leca, E. High doses of deferiprone may be associated with cerebellar syndrome. BMJ 2009, 338, a2319. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Nowroozzadeh, M.H. Posterior subcapsular opacity in two patients with thalassaemia major following deferiprone consumption. Clin. Exp. Optom. 2009, 92, 392–394. [Google Scholar] [CrossRef]
- Taneja, R.; Malik, P.; Sharma, M.; Agarwal, M.C. Multiple transfused thalassemia major: Ocular manifestations in a hospital-based population. Indian J. Ophthalmol. 2010, 58, 125–130. [Google Scholar] [PubMed]
- Masera, N.; Rescaldani, C.; Azzolini, M.; Vimercati, C.; Tavecchia, L.; Masera, G.; De Molfetta, V.; Arpa, P. Development of lens opacities with peculiar characteristics in patients affected by thalassemia major on chelating treatment with deferasirox (ICL670) at the Pediatric Clinic in Monza, Italy. Haematologica 2008, 93, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Keane, P.A.; Sadun, A.A.; Fawzi, A.A. Optical coherence tomography findings in deferasirox-related maculopathy. Retin Cases Brief Rep. 2010, 4, 229–232. [Google Scholar] [CrossRef]
- Farajipour, H.; Rahimian, S.; Taghizadeh, M. Curcumin: A new candidate for retinal disease therapy? J. Cell. Biochem. 2018, 120, 6886–6893. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Potential of the Bioflavonoids in the Prevention/Treatment of Ocular Disorders. J. Pharm. Pharmacol. 2010, 62, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.P.; Whitmore, S.S.; Flamme-Wiese, M.J.; Riker, M.J.; Wiley, L.A.; Tucker, B.A.; Stone, E.M.; Mullins, R.F.; Scheetz, T.E. Molecular characterization of foveal versus peripheral human retina by single-cell RNA sequencing. Exp. Eye Res. 2019, 184, 234–242. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharm. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today 2005, 10, 267–273. [Google Scholar] [CrossRef]
- de Jong, P.T.V.M. A Historical Analysis of the Quest for the Origins of Aging Macula Disorder, the Tissues Involved, and Its Terminology. Ophthalmol. Eye. Dis. 2016, 8, 5–14. [Google Scholar] [CrossRef]
- Weber, C.; Cole, D.J.; O’Regan, D.D.; Payne, M.C. Renormalization of myoglobin–ligand binding energetics by quantum many-body effects. PNAS 2014, 111, 5790–5795. [Google Scholar] [CrossRef] [PubMed]


| Proteins | Expression | Functions | Knock-Out Rodent Models | Human Pathologies | |
|---|---|---|---|---|---|
| Iron uptake/export | Transferrin (TF) | RPE, PR, MGC [8] | Extracellular transporter binding two ferric iron ions (Fe3+) [holoTF]. Kd = 1022M−1 | Hpx−/−: Decrease of the electroretinogram. Decrease TF, CP, TFR1 [17] | Congenital atransferrinemia |
| Transferrin receptor 1 (TFR1) | RPE, IS, OPL, INL, GCL, endothelial cells [8] | Transmembrane receptor of holoTF | ND | ND | |
| Lactoferrin (LF) | RPE [18] | Extracellular transporter binding two Fe3+ | Lf−/−: Higher susceptibility for laser induced choroidal neovascularization [19] | ND | |
| Lipocalin 2 (LCN2) or (neutrophil gelatinase-associated lipocalin (NGAL) or 24p3 | RPE, MGC, neural retina, microglia [20] | Extracellular transporter which binds Fe3+ by sequestering bacterial and mammalian siderophores (2,5-dihydroxybenzoic acid). | Lcn2−/−: Expression of LFT, TF et TFR1 unchanged (personal data) | ND | |
| 24p3R or (the solute carrier family 22 member 17 (SLC22A17) | RPE [21] | Transmembrane receptor of LCN2 under holo- and apo-forms | |||
| Megalin or (low density lipoprotein receptor-related protein 2 (LRP2) | RPE [22] | Transmembrane multiligands receptor (such lipocalin 2, lactoferrin, transferrin), co-receptor Cubulin | Lrp2F/F (FoxG1Cre+): myopia, hypertrophic RPE and retinal degeneration [22]. Increase FT and decrease TFR1 (personal data) | Donnai-Barrow syndrome: high myopia, retinal detachment | |
| Ferroportin (FPN) or (SLC40A1) | RPE, IS, OPL, IPL, MGC, REC [23] | Transmembrane transporter which exports ferrous iron (Fe2+) outside the cell in cooperation with ferroxidases | FpnC326S: HEPC-resistant FPN mice. Increase FT, iron deposits in RPE and choroid [24] | Hemochromatosis type 4 | |
| Ceruloplasmin (CP) | RPE, MGC [25] | Extracellular ferroxidase that oxidizes Fe2+ in Fe3+. Exists a glycosylphosphatidylinositol-anchored form | Cp−/−Hephsla/sla: Neovessels, deposits under RPE, loss of RPE and PR. Iron accumulation in RPE and PR. Increase FT and decrease TFR1. Cp−/−HephF/F (Bestrophin1Cre+): no iron deposits and no retinal dystrophy [26] | Aceruloplasminemia: iron deposits in drusen and RPE. Dry-AMD like phenotype [27] | |
| Hephaestin (HEPH) | RPE, PR, MGC [25] | Extracellular ferroxidase. 50% of homology with CP | ND | ||
| Amyloid-beta precursor protein (APP) | RPE, IS and OS, MGC, GCL [26] | Membrane ferroxidase | App−/−: Disturbances of synaptic development of secondary neurons [28]. | Associated with Alzheimer disease and cerebral amyloid angiopathy | |
| Zyklopen (ZP) | RPE, GCL [26,29] | Membrane ferroxidase | ND | ND | |
| DMT1 (Divalent Metal Transporter 1) or Natural resistance-associated macrophage protein (NRAM2) or SLC11A2 | IS, horizontal and rod bipolar cells [30] | Transmembrane import of Fe2 Iron exit from endosomal vesicle or cytosol under acidic pH (5.5). | ND | Microcytic hypochromic anemia with iron overload | |
| ZIP14 (Zinc transporter 14) or SLC39A14 | CEC, RPE, PR, MGC, GCL, REC [31] | Transmembrane zinc transporter which uptakes unbound Fe2+ in cytosol. Optimal at physiological pH (7.4). | ND | Hypermanganesemia with dystonia 2; Hyperostosis cranialis interna. | |
| ZIP8 (Zinc transporter 8) or SLC39A8 | Congenital disorder of glycosylation 2N | ||||
| Storage | Ferritin (FT) | Ubiquitous and highly express in RPE, IS, bipolar cells [8] | Cytosolic complex of 24 subunits of heavy (H) and light (L) chains, which can store 4, 500 Fe3+. The H subunits have ferroxidase activity. FT has also a nuclear localization | Hft+/−: Higher sensibility for stress [32] | HFT: Hemochromatosis type 5LFT: Hyperferritinemia with or without cataract; Neuroferritinopathy; L-ferritin deficiency. |
| Mitochondrial ferritin (FtMt) | All retina layers, with higher expression in RPE and ellipsoids of IS [30] | Mitochondria iron transporter. Share 79% of homology with HFT and has ferroxidase activity | ND | ND | |
| Regulation | Transferrin receptor 2 (TFR2) | RPE, IS, OPL, IPL [33] | Transmembrane receptor of holo-TF which regulates transcription of HEPC in cooperation with HFE under TF iron-saturation | ND | Hemochromatosis type 3 |
| Hereditary hemochromatosis protein (HFE) | RPE [33] | Membrane protein which bind β2M to TFR1 or TFR2 in function of TF iron-saturation | Hfe−/−: Hypertrophy/Hyperplasia of RPE, PR degeneration. Increase FT [34] | Hemochromatosis type 1: Dysmorphism of RPE, drusen and alteration of vision [30]. Variegate porphyria. Microvascular complications of diabetes 7 | |
| β-2-Microglobulin (β2M) | RPE, OS, IS, OPL, INL, IPL [33] | Membrane protein involved in HFE-TFR1/2 interaction | ND | Immunodeficiency 43; Amyloidosis 8. | |
| Bone Morphogenetic protein 6 (BMP6) | RPE, IS, OPL, IPL, GCL | Extracellular protein which regulates HEPC transcription. Bind Activin A receptor (Acvr1A) and BMP receptor type II (BMPR2) and HJV as coreceptor, all expressed in retina | Bmp6−/−: Iron accumulation in RPE and retina. RPE hypertrophy and PR degeneration. Decrease TFR1 and increase LFT [35] | ND | |
| Hemojuvelin (HJV) | RPE, PR, MGC, GCL [36] | Regulation of HEPC transcription | Hjv−/−: Neovessels in retina, gliosis, inner BRB leakage, PR degeneration. Increase LFT [37] | Hemochromatosis type 2A | |
| Transmembrane serine protease 6. (TMPRSS6) or Serine protease matriptase-2 | RPE, MGC GCL [38] | Membrane protein with serine protease activity which cleave HVJ | Tmprss6msk/msk: No visual alteration [38]. Increase HEPC, HJV, TFR1 | Iron-refractory iron deficiency anemia | |
| Hepcidin (HEPC) | RPE, IS, MGC, OPL [36] | Peptide hormone which transcription is activated by TF saturation or inflammation. Induces the degradation of FPN reducing iron export | Hamp−/−: Iron accumulation in RPE/choroid and in retina. Decrease TFR1 and increase FPN [39] | Hemochromatosis type 2B and juvenile | |
| Iron regulatory protein (IRP1) or cytoplasmic aconitase hydratase (ACO1) | Ubiquitous | Iron sensor protein with cluster iron-sulfur. Bind Iron responsive element (IRE) in target mRNA when intracellular iron levels are low. Under high iron condition, IRP1 is converted into an aconitase whereas IRP2 is degraded in proteasome | Ireb1+/−Ireb2−/−: No retinal alteration. Increase FPN and LFT [30] | ND | |
| IRP2 or Iron-responsive element-binding protein 2 (IREB2) | ND | ||||
| Hypoxia Inducible Factor (HIF) | RPE, PR ONL, INL, GCL [40,41] | Transcriptional regulator. Oxygen sensor sensitive to iron level. Bind Hypoxia responsive element (HRE) in target mRNA under hypoxia or when intracellular iron levels are low | ND | Familial erythrocytosis (HIF2α) |
| Deferoxamine | Deferiprone | Deferasirox | Transferrin | |
|---|---|---|---|---|
| Iron Binding | 1:1 | 3:1 | 2:1 | 2:1 |
| Route of administration | Sub-cutaneous (every 8–12h) Intravenous (IV) (5 days/week) | Oral (t.i.d) | Oral (q.d) | Intravenous [176] |
| Half-Life(after IV administration) | 20–30 min | 3–4 h | 8–16 h | 4–8 d [176] |
| Excretion | Urinary/fecal | Urinary | Fecal | Unknown |
| Usual Doses (mg/Kg/d) | 25–60 [177] | 75–100 [177] | 20–40 [177] | 100 [176] |
| Clinical Use | Acute iron intoxicationChronic iron overload | Chronic iron overload | Chronic iron overload | Atransferrinemia [178] Haematological stem cell transplant [176] |
| Ocular Side effects | Pigmentary retinopathy [173], visual loss [179], impaired night vision [180], optic neuritis [173] and cataract [172]. | Diplopia [181], cataract [182] and possible retinal toxicity [183]. | Lens opacities [184] and retinal disorders [185] | No adverse effects observed |
| Model Experiment | Physiopathology | Administration Mode | Therapeutic Action of Transferrin | References |
|---|---|---|---|---|
| Primary culture of Müller glial cells. | Iron exposure | Cell isolation from transgenic mice carrying the human transferrin gene (TghTF) | Cell number preservation. Lower necrosis revealed by lactate dehydrogenase release. Inhibition of mRNA TF diminution. | [46] |
| Primary culture of Müller glial cells | Iron exposure | Addition of apo- or holo-human TF | Dose-dependent cell number preservation by apo- but not holo-human TF | [46] |
| rd10 mice | Model of retinitis pigmentosa presenting iron accumulation in photoreceptors (PR) | Crossing rd10 mice with TghTF mice | Preservation of retinal histology (outer and inner nuclear layers thickness). Less apoptotic-positive retinal cells.Conservation of rods and cones morphology | [164] |
| rd10 mice | Model of retinitis pigmentosa presenting iron accumulation in PR | Daily intraperitoneal injections of apo-human TF | Dose-dependent preservation of retinal histology (outer and inner nuclear layers thickness). Less apoptotic-positive retinal cells. Conservation of rods and cones morphology | [164] |
| Light-induced degeneration | Model of acute degenerative retina | Intravitreal injection of apo-human TF before and after light-induced degeneration | Preservation of retinal histology and functions. Preservation of ONL thickness and PR morphology. Lower ONL apoptotic- positive cells.Regulation of iron homeostasis balance. Lower retinal iron accumulation and oxidative stress. Regulation of retina inflammation and diminution of microglial cells activation in outer retina. | [43] |
| Light-induced degeneration | Model of acute degenerative retina | Electrotransfer of cDNA of human TF for in oculo production | Preservation of retinal histology and ONL layer thickness. | [43] |
| P23H rats | Model of retinitis pigmentosa | Electrotransfer of cDNA of human TF for in oculo production | Preservation of retinal histology and ONL layer thickness. | [43] |
| Bone morphogenetic protein 6 mice | Model of hemochromatosis with retinal iron accumulation | Intraperitoneal and intravitreal injections of apo-human TF | Diminution of iron accumulation in retina pigment epithelium | [43] |
| Retinal explant of mice | Retinal detachment with iron exposure | Retinas from TghTF | Preservation of cones number and rod outer segments length. Lower necrosis Prevention of iron retinal accumulation | [105] |
| Retinal explant of rats | Retinal detachment with iron exposure | Addition of apo-human TF after iron exposure | Preservation of rhodopsin expression level and cones number Lower necrosis and apoptosis Prevention of retinal iron accumulation | [105] |
| Subretinal injection of hyaluronic acid in mice | Retinal detachment presenting iron accumulation in subretinal space | TghTF mice | Preservation of retinal histology, rods outer segments length and number of cones Diminution of retinal oedema and Müller glial cells activation Lower apoptosis and necrosis Regulation of pathways involved in biological functions | [105] |
| Subretinal injection of hyaluronic acid in rats | Retinal detachment presenting iron accumulation in subretinal space | Intravitreal injection of apo-hTF | Preservation of retinal histology, rods outer segments length Diminution of retinal oedema | [105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picard, E.; Daruich, A.; Youale, J.; Courtois, Y.; Behar-Cohen, F. From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology. Cells 2020, 9, 705. https://doi.org/10.3390/cells9030705
Picard E, Daruich A, Youale J, Courtois Y, Behar-Cohen F. From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology. Cells. 2020; 9(3):705. https://doi.org/10.3390/cells9030705
Chicago/Turabian StylePicard, Emilie, Alejandra Daruich, Jenny Youale, Yves Courtois, and Francine Behar-Cohen. 2020. "From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology" Cells 9, no. 3: 705. https://doi.org/10.3390/cells9030705
APA StylePicard, E., Daruich, A., Youale, J., Courtois, Y., & Behar-Cohen, F. (2020). From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology. Cells, 9(3), 705. https://doi.org/10.3390/cells9030705





