Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recombinant Fibronectin Modules
2.3. Stably Transfected HEK 293 Cell Lines
2.4. Cell Culture
2.5. Cell Treatments
2.6. Human IL-8 Enzyme-Linked Immunosorbent Assay
3. Results
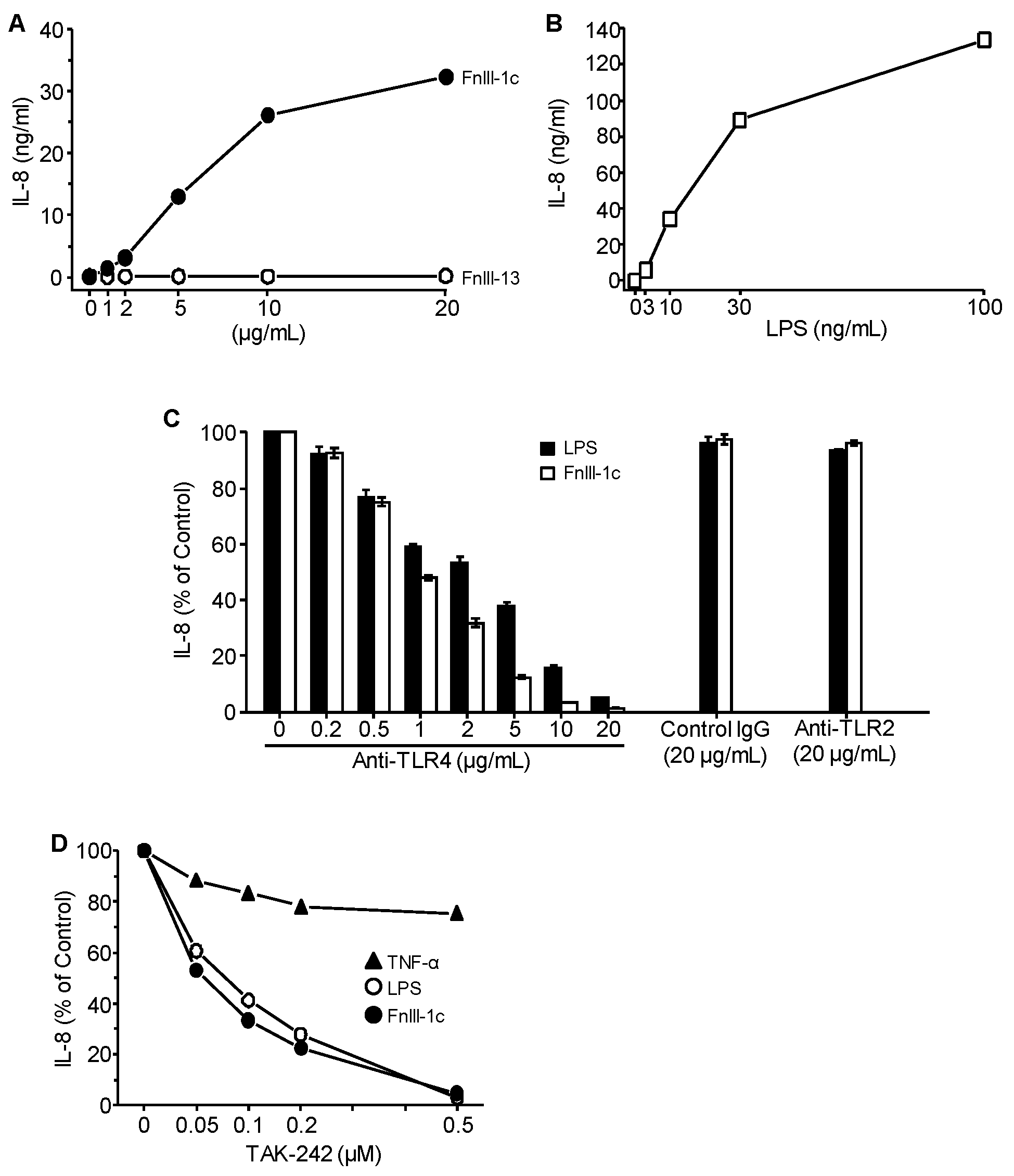
3.1. Cytokine Induction in Response to Either FnIII-1c or LPS is Dependent on TLR4
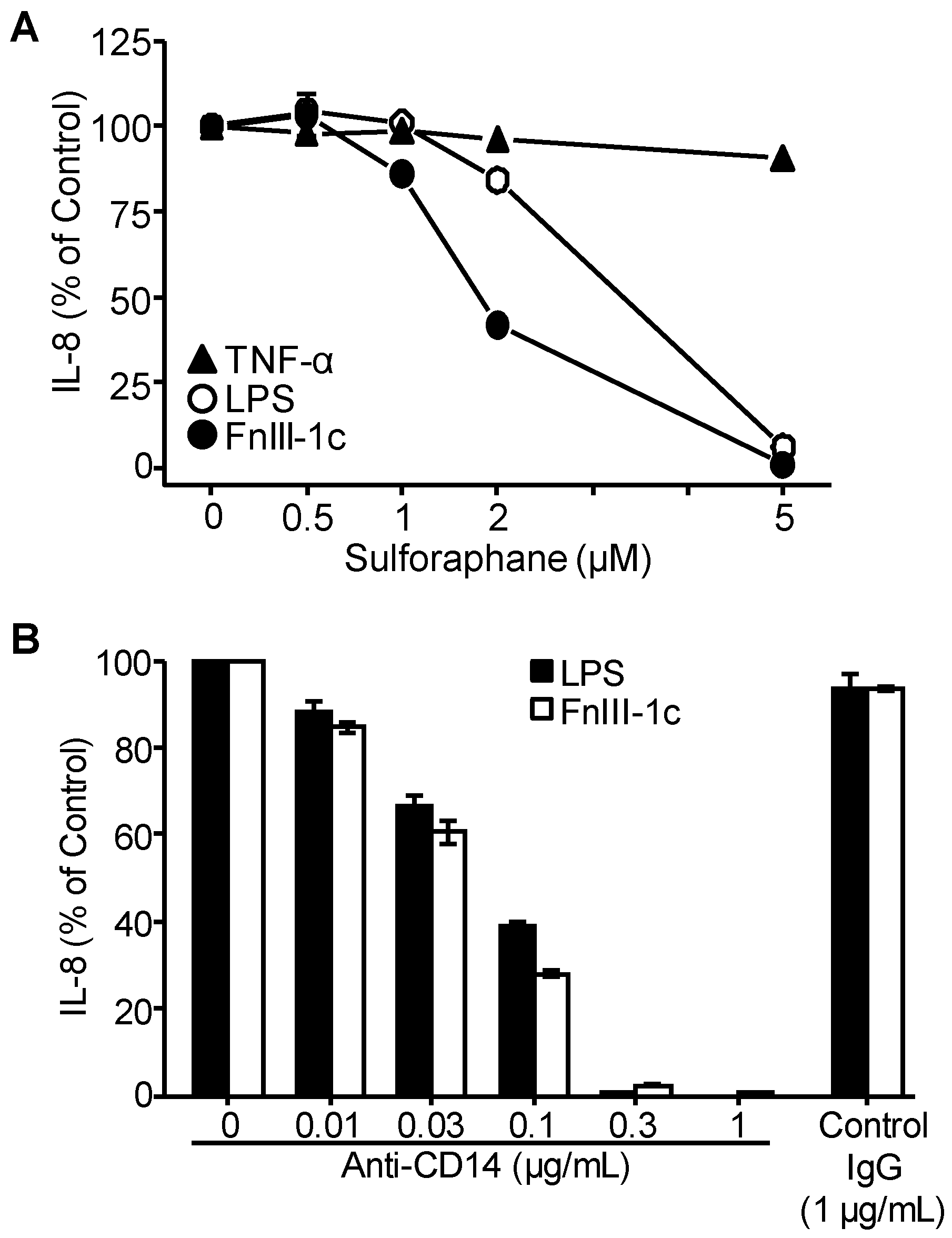
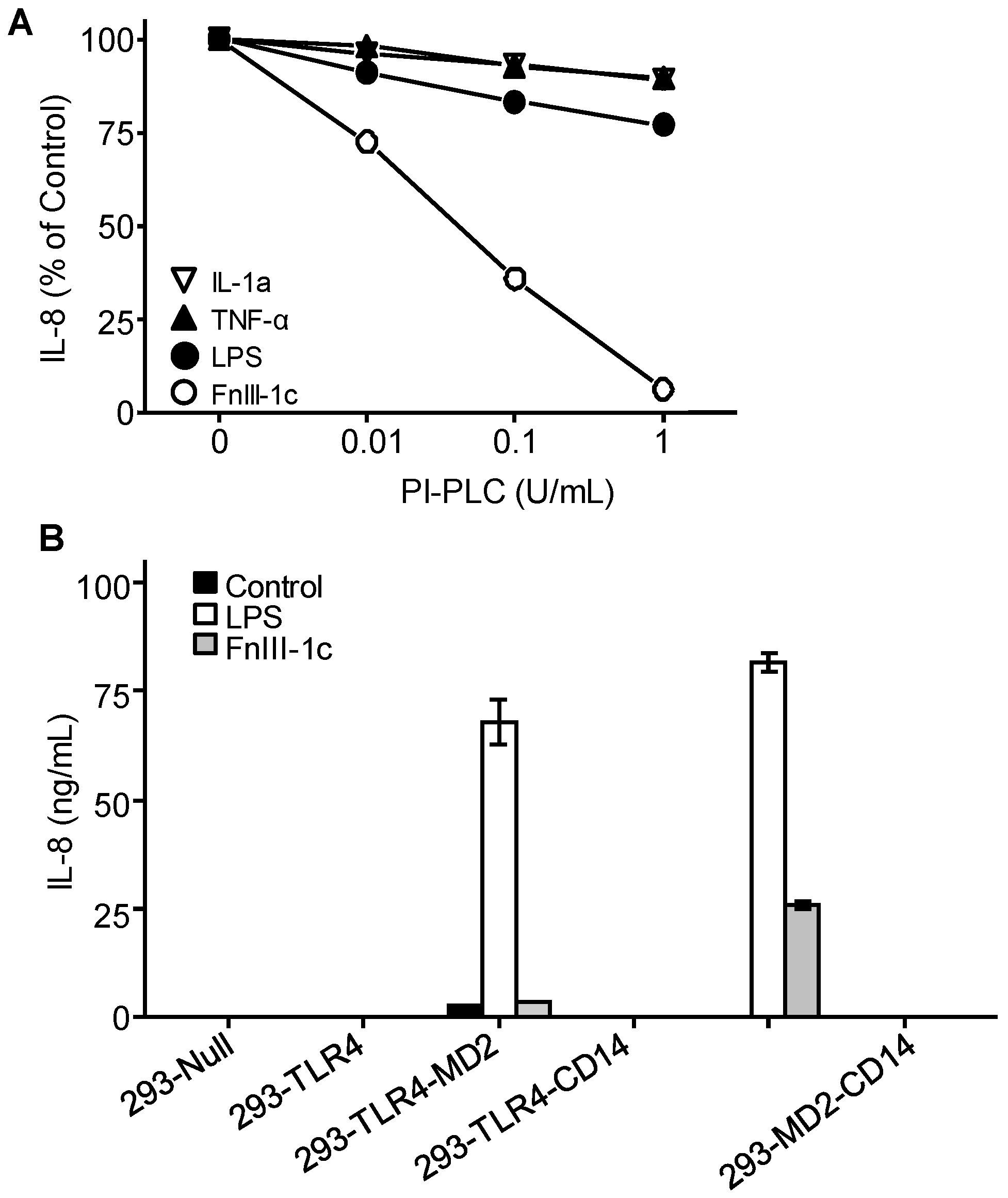
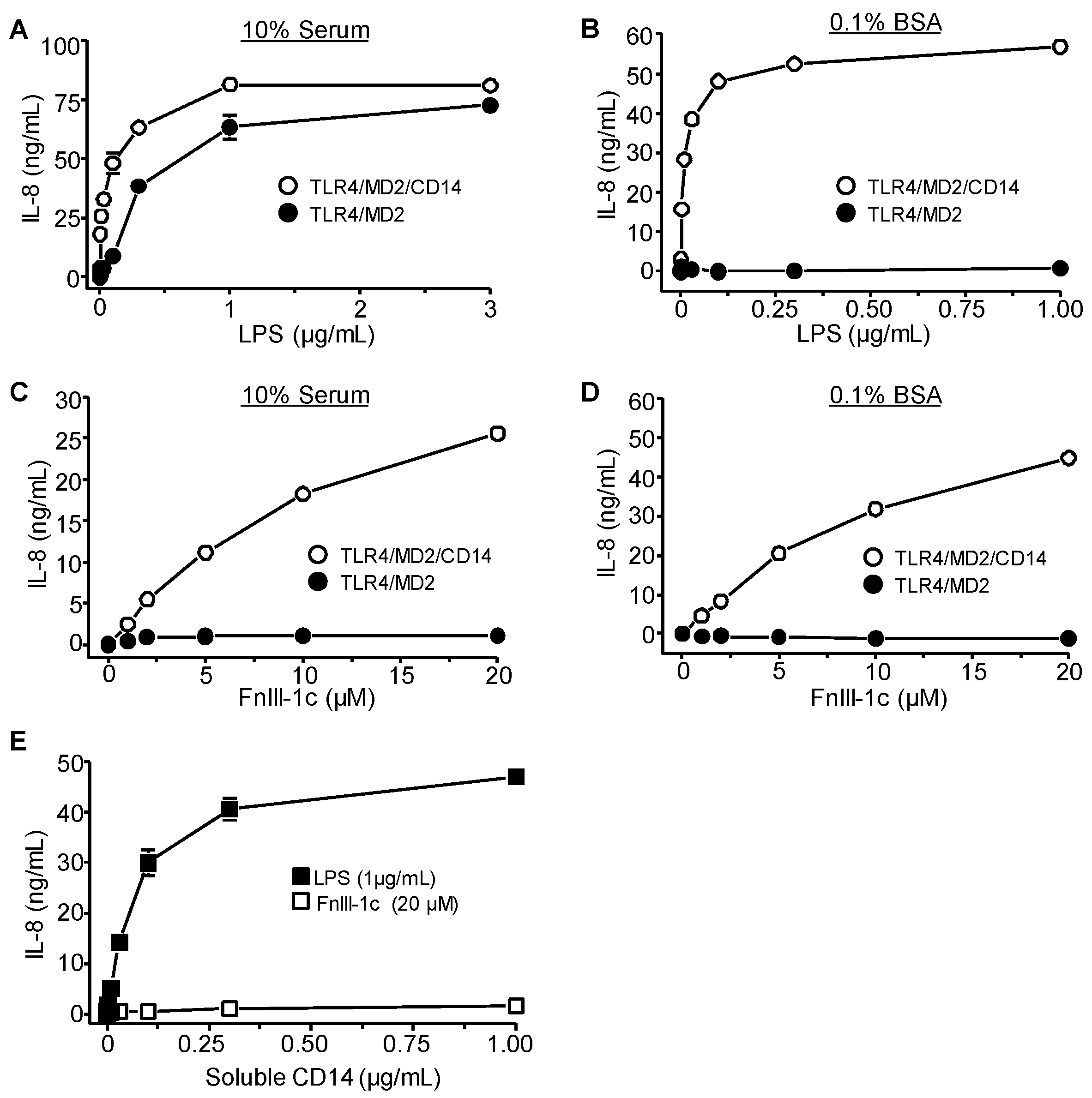
3.2. TLR4 Signaling in Response to Either FnIII-1c or LPS Depends on MD2 and CD14
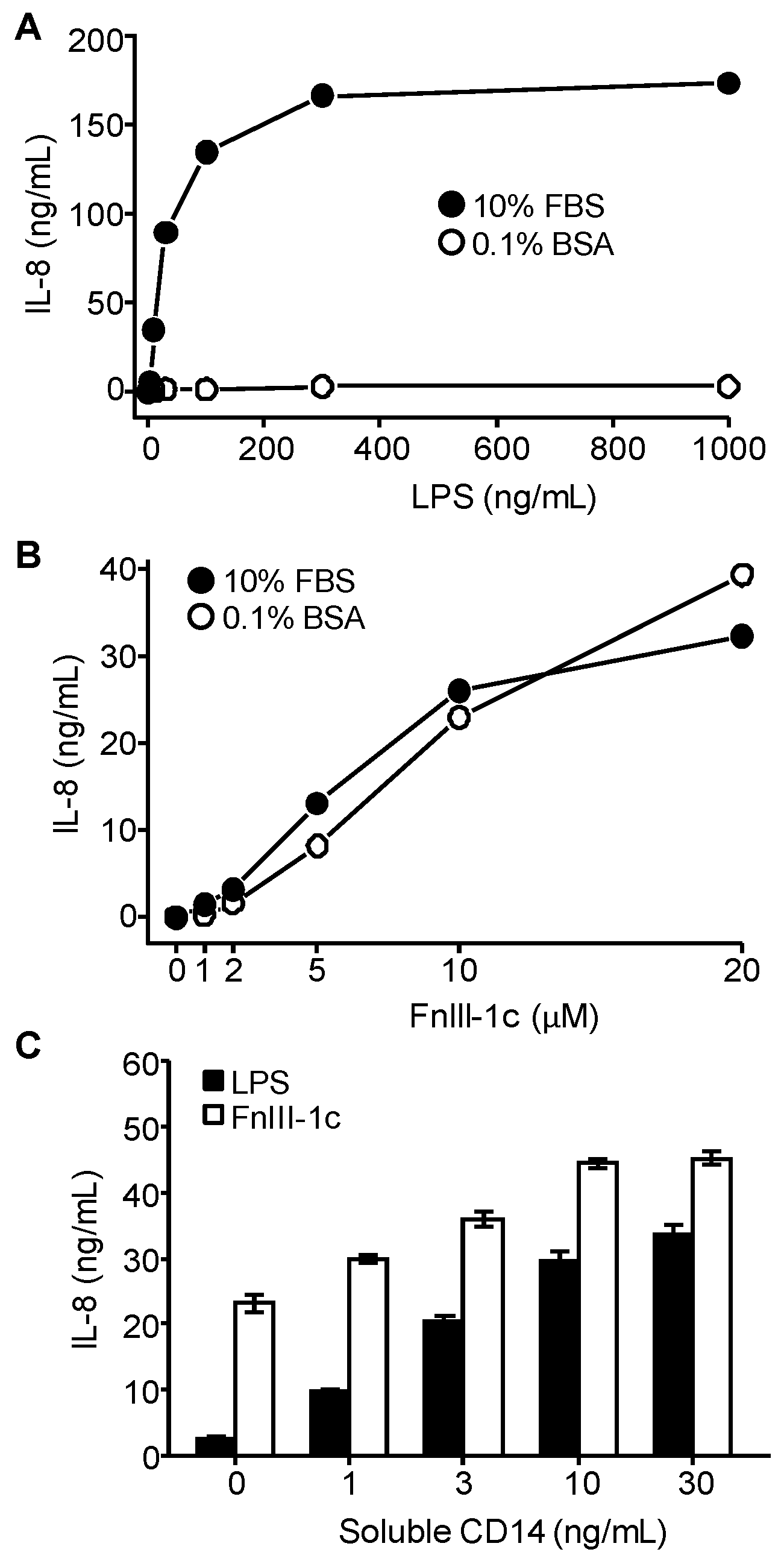
3.3. IL-8 Induction by LPS Is Serum-Dependent in Dermal Fibroblasts
3.4. IL-8 Induction by FnIII-1c Requires Membrane-Bound CD14 in Dermal Fibroblasts
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Broders-Bondon, F.; Nguyen Ho-Bouldoires, T.H.; Fernandez-Sanchez, M.E.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Insua-Rodriguez, J.; Oskarsson, T. The extracellular matrix in breast cancer. Adv. Drug. Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tivari, S.; Lu, H.; Disgupta, T.; De Lorenzo, M.S.; Wieder, R. Reawakening of dormant estroge-dependent human breast cancer cells by bone marrow stroma secretory senescence. Cell Commun. Signal. 2018, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Hielscher, A. Fibronectin: How its aberrant expression in tumors may improve therapeutic targeting. J.Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef]
- Maurer, L.M.; Ma, W.; Mosher, D.F. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem. Mol. Biol. 2015, 51, 213–227. [Google Scholar] [CrossRef]
- Klotzsch, E.; Smith, M.L.; Kubow, K.E.; Muntwyler, S.; Little, W.C.; Beyeler, F.; Gourdon, D.; Nelson, B.J.; Vogel, V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl. Acad. Sci. USA 2009, 106, 18267–18272. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Gourdon, D.; Little, W.C.; Kubow, K.E.; Eguiluz, R.A.; Luna-Morris, S.; Vogel, V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007, 5, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Nicosia, J.; Larouche, J.; Zhang, Y.; Bachman, H.; Brown, A.C.; Holmgren, L.; Barker, T.H. Detection of an integrin-binding mechanoswitch within fibronectin during tissue formation and fibrosis. ACS Nano 2017, 11, 7110–7117. [Google Scholar] [CrossRef]
- Chandler, E.M.; Saunders, M.P.; Yoon, C.J.; Gourdon, D.; Fischbach, C. Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys. Biol. 2011, 8, 015008. [Google Scholar] [CrossRef]
- Wang, K.; Andresen Eguiluz, R.C.; Wu, F.; Seo, B.R.; Fischbach, C.; Gourdon, D. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials 2015, 54, 63–71. [Google Scholar] [CrossRef]
- Gao, M.; Craig, D.; Lequin, O.; Campbell, I.D.; Vogel, V.; Schulten, K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc. Natl. Acad. Sci. USA 2003, 100, 14784–14789. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Zheng, M.; McKeown-Longo, P.J. The first type III repeat in fibronectin activates an inflammatory pathway in dermal fibroblasts. J. Biol. Chem. 2010, 285, 36255–36259. [Google Scholar] [CrossRef] [PubMed]
- Kelsh, R.; You, R.; Horzempa, C.; Zheng, M.; McKeown-Longo, P.J. Regulation of the innate immune response by fibronectin: Synergism between the III-1 and EDA domains. PLoS ONE 2014, 9, e102974. [Google Scholar] [CrossRef] [PubMed]
- Kelsh, R.M.; Ambesi, A.; Bertram, C.; McKeown-Longo, P.J. Integrin a4b1 and TLR4 cooperate to induce fibrotic gene expression in response to fibronectin’s EDA domain. J. Investig. Dermatol. 2017, 137, 2505–2512. [Google Scholar] [CrossRef]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G.; Freeze, H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. TLR4 as the mammalian endotoxin sensor. Curr. Top. Microbiol. Immunol. 2002, 270, 109–120. [Google Scholar]
- Gerwirtz, A.T. Intestinal epithelial toll-like receptors: To protect. And serve? Curr. Pharm. Des. 2003, 9, 1–5. [Google Scholar] [CrossRef]
- Hasan, U.A.; Trinchieri, G.; Vlach, J. Toll-like receptor signaling stimulates cell cycle entry and progress in fibroblasts. J. Biol. Chem. 2005, 280, 20620–20627. [Google Scholar] [CrossRef]
- Zeuke, S.; Ulmer, A.J.; Kusumoto, S.; Katus, H.A.; Heine, H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovasc. Res. 2002, 56, 126–134. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Cremer, I. Toll-like receptor stimulation in cancer: A pro- and anti-tumor double-edged sword. Immunobiology 2017, 222, 89–100. [Google Scholar] [CrossRef]
- Terhorst, D.; Kalali, B.N.; Ollert, M.; Ring, J.; Mempel, M. The role of toll-like receptors in host defenses and their relevance to dermatologic diseases. Am. J. Clin. Dermatol. 2010, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharaide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.; Zanoi, I. Toll-like receptor co-receptors as master regulators of the immune response. Mol. Immunol. 2015, 63, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Bazil, V.; Strominger, J.L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J. Immunol. 1991, 147, 1567–1574. [Google Scholar]
- Pahwa, R.; Devaraj, S.; Jialal, I. The effect of the accessory proteins, soluble CD14 and lipopolysaccharide-binding protein on Toll-like receptor 4 activity in human monocytes and adipocytes. Int. J. Obes. 2016, 2016, 907–911. [Google Scholar] [CrossRef]
- Kelsh, R.M.; McKeown-Longo, P.J. Topographical changes in extracellular matrix: Activation of TLR4 signaling and solid tumor progression. Trends Cancer Res. 2013, 9, 1–13. [Google Scholar]
- Roedig, H.; Damiescu, R.; Zeng-Brouwers, J.; Kutija, I.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Varga, J. Emerging roles of innate immune signaling and toll-like receptors in fibrosis and systemic sclerosis. Curr. Rheumatol. Rep. 2015, 17, 474. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Hocking, D.C.; Sottile, J.; McKeown-Longo, P. Activation of distinct a5 b1-mediated signaling pathways by fibronectin’s cell adhesion and matrix assembly domains. J. Cell Biol. 1998, 141, 241–253. [Google Scholar] [CrossRef]
- Klein, R.M.; Zheng, M.; Ambesi, A.; van de Water, L.; McKeown-Longo, P.J. Stimulation of extracellular matrix remodeling by the first type III repeat in fibronectin. J. Cell Sci. 2003, 116, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Read, M.A.; Cordle, S.R.; Veach, R.A.; Carlisle, C.D.; Hawiger, J. Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharide inhuman endothelial cells. Proc. Natl. Acad. Sci. USA 1993, 90, 9887–9891. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Varga, J. Endogenous ligands of TLR4 promote unresolving tissue fibrosis: Implications for systemic sclerosis and its targeted therapy. Immunol. Lett. 2018, 195, 9–17. [Google Scholar] [CrossRef]
- Turner, N.A. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J. Mol. Cell Carciol. 2016, 94, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Pathak, C. Human toll-like receptor 4 (hTLR4): Structural and functional dynamics in cancer. Int. J. Biol. Macromol. 2019, 122, 425–451. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Symmons, M.; Gay, N.J. Toll-like receptor signalling through macromolecular protein complexes. Mol. Immunol. 2015, 63, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E.; Gay, N.J.; Heymans, S.; Sacre, S.; Schaefer, L.; Midwood, K.S. Advances in Toll-like receptor biology: Modes of activation by dierse stimuli. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 359–379. [Google Scholar] [CrossRef]
- Taylor, K.R.; Trowbridge, J.M.; Rudisill, J.A.; Termeer, C.C.; Simon, J.C.; Gallo, R.L. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 2004, 279, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Zulliani-Alvarez, L.; Lim, J.M.P.; Midwood, K.S. Distinct microenvironment cues stimulate divergent TLR4-mediated signaling pathways in macrophages. Sci. Signal. 2016, 9, ra86. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Kagan, J.C. A cross discplinary perspective on the innate immune response to bacterial lipopolysaccharide. Mol. Cell 2014, 54, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Hocking, D.C.; Sottile, J.; McKeown-Longo, P.J. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J. Biol. Chem. 1994, 269, 19183–19187. [Google Scholar] [PubMed]
- Zhong, C.; Chrzanowska-Wodnicka, M.; Brown, J.; Shaub, A.; Belkin, A.M.; Burridge, K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 1998, 141, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Kubow, K.E.; Vukmirovic, R.; Zhe, L.; Kotzsch, E.; Smith, M.L.; Gourdon, D.; Luna, S.; Vogel, V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun. 2015, 6, 8026. [Google Scholar] [CrossRef]
- Mitsi, M.; Hong, Z.; Costello, C.E.; Nugent, M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry 2006, 45, 10319–10328. [Google Scholar] [CrossRef]
- Ortiz Franyuti, D.O.; Mitsi, M.; Vogel, V. Mechanical stretching of fibronectin fibers upregulates binding of Interleukin-7. Nano Lett. 2018, 18, 15–25. [Google Scholar] [CrossRef]
- Chabria, M.; Hertig, S.; Smith, M.L.; Vogel, V. Stretching fibronectin fibres disrupts binding of bacterial adhesins by physicially destroying an epitope. Nat. Commun. 2010, 1, 135. [Google Scholar] [CrossRef]
- Zheng, M.; Jones, D.M.; Horzempa, C.; Prasad, A.; McKeown-Longo, P.M. The first type III domain of fibronectin is associated with the expression of cytokines within the lung tumor microenvironment. J. Cancer 2011, 2, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Liu, S.; Wicha, M.S. Breast cancedr stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Investig. 2011, 121, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Hamilton, D.H.; Dominguez, C.; David, J.M.; McCampbell, K.K.; Palena, C. IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib. Oncotarget 2016, 7, 42031–42044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-N.; Chang, T.H.; Tsai, M.-F.; Wu, S.-G.; Tsai, T.-H.; Chen, H.-Y.; Yu, S.-L.; Yang, J.C.H.; Shih, J.-Y. IL-8 confers resistance to EGFR inhibitors by inducing stem cell properties in lung cancer. Oncotarget 2015, 6, 10415. [Google Scholar] [CrossRef]
- Britschgi, A.; Radimerski, T.; Bentires-Ali, M. Targeting PI3K, HER2 and the Il-8/JAK2 axis in metastatic breast cancer: Which combination makes the whole greater tahn the sum of its parts? Drug Resist. Updates 2013, 16, 68–72. [Google Scholar] [CrossRef]
- Fu, X.; Jesselsohn, R.; Pereira, R.; Hollingsworth, E.F.; Creighton, C.J.; Li, F.; Shea, M.; Nardone, A.; De Angelis, C.; Heiser, L.M.; et al. FOXA1 overexpression mediated endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6600–E6609. [Google Scholar] [CrossRef]
- Imafuji, H.; Matsuo, Y.; Ueda, G.; Omi, K.; Hayashi, Y.; Saito, K.; Tsuboi, K.; Morimoto, M.; Koide, S.; Ogawa, R.; et al. Acquisition of gemcitabine resistance enhances angiogenesis via upregulation of IL-8 production in pancreastic cancer. Oncol. Rep. 2019, 41, 3508–3516. [Google Scholar]
- Schinke, C.; Giricz, O.; Li, W.; Shastri, A.; Barreyro, L.; Bhagat, T.; Bhattacharyya, S.; Ramachandra, N.; Bartenstein, M.; Pellagatti, S. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood 2015, 125, 3144–3152. [Google Scholar] [CrossRef]
- Balla, M.M.; Desai, S.; Purwar, P.; Kuman, A.; Bhandarkar, P.; Shejul, Y.K.; Pramesh, C.S.; Laskar, S.; Pandey, B.N. Differential diagnosis of lung cancer, its metastasis and chronic obstructive pulmonary disease based on serum VEGF, IL-8 and MMP-9. Sci. Rep. 2016, 6, 36065. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo. Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Ambesi, A.; J. McKeown-Longo, P. Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin. Cells 2020, 9, 216. https://doi.org/10.3390/cells9010216
Zheng M, Ambesi A, J. McKeown-Longo P. Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin. Cells. 2020; 9(1):216. https://doi.org/10.3390/cells9010216
Chicago/Turabian StyleZheng, Mingzhe, Anthony Ambesi, and Paula J. McKeown-Longo. 2020. "Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin" Cells 9, no. 1: 216. https://doi.org/10.3390/cells9010216
APA StyleZheng, M., Ambesi, A., & J. McKeown-Longo, P. (2020). Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin. Cells, 9(1), 216. https://doi.org/10.3390/cells9010216




