Erebosis of Neurons May Exist in the Brain with Alzheimer’s Disease
Abstract
Highlights
- What are the main findings?
- Erebosis may be present in the neurons of mammalian brains and may be increased with aging and Alzheimer’s disease neuropathology.
- Phospho-tau may be a stimulus to induce erebosis in the brain.
- What is the implication of the main findings?
- Reducing erebosis may be a strategy to decrease cell loss/death in the brain of patients with Alzheimer’s disease.
- Erebosis may be a form of phospho-tau-induced neurotoxicity.
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals
2.2. Human Brain Sections
2.3. Tau Preformed Fibrils
2.4. Stereotaxic Surgery
2.5. Brain Harvesting
2.6. Immunohistochemical Staining
2.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.8. Quantification of Immunohistochemistry
2.9. Statistical Analysis
3. Results
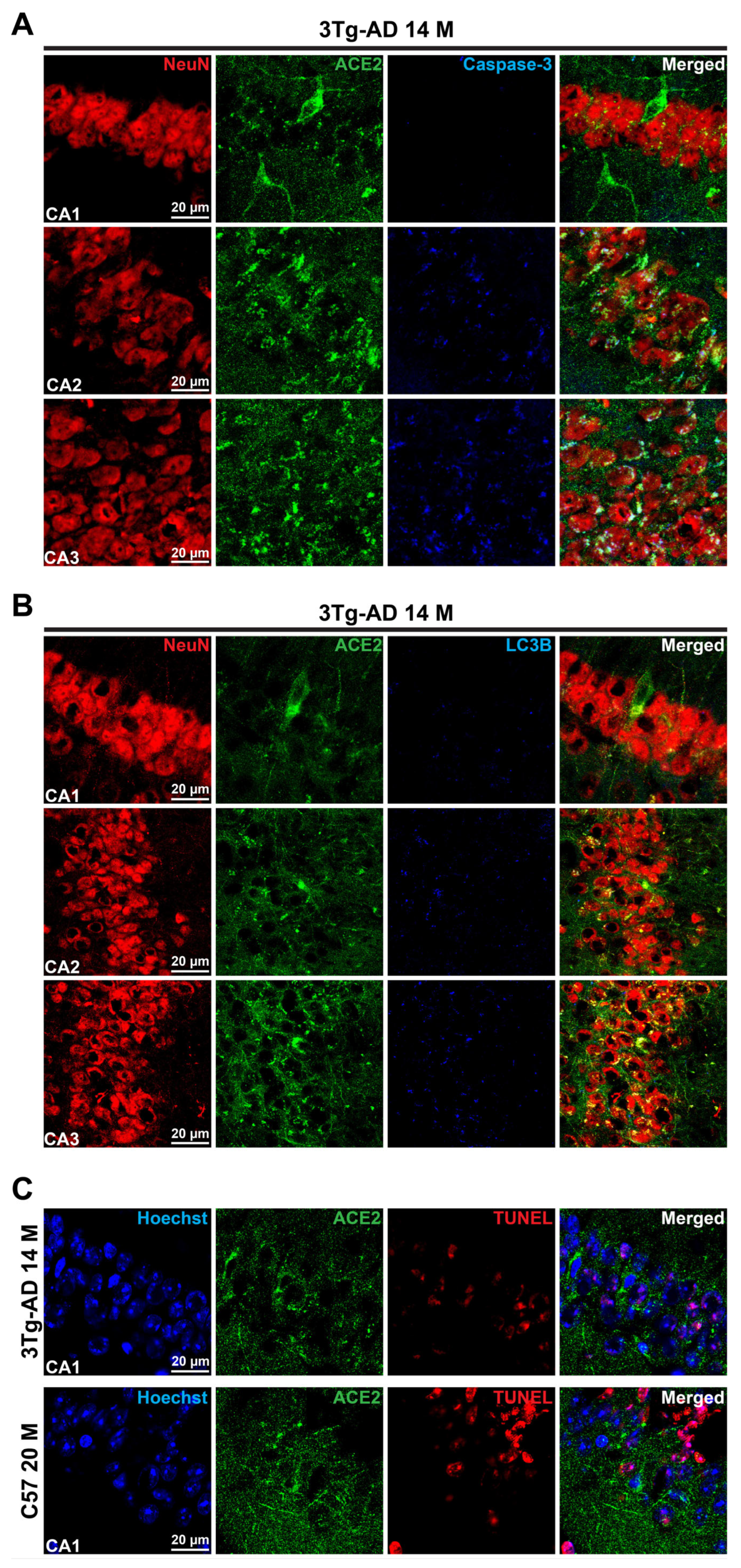
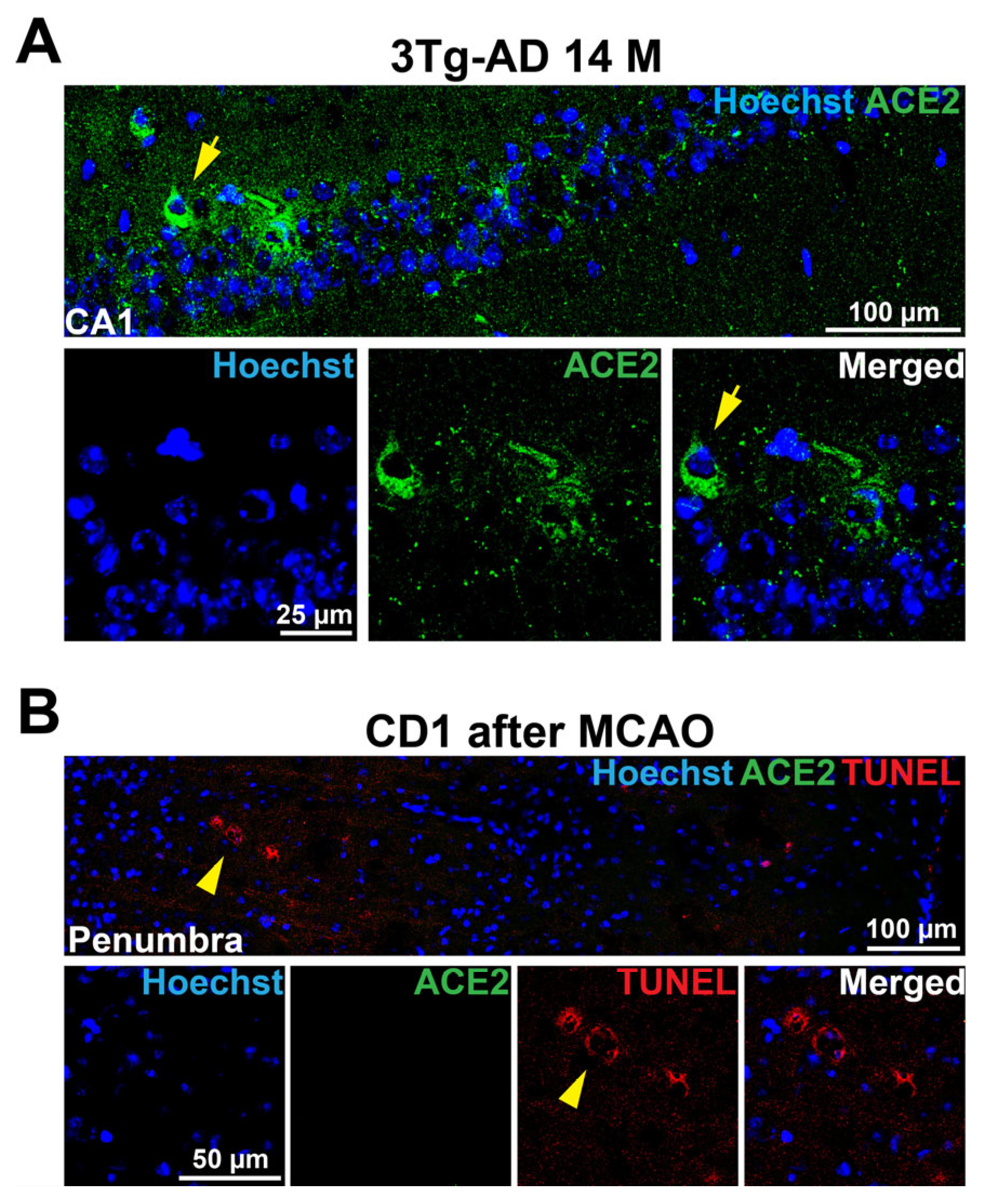
3.1. Erebosis May Exist in the Mouse Hippocampus and May Be Increased with Aging and AD Neuropathology
3.2. Phospho-Tau May Be an Inducer for Erebosis and Erebosis May Occur in the Neurons of Hippocampus
3.3. Accumulation of ACE2 May Be a Sign of Erebosis in the Brain
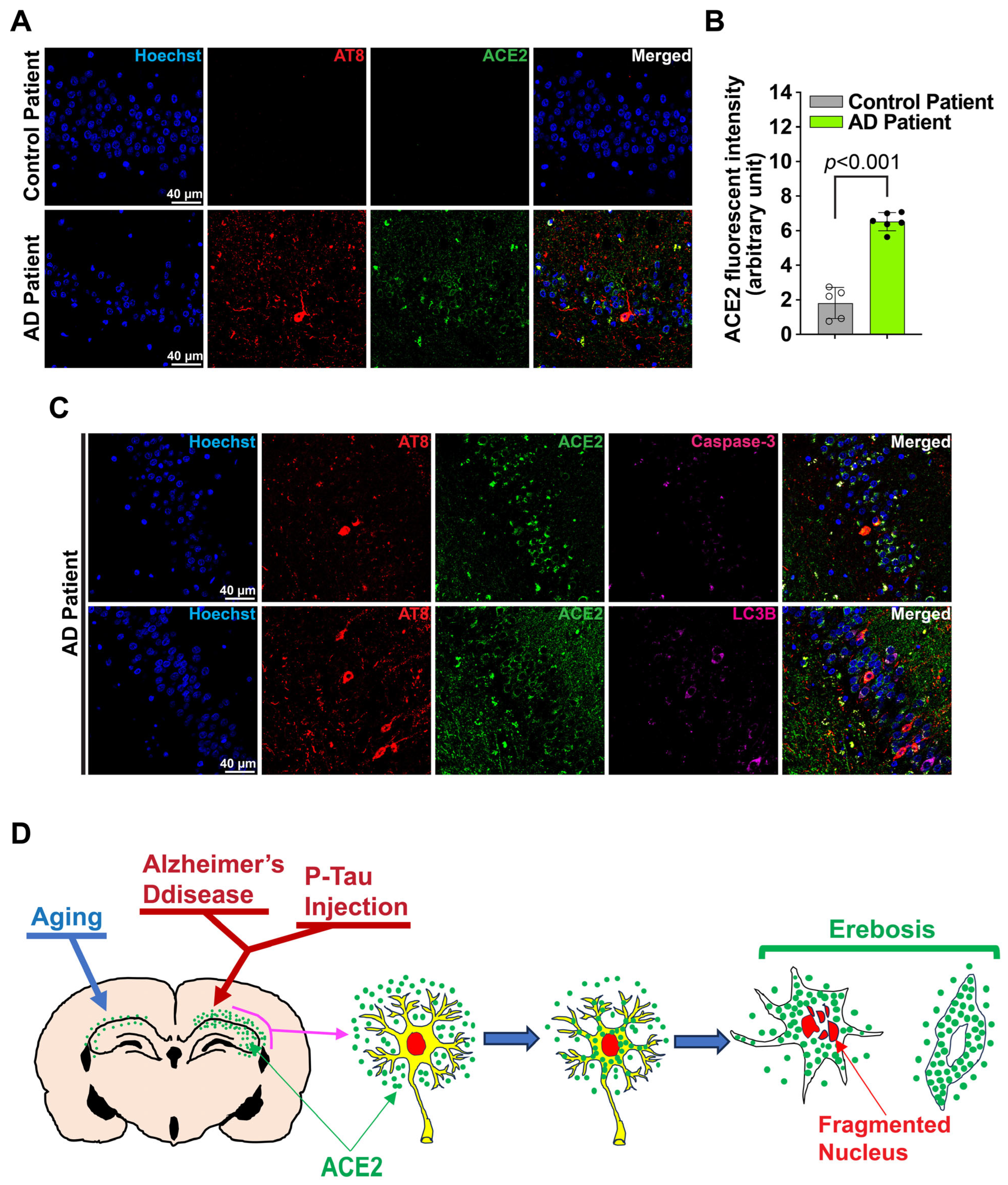
3.4. Erebosis May Occur in the Hippocampus of the Human Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, L.; Morris, J.C. Diagnosis. In Alzheimer Disease; Terry, R.D., Katzman, R., Bick, K., Eds.; Raven Press: New York, NY, USA, 1994; pp. 197–229. [Google Scholar]
- Vassar, R.; Citron, M. Abeta-generating enzymes: Recent advances in beta- and gamma-secretase research. Neuron. 2000, 27, 419–422. [Google Scholar] [CrossRef]
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. 2004, 23, 105–114. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Balusu, S.; Horre, K.; Thrupp, N.; Craessaerts, K.; Snellinx, A.; Serneels, L.; T’Syen, D.; Chrysidou, I.; Arranz, A.M.; Sierksma, A.; et al. Meg3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science 2023, 381, 1176–1182. [Google Scholar] [CrossRef]
- Keller, J.N. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing. Res. Rev. 2006, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Chen, T.; Song, S.; Hou, Y.; Feng, L.; Fan, C.; Li, M. Ferroptosis, Pyroptosis, and Cuproptosis in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3564–3587. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Ciesielski, H.M.; Nishida, H.; Takano, T.; Fukuhara, A.; Otani, T.; Ikegawa, Y.; Okada, M.; Nishimura, T.; Furuse, M.; Yoo, S.K. Erebosis, a new cell death mechanism during homeostatic turnover of gut enterocytes. PLoS Biol. 2022, 20, e3001586. [Google Scholar] [CrossRef]
- Park, W.; Wei, S.; Kim, B.S.; Kim, B.; Bae, S.J.; Chae, Y.C.; Ryu, D.; Ha, K.T. Diversity and complexity of cell death: A historical review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Li, J.; Shan, W.; Yang, J.; Zuo, Z. Endoplasmic reticulum stress-activated neuronal and microglial autophagy contributes to postoperative cognitive dysfunction in neonatal rats. Neurochem. Res. 2023, 48, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zuo, Z. Pyrrolidine dithiocarbamate ameliorates sepsis-associated encephalopathy by inhibiting autophagy and inflammation in the brain. Neurochem. Res. 2025, 50, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Sheng, W.; Zuo, Z. Metformin attenuates alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol. Biochem. Behav. 2012, 101, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, J.; Luo, Y.; Zhou, J.; Li, T.; Cao, L.; Peng, S.; Zuo, Z.; Wang, Z. Far infrared light irradiation enhances abeta clearance via increased exocytotic microglial atp and ameliorates cognitive deficit in Alzheimer’s disease-like mice. J. Neuroinflammation 2022, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Peng, K.Y.; Hu, Y.H.; Chang, C.C.; Chan, C.K.; Lai, T.S.; Lin, Y.H.; Wang, S.M.; Lu, C.C.; Liu, Y.C.; et al. Circulating plasma concentrations of ace2 in primary aldosteronism and cardiovascular outcomes. J. Clin. Endocrinol. Metab. 2022, 107, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shan, W.; Zuo, Z. Co-housing with alzheimer’s disease mice induces changes in gut microbiota and impairment of learning and memory in control mice. CNS Neurosci. Ther. 2024, 30, e14491. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of alzheimer’s disease-like pathology in 3xtg-ad mice. Aging Cell 2019, 18, e12873. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Mendell, A.L.; Creighton, S.D.; Wilson, H.A.; Jardine, K.H.; Isaacs, L.; Winters, B.D.; MacLusky, N.J. Inhibition of 5alpha reductase impairs cognitive performance, alters dendritic morphology and increases tau phosphorylation in the hippocampus of male 3xtg-ad mice. Neurosci. 2020, 429, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Grasl-Kraupp, B.; Ruttkay-Nedecky, B.; Koudelka, H.; Bukowska, K.; Bursch, W.; Schulte-Hermann, R. In situ detection of fragmented DNA (tunel assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatol. 1995, 21, 1465–1468. [Google Scholar] [CrossRef]
- Li, J.; Sun, N.; Hu, S.; Zuo, Z. Chronic high fat diet-induced cerebrovascular remodeling impairs recovery of blood flow after cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2025, 45, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Reveret, L.; Leclerc, M.; Emond, V.; Tremblay, C.; Loiselle, A.; Bourassa, P.; Bennett, D.A.; Hebert, S.S.; Calon, F. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein expression of angiotensin-converting enzyme 2 (ace2) is upregulated in brains with alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef]
- Cui, H.; Su, S.; Cao, Y.; Ma, C.; Qiu, W. The altered anatomical distribution of ace2 in the brain with alzheimer’s disease pathology. Front. Cell Dev. Biol. 2021, 9, 684874. [Google Scholar] [CrossRef]
- Sugawara, T.; Fujimura, M.; Morita-Fujimura, Y.; Kawase, M.; Chan, P.H. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal ca1 neurons in rats after transient global cerebral ischemia. J. Neurosci. 1999, 19, RC39. [Google Scholar] [CrossRef]
- Zola-Morgan, S.; Squire, L.R.; Amaral, D.G. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field ca1 of the hippocampus. J. Neurosci. 1986, 6, 2950–2967. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Tremblay, E.; Ottersen, O.P.; Meldrum, B.S. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980, 191, 79–97. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Ji, S.G.; Yin, H.Z.; Weiss, J.H. Differential vulnerability of ca1 versus ca3 pyramidal neurons after ischemia: Possible relationship to sources of Zn2+ accumulation and its entry into and prolonged effects on Mitochondria. J. Neurosci. 2017, 37, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef]
- Liu, D.; Yang, R.; Yan, X.; McAdoo, D.J. Hydroxyl radicals generated in vivo kill neurons in the rat spinal cord: Electrophysiological, histological, and neurochemical results. J. Neurochem. 1994, 62, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain renin-angiotensin system and microglial polarization: Implications for aging and neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, R.; Evans, C.E.; Chai, S.Y.; Good, M.A.; Kehoe, P.G.; Miners, J.S. Age-related reduction in brain ace-2 is not exacerbated by alzheimer’s disease pathology in mouse models of Alzheimer’s disease. Aging Brain 2023, 3, 100062. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Zhao, H.; Peng, S.; Zuo, Z. Chronic high fat diet induces cardiac hypertrophy and fibrosis in mice. Metabolism 2015, 64, 917–925. [Google Scholar] [CrossRef]
- Caponio, D.; Veverova, K.; Zhang, S.Q.; Shi, L.; Wong, G.; Vyhnalek, M.; Fang, E.F. Compromised autophagy and mitophagy in brain ageing and alzheimer’s diseases. Aging Brain 2022, 2, 100056. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zuo, Z. Erebosis of Neurons May Exist in the Brain with Alzheimer’s Disease. Cells 2025, 14, 1546. https://doi.org/10.3390/cells14191546
Li J, Zuo Z. Erebosis of Neurons May Exist in the Brain with Alzheimer’s Disease. Cells. 2025; 14(19):1546. https://doi.org/10.3390/cells14191546
Chicago/Turabian StyleLi, Jun, and Zhiyi Zuo. 2025. "Erebosis of Neurons May Exist in the Brain with Alzheimer’s Disease" Cells 14, no. 19: 1546. https://doi.org/10.3390/cells14191546
APA StyleLi, J., & Zuo, Z. (2025). Erebosis of Neurons May Exist in the Brain with Alzheimer’s Disease. Cells, 14(19), 1546. https://doi.org/10.3390/cells14191546





