Calpains at the Crossroads of Spinal Cord Physiology, Plasticity, and Pathology
Abstract
1. Introduction
2. Calpains in the Spinal Cord
3. Toward a Dynamic View of Spinal Plasticity: Are Calpains the Missing Piece?
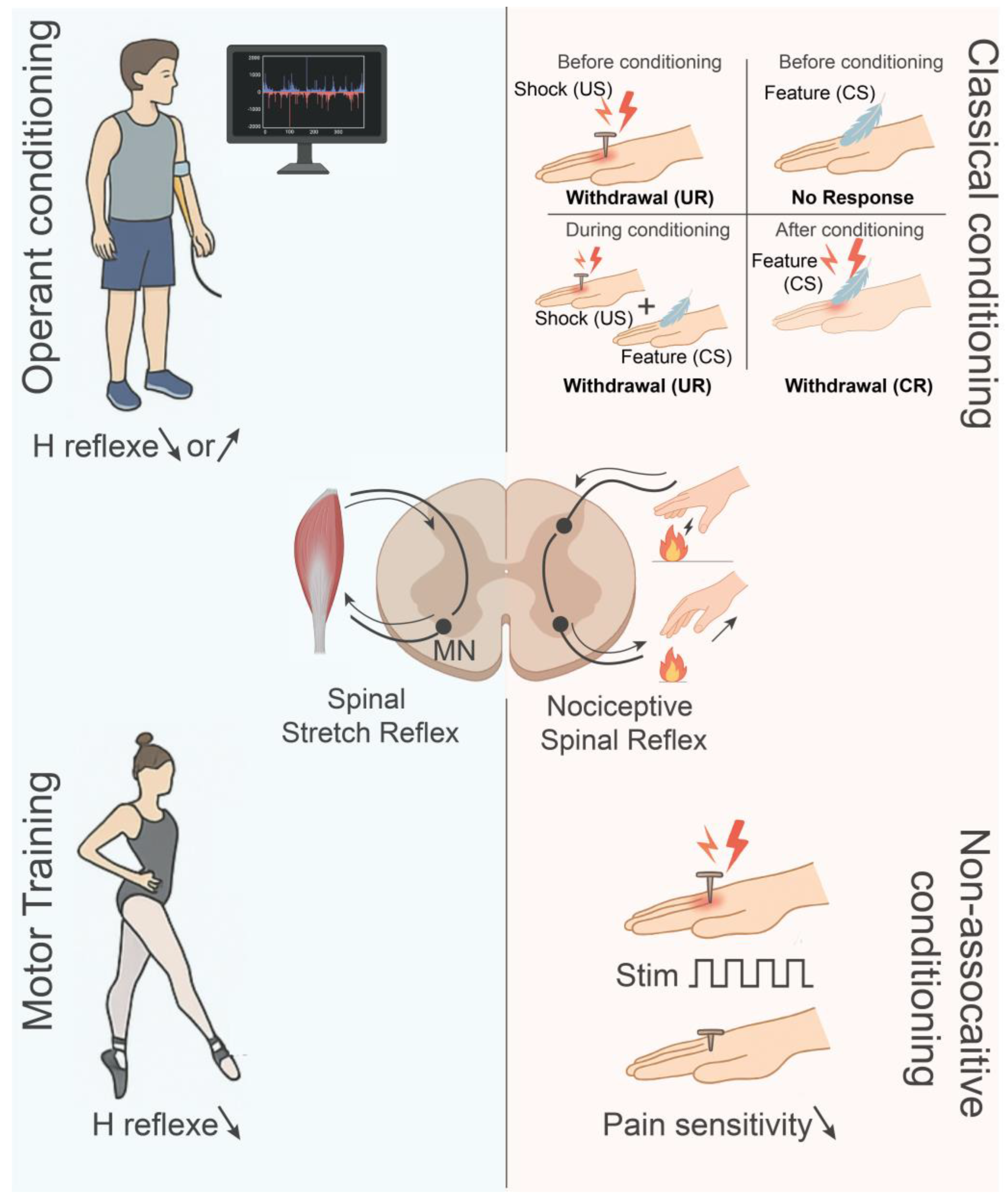
3.1. Forms and Paradigms of Spinal Plasticity
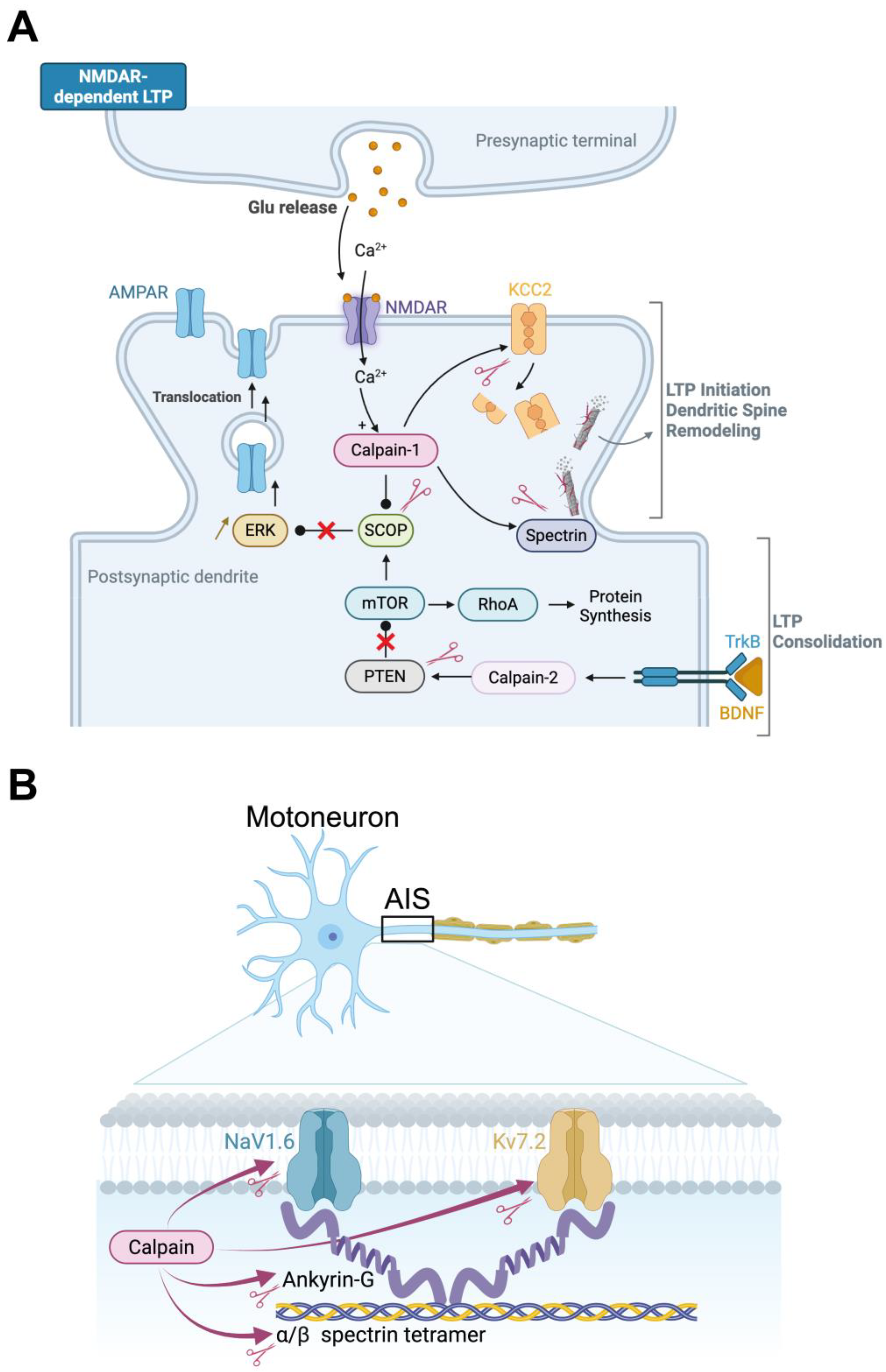
3.2. Molecular Mechanisms of Synaptic Plasticity
3.3. Calpains and Inhibitory Synaptic Plasticity
3.4. Calpains in Intrinsic Neuronal Plasticity
3.5. Interplay Between Neurotrophic Factors and Calpains
4. Breaking Down Barriers: Calpain-Driven Pathologies in the Spinal Cord
4.1. Spinal Cord Injury and Spasticity
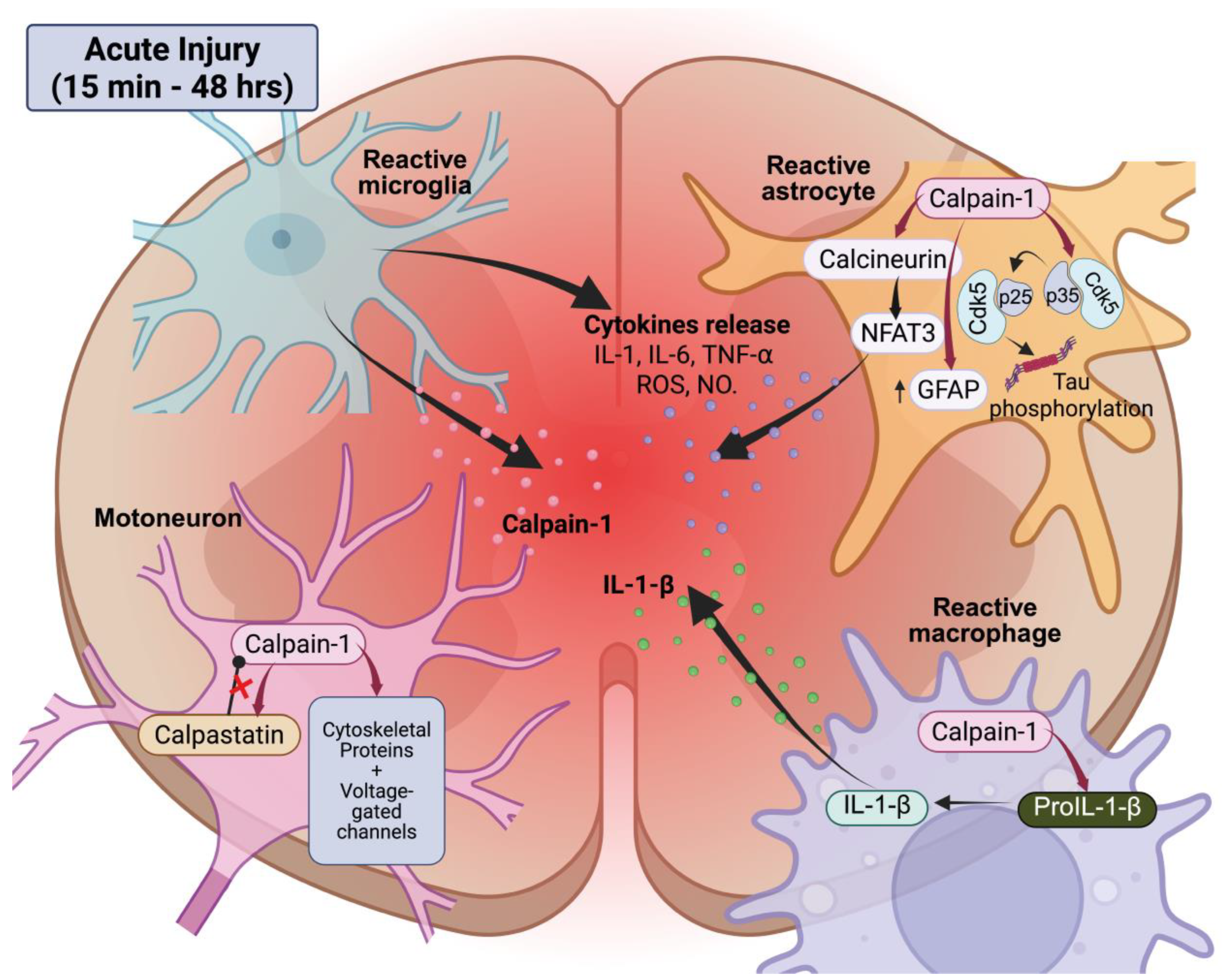
4.1.1. Acute Calpain Activation and Early Pathological Events
4.1.2. Chronic Calpain Activity and Functional Consequences
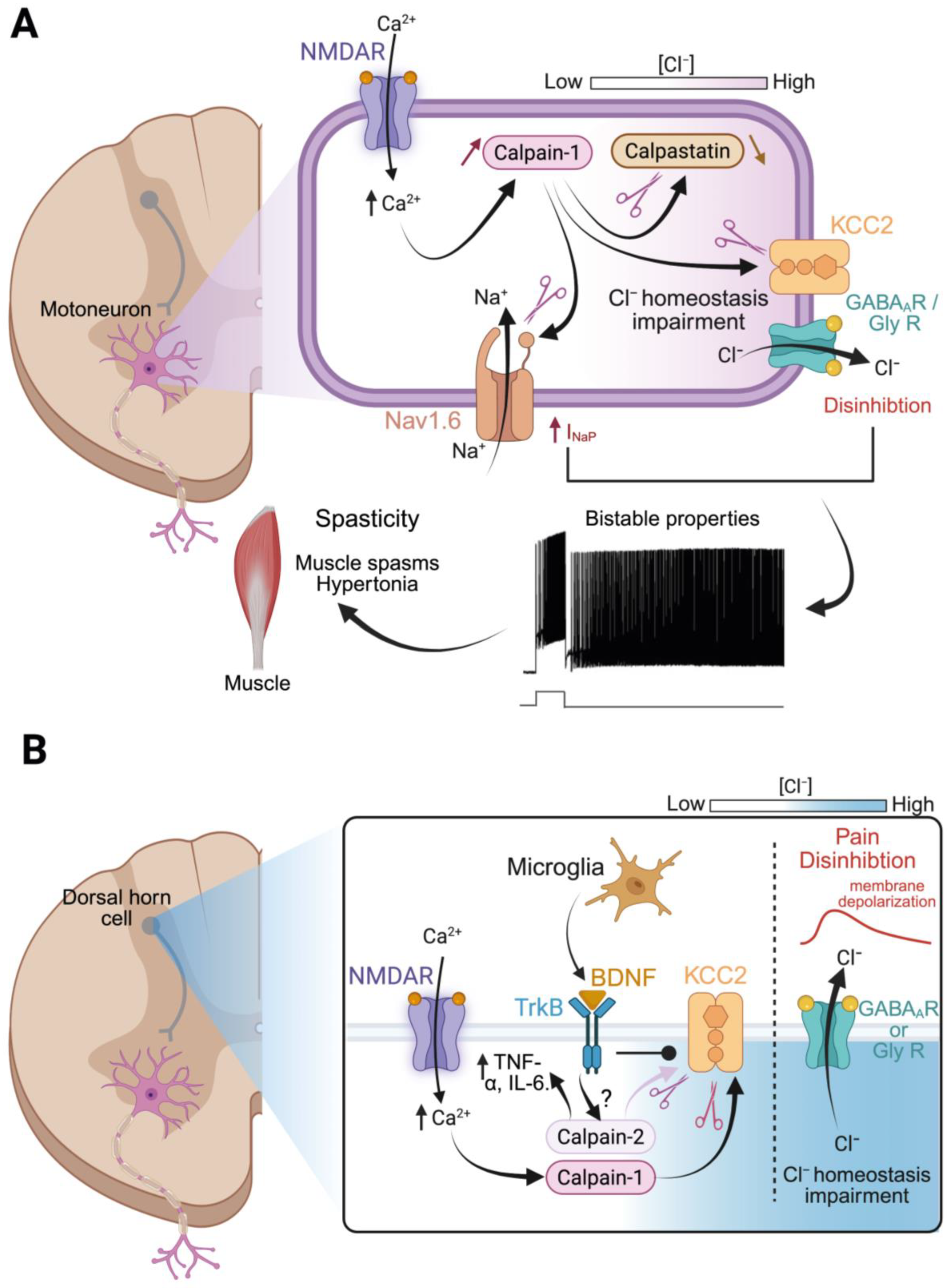
4.1.3. Mechanistic Links to Spasticity and Circuit Remodeling
4.2. Spinal Cord Injury and Pain
4.2.1. Calpain, KCC2, and Disinhibition
4.2.2. Calpains, Neuroimmune Interactions, and Modulation of Pain
4.3. Neurodegenerative Diseases
4.3.1. Amyotrophic Lateral Sclerosis
4.3.2. Multiple Sclerosis
5. Calpain Fingerprints as Biomarkers in Spinal Cord Disorders
5.1. Spectrin and GFAP Breakdown Fragments
5.2. Next-Generation Calpain-Mediated Biomarkers: Toward Disease Specificity
5.2.1. Traumatic Injury and Spasticity
5.2.2. Amyotrophic Lateral Sclerosis
5.2.3. Multiple Sclerosis
6. Disarming Calpain: Genetic and Pharmacological Tools for Spinal Cord Repair
6.1. Pharmacological Approaches
6.2. Gene Therapy
6.2.1. Calpastatin Overexpression: Broad-Spectrum Inhibition of CNS Calpains
6.2.2. Isoform-Specific Knockdown Approaches
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E.; Molinari, M. Calpain: A protease in search of a function? Biochem. Biophys. Res. Commun. 1998, 247, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.C.; Wang, K.K. Calpain in the CNS: From synaptic function to neurotoxicity. Sci. Signal 2008, 1, re1. [Google Scholar] [CrossRef]
- Wu, H.Y.; Lynch, D.R. Calpain and synaptic function. Mol. Neurobiol. 2006, 33, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Bi, X. Revisiting the calpain hypothesis of learning and memory 40 years later. Front. Mol. Neurosci. 2024, 17, 1337850. [Google Scholar] [CrossRef]
- Baudry, M.; Bi, X. Learning and memory: An emergent property of cell motility. Neurobiol. Learn. Mem. 2013, 104, 64–72. [Google Scholar] [CrossRef]
- Yamashima, T. Reconsider Alzheimer’s disease by the ‘calpain-cathepsin hypothesis’—A perspective review. Prog. Neurobiol. 2013, 105, 1–23. [Google Scholar] [CrossRef]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef]
- Ray, S.K.; Matzelle, D.D.; Sribnick, E.A.; Guyton, M.K.; Wingrave, J.M.; Banik, N.L. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J. Chem. Neuroanat. 2003, 26, 119–124. [Google Scholar] [CrossRef]
- Baudry, M.; Bi, X. Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration. Trends Neurosci. 2016, 39, 235–245. [Google Scholar] [CrossRef]
- Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.C.; Ray, S.K.; Gantt-Wilford, G.; Banik, N.L. Calpain expression varies among different rat and bovine central nervous system regions. J. Neurosci. Res. 1998, 53, 482–489. [Google Scholar] [CrossRef]
- Kumamoto, T.; Kleese, W.C.; Cong, J.Y.; Goll, D.E.; Pierce, P.R.; Allen, R.E. Localization of the Ca2+-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat. Rec. 1992, 232, 60–77. [Google Scholar] [CrossRef]
- Lane, R.D.; Allan, D.M.; Mellgren, R.L. A comparison of the intracellular distribution of mu-calpain, m-calpain, and calpastatin in proliferating human A431 cells. Exp. Cell Res. 1992, 203, 5–16. [Google Scholar] [CrossRef]
- Hood, J.L.; Brooks, W.H.; Roszman, T.L. Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 2004, 279, 43126–43135. [Google Scholar] [CrossRef]
- Hood, J.L.; Logan, B.B.; Sinai, A.P.; Brooks, W.H.; Roszman, T.L. Association of the calpain/calpastatin network with subcellular organelles. Biochem. Biophys. Res. Commun. 2003, 310, 1200–1212. [Google Scholar] [CrossRef]
- Garcia, M.; Bondada, V.; Geddes, J.W. Mitochondrial localization of mu-calpain. Biochem. Biophys. Res. Commun. 2005, 338, 1241–1247. [Google Scholar] [CrossRef]
- Saido, T.C.; Shibata, M.; Takenawa, T.; Murofushi, H.; Suzuki, K. Positive regulation of mu-calpain action by polyphosphoinositides. J. Biol. Chem. 1992, 267, 24585–24590. [Google Scholar] [CrossRef]
- Kamakura, K.; Ishiura, S.; Imajoh, S.; Nagata, N.; Sugita, H. Distribution of calcium-activated neutral protease inhibitor in the central nervous system of the rat. J. Neurosci. Res. 1992, 31, 543–548. [Google Scholar] [CrossRef]
- Li, J.; Grynspan, F.; Berman, S.; Nixon, R.; Bursztajn, S. Regional differences in gene expression for calcium activated neutral proteases (calpains) and their endogenous inhibitor calpastatin in mouse brain and spinal cord. J. Neurobiol. 1996, 30, 177–191. [Google Scholar] [CrossRef]
- Shinkai-Ouchi, F.; Shindo, M.; Doi, N.; Hata, S.; Ono, Y. Calpain-2 participates in the process of calpain-1 inactivation. Biosci. Rep. 2020, 40, BSR20200552. [Google Scholar] [CrossRef]
- Mendell, L.M. Modifiability of spinal synapses. Physiol. Rev. 1984, 64, 260–324. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Tennissen, A.M. Activity-dependent spinal cord plasticity in health and disease. Annu. Rev. Neurosci. 2001, 24, 807–843. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.W. Learning from the spinal cord: How the study of spinal cord plasticity informs our view of learning. Neurobiol. Learn. Mem. 2014, 108, 155–171. [Google Scholar] [CrossRef]
- Wolpaw, J.R. The negotiated equilibrium model of spinal cord function. J. Physiol. 2018, 596, 3469–3491. [Google Scholar] [CrossRef]
- Christiansen, L.; Lundbye-Jensen, J.; Perez, M.A.; Nielsen, J.B. How plastic are human spinal cord motor circuitries? Exp. Brain Res. 2017, 235, 3243–3249. [Google Scholar] [CrossRef]
- Bertrand, S.S.; Cazalets, J.R. Activity-dependent synaptic plasticity and metaplasticity in spinal motor networks. Curr. Pharm. Des. 2013, 19, 4498–4508. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Braitman, D.J.; Seegal, R.F. Adaptive plasticity in primate spinal stretch reflex: Initial development. J. Neurophysiol. 1983, 50, 1296–1311. [Google Scholar] [CrossRef]
- Wolpaw, J.R. The complex structure of a simple memory. Trends Neurosci. 1997, 20, 588–594. [Google Scholar] [CrossRef]
- Evatt, M.L.; Wolf, S.L.; Segal, R.L. Modification of human spinal stretch reflexes: Preliminary studies. Neurosci. Lett. 1989, 105, 350–355. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wolpaw, J.R. Operant conditioning of H-reflex in freely moving rats. J. Neurophysiol. 1995, 73, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Carp, J.S.; Tennissen, A.M.; Chen, X.Y.; Wolpaw, J.R. H-reflex operant conditioning in mice. J. Neurophysiol. 2006, 96, 1718–1727. [Google Scholar] [CrossRef]
- Thompson, A.K.; Chen, X.Y.; Wolpaw, J.R. Acquisition of a simple motor skill: Task-dependent adaptation plus long-term change in the human soleus H-reflex. J. Neurosci. 2009, 29, 5784–5792. [Google Scholar] [CrossRef]
- Wolpaw, J.R. What can the spinal cord teach us about learning and memory? Neuroscientist 2010, 16, 532–549. [Google Scholar] [CrossRef]
- Thompson, A.K.; Wolpaw, J.R. Restoring walking after spinal cord injury: Operant conditioning of spinal reflexes can help. Neuroscientist 2015, 21, 203–215. [Google Scholar] [CrossRef]
- Durkovic, R.G.; Prokowich, L.J. D-2-amino-5-phosphonovalerate, and NMDA receptor antagonist, blocks induction of associative long-term potentiation of the flexion reflex in spinal cat. Neurosci. Lett. 1998, 257, 162–164. [Google Scholar] [CrossRef]
- Madden, V.J.; Bellan, V.; Russek, L.N.; Camfferman, D.; Vlaeyen, J.W.S.; Moseley, G.L. Pain by Association? Experimental Modulation of Human Pain Thresholds Using Classical Conditioning. J. Pain 2016, 17, 1105–1115. [Google Scholar] [CrossRef]
- Groves, P.M.; Thompson, R.F. Habituation: A dual-process theory. Psychol. Rev. 1970, 77, 419–450. [Google Scholar] [CrossRef]
- Ji, R.R.; Kohno, T.; Moore, K.A.; Woolf, C.J. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci. 2003, 26, 696–705. [Google Scholar] [CrossRef]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef]
- Rossignol, S.; Frigon, A.; Barrière, G.; Martinez, M.; Barthélemy, D.; Bouyer, L.; Bélanger, M.; Provencher, J.; Chau, C.; Brustein, E.; et al. Chapter 16--spinal plasticity in the recovery of locomotion. Prog. Brain Res. 2011, 188, 229–241. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Roy, R.R. Robotic training and spinal cord plasticity. Brain Res. Bull. 2009, 78, 4–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Leon, R.D.; Kubasak, M.D.; Phelps, P.E.; Timoszyk, W.K.; Reinkensmeyer, D.J.; Roy, R.R.; Edgerton, V.R. Using robotics to teach the spinal cord to walk. Brain Res. Brain Res. Rev. 2002, 40, 267–273. [Google Scholar] [CrossRef]
- Leblond, H.; L’Esperance, M.; Orsal, D.; Rossignol, S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 2003, 23, 11411–11419. [Google Scholar] [CrossRef]
- Houle, J.D.; Côté, M.P. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann. N. Y. Acad. Sci. 2013, 1279, 154–163. [Google Scholar] [CrossRef]
- Hornby, T.G.; Zemon, D.H.; Campbell, D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys. Ther. 2005, 85, 52–66. [Google Scholar] [CrossRef]
- Harkema, S.J.; Schmidt-Read, M.; Lorenz, D.J.; Edgerton, V.R.; Behrman, A.L. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1508–1517. [Google Scholar] [CrossRef]
- Stevenson, V.; Gras, A.; Bardos, J.; Broughton, J. The high cost of spasticity in multiple sclerosis to individuals and society. Mult. Scler. 2015, 21, 1583–1592. [Google Scholar] [CrossRef]
- Courtine, G.; Gerasimenko, Y.; van den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef]
- Khalki, L.; Sadlaoud, K.; Lerond, J.; Coq, J.O.; Brezun, J.M.; Vinay, L.; Coulon, P.; Bras, H. Changes in innervation of lumbar motoneurons and organization of premotor network following training of transected adult rats. Exp. Neurol. 2018, 299, 1–14. [Google Scholar] [CrossRef]
- Cote, M.P.; Gandhi, S.; Zambrotta, M.; Houle, J.D. Exercise modulates chloride homeostasis after spinal cord injury. J. Neurosci. 2014, 34, 8976–8987. [Google Scholar] [CrossRef] [PubMed]
- Caron, G.; Bilchak, J.N.; Côté, M.P. Direct evidence for decreased presynaptic inhibition evoked by PBSt group I muscle afferents after chronic SCI and recovery with step-training in rats. J. Physiol. 2020, 598, 4621–4642. [Google Scholar] [CrossRef]
- Nielsen, J.; Crone, C.; Hultborn, H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 116–121. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jun, J.; Wang, J.; Bittar, A.; Chung, K.; Chung, J.M. Induction of long-term potentiation and long-term depression is cell-type specific in the spinal cord. Pain 2015, 156, 618–625. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.R.; Chen, H.; Zhou, J.J.; Jin, D.; Pan, H.L. Theta-Burst Stimulation of Primary Afferents Drives Long-Term Potentiation in the Spinal Cord and Persistent Pain via α2δ-1-Bound NMDA Receptors. J. Neurosci. 2022, 42, 513–527. [Google Scholar] [CrossRef]
- Pockett, S.; Figurov, A. Long-term potentiation and depression in the ventral horn of rat spinal cord in vitro. Neuroreport 1993, 4, 97–99. [Google Scholar] [CrossRef]
- Malenka, R.C.; Bear, M.F. LTP and LTD: An embarrassment of riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Sweatt, J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Huganir, R.L.; Nicoll, R.A. AMPARs and synaptic plasticity: The last 25 years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef]
- Lynch, G.; Baudry, M. The biochemistry of memory: A new and specific hypothesis. Science 1984, 224, 1057–1063. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Briz, V.; Hsu, Y.T.; Bi, X.; Baudry, M. A molecular brake controls the magnitude of long-term potentiation. Nat. Commun. 2014, 5, 3051. [Google Scholar] [CrossRef]
- Liu, X.G.; Sandkühler, J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci. Lett. 1995, 191, 43–46. [Google Scholar] [CrossRef]
- Svendsen, F.; Tjølsen, A.; Hole, K. AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. Neuroreport 1998, 9, 1185–1190. [Google Scholar] [CrossRef]
- Grau, J.W.; Crown, E.D.; Ferguson, A.R.; Washburn, S.N.; Hook, M.A.; Miranda, R.C. Instrumental learning within the spinal cord: Underlying mechanisms and implications for recovery after injury. Behav. Cogn. Neurosci. Rev. 2006, 5, 191–239. [Google Scholar] [CrossRef]
- Vinay, L.; Jean-Xavier, C. Plasticity of spinal cord locomotor networks and contribution of cation-chloride cotransporters. Brain Res. Rev. 2008, 57, 103–110. [Google Scholar] [CrossRef]
- Puskarjov, M.; Ahmad, F.; Kaila, K.; Blaesse, P. Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J. Neurosci. 2012, 32, 11356–11364. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, S.R.; Byun, H.S.; Chen, H.; Li, L.; Han, H.D.; Lopez-Berestein, G.; Sood, A.K.; Pan, H.L. N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 2012, 287, 33853–33864. [Google Scholar] [CrossRef]
- Plantier, V.; Sanchez-Brualla, I.; Dingu, N.; Brocard, C.; Liabeuf, S.; Gackiere, F.; Brocard, F. Calpain fosters the hyperexcitability of motoneurons after spinal cord injury and leads to spasticity. eLife 2019, 8, e51404. [Google Scholar] [CrossRef]
- Chamma, I.; Heubl, M.; Chevy, Q.; Renner, M.; Moutkine, I.; Eugène, E.; Poncer, J.C.; Lévi, S. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J. Neurosci. 2013, 33, 15488–15503. [Google Scholar] [CrossRef]
- Wang, W.; Gong, N.; Xu, T.L. Downregulation of KCC2 following LTP contributes to EPSP-spike potentiation in rat hippocampus. Biochem. Biophys. Res. Commun. 2006, 343, 1209–1215. [Google Scholar] [CrossRef]
- Silvestre de Ferron, B.; Vilpoux, C.; Kervern, M.; Robert, A.; Antol, J.; Naassila, M.; Pierrefiche, O. Increase of KCC2 in hippocampal synaptic plasticity disturbances after perinatal ethanol exposure. Addict. Biol. 2017, 22, 1870–1882. [Google Scholar] [CrossRef]
- Chevy, Q.; Heubl, M.; Goutierre, M.; Backer, S.; Moutkine, I.; Eugène, E.; Bloch-Gallego, E.; Lévi, S.; Poncer, J.C. KCC2 Gates Activity-Driven AMPA Receptor Traffic through Cofilin Phosphorylation. J. Neurosci. 2015, 35, 15772–15786. [Google Scholar] [CrossRef]
- Kuba, H.; Ishii, T.M.; Ohmori, H. Axonal site of spike initiation enhances auditory coincidence detection. Nature 2006, 444, 1069–1072. [Google Scholar] [CrossRef]
- Grubb, M.S.; Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010, 465, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, C.; Dargent, B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin. Cell Dev. Biol. 2014, 27, 44–51. [Google Scholar] [CrossRef]
- Jensen, D.B.; Klingenberg, S.; Dimintiyanova, K.P.; Wienecke, J.; Meehan, C.F. Intramuscular Botulinum toxin A injections induce central changes to axon initial segments and cholinergic boutons on spinal motoneurones in rats. Sci. Rep. 2020, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.T.; Whelan, P.J. Diversification of intrinsic motoneuron electrical properties during normal development and botulinum toxin-induced muscle paralysis in early postnatal mice. J. Neurophysiol. 2010, 103, 2833–2845. [Google Scholar] [CrossRef]
- Schafer, D.P.; Jha, S.; Liu, F.; Akella, T.; McCullough, L.D.; Rasband, M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009, 29, 13242–13254. [Google Scholar] [CrossRef]
- Reeves, T.M.; Greer, J.E.; Vanderveer, A.S.; Phillips, L.L. Proteolysis of submembrane cytoskeletal proteins ankyrin-G and αII-spectrin following diffuse brain injury: A role in white matter vulnerability at Nodes of Ranvier. Brain Pathol. 2010, 20, 1055–1068. [Google Scholar] [CrossRef]
- Benned-Jensen, T.; Christensen, R.K.; Denti, F.; Perrier, J.F.; Rasmussen, H.B.; Olesen, S.P. Live Imaging of Kv7.2/7.3 Cell Surface Dynamics at the Axon Initial Segment: High Steady-State Stability and Calpain-Dependent Excitotoxic Downregulation Revealed. J. Neurosci. 2016, 36, 2261–2266. [Google Scholar] [CrossRef]
- Brocard, C.; Plantier, V.; Boulenguez, P.; Liabeuf, S.; Bouhadfane, M.; Viallat-lieutaud, A.; Vinay, L.; Brocard, F. Cleavage of Na+ channels by calpain increases persistent Na+ current and promotes spasticity after spinal cord injury. Nat. Med. 2016, 22, 404–411. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Huie, J.R.; Ying, Z.; Ferguson, A.R.; Crown, E.D.; Baumbauer, K.M.; Edgerton, V.R.; Grau, J.W. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 2007, 148, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Baumbauer, K.M.; Young, E.E.; Joynes, R.L. Pain and learning in a spinal system: Contradictory outcomes from common origins. Brain Res. Rev. 2009, 61, 124–143. [Google Scholar] [CrossRef]
- Huie, J.R.; Garraway, S.M.; Baumbauer, K.M.; Hoy, K.C., Jr.; Beas, B.S.; Montgomery, K.S.; Bizon, J.L.; Grau, J.W. Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience 2012, 200, 74–90. [Google Scholar] [CrossRef]
- Bilchak, J.N.; Caron, G.; Côté, M.P. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 4858. [Google Scholar] [CrossRef]
- Beverungen, H.; Klaszky, S.C.; Klaszky, M.; Cote, M.P. Rehabilitation Decreases Spasticity by Restoring Chloride Homeostasis through the Brain-Derived Neurotrophic Factor-KCC2 Pathway after Spinal Cord Injury. J. Neurotrauma 2020, 37, 846–859. [Google Scholar] [CrossRef]
- Côté, M.P.; Azzam, G.A.; Lemay, M.A.; Zhukareva, V.; Houlé, J.D. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma 2011, 28, 299–309. [Google Scholar] [CrossRef]
- Skup, M.; Ziemlinska, E.; Gajewska-Wozniak, O.; Platek, R.; Maciejewska, A.; Czarkowska-Bauch, J. The impact of training and neurotrophins on functional recovery after complete spinal cord transection: Cellular and molecular mechanisms contributing to motor improvement. Acta Neurobiol. Exp. 2014, 74, 121–141. [Google Scholar] [CrossRef]
- Ziemlińska, E.; Kügler, S.; Schachner, M.; Wewiór, I.; Czarkowska-Bauch, J.; Skup, M. Overexpression of BDNF increases excitability of the lumbar spinal network and leads to robust early locomotor recovery in completely spinalized rats. PLoS ONE 2014, 9, e88833. [Google Scholar] [CrossRef]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 270–271. [Google Scholar] [CrossRef]
- Zadran, S.; Jourdi, H.; Rostamiani, K.; Qin, Q.; Bi, X.; Baudry, M. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J. Neurosci. 2010, 30, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo-Santos, A.; Vaz, S.H.; Parreira, S.; Rapaz-Lerias, S.; Caetano, A.P.; Buee-Scherrer, V.; Castren, E.; Valente, C.A.; Blum, D.; Sebastiao, A.M.; et al. Dysregulation of TrkB Receptors and BDNF Function by Amyloid-beta Peptide is Mediated by Calpain. Cereb. Cortex 2015, 25, 3107–3121. [Google Scholar] [CrossRef] [PubMed]
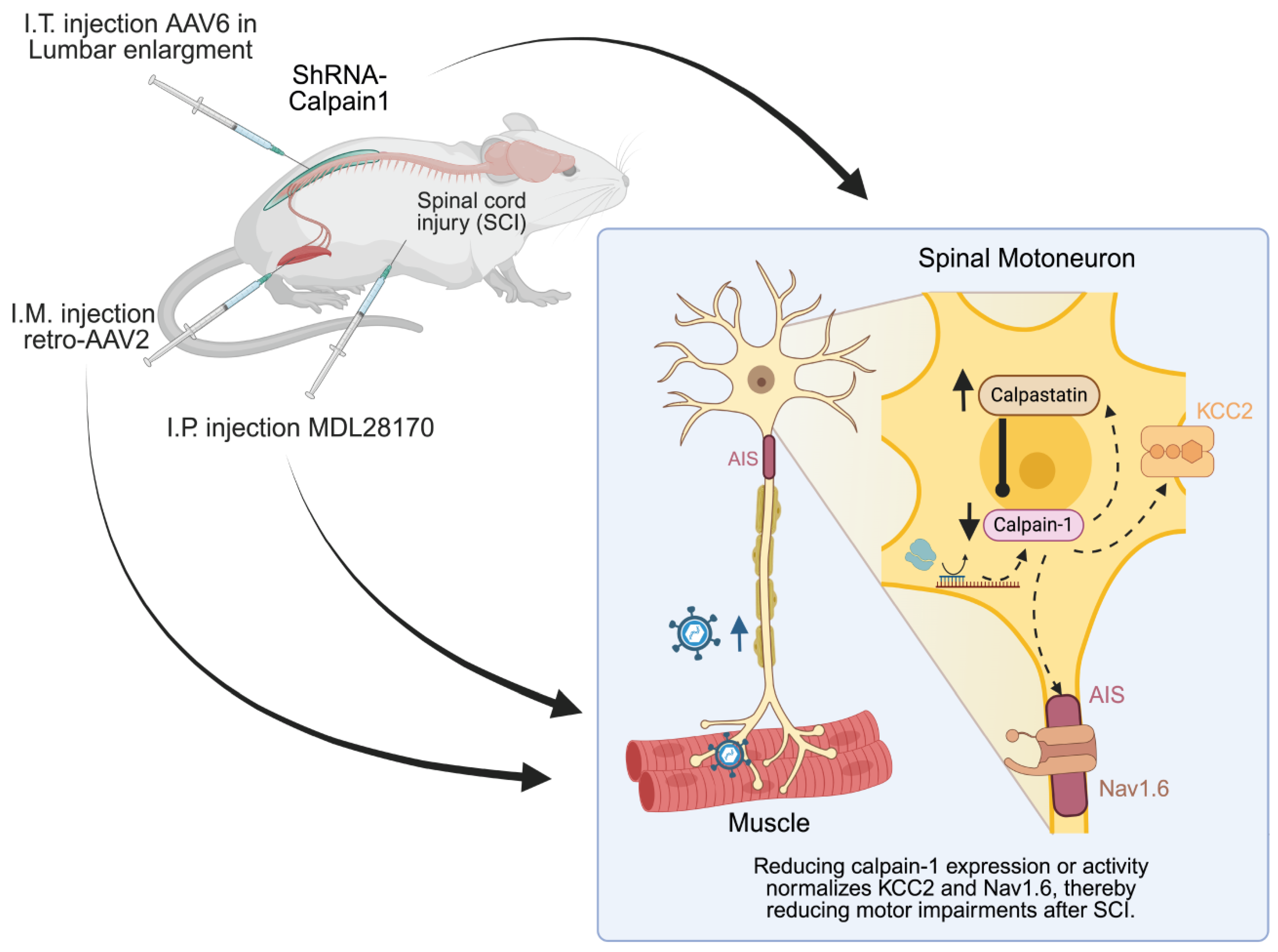
- Kerzonkuf, M.; Verneuil, J.; Brocard, C.; Dingu, N.; Trouplin, V.; Ramirez Franco, J.J.; Bartoli, M.; Brocard, F.; Bras, H. Knockdown of calpain1 in lumbar motoneurons reduces spasticity after spinal cord injury in adult rats. Mol. Ther. 2024, 32, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Hicks, A.L. Spasticity after spinal cord injury. Spinal Cord. 2005, 43, 577–586. [Google Scholar] [CrossRef]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef]
- Schumacher, P.A.; Eubanks, J.H.; Fehlings, M.G. Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience 1999, 91, 733–744. [Google Scholar] [CrossRef]
- Springer, J.E.; Azbill, R.D.; Kennedy, S.E.; George, J.; Geddes, J.W. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: Attenuation with riluzole pretreatment. J. Neurochem. 1997, 69, 1592–1600. [Google Scholar] [CrossRef]
- Ray, S.K.; Shields, D.C.; Saido, T.C.; Matzelle, D.C.; Wilford, G.G.; Hogan, E.L.; Banik, N.L. Calpain activity and translational expression increased in spinal cord injury. Brain Res. 1999, 816, 375–380. [Google Scholar] [CrossRef]
- Shields, D.C.; Schaecher, K.E.; Hogan, E.L.; Banik, N.L. Calpain activity and expression increased in activated glial and inflammatory cells in penumbra of spinal cord injury lesion. J. Neurosci. Res. 2000, 61, 146–150. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Li, Z.; Hogan, E.L.; Banik, N.L. Role of calpain in spinal cord injury: Increased calpain immunoreactivity in rat spinal cord after impact trauma. Neurochem. Res. 1996, 21, 441–448. [Google Scholar] [CrossRef]
- Li, Y.; Bondada, V.; Joshi, A.; Geddes, J.W. Calpain 1 and Calpastatin expression is developmentally regulated in rat brain. Exp. Neurol. 2009, 220, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Banik, N.L.; Matzelle, D.C.; Gantt-Wilford, G.; Osborne, A.; Hogan, E.L. Increased calpain content and progressive degradation of neurofilament protein in spinal cord injury. Brain Res. 1997, 752, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bramlett, H.M.; Moghieb, A.; Yu, D.; Wang, P.; Lin, F.; Bauer, C.; Selig, T.M.; Jaalouk, E.; Weissman, A.S.; et al. Temporal Profile and Severity Correlation of a Panel of Rat Spinal Cord Injury Protein Biomarkers. Mol. Neurobiol. 2018, 55, 2174–2184. [Google Scholar] [CrossRef]
- Du, S.; Rubin, A.; Klepper, S.; Barrett, C.; Kim, Y.C.; Rhim, H.W.; Lee, E.B.; Park, C.W.; Markelonis, G.J.; Oh, T.H. Calcium influx and activation of calpain I mediate acute reactive gliosis in injured spinal cord. Exp. Neurol. 1999, 157, 96–105. [Google Scholar] [CrossRef]
- Schultz, B.; Taday, J.; Menezes, L.; Cigerce, A.; Leite, M.C.; Gonçalves, C.A. Calpain-Mediated Alterations in Astrocytes Before and During Amyloid Chaos in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 84, 1415–1430. [Google Scholar] [CrossRef]
- Hung, K.S.; Hwang, S.L.; Liang, C.L.; Chen, Y.J.; Lee, T.H.; Liu, J.K.; Howng, S.L.; Wang, C.H. Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J. Neuropathol. Exp. Neurol. 2005, 64, 15–26. [Google Scholar] [CrossRef]
- Metwally, E.; Al-Abbadi, H.A.; Hashem, M.A.; Mahmoud, Y.K.; Ahmed, E.A.; Maaty, A.I.; Helal, I.E.; Ahmed, M.F. Selective Calpain Inhibition Improves Functional and Histopathological Outcomes in a Canine Spinal Cord Injury Model. Int. J. Mol. Sci. 2022, 23, 1772. [Google Scholar] [CrossRef]
- Yu, C.G.; Li, Y.; Raza, K.; Yu, X.X.; Ghoshal, S.; Geddes, J.W. Calpain 1 knockdown improves tissue sparing and functional outcomes after spinal cord injury in rats. J. Neurotrauma 2013, 30, 427–433. [Google Scholar] [CrossRef]
- Zai, L.J.; Wrathall, J.R. Cell proliferation and replacement following contusive spinal cord injury. Glia 2005, 50, 247–257. [Google Scholar] [CrossRef]
- Banik, N.L.; Matzelle, D.; Gantt-Wilford, G.; Hogan, E.L. Role of calpain and its inhibitors in tissue degeneration and neuroprotection in spinal cord injury. Ann. N. Y. Acad. Sci. 1997, 825, 120–127. [Google Scholar] [CrossRef]
- Välimäki, E.; Cypryk, W.; Virkanen, J.; Nurmi, K.; Turunen, P.M.; Eklund, K.K.; Åkerman, K.E.; Nyman, T.A.; Matikainen, S. Calpain Activity Is Essential for ATP-Driven Unconventional Vesicle-Mediated Protein Secretion and Inflammasome Activation in Human Macrophages. J. Immunol. 2016, 197, 3315–3325. [Google Scholar] [CrossRef]
- Carruth, L.M.; Demczuk, S.; Mizel, S.B. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J. Biol. Chem. 1991, 266, 12162–12167. [Google Scholar] [CrossRef]
- Podbielska, M.; Das, A.; Smith, A.W.; Chauhan, A.; Ray, S.K.; Inoue, J.; Azuma, M.; Nozaki, K.; Hogan, E.L.; Banik, N.L. Neuron-microglia interaction induced bi-directional cytotoxicity associated with calpain activation. J. Neurochem. 2016, 139, 440–455. [Google Scholar] [CrossRef]
- Alluri, H.; Grimsley, M.; Anasooya Shaji, C.; Varghese, K.P.; Zhang, S.L.; Peddaboina, C.; Robinson, B.; Beeram, M.R.; Huang, J.H.; Tharakan, B. Attenuation of Blood-Brain Barrier Breakdown and Hyperpermeability by Calpain Inhibition. J. Biol. Chem. 2016, 291, 26958–26969. [Google Scholar] [CrossRef]
- Tao, X.G.; Shi, J.H.; Hao, S.Y.; Chen, X.T.; Liu, B.Y. Protective Effects of Calpain Inhibition on Neurovascular Unit Injury through Downregulating Nuclear Factor-κB-related Inflammation during Traumatic Brain Injury in Mice. Chin. Med. J. 2017, 130, 187–198. [Google Scholar] [CrossRef]
- Khan, M.; Dhammu, T.S.; Singh, I.; Singh, A.K. Amelioration of spinal cord injury in rats by blocking peroxynitrite/calpain activity. BMC Neurosci. 2018, 19, 50. [Google Scholar] [CrossRef]
- Ray, S.K.; Matzelle, D.C.; Wilford, G.G.; Hogan, E.L.; Banik, N.L. E-64-d prevents both calpain upregulation and apoptosis in the lesion and penumbra following spinal cord injury in rats. Brain Res. 2000, 867, 80–89. [Google Scholar] [CrossRef]
- Wienecke, J.; Westerdahl, A.C.; Hultborn, H.; Kiehn, O.; Ryge, J. Global gene expression analysis of rodent motor neurons following spinal cord injury associates molecular mechanisms with development of postinjury spasticity. J. Neurophysiol. 2010, 103, 761–778. [Google Scholar] [CrossRef]
- Bose, P.; Parmer, R.; Thompson, F.J. Velocity-dependent ankle torque in rats after contusion injury of the midthoracic spinal cord: Time course. J. Neurotrauma 2002, 19, 1231–1249. [Google Scholar] [CrossRef]
- Bennett, D.J.; Sanelli, L.; Cooke, C.L.; Harvey, P.J.; Gorassini, M.A. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J. Neurophysiol. 2004, 91, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Plantier, V.; Brocard, F. Calpain as a new therapeutic target for treating spasticity after a spinal cord injury. Med. Sci. 2017, 33, 629–636. [Google Scholar] [CrossRef][Green Version]
- Dingu, N.; Bras, H.; Brocard, F. Calpain role in the pathophysiology of spasticity after spinal cord injury. In Cellular, Molecular, Physiological, and Behavioral Aspects of Spinal Cord Injury; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar][Green Version]
- Sadlaoud, K.; Khalki, L.; Brocard, F.; Vinay, L.; Boulenguez, P.; Bras, H. Alteration of glycinergic receptor expression in lumbar spinal motoneurons is involved in the mechanisms underlying spasticity after spinal cord injury. J. Chem. Neuroanat. 2020, 106, 101787. [Google Scholar] [CrossRef]
- Sadlaoud, K.; Tazerart, S.; Brocard, C.; Jean-Xavier, C.; Portalier, P.; Brocard, F.; Vinay, L.; Bras, H. Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J. Neurosci. 2010, 30, 3358–3369. [Google Scholar] [CrossRef]
- Bras, H.; Liabeuf, S. Differential effects of spinal cord transection on glycinergic and GABAergic synaptic signaling in sub-lesional lumbar motoneurons. J. Chem. Neuroanat. 2020, 113, 101847. [Google Scholar] [CrossRef]
- Hounsgaard, J.; Hultborn, H.; Jespersen, B.; Kiehn, O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp. Brain Res. 1984, 55, 391–394. [Google Scholar] [CrossRef]
- Li, Y.; Gorassini, M.A.; Bennett, D.J. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J. Neurophysiol. 2004, 91, 767–783. [Google Scholar] [CrossRef]
- Li, Y.; Bennett, D.J. Persistent Sodium and Calcium Currents Cause Plateau Potentials in Motoneurons of Chronic Spinal Rats. J. Neurophysiol. 2003, 90, 857–869. [Google Scholar] [CrossRef]
- Bouhadfane, M.; Tazerart, S.; Moqrich, A.; Vinay, L.; Brocard, F. Sodium-mediated plateau potentials in lumbar motoneurons of neonatal rats. J. Neurosci. 2013, 33, 15626–15641. [Google Scholar] [CrossRef]
- Duflocq, A.; Le, B.B.; Bullier, E.; Couraud, F.; Davenne, M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell Neurosci. 2008, 39, 180–192. [Google Scholar] [CrossRef]
- Drouillas, B.; Brocard, C.; Zanella, S.; Bos, R.; Brocard, F. Persistent Nav1.1 and Nav1.6 currents drive spinal locomotor functions through nonlinear dynamics. Cell Rep. 2023, 42, 113085. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, W. Sodium channel inactivation: Molecular determinants and modulation. Physiol. Rev. 2005, 85, 1271–1301. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Bezanilla, F.; Rojas, E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J. Gen. Physiol. 1973, 62, 375–391. [Google Scholar] [CrossRef]
- Chamma, I.; Chevy, Q.; Poncer, J.C.; Lévi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell Neurosci. 2012, 6, 5. [Google Scholar] [CrossRef]
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-chloride cotransporters and neuronal function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef]
- Watanabe, M.; Wake, H.; Moorhouse, A.J.; Nabekura, J. Clustering of neuronal K+-Cl− cotransporters in lipid rafts by tyrosine phosphorylation. J. Biol. Chem. 2009, 284, 27980–27988. [Google Scholar] [CrossRef]
- Mercado, A.; Broumand, V.; Zandi-Nejad, K.; Enck, A.H.; Mount, D.B. A C-terminal domain in KCC2 confers constitutive K+-Cl− cotransport. J. Biol. Chem. 2006, 281, 1016–1026. [Google Scholar] [CrossRef]
- Acton, B.A.; Mahadevan, V.; Mercado, A.; Uvarov, P.; Ding, Y.; Pressey, J.; Airaksinen, M.S.; Mount, D.B.; Woodin, M.A. Hyperpolarizing GABAergic transmission requires the KCC2 C-terminal ISO domain. J. Neurosci. 2012, 32, 8746–8751. [Google Scholar] [CrossRef]
- Yamashita, T.; Teramoto, S.; Kwak, S. Phosphorylated TDP-43 becomes resistant to cleavage by calpain: A regulatory role for phosphorylation in TDP-43 pathology of ALS/FTLD. Neurosci. Res. 2016, 107, 63–69. [Google Scholar] [CrossRef]
- Modol, L.; Mancuso, R.; Ale, A.; Francos-Quijorna, I.; Navarro, X. Differential effects on KCC2 expression and spasticity of ALS and traumatic injuries to motoneurons. Front. Cell Neurosci. 2014, 8, 7. [Google Scholar] [CrossRef]
- Verneuil, J.; Brocard, C.; Trouplin, V.; Villard, L.; Peyronnet-Roux, J.; Brocard, F. The M-current works in tandem with the persistent sodium current to set the speed of locomotion. PLoS Biol. 2020, 18, e3000738. [Google Scholar] [CrossRef]
- Bennett, D.J.; Li, Y.; Harvey, P.J.; Gorassini, M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001, 86, 1972–1982. [Google Scholar] [CrossRef]
- Bennett, D.J.; Li, Y.; Siu, M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J. Neurophysiol. 2001, 86, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, M.; Fuchs, A.; Low, P.; Bartsch, D.; Kiehn, O.; Bellardita, C. Early delivery and prolonged treatment with nimodipine prevents the development of spasticity after spinal cord injury in mice. Sci. Transl. Med. 2020, 12, aay0167. [Google Scholar] [CrossRef] [PubMed]
- Schön, C.; Paquet-Durand, F.; Michalakis, S. Cav1.4 L-Type Calcium Channels Contribute to Calpain Activation in Degenerating Photoreceptors of rd1 Mice. PLoS ONE 2016, 11, e0156974. [Google Scholar] [CrossRef]
- Abele, K.; Yang, J. Regulation of voltage-gated calcium channels by proteolysis. Sheng Li Xue Bao 2012, 64, 504–514. [Google Scholar]
- Shoshan-Barmatz, V.; Weil, S.; Meyer, H.; Varsanyi, M.; Heilmeyer, L.M. Endogenous, Ca2+-dependent cysteine-protease cleaves specifically the ryanodine receptor/Ca2+ release channel in skeletal muscle. J. Membr. Biol. 1994, 142, 281–288. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Jensen, M.P.; Norrbrink, C.; Trok, K.; Johannesen, I.L.; Jensen, T.S.; Werhagen, L. A prospective study of pain and psychological functioning following traumatic spinal cord injury. Spinal Cord. 2016, 54, 816–821. [Google Scholar] [CrossRef]
- Shiao, R.; Lee-Kubli, C.A. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics 2018, 15, 635–653. [Google Scholar] [CrossRef]
- Woolf, C.J.; Salter, M.W. Neuronal plasticity: Increasing the gain in pain. Science 2000, 288, 1765–1769. [Google Scholar] [CrossRef]
- Ultenius, C.; Linderoth, B.; Meyerson, B.A.; Wallin, J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci. Lett. 2006, 399, 85–90. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, J.; Xiong, L.; Zimmermann, M.; Yang, J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J. Physiol. 2008, 586, 5701–5715. [Google Scholar] [CrossRef]
- Sanchez-Brualla, I.; Boulenguez, P.; Brocard, C.; Liabeuf, S.; Viallat-Lieutaud, A.; Navarro, X.; Udina, E.; Brocard, F. Activation of 5-HT2A Receptors Restores KCC2 Function and Reduces Neuropathic Pain after Spinal Cord Injury. Neuroscience 2018, 387, 48–57. [Google Scholar] [CrossRef]
- Hasbargen, T.; Ahmed, M.M.; Miranpuri, G.; Li, L.; Kahle, K.T.; Resnick, D.; Sun, D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann. N. Y. Acad. Sci. 2010, 1198, 168–172. [Google Scholar] [CrossRef]
- Cramer, S.W.; Baggott, C.; Cain, J.; Tilghman, J.; Allcock, B.; Miranpuri, G.; Rajpal, S.; Sun, D.; Resnick, D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol. Pain. 2008, 4, 36. [Google Scholar] [CrossRef]
- Gagnon, M.; Bergeron, M.J.; Lavertu, G.; Castonguay, A.; Tripathy, S.; Bonin, R.P.; Perez-Sanchez, J.; Boudreau, D.; Wang, B.; Dumas, L.; et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013, 19, 1524–1528. [Google Scholar] [CrossRef]
- Davis, O.C.; Ferland, S.; Lorenzo, L.-E.; Murray-Lawson, C.; Shiers, S.; Yousuf, M.S.; Dedek, A.; Tsai, E.C.; Vines, E.; Horton, P.; et al. Decreased KCC2 expression in the human spinal dorsal horn associated with chronic pain and long-term opioid use. bioRxiv 2025. preprint. [Google Scholar] [CrossRef]
- Coull, J.A.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sík, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog. Brain Res. 2007, 161, 195–203. [Google Scholar] [CrossRef]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2 × 4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Inoue, K.; Salter, M.W. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005, 28, 101–107. [Google Scholar] [CrossRef]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Liao, G.J.; Yao, P.W.; Wang, S.K.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Calpain-2 Regulates TNF-α Expression Associated with Neuropathic Pain Following Motor Nerve Injury. Neuroscience 2018, 376, 142–151. [Google Scholar] [CrossRef]
- Chen, S.X.; Wang, S.K.; Yao, P.W.; Liao, G.J.; Na, X.D.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Early CALP2 expression and microglial activation are potential inducers of spinal IL-6 up-regulation and bilateral pain following motor nerve injury. J. Neurochem. 2018, 145, 154–169. [Google Scholar] [CrossRef]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef]
- Barbay, T.; Pecchi, E.; Ramirez-Franco, J.; Ivanov, A.; Brocard, F.; Rouach, N.; Bos, R. Functional contribution of astrocytic Kir4.1 channels to spasticity after spinal cord injury. Brain 2025, awaf147. [Google Scholar] [CrossRef]
- Zafra, F.; Lindholm, D.; Castrén, E.; Hartikka, J.; Thoenen, H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J. Neurosci. 1992, 12, 4793–4799. [Google Scholar] [CrossRef]
- Schwartz, J.P.; Nishiyama, N. Neurotrophic factor gene expression in astrocytes during development and following injury. Brain Res. Bull. 1994, 35, 403–407. [Google Scholar] [CrossRef]
- Albini, M.; Krawczun-Rygmaczewska, A.; Cesca, F. Astrocytes and brain-derived neurotrophic factor (BDNF). Neurosci. Res. 2023, 197, 42–51. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Gou-Fabregas, M.; Ramírez-Núñez, O.; Cacabelos, D.; Bahi, N.; Portero, M.; Garcera, A.; Soler, R.M. Calpain activation and CaMKIV reduction in spinal cords from hSOD1G93A mouse model. Mol. Cell Neurosci. 2014, 61, 219–225. [Google Scholar] [CrossRef]
- Stifanese, R.; Averna, M.; De Tullio, R.; Pedrazzi, M.; Milanese, M.; Bonifacino, T.; Bonanno, G.; Salamino, F.; Pontremoli, S.; Melloni, E. Role of calpain-1 in the early phase of experimental ALS. Arch. Biochem. Biophys. 2014, 562, 1–8. [Google Scholar] [CrossRef]
- De Marco, G.; Lomartire, A.; Manera, U.; Canosa, A.; Grassano, M.; Casale, F.; Fuda, G.; Salamone, P.; Rinaudo, M.T.; Colombatto, S.; et al. Effects of intracellular calcium accumulation on proteins encoded by the major genes underlying amyotrophic lateral sclerosis. Sci. Rep. 2022, 12, 395. [Google Scholar] [CrossRef]
- Camins, A.; Verdaguer, E.; Folch, J.; Pallàs, M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006, 12, 135–148. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Calcium, mitochondria, and the pathogenesis of ALS: The good, the bad, and the ugly. Front. Cell Neurosci. 2013, 7, 199. [Google Scholar] [CrossRef]
- Stifanese, R.; Averna, M.; De, T.R.; Pedrazzi, M.; Beccaria, F.; Salamino, F.; Milanese, M.; Bonanno, G.; Pontremoli, S.; Melloni, E. Adaptive modifications in the calpain/calpastatin system in brain cells after persistent alteration in Ca2+ homeostasis. J. Biol. Chem. 2010, 285, 631–643. [Google Scholar] [CrossRef]
- Rao, M.V.; Campbell, J.; Palaniappan, A.; Kumar, A.; Nixon, R.A. Calpastatin inhibits motor neuron death and increases survival of hSOD1(G93A) mice. J. Neurochem. 2016, 137, 253–265. [Google Scholar] [CrossRef]
- Chen, X.; Lv, S.; Liu, J.; Guan, Y.; Xu, C.; Ma, X.; Li, M.; Bai, X.; Liu, K.; Zhang, H.; et al. Exploring the Role of Axons in ALS from Multiple Perspectives. Cells 2024, 13, 2076. [Google Scholar] [CrossRef]
- Pant, H.C. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 1988, 256, 665–668. [Google Scholar] [CrossRef]
- Johnson, G.V.; Greenwood, J.A.; Costello, A.C.; Troncoso, J.C. The regulatory role of calmodulin in the proteolysis of individual neurofilament proteins by calpain. Neurochem. Res. 1991, 16, 869–873. [Google Scholar] [CrossRef]
- Yamashita, T.; Hideyama, T.; Hachiga, K.; Teramoto, S.; Takano, J.; Iwata, N.; Saido, T.C.; Kwak, S. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat. Commun. 2012, 3, 1307. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Chung, C.G.; Park, S.S.; Lee, D.; Kim, K.M.; Jeong, Y.; Kim, E.S.; Cho, J.H.; Jeon, Y.M.; Shen, C.J.; et al. Cytosolic calcium regulates cytoplasmic accumulation of TDP-43 through Calpain-A and Importin α3. eLife 2020, 9, 60132. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Aizawa, H.; Teramoto, S.; Akamatsu, M.; Kwak, S. Calpain-dependent disruption of nucleo-cytoplasmic transport in ALS motor neurons. Sci. Rep. 2017, 7, 39994. [Google Scholar] [CrossRef]
- Yamashita, T.; Hideyama, T.; Teramoto, S.; Kwak, S. The abnormal processing of TDP-43 is not an upstream event of reduced ADAR2 activity in ALS motor neurons. Neurosci. Res. 2012, 73, 153–160. [Google Scholar] [CrossRef]
- Wootz, H.; Hansson, I.; Korhonen, L.; Lindholm, D. XIAP decreases caspase-12 cleavage and calpain activity in spinal cord of ALS transgenic mice. Exp. Cell Res. 2006, 312, 1890–1898. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.C.; Lei, M.; Wang, Z.; Xiong, K. Regulatory role of calpain in neuronal death. Neural Regen. Res. 2018, 13, 556–562. [Google Scholar] [CrossRef]
- Deutsch, A.J.; Elbasiouny, S.M. Dysregulation of persistent inward and outward currents in spinal motoneurons of symptomatic SOD1-G93A mice. J. Physiol. 2024, 602, 3715–3736. [Google Scholar] [CrossRef]
- ElBasiouny, S.M.; Schuster, J.E.; Heckman, C.J. Persistent inward currents in spinal motoneurons: Important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2010, 121, 1669–1679. [Google Scholar] [CrossRef]
- Leroy, F.; Lamotte, D.I.B.; Imhoff-Manuel, R.D.; Zytnicki, D. Early intrinsic hyperexcitability does not contribute to motoneuron degeneration in amyotrophic lateral sclerosis. eLife 2014, 3, e04046. [Google Scholar] [CrossRef]
- Quinlan, K.A.; Schuster, J.E.; Fu, R.; Siddique, T.; Heckman, C.J. Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2011, 589, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.S.; Jensen, D.B.; Dimintiyanova, K.P.; Bonnevie, V.S.; Hedegaard, A.; Lehnhoff, J.; Moldovan, M.; Grondahl, L.; Meehan, C.F. Increased Axon Initial Segment Length Results in Increased Na+ Currents in Spinal Motoneurones at Symptom Onset in the G127X SOD1 Mouse Model of Amyotrophic Lateral Sclerosis. Neuroscience 2021, 468, 247–264. [Google Scholar] [CrossRef]
- Bonnevie, V.S.; Dimintiyanova, K.P.; Hedegaard, A.; Lehnhoff, J.; Grøndahl, L.; Moldovan, M.; Meehan, C.F. Shorter axon initial segments do not cause repetitive firing impairments in the adult presymptomatic G127X SOD-1 Amyotrophic Lateral Sclerosis mouse. Sci. Rep. 2020, 10, 1280. [Google Scholar] [CrossRef]
- Milinis, K.; Tennant, A.; Mills, R.J.; Al-Chalabi, A.; Burke, G.; Dick, D.J.; Ealing, J.; Hanemann, C.O.; Harrower, T.; McDermott, C.J.; et al. Development and validation of Spasticity Index-Amyotrophic Lateral Sclerosis. Acta Neurol. Scand. 2018, 138, 47–54. [Google Scholar] [CrossRef]
- Fuchs, A.; Ringer, C.; Bilkei-Gorzo, A.; Weihe, E.; Roeper, J.; Schutz, B. Downregulation of the potassium chloride cotransporter KCC2 in vulnerable motoneurons in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 1057–1070. [Google Scholar] [CrossRef]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple sclerosis--the plaque and its pathogenesis. N. Engl. J. Med. 2006, 354, 942–955. [Google Scholar] [CrossRef]
- Attfield, K.E.; Jensen, L.T.; Kaufmann, M.; Friese, M.A.; Fugger, L. The immunology of multiple sclerosis. Nat. Rev. Immunol. 2022, 22, 734–750. [Google Scholar] [CrossRef]
- Shields, D.C.; Banik, N.L. Pathophysiological role of calpain in experimental demyelination. J. Neurosci. Res. 1999, 55, 533–541. [Google Scholar] [CrossRef]
- Shields, D.C.; Schaecher, K.E.; Saido, T.C.; Banik, N.L. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc. Natl. Acad. Sci. USA 1999, 96, 11486–11491. [Google Scholar] [CrossRef]
- Diaz-Sanchez, M.; Williams, K.; DeLuca, G.C.; Esiri, M.M. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006, 111, 289–299. [Google Scholar] [CrossRef]
- Deshpande, R.V.; Goust, J.M.; Chakrabarti, A.K.; Barbosa, E.; Hogan, E.L.; Banik, N.L. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J. Biol. Chem. 1995, 270, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.C.; Tyor, W.R.; Deibler, G.E.; Banik, N.L. Increased calpain expression in experimental demyelinating optic neuritis: An immunocytochemical study. Brain Res. 1998, 784, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.V.; Goust, J.M.; Hogan, E.L.; Banik, N.L. Calpain secreted by activated human lymphoid cells degrades myelin. J. Neurosci. Res. 1995, 42, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Ray, S.K.; Das, A.; Nozaki, K.; Rohrer, B.; Banik, N.L. Calpain inhibition as a possible new therapeutic target in multiple sclerosis. AIMS Mol. Sci. 2017, 4, 446–462. [Google Scholar] [CrossRef]
- Banik, N.L.; Shields, D.C. A putative role for calpain in demyelination associated with optic neuritis. Histol. Histopathol. 1999, 14, 649–656. [Google Scholar] [CrossRef]
- Guyton, M.K.; Wingrave, J.M.; Yallapragada, A.V.; Wilford, G.G.; Sribnick, E.A.; Matzelle, D.D.; Tyor, W.R.; Ray, S.K.; Banik, N.L. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J. Neurosci. Res. 2005, 81, 53–61. [Google Scholar] [CrossRef]
- Schaecher, K.; Rocchini, A.; Dinkins, J.; Matzelle, D.D.; Banik, N.L. Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: Increased calpain activity begins with onset of disease. J. Neuroimmunol. 2002, 129, 1–9. [Google Scholar] [CrossRef]
- Banik, N.L.; Chou, C.H.; Deibler, G.E.; Krutzch, H.C.; Hogan, E.L. Peptide bond specificity of calpain: Proteolysis of human myelin basic protein. J. Neurosci. Res. 1994, 37, 489–496. [Google Scholar] [CrossRef]
- Guyton, M.K.; Das, A.; Samantaray, S.; Wallace, G.C.t.; Butler, J.T.; Ray, S.K.; Banik, N.L. Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J. Neurosci. Res. 2010, 88, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Hassen, G.W.; Feliberti, J.; Kesner, L.; Stracher, A.; Mokhtarian, F. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2006, 180, 135–146. [Google Scholar] [CrossRef]
- Mondello, S.; Robicsek, S.A.; Gabrielli, A.; Brophy, G.M.; Papa, L.; Tepas, J.; Robertson, C.; Buki, A.; Scharf, D.; Jixiang, M.; et al. αII-spectrin breakdown products (SBDPs): Diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma 2010, 27, 1203–1213. [Google Scholar] [CrossRef]
- Yang, Z.; Apiliogullari, S.; Fu, Y.; Istanbouli, A.; Kaur, S.; Jabbal, I.S.; Moghieb, A.; Irfan, Z.; Patterson, R.L.; Kurup, M.; et al. Association between Cerebrospinal Fluid and Serum Biomarker Levels and Diagnosis, Injury Severity, and Short-Term Outcomes in Patients with Acute Traumatic Spinal Cord Injury. Diagnostics 2023, 13, 1814. [Google Scholar] [CrossRef]
- Guéz, M.; Hildingsson, C.; Rosengren, L.; Karlsson, K.; Toolanen, G. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J. Neurotrauma 2003, 20, 853–858. [Google Scholar] [CrossRef]
- Acarin, L.; Villapol, S.; Faiz, M.; Rohn, T.T.; Castellano, B.; González, B. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia 2007, 55, 954–965. [Google Scholar] [CrossRef]
- Williams, S.T.; Smith, A.N.; Cianci, C.D.; Morrow, J.S.; Brown, T.L. Identification of the primary caspase 3 cleavage site in alpha II-spectrin during apoptosis. Apoptosis 2003, 8, 353–361. [Google Scholar] [CrossRef]
- Prchal, J.T.; Papayannopoulou, T.; Yoon, S.H. Patterns of spectrin transcripts in erythroid and non-erythroid cells. J. Cell Physiol. 1990, 144, 287–294. [Google Scholar] [CrossRef]
- Von Reyn, C.R.; Spaethling, J.M.; Mesfin, M.N.; Ma, M.; Neumar, R.W.; Smith, D.H.; Siman, R.; Meaney, D.F. Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J. Neurosci. 2009, 29, 10350–10356. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Winer, J.L.; Corbett, C.J.; Robinson, S. Erythropoietin Modulates Cerebral and Serum Degradation Products from Excess Calpain Activation following Prenatal Hypoxia-Ischemia. Dev. Neurosci. 2016, 38, 15–26. [Google Scholar] [CrossRef]
- Baucher, G.; Liabeuf, S.; Brocard, C.; Ponz, A.; Baumstarck, K.; Troude, L.; Leone, M.; Roche, P.H.; Brocard, F. The SpasT-SCI-T trial protocol: Investigating calpain-mediated sodium channel fragments as biomarkers for traumatic CNS injuries and spasticity prediction. PLoS ONE 2025, 20, e0319635. [Google Scholar] [CrossRef] [PubMed]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Masullo, M.; Quadalti, C.; Avoni, P.; Polischi, B.; Cherici, A.; Capellari, S.; Salvi, F.; et al. Plasma and CSF Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: A Cross-Sectional and Longitudinal Study. Front. Aging Neurosci. 2021, 13, 753242. [Google Scholar] [CrossRef] [PubMed]
- Halbgebauer, S.; Steinacker, P.; Verde, F.; Weishaupt, J.; Oeckl, P.; von Arnim, C.; Dorst, J.; Feneberg, E.; Mayer, B.; Rosenbohm, A.; et al. Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J. Neurol. Neurosurg. Psychiatry 2022, 93, 68–74. [Google Scholar] [CrossRef]
- Gaiottino, J.; Norgren, N.; Dobson, R.; Topping, J.; Nissim, A.; Malaspina, A.; Bestwick, J.P.; Monsch, A.U.; Regeniter, A.; Lindberg, R.L.; et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013, 8, e75091. [Google Scholar] [CrossRef]
- Kunz, S.; Niederberger, E.; Ehnert, C.; Coste, O.; Pfenninger, A.; Kruip, J.; Wendrich, T.M.; Schmidtko, A.; Tegeder, I.; Geisslinger, G. The calpain inhibitor MDL 28170 prevents inflammation-induced neurofilament light chain breakdown in the spinal cord and reduces thermal hyperalgesia. Pain 2004, 110, 409–418. [Google Scholar] [CrossRef]
- Kahn, O.I.; Dominguez, S.L.; Glock, C.; Hayne, M.; Vito, S.; Sengupta Ghosh, A.; Adrian, M.; Burgess, B.L.; Meilandt, W.J.; Friedman, B.A.; et al. Secreted neurofilament light chain after neuronal damage induces myeloid cell activation and neuroinflammation. Cell Rep. 2025, 44, 115382. [Google Scholar] [CrossRef]
- Majumder, V.; Gregory, J.M.; Barria, M.A.; Green, A.; Pal, S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol. 2018, 18, 90. [Google Scholar] [CrossRef]
- Khademullah, C.S.; De Koninck, Y. A novel assessment of fine-motor function reveals early hindlimb and detectable forelimb deficits in an experimental model of ALS. Sci. Rep. 2022, 12, 17010. [Google Scholar] [CrossRef]
- Schaecher, K.E.; Shields, D.C.; Banik, N.L. Mechanism of myelin breakdown in experimental demyelination: A putative role for calpain. Neurochem. Res. 2001, 26, 731–737. [Google Scholar] [CrossRef]
- Thomson, A.J.; Brazil, J.; Feighery, C.; Whelan, A.; Kellet, J.; Martin, E.A.; Hutchinson, M. CSF myelin basic protein in multiple sclerosis. Acta Neurol. Scand. 1985, 72, 577–583. [Google Scholar] [CrossRef]
- Imam, S.A.; Guyton, M.K.; Haque, A.; Vandenbark, A.; Tyor, W.R.; Ray, S.K.; Banik, N.L. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J. Neuroimmunol. 2007, 190, 139–145. [Google Scholar] [CrossRef]
- Saatman, K.E.; Murai, H.; Bartus, R.T.; Smith, D.H.; Hayward, N.J.; Perri, B.R.; McIntosh, T.K. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc. Natl. Acad. Sci. USA 1996, 93, 3428–3433. [Google Scholar] [CrossRef]
- Das, A.; Sribnick, E.A.; Wingrave, J.M.; Del Re, A.M.; Woodward, J.J.; Appel, S.H.; Banik, N.L.; Ray, S.K. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: Calpain inhibition provides functional neuroprotection. J. Neurosci. Res. 2005, 81, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Joshi, A.; Geddes, J.W. Intraspinal MDL28170 microinjection improves functional and pathological outcome following spinal cord injury. J. Neurotrauma 2008, 25, 833–840. [Google Scholar] [CrossRef]
- Yu, C.G.; Geddes, J.W. Sustained calpain inhibition improves locomotor function and tissue sparing following contusive spinal cord injury. Neurochem. Res. 2007, 32, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Uçeyler, N.; Tscharke, A.; Sommer, C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav. Immun. 2007, 21, 553–560. [Google Scholar] [CrossRef]
- Trager, N.; Smith, A.; Wallace Iv, G.; Azuma, M.; Inoue, J.; Beeson, C.; Haque, A.; Banik, N.L. Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J. Neurochem. 2014, 130, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Siklos, M.; BenAissa, M.; Thatcher, G.R. Cysteine proteases as therapeutic targets: Does selectivity matter? A systematic review of calpain and cathepsin inhibitors. Acta Pharm. Sin. B 2015, 5, 506–519. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Nham, A.; Sherbaf, A.; Quach, D.; Yahya, E.; Ranburger, D.; Bi, X.; Baudry, M. Calpain-2 as a therapeutic target in repeated concussion-induced neuropathy and behavioral impairment. Sci. Adv. 2020, 6, aba5547. [Google Scholar] [CrossRef]
- Baudry, M.; Wang, Y.; Bi, X.; Luo, Y.L.; Wang, Z.; Kamal, Z.; Shirokov, A.; Sullivan, E.; Lagasca, D.; Khalil, H.; et al. Identification and neuroprotective properties of NA-184, a calpain-2 inhibitor. Pharmacol. Res. Perspect. 2024, 12, e1181. [Google Scholar] [CrossRef]
- Wendt, A.; Thompson, V.F.; Goll, D.E. Interaction of calpastatin with calpain: A review. Biol. Chem. 2004, 385, 465–472. [Google Scholar] [CrossRef]
- Sorimachi, H.; Kinbara, K.; Kimura, S.; Takahashi, M.; Ishiura, S.; Sasagawa, N.; Sorimachi, N.; Shimada, H.; Tagawa, K.; Maruyama, K.; et al. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J. Biol. Chem. 1995, 270, 31158–31162. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.G.; Bondada, V.; Joshi, A.; Reneer, D.V.; Telling, G.C.; Saatman, K.E.; Geddes, J.W. Calpastatin Overexpression Protects against Excitotoxic Hippocampal Injury and Traumatic Spinal Cord Injury. J. Neurotrauma 2020, 37, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.L.; Vissel, B. CAST your vote: Is calpain inhibition the answer to ALS? J. Neurochem. 2016, 137, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Saido, T.C.; Kawashima, S.; Tani, E.; Yokota, M. Up- and down-regulation of calpain inhibitor polypeptide, calpastatin, in postischemic hippocampus. Neurosci. Lett. 1997, 227, 75–78. [Google Scholar] [CrossRef]
- Blomgren, K.; Hallin, U.; Andersson, A.L.; Puka-Sundvall, M.; Bahr, B.A.; McRae, A.; Saido, T.C.; Kawashima, S.; Hagberg, H. Calpastatin is up-regulated in response to hypoxia and is a suicide substrate to calpain after neonatal cerebral hypoxia-ischemia. J. Biol. Chem. 1999, 274, 14046–14052. [Google Scholar] [CrossRef]
- Zang, Y.; Chen, S.X.; Liao, G.J.; Zhu, H.Q.; Wei, X.H.; Cui, Y.; Na, X.D.; Pang, R.P.; Xin, W.J.; Zhou, L.J.; et al. Calpain-2 contributes to neuropathic pain following motor nerve injury via up-regulating interleukin-6 in DRG neurons. Brain Behav. Immun. 2015, 44, 37–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brocard, F.; Dingu, N. Calpains at the Crossroads of Spinal Cord Physiology, Plasticity, and Pathology. Cells 2025, 14, 1503. https://doi.org/10.3390/cells14191503
Brocard F, Dingu N. Calpains at the Crossroads of Spinal Cord Physiology, Plasticity, and Pathology. Cells. 2025; 14(19):1503. https://doi.org/10.3390/cells14191503
Chicago/Turabian StyleBrocard, Frédéric, and Nejada Dingu. 2025. "Calpains at the Crossroads of Spinal Cord Physiology, Plasticity, and Pathology" Cells 14, no. 19: 1503. https://doi.org/10.3390/cells14191503
APA StyleBrocard, F., & Dingu, N. (2025). Calpains at the Crossroads of Spinal Cord Physiology, Plasticity, and Pathology. Cells, 14(19), 1503. https://doi.org/10.3390/cells14191503






