NUP214 in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Methodology
3. The Structure and Function of the Nuclear Pore Complex and the NUP214 Molecule
3.1. The Structure of the Nuclear Pore Complex
- The general structure. The main structures is the spoke complex (also called the central scaffold/framework) that forms the central structure with a central pore that facilitates macromolecular passage between nucleus and cytoplasm. This central complex is flanked by the cytoplasmic and the nuclear rings. Filamentous structures project from both these rings; they are referred to as the cytoplasmic filaments and the nuclear basket, respectively. Based on this main structure of the nuclear pore complex, the nucleoporin proteins can be classified into various subsets as described below.
- Scaffold proteins. These proteins form the central framework or complex of the pore, and several of these proteins span both the cytoplasmic and nuclear side of the pore. Nucleoporins form the core of the complex.
- The transmembrane ring nucleoporins. These proteins are embedded within the nuclear envelope, and they thereby anchor the pore complex to the nuclear membrane.
- Peripheral proteins. The peripheral proteins are associated with the cytoplasmic filaments of the pore and with the nuclear basket on the nuclear side. These proteins function as important regulators of the selectivity/permeability of the pore.
- Proteins with FG motifs. Some authors prefer to distinguish a fourth separate subgroup among the peripheral proteins, namely nucleoporins that contain repetitive phenylalanine-glycine (FG) motifs (GLFG or FXFG motifs) interspersed between 20 charged amino acid residues. The FG domains are important both for anchoring of the pore complex to the nuclear membrane and for regulation of the transport through the pore complex. Approximately one-third of the nucleoporins contain FG domains. NUP214 is classified as a peripheral FG-containing nucleoporin.
3.2. The Structure and Function of NUP214
- NUP98 anchoring. NUP98 anchors NUP214 to the nuclear pore (see above), but NUP98 can in addition modulate the expression of genes involved in regulation of cellular development and cell proliferation [20].
- XPO1 colocalization. Overexpressed NUP214 colocalize together with XPO1 and importin 1; this leads to depletion of these two molecules from the nuclear pore complex and finally growth inhibition and induction of apoptosis [21].
- NUP214 knockdown. The functional effects of NUP214 have been investigated in various cellular models. Knockdown of NUP214 leads to cell cycle arrest in G2 phase in embryonic cells [22], whereas high levels/overexpression causes G0 phase arrest, nucleocytoplasmic transport defects, XPO1 depletion from the nuclear pore complex and apoptotic cell death [21]. These observations further illustrate the complexity of the NUP214 effects on fundamental cellular processes, but it is not known whether (any of) these effects are relevant in human AML.
- Scaffold for signaling proteins. Nucleoporins often function as scaffolds for proteins in intracellular signaling; and this is true also for NUP214. Transforming growth factor β (TGFβ) signaling can be mediated by an interaction between SMAD2 and NUP214, the same is true for SMAD3 and SMAD4 that also bind to NUP214 [23,24,25,26]. TGFβ can be involved in human leukemogenesis [27,28]. Furthermore, NUP98 is a binding partner both for NUP214 and RAE1, and this molecular complex can be recruited to the kinetochore as well as the spindle and spindle poles during mitosis [11,12].
- Noncoding RNA regulation. NUP214 expression is also regulated by noncoding RNAs; NUP214 expression can be downregulated by miR-133, and this mechanism may contribute to carcinogenesis [29].
4. Activation of HOX Genes in AML: A Common Characteristic of Several AML Subsets That Can Be Mediated by Nuclear Pore Molecules
5. The t(6;9)(p22;q34) Translocation and the DEK-NUP214 Fusion Protein
5.1. The Structure and Function of the DEK Proto-Oncogene Protein
5.1.1. The Structure and Function of the DEK Proto-Oncogene Protein
5.1.2. Cellular DEK Is a Histone Chaperone
5.1.3. Other Important Functions of the Cellular DEK Oncoprotein
- DNA/RNA binding. DEK can bind both DNA, RNA and chromatin proteins [54,55,64]; its binding affinity to both DNA and histones/chromatin is regulated by post-translational phosphorylation and acetylation [54,67]. It functions as a regulator of both histone acetyltransferases and deacetylases [58,59,60].
- Signaling target molecules and functional cellular effects. Among its targets/molecular interaction partners are RAD51 [62], STAT5 [73,74], mTOR [58], IRAK1 [69], p300 [79], p53 [73,74] and NCOR1 [58]. DEK is thereby involved in the regulation of several fundamental molecular processes including DNA repair [61,62], epigenetic regulation/histone acetylation [58,59,60], RNA processing and splicing [63,64,65,66], and p53 stabilization [73,74]. DEK becomes important for the cellular regulation of proliferation/cell cycle progression [64,69,70,71], survival/apoptosis (it has an antiapoptotic effect) [64,69,70,71,73], cellular quiescence [47,58,71,72], expression of several oncogenes and tumor suppressor genes [64], and cellular adhesion/migration [69,70,71].
- Extracellular release. Extracellular release of DEK can be a part of the communication between neighboring cells (see Section 5.2) [81,82,83].
5.2. Extracellular Release of a Soluble Form of DEK
5.3. Expression of Normal DEK in AML
5.4. The Structure of the DEK-NUP214 Fusion Protein
5.5. Biological Mechanisms Involved in the Leukemic Transformation of AML Cells with t(6;9)
5.5.1. Effects on the Level of and the Interaction with the Full-Length DEK Protein
5.5.2. Effects of the Fusion Protein on Intracellular Mediators; Effects on mTOR, NFκB, STAT5 and p53
5.5.3. Effects by the NUP214 Derived Part of the Fusion Protein: Effects of Fusion Protein Binding to the Nuclear Transporter XPO1
5.5.4. The Multiple Molecular Effects of DEK-NUP214 Influence Multiple Cellular Functions Including Transcription/Translation, DNA Repair, Metabolism and Proliferation/Survival
5.6. Clinical and Biological Characteristics of DEK-NUP214 AML
- Leukemization. Peripheral blood leukemization is relatively common (median level of AML blasts in peripheral blood 28%), but the total peripheral blood white blood cell count shows a considerable variation (median 12.9 × 109/L, range 0.5–181 × 109/L) between patients [46]. Wide variations have been observed also in other studies [112,113,114].
- Bone marrow failure. Anemia (median levels 8.4 g/100 mL) and thrombocytopenia (median level 45 × 109/L) are common [46]. Similar cytopenias have been observed in other studies [113,114]. Furthermore, the median level of bone marrow blasts was 58% (range 4–99%) in one study [46]. Similar variations have also been observed in other studies [112,114].
- AML cell morphology. Up to half of the patients have AML cells with M2 phenotype according to the FAB classification, but the M4/M5 phenotype is also common [46,114,115,117]. Basophilia defined as ≥2% basophils in the bone marrow can be observed for a subset of patients, the same is true for Auer rods and dysplasia of single or multiple lineages [46]. A small study including 16 adult patients described trilineage dysplasia for 81% of patients [112]. Similar results have been described in other studies [46,113,114]. Finally, one previous study also described a patient with a morphological phenotype resembling acute promyelocytic leukemia and paraneoplastic manifestations consistent with Still’s syndrome [118].
- Molecular signs of differentiation. The AML blasts express CD9, CD13, CD33 and HLA-DR for almost all patients, expression of CD38, CD45 and CD117 is also common [46,112,113,114]. CD15 and CD34 are expressed for a majority of patients [46,112,113]. The immunophenotype is suggestive but not diagnostic for this AML variant, and for the evaluation of minimal residual disease (MRD), genetic PCR diagnosis therefore has to be used [111].
- Other genetic abnormalities. The t(6;9) translocation seems to be the sole cytogenetic abnormality for the majority of patients [46,112,113,114]. Complex karyotypes are uncommon [112,113]. A majority of both pediatric and adult patients seem to have FLT3-ITD, but genetic abnormalities in the FLT3 tyrosine kinase domain are uncommon/absent [46].
5.7. The Possible Prognostic Impact of the DEK-NUP214 Fusion for AML Patients Receiving Intensive Antileukemic Treatment: Adverse Prognosis After Intensive Antileukemic Chemotherapy
- Remission induction. Complete hematological remission was achieved for 71% of pediatric patients and 58% of the adults; these rates compared similar to the corresponding rates for young adults with intermediate (76%) and unfavorable prognosis (55%) in the SWOG/ECOG S9034/3489 study [119].
- Survival analyses. The 5-year survival estimate was 28% for pediatric and 9% for adult patients. The overall survival was comparable to the overall survival for young adults with unfavorable (12%) disease in the SWOG/ECOG S9034/3489 study [119]. Furthermore, overall and AML-free survival did not differ between pediatric and adult patients.
- Independent prognostic factors. Multivariate analyses showed that the white blood cell count remained significant for decreased overall survival and high bone marrow blast count for decreased AML-free survival [119].
5.8. The Possible Prognostic Impact of the DEK-NUP214 Fusion for AML Patients Receiving Intensive Antileukemic Treatment: Improved Prognosis After Allogeneic Stem Cell Transplantation
- Relapse and survival. Patients transplanted in first complete remission had lower relapse risk as well as higher AML-free and overall survival than patients transplanted with more advanced disease (i.e., second or later remission, active residual disease). Furthermore, for patients transplanted in first remission the relapse incidence was 19%, two-year AML-free survival 57% and two-year overall survival 61%.
- Prognostic classification. The results for patients transplanted in first remission are comparable to patients with favorable to intermediate risk disease [122], whereas other patients classified as having high-risk disease show two-year relapse rates up to 60% after allogeneic stem cell transplantation (for references and more detailed comments, see [123]).
- FLT3-ITD. The presence of FLT3-ITD did not significantly influence the outcome in this study, but it should be emphasized that information about FLT3 status was only available for approximately one-third of the patients.
5.9. The Possible Prognostic Impact of the DEK-NUP214 Fusion Pediatric AML Patients Receiving Intensive Antileukemic Adverse Prognosis Improved by Allogeneic Stem Cell Transplantation
5.10. Should MDS Patients with t(6;9) Rather Be Classified as AML Patients?
6. The del(9)(q34.11q34.13) Abnormality with the SET (SET Nuclear Proto-Oncogene)-NUP214 Fusion Protein
6.1. The Structure and Function of the Fusion Partner SET (SET Nuclear Proto-Oncogene)
6.2. The Histone Chaperone Function of SET: An Important Function That Is Shared with DEK
6.3. The Structure and Functions of the SET-NUP214 Fusion Protein
6.4. Patient Characteristics and Biological Characteristics of Non-T Acute Leukemia Cells with SET-NUP214 Fusion
- Age. There is a dominance of males among published cases (16 out of 20 published cases) and most of these 20 patients were relatively young (median age 32 years, range 12–50 years).
- Differentiation and stemness. Many of these T-ALLs show expression of the myeloid markers CD13 and CD33 and several AMLs with aberrant cytoplasmic CD3 have also been described. The non-T cell variants of SET-NUP214 positive acute leukemias show a considerable diversity according to the WHO classification and include AML, acute undifferentiated leukemia, mixed phenotype acute leukemia and blast phase of CML. This is therefore a very heterogeneous AML group with regard to differentiation/phenotype; whereas, in contrast, the large majority of T-ALL with SET-NUP214 fusion share a common characteristic—TCRγδ expression [133]. Finally, the fusion protein modulate vitamin D receptor functions, including its function as a transcription factor [181], and vitamin D is also important for maintaining stemness both in normal and AML leukemogenesis [182].
- Leukemization. These patients show a wide variation with regard to leukemization; both decreased leukocyte levels as well as hyperleukocytosis have been described. A recent review described a median white blood cell count of 38.8 × 109/L (range 0.56–283 × 109/L).
6.5. Does the Fusion Protein SET-NUP214 Have Any Prognostic Impact in Human AML?
7. Episomal Amplification of Chromosome Arm 9q with Formation of the NUP214-ABL1 Fusion Protein
7.1. The Structure and Function of ABL1
7.2. The Genetic Abnormalities in NUP-ABL1 Fusion AML
7.3. The Clinical Characteristics of NUP214-ABL1 Fusion AML
- Age. This genetic abnormality has been detected both in pediatric and adult AML patients, but the abnormality has also been detected in patients with high-risk MDS.
- Differentiation of the AML cells. Cases with morphological signs of differentiation have been reported. The AML cells can express CD4, CD7, CD11b, CD11c, CD13, CD33, CD34, CD38, CD45, CD56, CD64, CD117, CD123, HLA-DR and myeloperoxidase.
- Diagnosis. This is a cryptic fusion; the diagnosis is made by molecular genetic analysis. The breakpoints vary between patients. Fusion genes with NUP214 breakpoints in exon 29 and 34 have been detected.
- Genetics. The abnormality can be detected in patients with normal karyotype as well as patient with additional/complex cytogenetic abnormalities. Mutations of BRCA2, GATA1, EGLN1, TP53, IDH2 and RAD50 as well as whole deletions of DDX41, NPM1 and RAD50 have been detected in combination with the NUP214-ABL1 fusion.
- Prognosis. Long-term AML-free survival has been described in one patient.
8. The der(5)t(5;9)(q35;q34) with the Sequestosome-1(SQSTM1)-NUP214 Fusion Protein
9. NUP214-RAC1 Fusion
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acids |
| ABC | ATP synthase (ATP)-binding cassette transporters |
| ABD | Actin-binding domain |
| ABL | ABL proto-oncogene 1, non-receptor tyrosine kinase |
| ADP | Adenosine diphosphate |
| AF9/MLLT3 | MLLT3 super elongation complex subunit |
| AKT | AKT serine/threonine kinase |
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| ASXL1 | ASXL transcriptional regulator 1 |
| ATP | Adenosine triphosphate |
| BMI1 | BMI1 proto-oncogene, polycomb ring finger |
| BRCA1/2 | BRCA1/2 DNA repair associated |
| CD | Cluster of differentiation |
| CDKN2C | Cyclin dependent kinase inhibitor 2C |
| CDX | Caudal type homeobox |
| CK2 | Casein kinase 2 |
| CREB | cAMP responsive element binding protein |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | CXC chemokine receptor |
| DDX41 | DEAD-box helicase 41 |
| DEK | DEK proto-oncogene |
| DOTIL | Disruptor of telomeric-silencing 1-like |
| DNA | Deoxyribonucleic acid |
| EBMT | European society for blood and marrow transplantation |
| ECOG | Eastern cooperative oncology group |
| EGLN1 | Egl-9 family hypoxia inducible factor 1 |
| EIF4E | Eukaryotic translation initiation factor 4E |
| EYA3 | EYA transcriptional coactivator and phosphatase 3 |
| FAB | French-American-British |
| FG | Phenylalanine-Glycine |
| FLT | Fms related receptor tyrosine kinase 3 |
| FOXC1 | Forkhead box C1 |
| FOXO1 | Forkhead box o1 |
| FYN | FYN proto-oncogene, Src family tyrosine kinase |
| GATA1 | GATA binding protein 1 |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GSK3 | Glycogen synthase kinase-3 |
| GTP | Guanosine triphosphate |
| HIF1α | Hypoxia inducible factor 1 subunit α |
| HIST2H4 | H4 clustered histone 14 (also referred to as H4C14) |
| HLB | HMG-like box |
| HOX | Homeobox |
| IDH2 | Isocitrate dehydrogenase 2 |
| IL | Interleukin |
| Ins | Insertion |
| ITD | Internal tandem duplication |
| IRAK1 | Interleukin 1 receptor associated kinase 1 |
| JAK | Janus kinase |
| kDA | Kilo Dalton |
| KLF4 | Krüppel-like factor 4 |
| KMT2A | Lysine methyltransferase 2A |
| MDS | Myelodysplastic syndrome |
| MEIS | Myeloid ecotropic viral integration site |
| miR | Micro RNA |
| MLL/KMT2A | Mixed lineage leukemia gene/Lysine methyltransferase 2A |
| MRD | Minimal residual disease |
| mTOR | Mechanistic (previously mammalian) target of rapamycin |
| NCOR1 | Nuclear receptor corepressor 1 |
| NES | Nuclear export signal |
| NFκB | Nuclear Factor kappa-light-chain-enhancer of activated B cells) |
| NFX1 | Nuclear transcription factor, X-box binding 1 |
| NK | Natural killer |
| NLS | Nuclear localization signal |
| NPM1 | Nuclophosmin 1 |
| NUP | Nucleoporin |
| P70S6K/RP70S6K | ribosomal protein S6 kinase B1 |
| PB1 | Phox-BEM1 |
| PCR | Polymerase chain reaction |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta |
| PP2A | Protein phosphatase 2 phosphatase activator |
| PRDM2 | PR/SET domain 2 (also referred to as RIZ) |
| PRL | Proline risch linker |
| RAC1 | Rac family small GTPase 1 |
| RAD50 | RAD50 double strand break repair protein |
| RAD51 | RAD51 recombinase |
| RAE1 | Ribonucleic acid export 1 |
| RNA | Ribonucleic acid |
| SAF | Scaffold attachment factor protein domain |
| SAP domain | SAF-A and -B, Acibus, PIAS domain |
| SESN1 | Sestrin 1 |
| SET | SET nuclear proto-oncogene |
| SH2/3 | Src homology 2/3 |
| SETBP | SET binding protein |
| SMAD | Suppressor of Mothers against Decapentaplegic |
| SQSTM1 | Sequestome 1 |
| SRC | SRC proto-oncogene, non-receptor tyrosine kinase |
| STAT | Signal transducer and activator of transcription |
| SWOG | Southwest oncology group |
| TB | TGFβ-binding protein-like |
| TCR | T cell receptor |
| TGFβ | Transforming growth factor β |
| VEGF | Vascular endothelial growth factor |
| XPO1 | Exportin 1 |
References
- Petrovic, S.; Mobbs, G.W.; Bley, C.J.; Nie, S.; Patke, A.; Hoelz, A. Structure and Function of the Nuclear Pore Complex. Cold Spring Harb. Perspect. Biol. 2022, 14, a041264. [Google Scholar] [CrossRef]
- Lin, D.H.; Hoelz, A. The Structure of the Nuclear Pore Complex (An Update). Annu. Rev. Biochem. 2019, 88, 725–783. [Google Scholar] [CrossRef] [PubMed]
- Zaitsava, H.; Gachowska, M.; Bartoszewska, E.; Kmiecik, A.; Kulbacka, J. The Potential of Nuclear Pore Complexes in Cancer Therapy. Molecules 2024, 29, 4832. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Selheim, F.; Hernandez-Valladares, M.; Reikvam, H. XPO1/Exportin-1 in Acute Myelogenous Leukemia; Biology and Therapeutic Targeting. Biomolecules 2025, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Savona, M.; Baz, R.; Andreeff, M.; Gabrail, N.; Gutierrez, M.; Savoie, L.; Mau-Sorensen, P.M.; Wagner-Johnston, N.; Yee, K.; et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood 2017, 129, 3165–3174. [Google Scholar] [CrossRef]
- Taylor, J.; Mi, X.; Penson, A.V.; Paffenholz, S.V.; Alvarez, K.; Sigler, A.; Chung, S.S.; Rampal, R.K.; Park, J.H.; Stein, E.M.; et al. Safety and activity of selinexor in patients with myelodysplastic syndromes or oligoblastic acute myeloid leukaemia refractory to hypomethylating agents: A single-centre, single-arm, phase 2 trial. Lancet Haematol. 2020, 7, e566–e574. [Google Scholar] [CrossRef]
- Janssen, J.J.W.M.; Löwenberg, B.; Manz, M.; Biemond, B.J.; Westerweel, P.E.; Klein, S.K.; Fehr, M.; Sinnige, H.A.M.; Efthymiou, A.; Legdeur, M.C.J.C.; et al. Addition of the nuclear export inhibitor selinexor to standard intensive treatment for elderly patients with acute myeloid leukemia and high risk myelodysplastic syndrome. Leukemia 2022, 36, 2189–2195. [Google Scholar] [CrossRef]
- Moore, M.A.; Chung, K.Y.; Plasilova, M.; Schuringa, J.J.; Shieh, J.H.; Zhou, P.; Morrone, G. NUP98 dysregulation in myeloid leukemogenesis. Ann. N. Y. Acad. Sci. 2007, 1106, 114–142. [Google Scholar] [CrossRef]
- Gough, S.M.; Slape, C.I.; Aplan, P.D. NUP98 gene fusions and hematopoietic malignancies: Common themes and new biologic insights. Blood 2011, 118, 6247–6257. [Google Scholar] [CrossRef]
- Charles Cano, F.; Kloos, A.; Hebalkar, R.Y.; Plenge, T.; Geffers, R.; Kirchhoff, H.; Kattre, N.; Görlich, K.; Büsche, G.; Shcherbata, H.R.; et al. XPO1-dependency of DEK:NUP214 leukemia. Leukemia 2025, 39, 1102–1113. [Google Scholar] [CrossRef]
- Chatel, G.; Fahrenkrog, B. Dynamics and diverse functions of nuclear pore complex proteins. Nucleus 2012, 3, 162–171. [Google Scholar] [CrossRef]
- Chatel, G.; Fahrenkrog, B. Nucleoporins: Leaving the nuclear pore complex for a successful mitosis. Cell. Signal. 2011, 23, 1555–1562. [Google Scholar] [CrossRef]
- Mendes, A.; Fahrenkrog, B. NUP214 in Leukemia: It’s More than Transport. Cells 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Paulillo, S.M.; Powers, M.A.; Ullman, K.S.; Fahrenkrog, B. Changes in nucleoporin domain topology in response to chemical effectors. J. Mol. Biol. 2006, 363, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gaik, M.; Flemming, D.; von Appen, A.; Kastritis, P.; Mücke, N.; Fischer, J.; Stelter, P.; Ori, A.; Bui, K.H.; Baßler, J.; et al. Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold. J. Cell Biol. 2015, 208, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.H.; von Appen, A.; DiGuilio, A.L.; Ori, A.; Sparks, L.; Mackmull, M.T.; Bock, T.; Hagen, W.; Andrés-Pons, A.; Glavy, J.S.; et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell 2013, 155, 1233–1243. [Google Scholar] [CrossRef]
- Port, S.A.; Monecke, T.; Dickmanns, A.; Spillner, C.; Hofele, R.; Urlaub, H.; Ficner, R.; Kehlenbach, R.H. Structural and Functional Characterization of CRM1-Nup214 Interactions Reveals Multiple FG-Binding Sites Involved in Nuclear Export. Cell Rep. 2015, 13, 690–702. [Google Scholar] [CrossRef]
- Sheikhi, M.; Siyadat, P.; Rostami, M.; Sadeghian, M.H.; Zahiri, E.; Ghorbani, M.; Ayatollahi, H.; Ayatollahi, A.; Hemmatan Attarbashi, R.; Khoshnegah, Z. Prognostic importance of NUP98-rearrangements in acute myeloid leukemia: A systematic review and meta-analysis. Casp. J. Intern. Med. 2024, 15, 579–588. [Google Scholar]
- Rasouli, M.; Troester, S.; Grebien, F.; Goemans, B.F.; Zwaan, C.M.; Heidenreich, O. NUP98 oncofusions in myeloid malignancies: An update on molecular mechanisms and therapeutic opportunities. Hemasphere 2024, 8, e70013. [Google Scholar] [CrossRef]
- Kalverda, B.; Pickersgill, H.; Shloma, V.V.; Fornerod, M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 2010, 140, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Bonten-Surtel, J.; Grosveld, G. Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis. Mol. Cell Biol. 1998, 18, 1236–1247. [Google Scholar] [CrossRef]
- van Deursen, J.; Boer, J.; Kasper, L.; Grosveld, G. G2 arrest and impaired nucleocytoplasmic transport in mouse embryos lacking the proto-oncogene CAN/Nup214. EMBO J. 1996, 15, 5574–5583. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L. Specific nucleoporin requirement for Smad nuclear translocation. Mol. Cell Biol. 2010, 30, 4022–4034. [Google Scholar] [CrossRef]
- Yao, X.; Chen, X.; Cottonham, C.; Xu, L. Preferential utilization of Imp7/8 in nuclear import of Smads. J. Biol. Chem. 2008, 283, 22867–22874. [Google Scholar] [CrossRef]
- Xu, L.; Kang, Y.; Cöl, S.; Massagué, J. Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFbeta signaling complexes in the cytoplasm and nucleus. Mol. Cell 2002, 10, 271–282. [Google Scholar] [CrossRef]
- Xu, L.; Alarcón, C.; Cöl, S.; Massagué, J. Distinct domain utilization by Smad3 and Smad4 for nucleoporin interaction and nuclear import. J. Biol. Chem. 2003, 278, 42569–42577. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, F.W.; Akel, S.; Bartelmez, S.H. Autocrine transforming growth factor-beta regulation of hematopoiesis: Many outcomes that depend on the context. Oncogene 2005, 24, 5751–5763. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Bergmann, S.; Pandolfi, P.P. Deregulated TGF-beta signaling in leukemogenesis. Oncogene 2005, 24, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, S.; Roy, K.S.; Ganguly, A.; Sarkar, S.; Panda, C.K.; Bhattacharyya, D.; Bhattacharyya, N.P.; Roychoudhury, S. Inhibition of nucleoporin member Nup214 expression by miR-133b perturbs mitotic timing and leads to cell death. Mol. Cancer 2015, 14, 42. [Google Scholar] [CrossRef]
- Argiropoulos, B.; Humphries, R.K. Hox genes in hematopoiesis and leukemogenesis. Oncogene 2007, 26, 6766–6776. [Google Scholar] [CrossRef]
- Khan, I.; Amin, M.A.; Eklund, E.A.; Gartel, A.L. Regulation of HOX gene expression in AML. Blood Cancer J. 2024, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Mura, S.; Otani, M.; Miyamoto, Y.; Nogami, J.; Maehara, K.; Harada, A.; Tachibana, T.; Yoneda, Y.; Ohkawa, Y. Chromatin-bound CRM1 recruits SET-Nup214 and NPM1c onto HOX clusters causing aberrant HOX expression in leukemia cells. eLife 2019, 8, e46667. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Mura, S.; Yamada, K.; Sangel, P.; Hirata, S.; Maehara, K.; Kawakami, K.; Tachibana, T.; Ohkawa, Y.; Kimura, H.; et al. Chromatin-prebound Crm1 recruits Nup98-HoxA9 fusion to induce aberrant expression of Hox cluster genes. Elife 2016, 5, e09540. [Google Scholar] [CrossRef] [PubMed]
- Shimosato, Y.; Yamamoto, K.; Jia, Y.; Zhang, W.; Shiba, N.; Hayashi, Y.; Ito, S.; Kitamura, T.; Goyama, S. NPM1-fusion proteins promote myeloid leukemogenesis through XPO1-dependent HOX activation. Leukemia 2025, 39, 75–86. [Google Scholar]
- Scholl, C.; Bansal, D.; Döhner, K.; Eiwen, K.; Huntly, B.J.; Lee, B.H.; Rücker, F.G.; Schlenk, R.F.; Bullinger, L.; Döhner, H.; et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J. Clin. Investig. 2007, 117, 1037–1048. [Google Scholar] [CrossRef]
- Darvishi, M.; Mashati, P.; Khosravi, A. The clinical significance of CDX2 in leukemia: A new perspective for leukemia research. Leuk. Res. 2018, 72, 45–51. [Google Scholar] [CrossRef]
- Rawat, V.P.S.; Götze, M.; Rasalkar, A.; Vegi, N.M.; Ihme, S.; Thoene, S.; Pastore, A.; Bararia, D.; Döhner, H.; Döhner, K.; et al. The microRNA miR-196b acts as a tumor suppressor in Cdx2-driven acute myeloid leukemia. Haematologica 2020, 105, e285–e289. [Google Scholar]
- Rawat, V.P.; Cusan, M.; Deshpande, A.; Hiddemann, W.; Quintanilla-Martinez, L.; Humphries, R.K.; Bohlander, S.K.; Feuring-Buske, M.; Buske, C. Ectopic expression of the homeobox gene Cdx2 is the transforming event in a mouse model of t(12;13)(p13;q12) acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2004, 101, 817–822. [Google Scholar] [CrossRef]
- Rawat, V.P.; Thoene, S.; Naidu, V.M.; Arseni, N.; Heilmeier, B.; Metzeler, K.; Petropoulos, K.; Deshpande, A.; Quintanilla-Martinez, L.; Bohlander, S.K.; et al. Overexpression of CDX2 perturbs HOX gene expression in murine progenitors depending on its N-terminal domain and is closely correlated with deregulated HOX gene expression in human acute myeloid leukemia. Blood 2008, 111, 309–319. [Google Scholar] [CrossRef][Green Version]
- Koo, S.; Huntly, B.J.; Wang, Y.; Chen, J.; Brumme, K.; Ball, B.; McKinney-Freeman, S.L.; Yabuuchi, A.; Scholl, C.; Bansal, D.; et al. Cdx4 is dispensable for murine adult hematopoietic stem cells but promotes MLL-AF9-mediated leukemogenesis. Haematologica 2010, 95, 1642–1650. [Google Scholar][Green Version]
- Bansal, D.; Scholl, C.; Fröhling, S.; McDowell, E.; Lee, B.H.; Döhner, K.; Ernst, P.; Davidson, A.J.; Daley, G.Q.; Zon, L.I.; et al. Cdx4 dysregulates Hox gene expression and generates acute myeloid leukemia alone and in cooperation with Meis1a in a murine model. Proc. Natl. Acad. Sci. USA 2006, 103, 16924–16929. [Google Scholar] [CrossRef] [PubMed]
- Fröhling, S.; Scholl, C.; Bansal, D.; Huntly, B.J. HOX gene regulation in acute myeloid leukemia: CDX marks the spot? Cell Cycle 2007, 6, 2241–2245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faber, K.; Bullinger, L.; Ragu, C.; Garding, A.; Mertens, D.; Miller, C.; Martin, D.; Walcher, D.; Döhner, K.; Döhner, H.; et al. CDX2-driven leukemogenesis involves KLF4 repression and deregulated PPARγ signaling. J. Clin. Investig. 2013, 123, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Iwasaki, M.; Ficara, F.; Lin, C.; Matheny, C.; Wong, S.; Smith, K.S.; Cleary, M.L. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell 2010, 17, 597–608. [Google Scholar] [CrossRef]
- Chen, C.W.; Armstrong, S.A. Targeting DOT1L and HOX gene expression in MLL-rearranged leukemia and beyond. Exp. Hematol. 2015, 43, 673–684. [Google Scholar] [CrossRef]
- Slovak, M.L.; Gundacker, H.; Bloomfield, C.D.; Dewald, G.; Appelbaum, F.R.; Larson, R.A.; Tallman, M.S.; Bennett, J.M.; Stirewalt, D.L.; Meshinchi, S.; et al. A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare ‘poor prognosis’ myeloid malignancies. Leukemia 2006, 20, 1295–1297. [Google Scholar] [CrossRef]
- Wilcher, K.E.; Page, E.R.H.; Privette Vinnedge, L.M. The impact of the chromatin binding DEK protein in hematopoiesis and acute myeloid leukemia. Exp. Hematol. 2023, 123, 18–27. [Google Scholar] [CrossRef]
- Sandén, C.; Gullberg, U. The DEK oncoprotein and its emerging roles in gene regulation. Leukemia 2015, 29, 1632–1636. [Google Scholar] [CrossRef]
- Capitano, M.L.; Broxmeyer, H.E. A role for intracellular and extracellular DEK in regulating hematopoiesis. Curr. Opin. Hematol. 2017, 24, 300–306. [Google Scholar] [CrossRef]
- Riveiro-Falkenbach, E.; Soengas, M.S. Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin. Cancer Res. 2010, 16, 2932–2938. [Google Scholar] [CrossRef]
- Privette Vinnedge, L.M.; Kappes, F.; Nassar, N.; Wells, S.I. Stacking the DEK: From chromatin topology to cancer stem cells. Cell Cycle 2013, 12, 51–66. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Mor-Vaknin, N.; Kappes, F.; Legendre, M.; Saha, A.K.; Ou, X.; O’Leary, H.; Capitano, M.; Cooper, S.; Markovitz, D.M. Concise review: Role of DEK in stem/progenitor cell biology. Stem Cells 2013, 31, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Kappes, F.; Scholten, I.; Richter, N.; Gruss, C.; Waldmann, T. Functional domains of the ubiquitous chromatin protein DEK. Mol. Cell Biol. 2004, 24, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Kappes, F.; Damoc, C.; Knippers, R.; Przybylski, M.; Pinna, L.A.; Gruss, C. Phosphorylation by protein kinase CK2 changes the DNA binding properties of the human chromatin protein DEK. Mol. Cell Biol. 2004, 24, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.; Scholten, I.; Kappes, F.; Hu, H.G.; Knippers, R. The DEK protein—An abundant and ubiquitous constituent of mammalian chromatin. Gene 2004, 343, 1–9. [Google Scholar] [CrossRef]
- Kujirai, T.; Echigoya, K.; Kishi, Y.; Saeki, M.; Ito, T.; Kato, J.; Negishi, L.; Kimura, H.; Masumoto, H.; Takizawa, Y.; et al. Structural insights into how DEK nucleosome binding facilitates H3K27 trimethylation in chromatin. Nat. Struct. Mol. Biol. 2025, 32, 1183–1192. [Google Scholar] [CrossRef]
- Fornerod, M.; Boer, J.; van Baal, S.; Jaeglé, M.; von Lindern, M.; Murti, K.G.; Davis, D.; Bonten, J.; Buijs, A.; Grosveld, G. Relocation of the carboxyterminal part of CAN from the nuclear envelope to the nucleus as a result of leukemia-specific chromosome rearrangements. Oncogene 1995, 10, 1739–1748. [Google Scholar]
- Chen, Z.; Huo, D.; Li, L.; Liu, Z.; Li, Z.; Xu, S.; Huang, Y.M.; Wu, W.; Zhou, C.; Liu, Y.; et al. Nuclear DEK preserves hematopoietic stem cells potential via NCoR1/HDAC3-Akt1/2-mTOR axis. J. Exp. Med. 2021, 218, e20201974. [Google Scholar] [CrossRef]
- Hollenbach, A.D.; McPherson, C.J.; Mientjes, E.J.; Iyengar, R.; Grosveld, G. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 2002, 115, 3319–3330. [Google Scholar] [CrossRef]
- Ko, S.I.; Lee, I.; Kim, J.Y.; Kim, S.M.; Kim, D.W.; Lee, K.S.; Woo, K.M.; Baek, J.H.; Choo, J.K.; Seo, S.B. Regulation of histone acetyltransferase activity of p300 and PCAF by proto-oncogene protein DEK. FEBS Lett. 2006, 580, 3217–3222. [Google Scholar] [CrossRef]
- Kavanaugh, G.M.; Wise-Draper, T.M.; Morreale, R.J.; Morrison, M.A.; Gole, B.; Schwemberger, S.; Tichy, E.D.; Lu, L.; Babcock, G.F.; Wells, J.M.; et al. The human DEK oncogene regulates DNA damage response signaling and repair. Nucleic Acids Res. 2011, 39, 7465–7476. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Gole, B.; Willis, N.A.; Soria, R.; Starnes, L.M.; Krumpelbeck, E.F.; Jegga, A.G.; Ali, A.M.; Guo, H.; Meetei, A.R.; et al. DEK is required for homologous recombination repair of DNA breaks. Sci. Rep. 2017, 7, 44662. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, N.; Prell, M.; Königs, H.; Hermanns-Sachweh, B.; Lüscher, B.; Kappes, F. Bacterial Growth Inhibition Screen (BGIS): Harnessing recombinant protein toxicity for rapid and unbiased interrogation of protein function. FEBS Lett. 2021, 595, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Y.; Zhang, Y.; Xing, Y.; Suo, J. DEK modulates both expression and alternative splicing of cancerrelated genes. Oncol. Rep. 2022, 47, 111. [Google Scholar] [CrossRef]
- Yue, L.; Wan, R.; Luan, S.; Zeng, W.; Cheung, T.H. Dek Modulates Global Intron Retention during Muscle Stem Cells Quiescence Exit. Dev. Cell 2020, 53, 661–676. [Google Scholar] [CrossRef]
- McGarvey, T.; Rosonina, E.; McCracken, S.; Li, Q.; Arnaout, R.; Mientjes, E.; Nickerson, J.A.; Awrey, D.; Greenblatt, J.; Grosveld, G.; et al. The acute myeloid leukemia- associated protein, DEK, forms a splicing-dependent interaction with exon- product complexes. J. Cell Biol. 2000, 50, 309–320. [Google Scholar] [CrossRef]
- Cleary, J.; Sitwala, K.V.; Khodadoust, M.S.; Kwok, R.P.; Mor-Vaknin, N.; Cebrat, M.; Cole, P.A.; Markovitz, D.M. p300/CBP-associated factor drives DEK into interchromatin granule clusters. J. Biol. Chem. 2005, 280, 31760–31767. [Google Scholar] [CrossRef]
- Tabbert, A.; Kappes, F.; Knippers, R.; Kellermann, J.; Lottspeich, F.; Ferrando-May, E. Hypophosphorylation of the architectural chromatin protein DEK in death-receptor-induced apoptosis revealed by the isotope coded protein label proteomic platform. Proteomics 2006, 6, 5758–5772. [Google Scholar] [CrossRef]
- Adams, A.K.; Bolanos, L.C.; Dexheimer, P.J.; Karns, R.A.; Aronow, B.J.; Komurov, K.; Jegga, A.G.; Casper, K.A.; Patil, Y.J.; Wilson, K.M.; et al. IRAK1 is a novel DEK transcriptional target and is essential for head and neck cancer cell survival. Oncotarget 2015, 6, 43395–43407. [Google Scholar] [CrossRef]
- Pease, N.A.; Shephard, M.S.; Sertorio, M.; Waltz, S.E.; Vinnedge, L.M.P. DEK Expression in Breast Cancer Cells Leads to the Alternative Activation of Tumor Associated Macrophages. Cancers 2020, 12, 1936. [Google Scholar] [CrossRef]
- Serrano-Lopez, J.; Nattamai, K.; Pease, N.A.; Shephard, M.S.; Wellendorf, A.M.; Sertorio, M.; Smith, E.A.; Geiger, H.; Wells, S.I.; Cancelas, J.A.; et al. Loss of DEK induces radioresistance of murine restricted hematopoietic progenitors. Exp. Hematol. 2018, 59, 40–50.e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Jia, X.Z.; Lu, Q.Y.; Cai, S.; Huang, X.T.; Yang, S.H.; Wood, C.; Wang, Y.H.; Zhou, J.J.; Chen, Y.D.; et al. Exosomal DEK removes chemoradiotherapy resistance by triggering quiescence exit of breast cancer stem cells. Oncogene 2022, 41, 2624–2637. [Google Scholar] [CrossRef] [PubMed]
- Wise-Draper, T.M.; Allen, H.V.; Jones, E.E.; Habash, K.B.; Matsuo, H.; Wells, S.I. Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol. Cell Biol. 2006, 26, 7506–7519. [Google Scholar] [PubMed]
- Pei, H.Z.; Guo, Y.; Zhao, Y.; Zhang, D.; Chang, Z.; Zhou, J.; Baek, S.H.; Zhao, Z.J.; Chen, C.; Chen, Y. FLT3 inhibitors induce p53 instability, driven by STAT5/MDM2/p53 competitive interactions in acute myeloid leukemia. Cancer Lett. 2025, 611, 217446. [Google Scholar]
- Brenner, A.K.; Bruserud, Ø. Functional Toll-Like Receptors (TLRs) Are Expressed by a Majority of Primary Human Acute Myeloid Leukemia Cells and Inducibility of the TLR Signaling Pathway Is Associated with a More Favorable Phenotype. Cancers 2019, 11, 973. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Reikvam, H.; Brenner, A.K. Toll-like Receptor 4, Osteoblasts and Leukemogenesis; the Lesson from Acute Myeloid Leukemia. Molecules 2022, 27, 735. [Google Scholar] [CrossRef]
- Caiado, F.; Manz, M.G. IL-1 in aging and pathologies of hematopoietic stem cells. Blood 2024, 144, 368–377. [Google Scholar] [CrossRef]
- Logan, G.E.; Mor-Vaknin, N.; Braunschweig, T.; Jost, E.; Schmidt, P.V.; Markovitz, D.M.; Mills, K.I.; Kappes, F.; Percy, M.J. DEK oncogene expression during normal hematopoiesis and in Acute Myeloid Leukemia (AML). Blood Cells Mol. Dis. 2015, 54, 123–131. [Google Scholar]
- Zhang, Y.; Liu, J.; Wang, S.; Luo, X.; Li, Y.; Lv, Z.; Zhu, J.; Lin, J.; Ding, L.; Ye, Q. The DEK oncogene activates VEGF expression and promotes tumor angiogenesis and growth in HIF-1α-dependent and -independent manners. Oncotarget 2016, 7, 23740–23756. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Saha, A.; Legendre, M.; Carmona-Rivera, C.; Amin, M.A.; Rabquer, B.J.; Gonzales-Hernandez, M.J.; Jorns, J.; Mohan, S.; Yalavarthi, S.; et al. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat. Commun. 2017, 8, 14252. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Kappes, F.; Dick, A.E.; Legendre, M.; Damoc, C.; Teitz-Tennenbaum, S.; Kwok, R.; Ferrando-May, E.; Adams, B.S.; Markovitz, D.M. DEK in the synovium of patients with juvenile idiopathic arthritis: Characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheumatol. 2011, 63, 556–567. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Faulkner, N.; Legendre, M.; Khodadoust, M.S.; Kappes, F.; Ruth, J.H.; Koch, A.; Glass, D.; et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol. Cell Biol. 2006, 26, 9484–9496. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Kappes, F.; Mundade, A.; Deutzmann, A.; Rosmarin, D.M.; Legendre, M.; Chatain, N.; Al-Obaidi, Z.; Adams, B.S.; Ploegh, H.L.; et al. Intercellular trafficking of the nuclear oncoprotein DEK. Proc. Natl. Acad. Sci. USA 2013, 110, 6847–6852. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Callister, M.E.; Mumby, S.; Quinlan, G.J.; Welsh, K.I.; duBois, R.M.; Evans, T.W. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur. Respir. J. 2004, 23, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Hasegawa, M.; Takehara, K.; Sato, S. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J. Rheumatol. 2004, 31, 1112–1120. [Google Scholar]
- Capitano, M.L.; Mor-Vaknin, N.; Saha, A.K.; Cooper, S.; Legendre, M.; Guo, H.; Contreras-Galindo, R.; Kappes, F.; Sartor, M.A.; Lee, C.T.; et al. Secreted nuclear protein DEK regulates hematopoiesis through CXCR2 signaling. J. Clin. Investig. 2019, 129, 2555–2570. [Google Scholar] [CrossRef]
- Capitano, M.L.; Sammour, Y.; Ropa, J.; Legendre, M.; Mor-Vaknin, N.; Markovitz, D.M. DEK, a nuclear protein, is chemotactic for hematopoietic stem/progenitor cells acting through CXCR2 and Gαi signaling. J. Leukoc. Biol. 2022, 112, 449–456. [Google Scholar] [CrossRef]
- Tang, W.; Li, Z.; Li, X.; Huo, Z. High CXCR2 expression predicts poor prognosis in adult patients with acute myeloid leukemia. Ther. Adv. Hematol. 2020, 11, 2040620720958586. [Google Scholar] [CrossRef]
- Cao, H.; Tadros, V.; Hiramoto, B.; Leeper, K.; Hino, C.; Xiao, J.; Pham, B.; Kim, D.H.; Reeves, M.E.; Chen, C.S.; et al. Targeting TKI-Activated NFKB2-MIF/CXCLs-CXCR2 Signaling Pathways in FLT3 Mutated Acute Myeloid Leukemia Reduced Blast Viability. Biomedicines 2022, 10, 1038. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Zhang, Y.; Li, M.; Chen, J. Prognostic role of chemokine-related genes in acute myeloid leukemia. PeerJ 2024, 12, e1. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, J.; Chen, X.; Hu, Y.; Xie, W.; Yao, J.; Huang, S. Risk Stratification in Acute Myeloid Leukemia Using CXCR Gene Signatures: A Bioinformatics Analysis. Front. Oncol. 2020, 10, 584766. [Google Scholar] [CrossRef]
- Casas, S.; Nagy, B.; Elonen, E.; Aventín, A.; Larramendy, M.L.; Sierra, J.; Ruutu, T.; Knuutila, S. Aberrant expression of HOXA9, DEK, CBL and CSF1R in acute myeloid leukemia. Leuk. Lymphoma 2003, 44, 1935–1941. [Google Scholar] [CrossRef]
- Larramendy, M.L.; Niini, T.; Elonen, E.; Nagy, B.; Ollila, J.; Vihinen, M.; Knuutila, S. Overexpression of translocation-associated fusion genes of FGFRI, MYC, NPMI, and DEK, but absence of the translocations in acute myeloid leukemia. A microarray analysis. Haematologica 2002, 87, 569–577. [Google Scholar] [PubMed]
- Chiriches, C.; Khan, D.; Wieske, M.; Guillen, N.; Rokicki, M.; Guy, C.; Wilson, M.; Heesom, K.J.; Ottmann, O.G.; Ruthardt, M. Activation of signaling pathways in models of t(6;9)-acute myeloid leukemia. Ann. Hematol. 2022, 101, 2179–2193. [Google Scholar] [CrossRef] [PubMed]
- Chiriches, C.; Nicolaisen, N.; Wieske, M.; Elhaddad, H.; Mehmetbeyoglu, E.; Alvares, C.; Becher, D.; Hole, P.; Ottmann, O.G.; Ruthardt, M. Understanding a high-risk acute myeloid leukemia by analyzing the interactome of its major driver mutation. PLoS Genet. 2022, 18, e1010463. [Google Scholar] [CrossRef] [PubMed]
- Kaya, F.; Bewicke-Copley, F.; Miettinen, J.J.; Casado, P.; Leddy, E.; Deniz, Ö.; Lavallée, V.P.; Philippe, C.; Zheng, J.; Grebien, F.; et al. DEK::NUP214 acts as an XPO1-dependent transcriptional activator of essential leukemia genes. Leukemia 2025, 39, 1526–1531. [Google Scholar] [CrossRef]
- Saito, S.; Cigdem, S.; Okuwaki, M.; Nagata, K. Leukemia-Associated Nup214 Fusion Proteins Disturb the XPO1-Mediated Nuclear-Cytoplasmic Transport Pathway and Thereby the NF-κB Signaling Pathway. Mol. Cell Biol. 2016, 36, 1820–1835. [Google Scholar] [CrossRef]
- Matsushima, T.; Saitoh, T.; Karasawa, M.; Takizawa, M.; Miyawaki, S.; Nojima, Y.; Murakami, H. Effect of cytokines on growth and differentiation of leukaemic cells with translocation t(6;9)(p23;q34). Br. J. Haematol. 2001, 115, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Sandén, C.; Ageberg, M.; Petersson, J.; Lennartsson, A.; Gullberg, U. Forced expression of the DEK-NUP214 fusion protein promotes proliferation dependent on upregulation of mTOR. BMC Cancer 2013, 13, 440. [Google Scholar] [CrossRef]
- Moore, M.A.; Dorn, D.C.; Schuringa, J.J.; Chung, K.Y.; Morrone, G. Constitutive activation of Flt3 and STAT5A enhances self-renewal and alters differentiation of hematopoietic stem cells. Exp. Hematol. 2007, 35 (Suppl. S1), 105–116. [Google Scholar] [CrossRef]
- Chougule, R.A.; Kazi, J.U.; Rönnstrand, L. FYN expression potentiates FLT3-ITD induced STAT5 signaling in acute myeloid leukemia. Oncotarget 2016, 7, 9964–9974. [Google Scholar] [CrossRef]
- Qin, H.; Malek, S.; Cowell, J.K.; Ren, M. Transformation of human CD34+ hematopoietic progenitor cells with DEK-NUP214 induces AML in an immunocompromised mouse model. Oncogene 2016, 35, 5686–5691. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yabe, M.; Zhang, X.; Kim, Y.; Wu, X.; Wei, P.; Chi, S.; Zheng, L.; Garcia-Manero, G.; Shao, L.; et al. Myelodysplastic syndrome with t(6;9)(p22;q34.1)/DEK-NUP214 better classified as acute myeloid leukemia? A multicenter study of 107 cases. Mod. Pathol. 2021, 34, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Potluri, S.; Kellaway, S.G.; Coleman, D.J.L.; Keane, P.; Imperato, M.R.; Assi, S.A.; Cockerill, P.N.; Bonifer, C. Gene regulation in t(6;9) DEK::NUP214 Acute Myeloid Leukemia resembles that of FLT3-ITD/NPM1 Acute Myeloid Leukemia but with an altered HOX/MEIS axis. Leukemia 2024, 38, 403–407. [Google Scholar] [CrossRef]
- Oancea, C.; Rüster, B.; Henschler, R.; Puccetti, E.; Ruthardt, M. The t(6;9) associated DEK/CAN fusion protein targets a population of long-term repopulating hematopoietic stem cells for leukemogenic transformation. Leukemia 2010, 24, 1910–1919. [Google Scholar] [CrossRef]
- Ageberg, M.; Drott, K.; Olofsson, T.; Gullberg, U.; Lindmark, A. Identification of a novel and myeloid specific role of the leukemia-associated fusion protein DEK-NUP214 leading to increased protein synthesis. Genes Chromosomes Cancer 2008, 47, 276–287. [Google Scholar] [CrossRef]
- Oancea, C.; Rüster, B.; Brill, B.; Roos, J.; Heinssmann, M.; Bug, G.; Mian, A.A.; Guillen, N.A.; Kornblau, S.M.; Henschler, R.; et al. STAT activation status differentiates leukemogenic from non-leukemogenic stem cells in AML and is suppressed by arsenic in t(6;9)-positive AML. Genes Cancer 2014, 5, 378–392. [Google Scholar] [CrossRef]
- Heuser, M.; Sly, L.M.; Argiropoulos, B.; Kuchenbauer, F.; Lai, C.; Weng, A.; Leung, M.; Lin, G.; Brookes, C.; Fung, S.; et al. Modeling the functional heterogeneity of leukemia stem cells: Role of STAT5 in leukemia stem cell self-renewal. Blood 2009, 114, 3983–3993. [Google Scholar] [CrossRef]
- Schepers, H.; Wierenga, A.T.; Vellenga, E.; Schuringa, J.J. STAT5-mediated self-renewal of normal hematopoietic and leukemic stem cells. JAKSTAT 2012, 1, 13–22. [Google Scholar] [CrossRef]
- Wingelhofer, B.; Maurer, B.; Heyes, E.C.; Cumaraswamy, A.A.; Berger-Becvar, A.; de Araujo, E.D.; Orlova, A.; Freund, P.; Ruge, F.; Park, J.; et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia 2018, 32, 1135–1146. [Google Scholar] [CrossRef]
- Garçon, L.; Libura, M.; Delabesse, E.; Valensi, F.; Asnafi, V.; Berger, C.; Schmitt, C.; Leblanc, T.; Buzyn, A.; Macintyre, E. DEK-CAN molecular monitoring of myeloid malignancies could aid therapeutic stratification. Leukemia 2005, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ashok Kumar, J.; Sitaram, U.; Neeraj, S.; Nancy, A.; Balasubramanian, P.; Abraham, A.; Mathews, V.; Viswabandya, A.; George, B.; et al. The t(6;9)(p22;q34) in myeloid neoplasms: A retrospective study of 16 cases. Cancer Genet. Cytogenet. 2010, 203, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Oyarzo, M.P.; Lin, P.; Glassman, A.; Bueso-Ramos, C.E.; Luthra, R.; Medeiros, L.J. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations. Am. J. Clin. Pathol. 2004, 122, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Alsabeh, R.; Brynes, R.K.; Slovak, M.L.; Arber, D.A. Acute myeloid leukemia with t(6;9) (p23;q34): Association with myelodysplasia, basophilia, and initial CD34 negative immunophenotype. Am. J. Clin. Pathol. 1997, 107, 430–437. [Google Scholar]
- Ishiyama, K.; Takami, A.; Kanda, Y.; Nakao, S.; Hidaka, M.; Maeda, T.; Naoe, T.; Taniguchi, S.; Kawa, K.; Nagamura, T.; et al. Prognostic factors for acute myeloid leukemia patients with t(6;9)(p23;q34) who underwent an allogeneic hematopoietic stem cell transplant. Leukemia 2012, 26, 1416–1419. [Google Scholar] [CrossRef][Green Version]
- Ishiyama, K.; Takami, A.; Kanda, Y.; Nakao, S.; Hidaka, M.; Maeda, T.; Naoe, T.; Taniguchi, S.; Kawa, K.; Nagamura, T.; et al. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9)(p23;q34) dramatically improves the patient prognosis: A matched-pair analysis. Leukemia 2012, 26, 461–464. [Google Scholar] [CrossRef]
- Tarlock, K.; Alonzo, T.A.; Moraleda, P.P.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Ravindranath, Y.; Lange, B.; Woods, W.G.; Gamis, A.S.; et al. Acute myeloid leukaemia (AML) with t(6;9)(p23;q34) is associated with poor outcome in childhood AML regardless of FLT3-ITD status: A report from the Children’s Oncology Group. Br. J. Haematol. 2014, 166, 254–259. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.X.; Liu, F.H.; Wang, S.G. Acute myeloid leukemia with DEK::NUP214 fusion resembling acute promyelocytic leukemia, initially presenting as sweet syndrome: A case report and literature review. J. Int. Med. Res. 2025, 53, 3000605251327476. [Google Scholar]
- Slovak, M.L.; Kopecky, K.J.; Cassileth, P.A.; Harrington, D.H.; Theil, K.S.; Mohamed, A.; Paietta, E.; Willman, C.L.; Head, D.R.; Rowe, J.M.; et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000, 96, 4075–4083. [Google Scholar]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- Díaz-Beyá, M.; Labopin, M.; Maertens, J.; Aljurf, M.; Passweg, J.; Dietrich, B.; Schouten, H.; Socié, G.; Schaap, N.; Schwerdtfeger, R.; et al. Allogeneic stem cell transplantation in AML with t(6;9)(p23;q34);DEK-NUP214 shows a favourable outcome when performed in first complete remission. Br. J. Haematol. 2020, 189, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Mrózek, K.; Marcucci, G.; Nicolet, D.; Maharry, K.S.; Becker, H.; Whitman, S.P.; Metzeler, K.H.; Schwind, S.; Wu, Y.Z.; Kohlschmidt, J.; et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 4515–4523. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Tallman, M.S. Outcomes of allogeneic stem cell transplantation for patients with t(6:9) AML- A strong case for allogeneic stem cell transplantation in first complete remission. Br. J. Haematol. 2020, 189, 806–808. [Google Scholar] [CrossRef]
- Csizmar, C.M.; Saliba, A.N.; Greipp, P.T.; Alkhateeb, H.; Begna, K.H.; Foran, J.M.; Gangat, N.; Hogan, W.J.; Hook, C.C.; Litzow, M.R.; et al. FLT3 inhibitors potentially improve response rates in acute myeloid leukemia harboring t(6;9)(DEK::NUP214): The Mayo Clinic experience. Haematologica 2024, 109, 3785–3789. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef]
- Sandahl, J.D.; Coenen, E.A.; Forestier, E.; Harbott, J.; Johansson, B.; Kerndrup, G.; Adachi, S.; Auvrignon, A.; Beverloo, H.B.; Cayuela, J.M.; et al. t(6;9)(p22;q34)/DEK-NUP214-rearranged pediatric myeloid leukemia: An international study of 62 patients. Haematologica 2014, 99, 865–872. [Google Scholar] [CrossRef]
- Cuneo, A.; Kerim, S.; Vandenberghe, E.; Van Orshoven, A.; Rodhain, J.; Bosly, A.; Zachee, P.; Louwagie, A.; Michaux, J.L.; Dal Cin, P.; et al. Translocation t(6;9) occurring in acute myelofibrosis, myelodysplastic syndrome, and acute nonlymphocytic leukemia suggests multipotent stem cell involvement. Cancer Genet. Cytogenet. 1989, 42, 209–219. [Google Scholar] [CrossRef]
- Shapira, M.Y.; Hirshberg, B.; Amir, G.; Rund, D. 6;9 translocation in myelodysplastic syndrome. Cancer Genet. Cytogenet. 1999, 112, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.S.; Raza, A.; Schumer, J.; Sait, S.N.; Block, A.W.; Snyderman, M.; Sandberg, A.A. Translocation t(6;9)(p22.3;q34) in myelodysplastic syndrome--refractory anemia with excess blasts. Cancer Genet. Cytogenet. 1987, 29, 135–138. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, Q.R.; Lu, X.X.; Zhang, L.J.; Wang, X.X.; Zhang, H.Y. The characteristics and prognostic significance of the SET-CAN/NUP214 fusion gene in hematological malignancies: A systematic review. Medicine 2022, 101, e29294. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, H.; Fan, S. SET-CAN/NUP214 fusion gene in leukemia: General features and clinical advances. Front. Oncol. 2023, 13, 1269531. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelali, R.; Roggy, A.; Leguay, T.; Cieslak, A.; Renneville, A.; Touzart, A.; Banos, A.; Randriamalala, E.; Caillot, D.; Lioure, B.; et al. SET-NUP214 is a recurrent γδ lineage-specific fusion transcript associated with corticosteroid/chemotherapy resistance in adult T-ALL. Blood 2014, 123, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Q.; Cen, J.; Xu, C.; Tao, T.T.; Xie, J.; Shen, W.; Gong, Y.; Pan, J.; Yao, L. Blast phase of chronic myeloid leukemia with concurrent BCR::ABL1 and SET::NUP214: A report of two cases. Mol. Carcinog. 2023, 62, 117–121. [Google Scholar] [CrossRef]
- Xu, X.; Zhai, Q.; Jin, H.; Yu, Y.; Han, D.; Zhang, H.; Fu, K.; Meng, B. SET-NUP214 Fusion Gene Involved Early T-Cell Precursor Acute Lymphoblastic Leukemia in Adult with B Marker Expression. Int. J. Gen. Med. 2021, 14, 659–664. [Google Scholar] [CrossRef]
- Sousa, L.O.; Sobral, L.M.; de Almeida, L.O.; Garcia, C.B.; Greene, L.J.; Leopoldino, A.M. SET protein modulates H4 histone methylation status and regulates miR-137 level in oral squamous cell carcinoma. Epigenomics 2020, 12, 475–485. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.C.; Liu, Y.F.; Qi, J.; Zhang, M. Construction of SET overexpression vector and its effects on the proliferation and apoptosis of 293T cells. Mol. Med. Rep. 2016, 13, 4329–4334. [Google Scholar] [CrossRef]
- Makishima, H.; Yoshida, K.; Nguyen, N.; Przychodzen, B.; Sanada, M.; Okuno, Y.; Ng, K.P.; Gudmundsson, K.O.; Vishwakarma, B.A.; Jerez, A.; et al. Somatic SETBP1 mutations in myeloid malignancies. Nat. Genet. 2013, 45, 942–946. [Google Scholar] [CrossRef]
- Cristóbal, I.; Garcia-Orti, L.; Cirauqui, C.; Alonso, M.M.; Calasanz, M.J.; Odero, M.D. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia 2011, 25, 606–614. [Google Scholar] [CrossRef]
- Cristóbal, I.; Blanco, F.J.; Garcia-Orti, L.; Marcotegui, N.; Vicente, C.; Rifon, J.; Novo, F.J.; Bandres, E.; Calasanz, M.J.; Bernabeu, C.; et al. SETBP1 overexpression is a novel leukemogenic mechanism that predicts adverse outcome in elderly patients with acute myeloid leukemia. Blood 2010, 115, 615–625. [Google Scholar] [CrossRef]
- Li, M.; Makkinje, A.; Damuni, Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 1996, 271, 11059–11062. [Google Scholar] [CrossRef]
- Oakley, K.; Han, Y.; Vishwakarma, B.A.; Chu, S.; Bhatia, R.; Gudmundsson, K.O.; Keller, J.; Chen, X.; Vasko, V.; Jenkins, N.A.; et al. Setbp1 promotes the self-renewal of murine myeloid progenitors via activation of Hoxa9 and Hoxa10. Blood 2012, 119, 6099–6108. [Google Scholar] [CrossRef]
- Inoue, D.; Kitaura, J.; Matsui, H.; Hou, H.A.; Chou, W.C.; Nagamachi, A.; Kawabata, K.C.; Togami, K.; Nagase, R.; Horikawa, S.; et al. SETBP1 mutations drive leukemic transformation in ASXL1-mutated MDS. Leukemia 2015, 29, 847–857. [Google Scholar] [CrossRef]
- Wang, D.; Kon, N.; Lasso, G.; Jiang, L.; Leng, W.; Zhu, W.G.; Qin, J.; Honig, B.; Gu, W. Acetylation-regulated interaction between p53 and SET reveals a widespread regulatory mode. Nature 2016, 538, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Kim, K.B.; Kang, J.Y.; Kim, S.R.; Jung, H.S.; Seo, S.B. Inhibition of FoxO1 acetylation by INHAT subunit SET/TAF-Iβ induces p21 transcription. FEBS Lett. 2014, 588, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kornblau, S.M.; Kobayashi, T.; Gambel, A.; Claxton, D.; Deisseroth, A.B. High levels of constitutive WAF1/Cip1 protein are associated with chemoresistance in acute myelogenous leukemia. Clin. Cancer Res. 1995, 1, 1051–1057. [Google Scholar]
- Shamloo, B.; Usluer, S. p21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Selheim, F.; Hernandez-Valladares, M.; Reikvam, H. Monocytic Differentiation in Acute Myeloid Leukemia Cells: Diagnostic Criteria, Biological Heterogeneity, Mitochondrial Metabolism, Resistance to and Induction by Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 6356. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Chu, G.; Lai, G.; Zhang, B.; Wang, L.; Zhao, Y. miR-137 downregulates c-kit expression in acute myeloid leukemia. Leuk. Res. 2017, 57, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.S.; Yang, Y.; Li, Y.; Lv, J.L.; Cheng, Y. TRIM25 contributes to the malignancy of acute myeloid leukemia and is negatively regulated by microRNA-137. Open Med. 2020, 16, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Senda, M.; Akai, Y.; Sato, L.; Suzuki, T.; Nagai, R.; Senda, T.; Horikoshi, M. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc. Natl. Acad. Sci. USA 2007, 104, 4285–4290. [Google Scholar] [CrossRef] [PubMed]
- Eitoku, M.; Sato, L.; Senda, T.; Horikoshi, M. Histone chaperones: 30 years from isolation to elucidation of the mechanisms of nucleosome assembly and disassembly. Cell Mol. Life Sci. 2008, 65, 414–444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagata, K.; Kawase, H.; Handa, H.; Yano, K.; Yamasaki, M.; Ishimi, Y.; Okuda, A.; Kikuchi, A.; Matsumoto, K. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 4279–4283. [Google Scholar] [CrossRef]
- Kutney, S.N.; Hong, R.; Macfarlan, T.; Chakravarti, D. A signaling role of histone-binding proteins and INHAT subunits pp32 and Set/TAF-Ib in integrating chro matin hypoacetylation and transcriptional repression. J. Biol. Chem. 2004, 279, 30850–30855. [Google Scholar] [CrossRef]
- Schneider, R.; Bannister, A.J.; Weise, C.; Kouzarides, T. Direct binding of INHAT to H3 tails disrupted by modifications. J. Biol. Chem. 2004, 279, 23859–23862. [Google Scholar] [CrossRef]
- Seo, S.B.; McNamara, P.; Heo, S.; Turner, A.; Lane, W.S.; Chakravarti, D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 2001, 104, 119–130. [Google Scholar] [CrossRef]
- Miyamoto, S.; Suzuki, T.; Muto, S.; Aizawa, K.; Kimura, A.; Mizuno, Y.; Nagino, T.; Imai, Y.; Adachi, N.; Horikoshi, M. Positive and negative regulation of the cardiovascular transcription factor KLF5 by p300 and the oncogenic regulator SET through interaction and acetylation onthe DNA-bindingdomain. Mol. Cell Biol. 2003, 23, 8528–8541. [Google Scholar] [CrossRef]
- Suzuki, T.; Muto, S.; Miyamoto, S.; Aizawa, K.; Horikoshi, M.; Nagai, R. Functional interaction of the DNA binding transcription factor Sp1 through its DNA-binding domain with the histone chaperone TAF-I. J. Biol. Chem. 2003, 278, 28758–28764. [Google Scholar] [CrossRef]
- Loven, M.A.; Muster, N.; Yates, J.R.; Nardulli, A.M. A novel estrogen receptor a-associated protein, template-activating factor Ib, inhibits acetylation and trans activation. Mol. Endocrinol. 2003, 17, 67–78. [Google Scholar] [CrossRef]
- Telese, F.; Bruni, P.; Donizetti, A.; Gianni, D.D.; Ambrosio, C.; Scaloni, A.; Zambrano, N.; Rosenfeld, M.G.; Russo, T. Transcription regulation by the adaptor protein Fe65 and the nucleosome assembly factor SET. EMBO Rep. 2005, 6, 77–82. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.G.; Song, J.; Kim, S.J.; Rha, S.Y.; Lee, K.A.; Park, T.S.; Choi, J.R. Molecular characterization of alternative SET-NUP214 fusion transcripts in a case of acute undifferentiated leukemia. Cancer Genet. Cytogenet. 2010, 201, 73–80. [Google Scholar] [CrossRef]
- Van Vlierberghe, P.; van Grotel, M.; Tchinda, J.; Lee, C.; Beverloo, H.B.; van der Spek, P.J.; Stubbs, A.; Cools, J.; Nagata, K.; Fornerod, M.; et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood 2008, 111, 4668–4680. [Google Scholar] [CrossRef]
- Quentmeier, H.; Schneider, B.; Röhrs, S.; Romani, J.; Zaborski, M.; Macleod, R.A.; Drexler, H.G. SET-NUP214 fusion in acute myeloid leukemia- and T-cell acute lymphoblastic leukemia-derived cell lines. J. Hematol. Oncol. 2009, 2, 3. [Google Scholar] [CrossRef]
- Kraemer, D.; Wozniak, R.W.; Blobel, G.; Radu, A. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc. Natl. Acad. Sci. USA 1994, 91, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- von Lindern, M.; van Baal, S.; Wiegant, J.; Raap, A.; Hagemeijer, A.; Grosveld, G. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3’ half to different genes: Characterization of the set gene. Mol. Cell Biol. 1992, 12, 3346–3355. [Google Scholar] [PubMed]
- Port, S.A.; Mendes, A.; Valkova, C.; Spillner, C.; Fahrenkrog, B.; Kaether, C.; Kehlenbach, R.H. The Oncogenic Fusion Proteins SET-Nup214 and Sequestosome-1 (SQSTM1)-Nup214 Form Dynamic Nuclear Bodies and Differentially Affect Nuclear Protein and Poly(A)+ RNA Export. J. Biol. Chem. 2016, 291, 23068–23083. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.; Jühlen, R.; Bousbata, S.; Fahrenkrog, B. Disclosing the Interactome of Leukemogenic NUP98-HOXA9 and SET-NUP214 Fusion Proteins Using a Proteomic Approach. Cells 2020, 9, 1666. [Google Scholar] [CrossRef]
- Mendes, A.; Jühlen, R.; Martinelli, V.; Fahrenkrog, B. Targeted CRM1-inhibition perturbs leukemogenic NUP214 fusion proteins and exerts anti-cancer effects in leukemia cell lines with NUP214 rearrangements. Oncotarget 2020, 11, 3371–3386. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Ryningen, A.; Olsnes, A.M.; Stordrange, L.; Øyan, A.M.; Kalland, K.H.; Gjertsen, B.T. Subclassification of patients with acute myelogenous leukemia based on chemokine responsiveness and constitutive chemokine release by their leukemic cells. Haematologica 2007, 92, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Cigdem, S.; Saito, S.; Nishikata, D.; Nagata, K.; Okuwaki, M. SET-NUP214 and MLL cooperatively regulate the promoter activity of the HoxA10 gene. Genes Cells 2021, 26, 830–837. [Google Scholar] [PubMed]
- Antunes, E.T.B.; Ottersbach, K. The MLL/SET family and haematopoiesis. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194579. [Google Scholar]
- Xu, J.; Du, W. HoxBlinc: A key driver of chromatin dynamics in NUP98 fusion-driven leukemia. J. Clin. Investig. 2025, 135, e191355. [Google Scholar] [CrossRef]
- Saito, S.; Nouno, K.; Shimizu, R.; Yamamoto, M.; Nagata, K. Impairment of erythroid and megakaryocytic differentiation by a leukemia-associated and t(9;9)-derived fusion gene product, SET/TAF-Ibeta-CAN/Nup214. J. Cell Physiol. 2008, 214, 322–333. [Google Scholar]
- Ozbek, U.; Kandilci, A.; van Baal, S.; Bonten, J.; Boyd, K.; Franken, P.; Fodde, R.; Grosveld, G.C. SET-CAN, the product of the t(9;9) in acute undifferentiated leukemia, causes expansion of early hematopoietic progenitors and hyperproliferation of stomach mucosa in transgenic mice. Am. J. Pathol. 2007, 171, 654–666. [Google Scholar] [CrossRef]
- Kandilci, A.; Mientjes, E.; Grosveld, G. Effects of SET and SET-CAN on the differentiation of the human promonocytic cell line U937. Leukemia 2004, 18, 337–340. [Google Scholar]
- Oka, M.; Otani, M.; Miyamoto, Y.; Oshima, R.; Adachi, J.; Tomonaga, T.; Asally, M.; Nagaoka, Y.; Tanaka, K.; Toyoda, A.; et al. Phase-separated nuclear bodies of nucleoporin fusions promote condensation of MLL1/CRM1 and rearrangement of 3D genome structure. Cell Rep 2023, 42, 112884. [Google Scholar]
- Menchits, Y.; Salimova, T.; Komkov, A.; Abramov, D.; Konyukhova, T.; Abasov, R.; Raykina, E.; Itov, A.; Gaskova, M.; Borkovskaia, A.; et al. Unusual Presentation of SET::NUP214-Associated Concomitant Hematological Neoplasm in a Child-Diagnostic and Treatment Struggle. Int. J. Mol. Sci. 2023, 24, 14451. [Google Scholar]
- Jeong, I.H.; An, G.D.; Lim, H.H.; Woo, K.S.; Kim, K.H.; Kim, J.M.; Lee, J.H.; Han, J.Y. A Rare Case of Acute Myeloid Leukemia With SET-NUP214 Fusion and Massive Hyperdiploidy. Ann. Lab. Med. 2019, 39, 403–405. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Sakaguchi, N.; Okada, T.; Makishima, M.; Ozono, K.; Michigami, T. Oncogenic nucleoporin CAN/Nup214 interacts with vitamin D receptor and modulates its function. J. Cell Biochem. 2009, 106, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Paubelle, E.; Zylbersztejn, F.; Maciel, T.T.; Carvalho, C.; Mupo, A.; Cheok, M.; Lieben, L.; Sujobert, P.; Decroocq, J.; Yokoyama, A.; et al. Vitamin D Receptor Controls Cell Stemness in Acute Myeloid Leukemia and in Normal Bone Marrow. Cell Rep. 2020, 30, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, D.; Zhang, R.; Chen, X.; Ma, Q.; Wei, J.; Zhai, W.; Pang, A.; He, Y.; Jiang, E.; et al. The outcome of acute leukemia patients with SET-NUP214 fusion after allogeneic stem cell transplantation. Front. Oncol. 2023, 13, 1256043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, X.; Meng, T.; You, H.; Dong, Y.; Xing, J.; Chen, S. SET protein overexpression contributes to paclitaxel resistance in MCF-7/S cells through PI3K/Akt pathway. J. Drug Target. 2017, 25, 255–263. [Google Scholar] [CrossRef]
- Johnson, S.M.; Haberberger, J.; Galeotti, J.; Ramkissoon, L.; Coombs, C.C.; Richardson, D.R.; Foster, M.C.; Duncan, D.; Montgomery, N.D.; Ferguson, N.L.; et al. Comprehensive genomic profiling reveals molecular subsets of ASXL1-mutated myeloid neoplasms. Leuk Lymphoma 2024, 65, 209–218. [Google Scholar] [CrossRef]
- Pacharne, S.; Dovey, O.M.; Cooper, J.; Gu, M.; Friedrich, M.J.; Rajan, S.S.; Barenboim, M.; Collord, G.; Vijayabaskar, M.S.; Ponstingl, H.; et al. SETBP1 overexpression acts in the place of class-defining mutations to drive FLT3-ITD-mutant AML. Blood Adv. 2021, 5, 2412–2425. [Google Scholar] [CrossRef]
- Arriazu, E.; Pippa, R.; Odero, M.D. Protein Phosphatase 2A as a Therapeutic Target in Acute Myeloid Leukemia. Front. Oncol. 2016, 6, 78. [Google Scholar] [CrossRef]
- De Keersmaecker, K.; Versele, M.; Cools, J.; Superti-Furga, G.; Hantschel, O. Intrinsic differences between the catalytic properties of the oncogenic NUP214-ABL1 and BCR-ABL1 fusion protein kinases. Leukemia 2008, 22, 2208–2216. [Google Scholar] [CrossRef]
- Wang, J.Y. The capable ABL: What is its biological function? Mol. Cell Biol. 2014, 34, 1188–1197. [Google Scholar] [CrossRef]
- Simon, D.N.; Wilson, K.L. Thenucleoskeletonasagenome-associated dynamic ‘network of networks. Nat. Rev. Mol. Cell Biol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Lewis, J.M.; Baskaran, R.; Taagepera, S.; Schwartz, M.A.; Wang, J.Y. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic nuclear transport. Proc. Natl. Acad. Sci. USA 1996, 93, 15174–15179. [Google Scholar] [CrossRef] [PubMed]
- Colicelli, J. ABL tyrosine kinases: Evolution of function, regulation, and specificity. Sci. Signal. 2010, 3, re6. [Google Scholar] [CrossRef] [PubMed]
- Woodring, P.J.; Hunter, T.; Wang, J.Y. 2001. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J. Biol. Chem. 2001, 276, 27104–27110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y. Controlling Abl: Auto-inhibition and co-inhibition? Nat. Cell Biol. 2004, 6, 3–7. [Google Scholar] [CrossRef]
- Welch, P.J.; Wang, J.Y. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell 1993, 75, 779–790. [Google Scholar] [CrossRef]
- Shaul, Y.; Ben-Yehoyada, M. Roleof c-Abl in the DNAdamagestress response. Cell Res. 2005, 15, 33–35. [Google Scholar] [CrossRef]
- Nagar, B.; Hantschel, O.; Young, M.A.; Scheffzek, K.; Veach, D.; Bornmann, W.; Clarkson, B.; Superti-Furga, G.; Kuriyan, J. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 2003, 112, 859–871. [Google Scholar] [CrossRef]
- Miao, Y.J.; Wang, J.Y. Binding of A/T-rich DNA by three high mobility group-like domains in c-Abl tyrosine kinase. J. Biol. Chem. 1996, 271, 22823–22830. [Google Scholar] [CrossRef]
- Buratowski, S. The CTD code. Nat. Struct. Biol. 2003, 10, 679–680. [Google Scholar] [CrossRef]
- Kaidi, A.; Jackson, S.P. KAT5 tyrosine phosphorylation couples chro matin sensing to ATM signalling. Nature 2013, 498, 70–74. [Google Scholar] [CrossRef]
- De Braekeleer, E.; Douet-Guilbert, N.; Rowe, D.; Bown, N.; Morel, F.; Berthou, C.; Férec, C.; De Braekeleer, M. ABL1 fusion genes in hematological malignancies: A review. Eur. J. Haematol. 2011, 86, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.H.; Yang, Q.M. NUP214 fusion genes in acute leukemia (Review). Oncol. Lett. 2014, 8, 959–962. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Mota, F.; Gamba, F.T.; de Carvalho Pires, M.G.; de Toledo, S.R.C.; Gouveia, J.T.; Oliveira, I.D.; da Silva Santos, N.; Delbuono, E.; Rhein, B.N.; da Costa Guimarães, R.F.; et al. NUP214::ABL1: A Ph-like fusion found in a pediatric acute myeloid leukemia patient with normal karyotype. Pediatr. Blood Cancer 2023, 70, e30203. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; He, J.J.; Zhu, Q.Y.; Wang, L.; Li, J.H.; Huang, J.S.; Xie, W.Z.; Zhu, H.H.; Jin, J. Case Report: The First Report of NUP214-ABL1 Fusion Gene in Acute Myeloid Leukemia Patient Detected by Next-Generation Sequencing. Front. Oncol. 2021, 11, 706798. [Google Scholar] [CrossRef]
- Ikeda, D.; Chi, S.; Uchiyama, S.; Nakamura, H.; Guo, Y.M.; Yamauchi, N.; Yuda, J.; Minami, Y. Molecular Classification and Overcoming Therapy Resistance for Acute Myeloid Leukemia with Adverse Genetic Factors. Int. J. Mol. Sci. 2022, 23, 5950. [Google Scholar] [CrossRef]
- George, T.I.; Bajel, A. Diagnosis of rare subtypes of acute myeloid leukaemia and related neoplasms. Pathology 2021, 53, 312–327. [Google Scholar] [CrossRef]
- Wakefield, C.; Hansen Smith, M.; Dashkevych, U.; Proytcheva, M.; Khurana, S. Clinical, Phenotypic and Molecular Characterization of NUP214-ABL1 Fusion Positive Myeloid Malignancies. J. Med. Cases 2024, 15, 250–255. [Google Scholar] [CrossRef]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Saito, T.; Komatsu, M. p62/SQSTM1: ‘Jack of all trades’ in health and cancer. FEBS J. 2019, 286, 8–23. [Google Scholar]
- Trocoli, A.; Bensadoun, P.; Richard, E.; Labrunie, G.; Merhi, F.; Schläfli, A.M.; Brigger, D.; Souquere, S.; Pierron, G.; Pasquet, J.M.; et al. p62/SQSTM1 upregulation constitutes a survival mechanism that occurs during granulocytic differentiation of acute myeloid leukemia cells. Cell Death Differ. 2014, 21, 1852–1861. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yin, J.; Wang, C.; Yang, M.; Gu, J.; He, M.; Xu, H.; Fu, W.; Zhang, W.; et al. A mitophagy inhibitor targeting p62 attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Lett. 2021, 510, 24–36. [Google Scholar] [CrossRef]
- Mathew, R.; Karp, C.M.; Beaudoin, B.; Vuong, N.; Chen, G.; Chen, H.Y.; Bray, K.; Reddy, A.; Bhanot, G.; Gelinas, C.; et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009, 137, 1062–1075. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Deng, T.; Hu, B.; Wang, X.; Ding, S.; Lin, L.; Yan, Y.; Peng, X.; Zheng, X.; Liao, M.; Jin, Y.; et al. TRAF6 autophagic degradation by avibirnavirus VP3 inhibits antiviral innate immunity via blocking NFKB/NF-κB activation. Autophagy 2022, 18, 2781–2798. [Google Scholar] [CrossRef] [PubMed]
- Thakar, K.; Karaca, S.; Port, S.A.; Urlaub, H.; Kehlenbach, R.H. Identification of CRM1-dependent Nuclear Export Cargos Using Quantitative Mass Spectrometry. Mol. Cell Proteom. 2013, 12, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Alcober-Boquet, L.; Zang, T.; Pietsch, L.; Suess, E.; Hartmann, M.; Proschak, E.; Gross, L.Z.F.; Sacerdoti, M.; Zeuzem, S.; Rogov, V.V.; et al. The PB1 and the ZZ domain of the autophagy receptor p62/SQSTM1 regulate the interaction of p62/SQSTM1 with the autophagosome protein LC3B. Protein Sci. 2024, 33, e4840. [Google Scholar] [CrossRef] [PubMed]
- Lavau, C.P.; Aumann, W.K.; Sze, S.K.; Gupta, V.; Ripple, K.; Port, S.A.; Kehlenbach, R.H.; Wechsler, D.S. The SQSTM1-NUP214 fusion protein interacts with Crm1, activates Hoxa and Meis1 genes, and drives leukemogenesis in mice. PLoS ONE 2020, 15, e0232036. [Google Scholar] [CrossRef]
- Gorello, P.; La Starza, R.; Di Giacomo, D.; Messina, M.; Puzzolo, M.C.; Crescenzi, B.; Santoro, A.; Chiaretti, S.; Mecucci, C. SQSTM1-NUP214: A new gene fusion in adult T-cell acute lymphoblastic leukemia. Haematologica 2010, 95, 2161–2163. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.C.; Cifani, P.; Drill, E.; He, J.; Still, E.; Zhong, S.; Balasubramanian, S.; Pavlick, D.; Yilmazel, B.; Knapp, K.M.; et al. Genomics of primary chemoresistance and remission induction failure in paediatric and adult acute myeloid leukaemia. Br. J. Haematol. 2017, 176, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Yamamoto, Y.; Iba, S.; Okamoto, A.; Tokuda, M.; Inaguma, Y.; Yanada, M.; Morishima, S.; Kanie, T.; Tsuzuki, M.; et al. NUP214-RAC1 and RAC1-COL12A1 Fusion in Complex Variant Translocations Involving Chromosomes 6, 7 and 9 in an Acute Myeloid Leukemia Case with DEK- NUP214. Cytogenet. Genome Res. 2015, 146, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Echeverria, E.; Lenicov, F.R.; Cardama, G.; Gonzalez, N.; Davio, C.; Fernández, N.; Menna, P.L. Pharmacological Rac1 inhibitors with selective apoptotic activity in human acute leukemic cell lines. Oncotarget 2017, 8, 98509–98523. [Google Scholar] [CrossRef]
- Azlan, A.; Khor, K.Z.; Rajasegaran, Y.; Rosli, A.A.; Said, M.S.M.; Yusoff, N.M.; Moses, E.J. RUNX1/ETO regulates reactive oxygen species (ROS) levels in t(8,21) acute myeloid leukaemia via FLT3 and RAC1. Med. Oncol. 2023, 40, 208. [Google Scholar] [CrossRef]
- Nimmagadda, S.C.; Frey, S.; Edelmann, B.; Hellmich, C.; Zaitseva, L.; König, G.M.; Kostenis, E.; Bowles, K.M.; Fischer, T. Bruton’s tyrosine kinase and RAC1 promote cell survival in MLL-rearranged acute myeloid leukemia. Leukemia 2018, 32, 846–849. [Google Scholar] [CrossRef]
- Wang, J.; Rao, Q.; Wang, M.; Wei, H.; Xing, H.; Liu, H.; Wang, Y.; Tang, K.; Peng, L.; Tian, Z.; et al. Overexpression of Rac1 in leukemia patients and its role in leukemia cell migration and growth. Biochem. Biophys. Res. Commun. 2009, 386, 769–774. [Google Scholar] [CrossRef]
- Rørvik, S.D.; Torkildsen, S.; Bruserud, Ø.; Tvedt, T.H.A. Acute myeloid leukemia with rare recurring translocations-an overview of the entities included in the international consensus classification. Ann. Hematol. 2024, 103, 1103–1119. [Google Scholar] [CrossRef]
- Shiba, N.; Ichikawa, H.; Taki, T.; Park, M.J.; Jo, A.; Mitani, S.; Kobayashi, T.; Shimada, A.; Sotomatsu, M.; Arakawa, H.; et al. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosomes Cancer 2013, 52, 683–693. [Google Scholar] [CrossRef]
- Yuan, X.; Ni, L.; Chen, T.; Wu, Y.; Lai, X.; Liu, L.; Xu, Z.; Xu, Y.; Yang, T.; Lu, Y.; et al. NUP98 Rearrangement Dynamics Predict Outcomes in Adult Patients With Acute Myeloid Leukemia Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: A Multicenter Real-World Study. Transplant. Cell Ther. 2025, S2666–6367, 01324–01327. [Google Scholar] [CrossRef]
- Kojima, K.; Kornblau, S.M.; Ruvolo, V.; Dilip, A.; Duvvuri, S.; Davis, R.E.; Zhang, M.; Wang, Z.; Coombes, K.R.; Zhang, N.; et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood 2013, 121, 4166–4174. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Senga, S.S.; Grose, R.P. Hallmarks of cancer-the new testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Du, W.; Xu, A.; Huang, Y.; Cao, J.; Zhu, H.; Yang, B.; Shao, X.; He, Q.; Ying, M. The role of autophagy in targeted therapy for acute myeloid leukemia. Autophagy 2021, 17, 2665–2679. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Kociszewska, K.; Grosicka, O.; Grosicki, S. The role of autophagy in acute myeloid leukemia development. Expert Rev. Anticancer Ther. 2023, 23, 5–18. [Google Scholar] [CrossRef]
- Khan, A.; Singh, V.K.; Thakral, D.; Gupta, R. Autophagy in acute myeloid leukemia: A paradoxical role in chemoresistance. Clin. Transl. Oncol. 2022, 24, 1459–1469. [Google Scholar] [CrossRef]
- Ramos, D.F.V.; Mancuso, R.I.; Contieri, B.; Duarte, A.; Paiva, L.; de Melo Carrilho, J.; Saad, S.T.O.; Lazarini, M. Rac GTPases in acute myeloid leukemia cells: Expression profile and biological effects of pharmacological inhibition. Toxicol. Appl. Pharmacol. 2022, 442, 115990. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Sun, M.; Deng, X.; Wu, X.; Ma, Y.; Li, M.; Shuoa, S.M.; You, Q.; Miao, L. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun. 2020, 40, 69–80. [Google Scholar] [CrossRef]
- Dhayni, K.; Zibara, K.; Issa, H.; Kamel, S.; Bennis, Y. Targeting CXCR1 and CXCR2 receptors in cardiovascular diseases. Pharmacol. Ther. 2022, 237, 108257. [Google Scholar] [CrossRef]
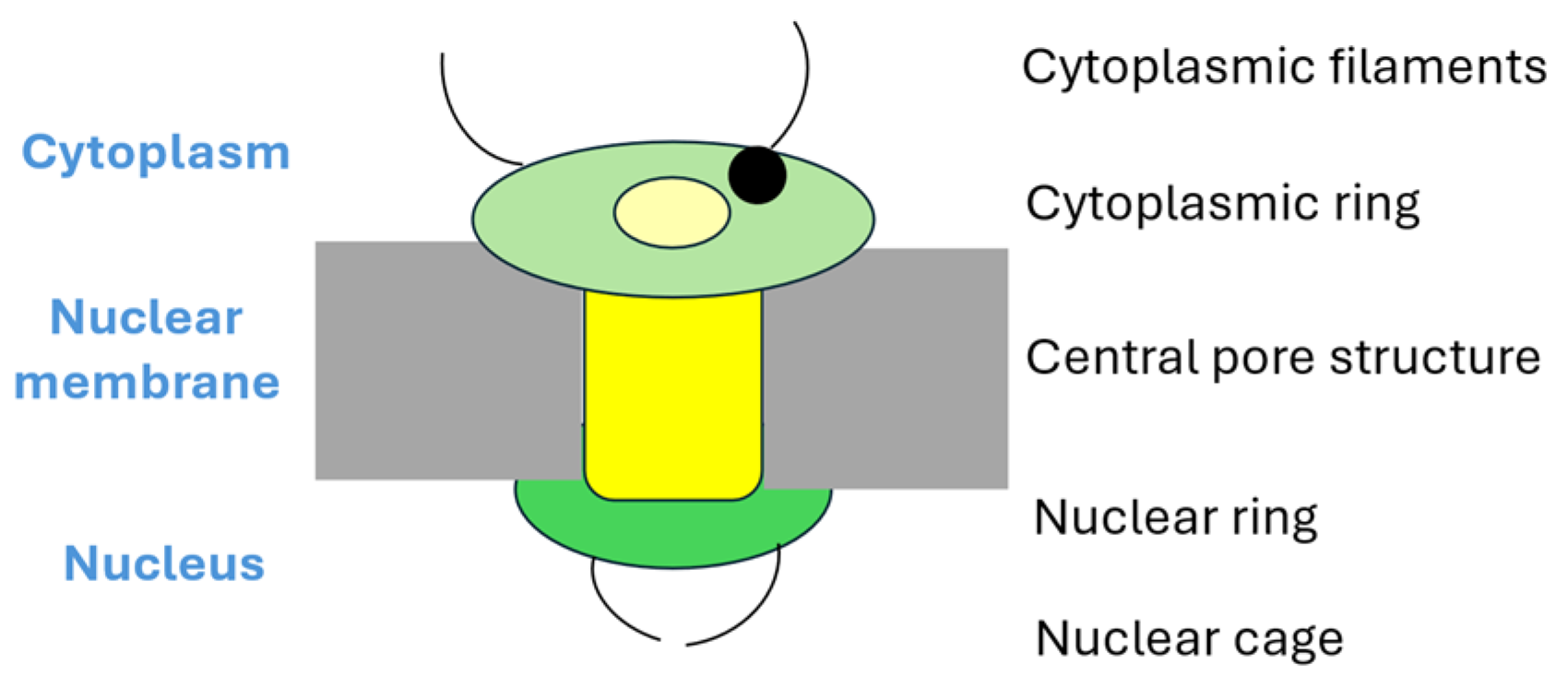
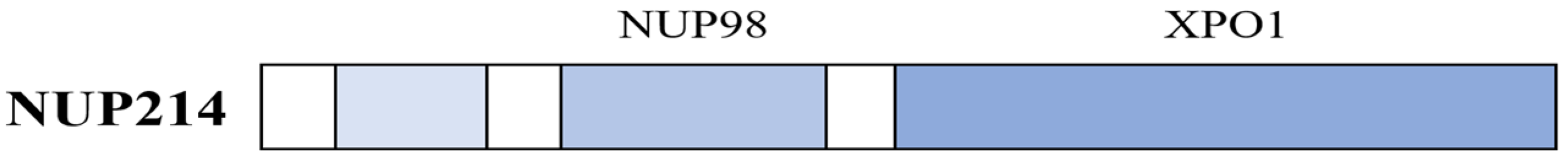
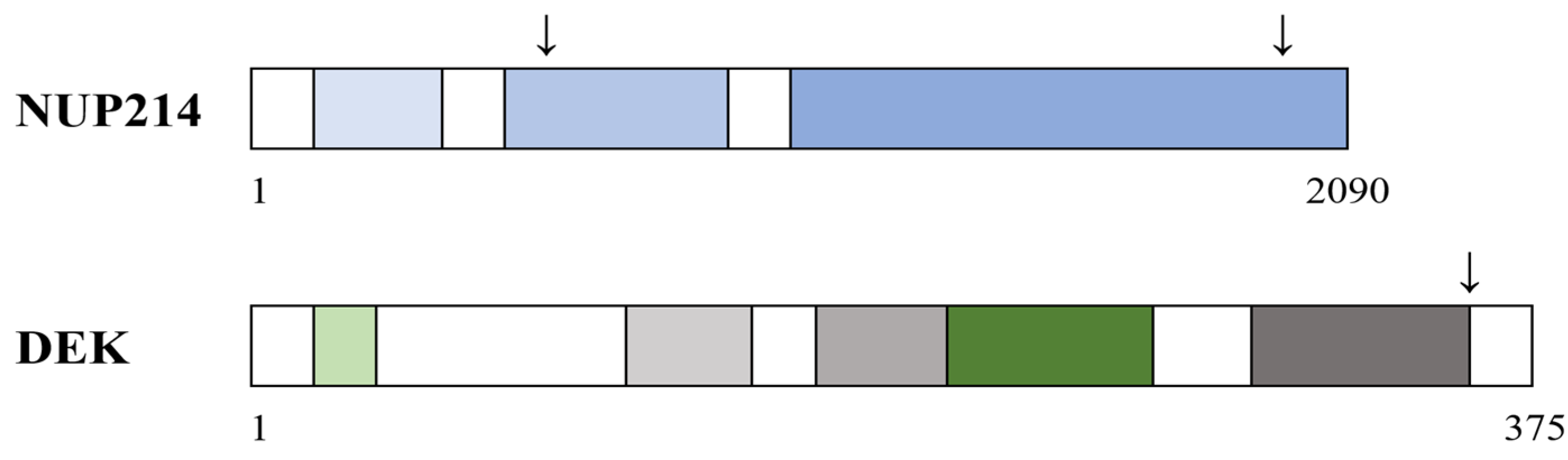


| Molecular Region (Amino Acids) or Characteristic | The Name and Functional Characteristic of Each Molecular Region |
|---|---|
| AA 29–50, 248–246, 241–254, 299–310 | The four acidic regions The (i) first region is located on the aminoterminal (N) side of the histone binding region, (ii) the two next regions are located between the SAP and the DNA binding carboxy(C)-terminal DNA binding domain where in the region that has RNA binding activity and a nuclear localization signal, and (iii) the last region is located on the aminoterminal side of the C-terminal DNA binding domain [55]. |
| AA 52–60 | The histone binding region; a region important for the histone chaperone function. |
| A pseudo-SAP domain is localized close to the aminoterminal side of the SAP domain [47]. | |
| AA 149–187 | This aminoterminal SAP domain has a DNA binding motif [47,53,54]. |
| AA 270–350 | DNA binding and multimerization region The C-terminal DNA binding domain is located in this part of the molecule that partly overlaps with the multimerization activity [53,54,55]. These activities are localized to the amino acid residues 270–350, and multimerization is in addition dependent on phosphorylation by CK2 [53]. |
| Phosphorylation | DEK is a phosphoprotein [57] with several phosphorylation sites of which most are serine and threonine residues clustered in the carboxy-terminal region [53,54,55]. Multimerization as well as DNA binding by the C-terminal DNA binding domain are influenced by the phosphorylation status of DEK, and the multimerization is dependent on phosphorylation by CK2 [53,54,55]. The cellular levels of DEK are not altered but the DEK phosphorylation status varies during the cell cycle [55]. |
| DNA binding, posttranscriptional modulation with nuclear relocalization and DNA repair |
| DEK is a DNA binding protein; its affinity to DNA depends on the phosphorylation status in its C-terminal part, but this phosphorylation does not influence its binding to chromatin [54]. Its DNA binding activity is also regulated by acetylation within the first 70 N-terminal amino acids that decreases the DNA affinity and leads to nuclear relocalization to RNA-processing structures [67]. DEK is important for several forms of DNA repair; the effects are partly mediated by RAD51 interactions [61,62]. |
| Chromatin modulation and histone interactions |
| DEK shows hypophosphorylation and enhanced chromatin binding during apoptosis [68]. DEK can modulate histone H3 and to a lesser extent H4 acetylation through interactions with both histone deacetylases and histone acetyltransferases [58,59,60]. The acidic domain of DEK interacts with histones; DEK thereby inhibits histone transferase activity (e.g., p300 activity), induces histone hypoacetylation and reduces gene transcription [60]. |
| Transcription/splicing/translation |
| DEK has an RNA binding domain that is possibly important for its role in RNA splicing [63,64,65,66], including altered splicing of genes involved in regulation of apoptosis and cell cycle progression [64]. It can decrease the expression of quiescence-associated proteins due to its modulation of histone acetylation/epigenetic regulation [58]. Studies in cancer cell lines have shown that DEK regulates the expression of several cancer-associated genes, including both oncogenes and tumor suppressors [64]. |
| Cellular effects: proliferation, adhesion, programmed cell death, quiescence, metabolism, signaling |
| DEK modulates a wide range of genes involved in the regulation of cellular proliferation, adhesion/migration and DNA repair [62,69,70,71]. Animal studies suggest that DEK expression promote exit from quiescence [47,72]. DEK has an antiapoptotic effect; DEK depletion causes apoptosis and induces increased stabilization and transcriptional activity of p53 in cancer cell lines [73]. However, at least in certain models of FLT3-ITD AML, the DEK-mediated destabilization of p53 may be counteracted by STAT5-mediated reduction in p53 degradation [74]. The phosphorylation of DEK is altered as an early event during apoptosis [68]. DEK is involved in DNA repair, and the loss of DEK therefore induces genomic instability [62]. DEK preserves the self-renewing capacity of hematopoietic stem cells through increased quiescence and decreased mitochondrial metabolism; this effect partly depends on mTOR, but DEK also recruits the corepressor NCOR1 to reduce histone 3 acetylation and it thereby modulates expression of quiescence-associated genes [58]. Increased expression of the serine/threonine kinase IRAK1 in cancer cell lines [69]; this mediator is a downstream mediator of Toll-like receptors and the IL1 receptor that both are regulators of AML cell proliferation [75,76,77]. DEK has an antiapoptotic effect in cancer cell lines through increased IRAK1 expression [69]. DEK knockdown induces quiescence in normal hematopoietic cells and thereby renders these cells more resistant to irradiation [47,71]. |
| DEK in malignant cells and cancer-supporting bone marrow cells that are relevant for AML |
| DEK mRNA and protein levels are low in primary AML cells compared with normal bone marrow cells [78]. DEK appears important for AML-supporting bone marrow stromal cells—it can stimulate cancer-associated angiogenesis and seems to stimulate M2-like macrophage polarization [70,79]. DEK expression in human breast cancer seems to induce a M2 macrophage phenotypes [70]. DEK increases VEGF expression in cancer cells through direct binding to the DEK-responsive element of the VEGF promoter and in addition through recruitment of HIF1α and the histone acetyltransferase p300 to the VEGF promoter [79]. DEK protein level, VEGF expression and microvessel density are correlated in human breast cancer [79]. |
| Extracellular release |
| DEK is released extracellularly and functions as a chemokine or as a component of the neutrophil traps [80,81,82,83]. |
| Transcriptional regulation and gene expression |
| Transformation of human CD34+ cells by the fusion proteins causes increased HOXA and HOXB expression [102,103,104]. Primary AML cells with t(6;9) seem to have increased expression of EYA3, SESN1, PRDM2, and HIST2H4; these effects were observed together with the overexpression of HOXA and HOXB genes [103]. AML cells with t(6;9) AML show similarities in their gene regulation network with NPM1-Ins and FLT3-ITD positive AML, but they differs from these two variants with regard to the regulation of HOX genes [104]. The t(6;9) fusion proteins bind to and function as a XPO1-dependent transcriptional activator; this mechanism causes increased expression of several genes including HOX genes and FOXC1 that are important in AML leukemogenesis [89]. The t(6;9) fusion protein also increases the expression of several other genes that seem to be involved in the process of AML leukemogenesis [96]. |
| Molecular interactions and localization (chromatin, DNA repair, transcription, RNA function) |
| The t(6;9) fusion protein can bind to the nuclear pore protein XPO1; this is similar to the SET-NUP214 fusion protein (see Section 6) [94,95]. The fusion protein thereby functions as a XPO1-dependent transcriptional activator [96]. The DEK-NUP214 protein interacts with XPO1-mediated nuclear export of several proteins involved in leukemogenesis, including cyclin B1 and members of the NFκB signaling pathway [97]. The SET-NUP214 protein has a similar effect [97]. The fusion protein can also bind to several proteins involved in RNA processing and splicing, ribosome biogenesis, epigenetic regulation of gene expression, chromatine remodeling and DNA repair [95]. Some of these proteins have also been identified as DEK interacting proteins, whereas other DEK targeted proteins could not be identified as targets of the fusion protein (e.g., the kinase CK2 and histone H3) [47,95]. The fusion protein causes an altered localization of normal NUP214 away from the perinuclear region/nuclear pore towards an intranuclear microspeckled pattern [95]. The chromatin localization of full-length DEK was not altered by the fusion protein, except that binding to histone H3 was no longer detected [94,95]. The interactome of the DEK-NUP14 fusion protein includes mainly proteins that are involved in RNA metabolism and regulation of gene expression, but several genes involved in regulation of leukocyte activation, apoptosis and gene expression are also included in the interactome [95]. |
| Intracellular signaling |
| Increased AML cell proliferation due to posttranscriptional upregulation of mTOR and increased mTOR1 activity [98,99]. The DEK-NUP214 fusion protein inhibits NFκB signaling [97]. The DEK-NUP214 fusion protein activates JAK2 and thereby upregulates STAT3 and STAT5 [100]. Studies in the human cell line FKH1 and cells transduced with the fusion protein showed that activation of STAT5, mTOR (i.e., mTOR1 activity with p70S6K phosphorylation); SRC family kinases were needed for continued growth and survival [95,99]. STAT5 activation can be further enhanced by genetic FLT3 abnormalities [100,101]. |
| Cellular metabolism |
| The increased mTORC1 activity also leads to a metabolic shift towards oxidative phosphorylation [102]. |
| Cellular functions: leukemogenesis and AML cell proliferation |
| AML cells with t(6;9) show strong proliferative responses to G-CSF, GM-CSF and IL3 [98], but it should be emphasized that this study included very few patients. Murine studies suggest that the fusion protein causes cellular transformation and leukemic disease especially when long-term hematopoietic stem cells were transduced with the fusion protein; transduction of other immature hematopoietic stem cell subsets was less efficient [105]. Forced expression of the DEK-NUP214 fusion protein has a proliferation-promoting effect in AML cell lines; this effect seems to be caused by increased mTOR1 but not mTOR2 activity because it is associated with increased phosphorylation of the mTOR1 target p70S6K protein but not the mTOR2 target AKT at Ser 473 [99]. The fusion protein causes a global increase in protein synthesis, an effect that seems to be restricted to cells of the myeloid lineage and it correlates with the phosphorylation status of the translation initiating protein EIF4E [99,106]. |
| The NUP214’s fusion partner | Most of the SET and DEK full-length molecules are included in the fusion molecules. Both fusion partners functions as H3/H4 histone chaperones and transcriptional regulators. Both partners function as p53 regulators. |
| The NUP part of the fusion gene/protein | The C-terminal XPO1 binding part of NUP214 is included in the fusion molecules Important effects on gene transcription are mediated by the binding to XPO1 by the NUP214 part of the fusion molecule. Their influence of HOX gene expression is probably mediated by NUP214. Both fusion molecules modulates nuclear export. |
| Prognostic impact | NUP-DEK is associated with adverse prognosis in human AML. NUP-SET is also associated with chemoresistance/adverse prognosis, but this is only documented in ALL. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruserud, Ø.; Reikvam, H. NUP214 in Acute Myeloid Leukemia. Cells 2025, 14, 1461. https://doi.org/10.3390/cells14181461
Bruserud Ø, Reikvam H. NUP214 in Acute Myeloid Leukemia. Cells. 2025; 14(18):1461. https://doi.org/10.3390/cells14181461
Chicago/Turabian StyleBruserud, Øystein, and Håkon Reikvam. 2025. "NUP214 in Acute Myeloid Leukemia" Cells 14, no. 18: 1461. https://doi.org/10.3390/cells14181461
APA StyleBruserud, Ø., & Reikvam, H. (2025). NUP214 in Acute Myeloid Leukemia. Cells, 14(18), 1461. https://doi.org/10.3390/cells14181461







