From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Sequencing
2.2.1. RNA Extraction
2.2.2. RNA Sequencing and Read Processing
2.2.3. Differential Gene Expression Analysis
2.2.4. Functional Profiling of Metabolically Stimulated Differentially Expressed Genes
2.2.5. Candidate Gene Expression
2.3. Isolation and Purification of Bat Primary Myoblasts
2.4. Cell Immortalization
2.5. Myotube Differentiation
2.6. Myoblast Proliferation Assay
2.7. Chromosome Counting
2.8. Immunofluorescence
3. Results
3.1. Functional and Molecular Specializations Supporting Flight in Pteronotus parnellii
3.1.1. Candidate Gene Expression Reveals Fiber-Type Heterogeneity
3.1.2. DEG Analyses Reveal Flight Muscle Activation of Regeneration and Metabolism
3.2. Isolation of PAX7+ Cells from Flight Muscle and Verification of Myogenic Cells
3.3. Establishment of Immortalized Bat Myoblast (iBatM) Cell Lines
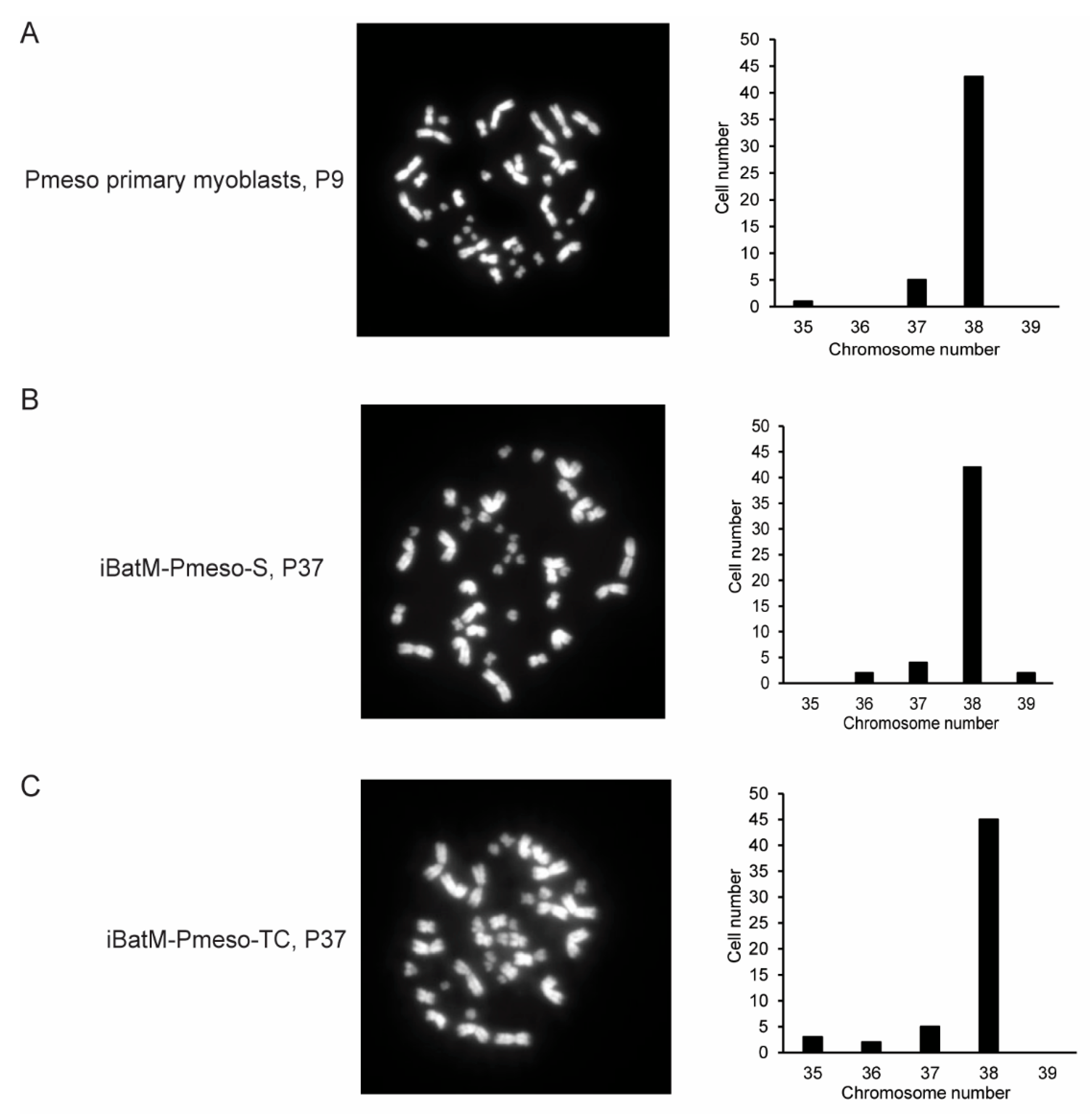
3.4. Immortalized Bat Myoblasts Are Genetically Stable
3.5. Immortalized Bat Myoblasts Retain the Proliferation Capacity of Bat Primary Myoblasts
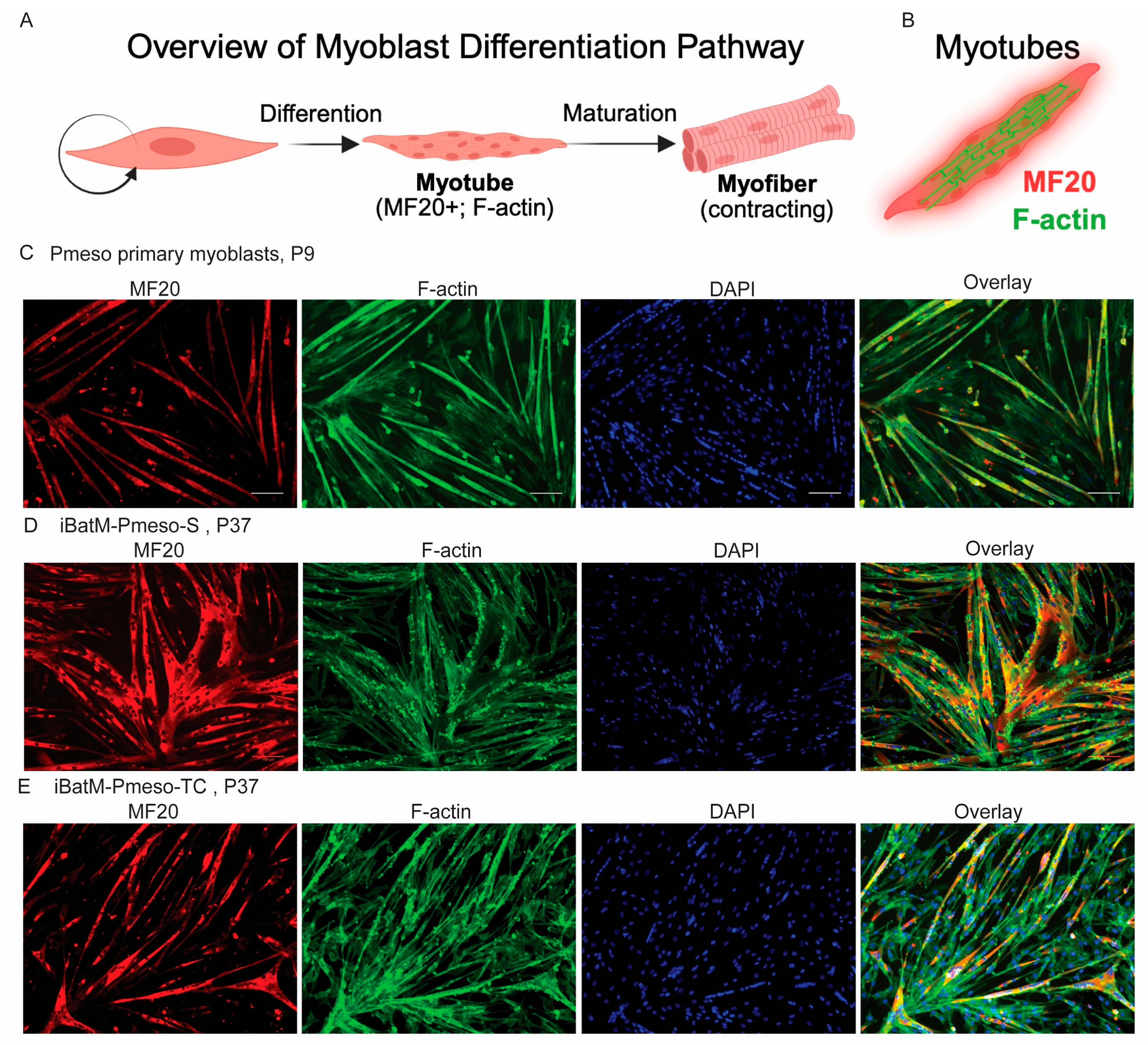
3.6. Immortalized Bat Myoblasts Retain the Differentiation Capacity of Bat Primary Myoblasts
3.7. Immortalized Bat Myoblasts Enable Functional Profiling of Flight Muscle Under Metabolic Overload
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hTERT | human telomerase reverse transcriptase |
| CDK4 | cyclin-dependent kinase 4 |
| CDKI | cyclin-dependent kinase inhibitor |
| rh EGF | recombinant human epidermal growth factor |
| rh FGF-b | recombinant human fibroblast growth factor basic protein |
| P/S | Penicillin/Streptomycin |
| P. mesoamericanus | Pteronotus mesoamericanus |
| P. parnellii | Pteronotus parnellii |
| iPSC | induced pluripotent stem cells |
References
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wosczyna, M.N.; Konishi, C.T.; Perez Carbajal, E.E.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035.e5. [Google Scholar] [CrossRef]
- Richler, C.; Yaffe, D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev. Biol. 1970, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc. Natl. Acad. Sci. USA 1968, 61, 477–483. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Stadler, G.; Chen, J.C.; Wagner, K.; Robin, J.D.; Shay, J.W.; Emerson, C.P., Jr.; Wright, W.E. Establishment of clonal myogenic cell lines from severely affected dystrophic muscles—CDK4 maintains the myogenic population. Skelet. Muscle 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Manchon, J.; Capo, J.; Martinez-Pineiro, A.; Juanola, E.; Pesovic, J.; Mosqueira-Martin, L.; Gonzalez-Imaz, K.; Maestre-Mora, P.; Odria, R.; Cerro-Herreros, E.; et al. Immortalized human myotonic dystrophy type 1 muscle cell lines to address patient heterogeneity. iScience 2024, 27, 109930. [Google Scholar] [CrossRef]
- Lopez, S.M.; Balog-Alvarez, C.; Canessa, E.H.; Hathout, Y.; Brown, K.J.; Vitha, S.; Bettis, A.K.; Boehler, J.; Kornegay, J.N.; Nghiem, P.P. Creation and characterization of an immortalized canine myoblast cell line: Myok9. Mamm. Genome 2020, 31, 95–109. [Google Scholar] [CrossRef]
- Tone, Y.; Mamchaoui, K.; Tsoumpra, M.K.; Hashimoto, Y.; Terada, R.; Maruyama, R.; Gait, M.J.; Arzumanov, A.A.; McClorey, G.; Imamura, M.; et al. Immortalized Canine Dystrophic Myoblast Cell Lines for Development of Peptide-Conjugated Splice-Switching Oligonucleotides. Nucleic Acid Ther. 2021, 31, 172–181. [Google Scholar] [CrossRef]
- Lathuiliere, A.; Vernet, R.; Charrier, E.; Urwyler, M.; Von Rohr, O.; Belkouch, M.C.; Saingier, V.; Bouvarel, T.; Guillarme, D.; Engel, A.; et al. Immortalized human myoblast cell lines for the delivery of therapeutic proteins using encapsulated cell technology. Mol. Ther. Methods Clin. Dev. 2022, 26, 441–458. [Google Scholar] [CrossRef]
- Long, X.; Chen, W.; Liu, G.; Hu, W.; Tan, Q. Establishment and characterization of a skeletal myoblast cell line of grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2023, 49, 1043–1061. [Google Scholar] [CrossRef]
- Baik, J.; Ortiz-Cordero, C.; Magli, A.; Azzag, K.; Crist, S.B.; Yamashita, A.; Kiley, J.; Selvaraj, S.; Mondragon-Gonzalez, R.; Perrin, E.; et al. Establishment of Skeletal Myogenic Progenitors from Non-Human Primate Induced Pluripotent Stem Cells. Cells 2023, 12, 1147. [Google Scholar] [CrossRef]
- Ikeda, D.; Otsuka, Y.; Kan-No, N. Development of a novel Japanese eel myoblast cell line for application in cultured meat production. Biochem. Biophys. Res. Commun. 2024, 734, 150784. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Zheng, H.; Zhao, Y.; Zhao, Z.; Wang, J.; Zhang, Y.; Li, Y.; Wang, S.; Liu, Y.; Xue, C.; et al. A spontaneously immortalized muscle stem cell line (EfMS) from brown-marbled grouper for cell-cultured fish meat production. Commun. Biol. 2024, 7, 1697. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lin, S.; Wang, X.; Jiao, Z.; Li, G.; An, L.; Zhang, Z.; Zhang, L. Establishment and Characterization of a Chicken Myoblast Cell Line. Int. J. Mol. Sci. 2024, 25, 8340. [Google Scholar] [CrossRef] [PubMed]
- Peruffo, A.; Bassan, I.; Gonella, A.; Maccatrozzo, L.; Otero-Sabio, C.; Iannuzzi, L.; Perucatti, A.; Pistucci, R.; Giacomello, M.; Centelleghe, C. Establishment and characterization of the Cuvier’s beaked whale (Ziphius cavirostris) myogenic cell line. Res. Vet. Sci. 2025, 182, 105471. [Google Scholar] [CrossRef]
- Giannini, N.P.; Cannell, A.; Amador, L.I.; Simmons, N.B. Palaeoatmosphere facilitates a gliding transition to powered flight in the Eocene bat, Onychonycteris finneyi. Commun. Biol. 2024, 7, 365. [Google Scholar] [CrossRef]
- Matthew, F.; Jones, K.C.B.N.B.S. Phylogeny and systematics of early Paleogene bats. J. Mamm. Evol. 2024, 31, 18. [Google Scholar] [CrossRef]
- Rummel, A.D.; Swartz, S.M.; Marsh, R.L. Thermal Stability of Contractile Proteins in Bat Wing Muscles Explains Differences in Temperature Dependence of Whole-Muscle Shortening Velocity. Physiol. Biochem. Zool. 2023, 96, 100–105. [Google Scholar] [CrossRef]
- Sears, K.E.; Behringer, R.R.; Rasweiler, J.J.t.; Niswander, L.A. Development of bat flight: Morphologic and molecular evolution of bat wing digits. Proc. Natl. Acad. Sci. USA 2006, 103, 6581–6586. [Google Scholar] [CrossRef]
- Cretekos, C.J.; Wang, Y.; Green, E.D.; Martin, J.F.; Rasweiler, J.J.t.; Behringer, R.R. Regulatory divergence modifies limb length between mammals. Genes Dev. 2008, 22, 141–151. [Google Scholar] [CrossRef]
- Tokita, M.; Abe, T.; Suzuki, K. The developmental basis of bat wing muscle. Nat. Commun. 2012, 3, 1302. [Google Scholar] [CrossRef]
- Eckalbar, W.L.; Schlebusch, S.A.; Mason, M.K.; Gill, Z.; Parker, A.V.; Booker, B.M.; Nishizaki, S.; Muswamba-Nday, C.; Terhune, E.; Nevonen, K.A.; et al. Transcriptomic and epigenomic characterization of the developing bat wing. Nat. Genet. 2016, 48, 528–536. [Google Scholar] [CrossRef]
- Feigin, C.Y.; Moreno, J.A.; Ramos, R.; Mereby, S.A.; Alivisatos, A.; Wang, W.; van Amerongen, R.; Camacho, J.; Rasweiler, J.J.t.; Behringer, R.R.; et al. Convergent deployment of ancestral functions during the evolution of mammalian flight membranes. Sci. Adv. 2023, 9, eade7511. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Feregrino, C.; Aldrovandi, S.; Lo, B.W.; Monaco, A.A.; Ringel, A.R.; Morales, A.E.; Zehnder, T.; Behncke, R.Y.; Glaser, J.; et al. Comparative single-cell analyses reveal evolutionary repurposing of a conserved gene programme in bat wing development. Nat. Ecol. Evol. 2025. [Google Scholar] [CrossRef]
- Lyu, X.; Bai, J.; Jiang, J.B.; Sun, C.J.; Chen, P.; Liu, Q.; Ma, Y.S.; Liu, Z. Single-cell expression profiling of bat wing development. Nat. Commun. 2025, 16, 6612. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Liang, L.; Zhu, Z.H.; Zhou, W.P.; Irwin, D.M.; Zhang, Y.P. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. USA 2010, 107, 8666–8671. [Google Scholar] [CrossRef]
- Zhang, G.; Cowled, C.; Shi, Z.; Huang, Z.; Bishop-Lilly, K.A.; Fang, X.; Wynne, J.W.; Xiong, Z.; Baker, M.L.; Zhao, W.; et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 2013, 339, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Jin, J.P. Evolution of Flight Muscle Contractility and Energetic Efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.N.; Ansari, M.Y.; Capshaw, G.; Galazyuk, A.; Lauer, A.M.; Moss, C.F.; Sears, K.E.; Stewart, M.; Teeling, E.C.; Wilkinson, G.S.; et al. Bats as instructive animal models for studying longevity and aging. Ann. N. Y. Acad. Sci. 2024, 1541, 10–23. [Google Scholar] [CrossRef]
- Johnson, A.A.; Shokhirev, M.N.; Shoshitaishvili, B. Revamping the evolutionary theories of aging. Ageing Res. Rev. 2019, 55, 100947. [Google Scholar] [CrossRef]
- Hua, R.; Ma, Y.S.; Yang, L.; Hao, J.J.; Hua, Q.Y.; Shi, L.Y.; Yao, X.Q.; Zhi, H.Y.; Liu, Z. Experimental evidence for cancer resistance in a bat species. Nat. Commun. 2024, 15, 1401. [Google Scholar] [CrossRef]
- Athar, F.; Zheng, Z.; Riquier, S.; Zacher, M.; Lu, J.Y.; Zhao, Y.; Volobaev, V.; Alcock, D.; Galazyuk, A.; Cooper, L.N.; et al. Limited cell-autonomous anticancer mechanisms in long-lived bats. Nat. Commun. 2025, 16, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Cui, J.; Irving, A.T.; Wang, L.F. Unique Loss of the PYHIN Gene Family in Bats Amongst Mammals: Implications for Inflammasome Sensing. Sci. Rep. 2016, 6, 21722. [Google Scholar] [CrossRef]
- Ahn, M.; Chen, V.C.; Rozario, P.; Ng, W.L.; Kong, P.S.; Sia, W.R.; Kang, A.E.Z.; Su, Q.; Nguyen, L.H.; Zhu, F.; et al. Bat ASC2 suppresses inflammasomes and ameliorates inflammatory diseases. Cell 2023, 186, 2144–2159.e22. [Google Scholar] [CrossRef]
- Moreno Santillan, D.D.; Lama, T.M.; Gutierrez Guerrero, Y.T.; Brown, A.M.; Donat, P.; Zhao, H.; Rossiter, S.J.; Yohe, L.R.; Potter, J.H.; Teeling, E.C.; et al. Large-scale genome sampling reveals unique immunity and metabolic adaptations in bats. Mol. Ecol. 2021, 30, 6449–6467. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.E.; Dong, Y.; Brown, T.; Baid, K.; Kontopoulos, D.; Gonzalez, V.; Huang, Z.; Ahmed, A.W.; Bhuinya, A.; Hilgers, L.; et al. Bat genomes illuminate adaptations to viral tolerance and disease resistance. Nature 2025, 638, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Faure, P.A.; Re, D.E.; Clare, E.L. Wound Healing in the Flight Membranes of Big Brown Bats. J. Mammal. 2009, 90, 1148–1156. [Google Scholar] [CrossRef]
- Ma, S.; Upneja, A.; Galecki, A.; Tsai, Y.M.; Burant, C.F.; Raskind, S.; Zhang, Q.; Zhang, Z.D.; Seluanov, A.; Gorbunova, V.; et al. Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity. eLife 2016, 5, e19130. [Google Scholar] [CrossRef]
- Stawski, C.; Willis, C.K.R.; Geiser, F. The importance of temporal heterothermy in bats. J. Zool. 2014, 292, 86–100. [Google Scholar] [CrossRef]
- Bernal-Rivera, A.; Cuellar-Valencia, O.M.; Calvache-Sánchez, C.; Murillo-García, O.E. Morphological, anatomical, and physiological signs of senescence in the great fruit-eating bat (Artibeus lituratus). Acta Chiropterolog. 2002, 24, 405–413. [Google Scholar] [CrossRef]
- Irving, A.T.; Ng, J.H.; Boyd, V.; Dutertre, C.A.; Ginhoux, F.; Dekkers, M.H.; Meers, J.; Field, H.E.; Crameri, G.; Wang, L.F. Optimizing dissection, sample collection and cell isolation protocols for frugivorous bats. Methods Ecol. Evol. 2020, 11, 150–158. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Rissino, J.D.; Nagamachi, C.Y.; Pieczarka, J.C.; Noronha, R.C.R. Isolation and establishment of skin-derived and mesenchymal cells from south American bat Artibeus planirostris (Chiroptera—Phyllostomidae). Tissue Cell 2021, 71, 101507. [Google Scholar] [CrossRef]
- Deng, F.; Morales-Sosa, P.; Bernal-Rivera, A.; Wang, Y.; Tsuchiya, D.; Javier, J.E.; Rohner, N.; Zhao, C.; Camacho, J. Establishing Primary and Stable Cell Lines from Frozen Wing Biopsies for Cellular, Physiological, and Genetic Studies in Bats. Curr. Protoc. 2024, 4, e1123. [Google Scholar] [CrossRef]
- Jagannathan, N.S.; Koh, J.Y.P.; Lee, Y.; Sobota, R.M.; Irving, A.T.; Wang, L.F.; Itahana, Y.; Itahana, K.; Tucker-Kellogg, L. Multi-omic analysis of bat versus human fibroblasts reveals altered central metabolism. eLife 2024, 13, e94007. [Google Scholar] [CrossRef] [PubMed]
- Alcock, D.; Power, S.; Hogg, B.; Sacchi, C.; Kacprzyk, J.; McLoughlin, S.; Bertelsen, M.F.; Fletcher, N.F.; O’Riain, A.; Teeling, E.C. Generating bat primary and immortalised cell-lines from wing biopsies. Sci. Rep. 2024, 14, 27633. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.; Word, C.; Guerra-Pilaquinga, N.; Mazinani, M.; Fawcett, S.; Portfors, C.; Falzarano, D.; Kell, A.M.; Jangra, R.K.; Banerjee, A.; et al. Expanding the bat toolbox: Carollia perspicillata bat cell lines and reagents enable the characterization of viral susceptibility and innate immune responses. PLoS Biol. 2025, 23, e3003098. [Google Scholar] [CrossRef]
- Bai, L.; Tani, T.; Kobayashi, T.; Nouda, R.; Kanai, Y.; Sano, Y.; Takami, K.; Tomita, H.; Sugano, E.; Ozaki, T.; et al. Establishment of immortalized Egyptian Rousettus bat cell lines. FEBS Open Bio 2024, 14, 598–612. [Google Scholar] [CrossRef]
- Dejosez, M.; Marin, A.; Hughes, G.M.; Morales, A.E.; Godoy-Parejo, C.; Gray, J.L.; Qin, Y.; Singh, A.A.; Xu, H.; Juste, J.; et al. Bat pluripotent stem cells reveal unusual entanglement between host and viruses. Cell 2023, 186, 957–974.e928. [Google Scholar] [CrossRef]
- Banerjee, A.; Rapin, N.; Miller, M.; Griebel, P.; Zhou, Y.; Munster, V.; Misra, V. Generation and Characterization of Eptesicus fuscus (Big brown bat) kidney cell lines immortalized using the Myotis polyomavirus large T-antigen. J. Virol. Methods 2016, 237, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Violet-Lozano, L.; Paredes-Galarza, B.; Gasparetto, R.; Mangini, A.T.; Timm, F.B.; Melgarejo, A.S.; Prandi, B.A.; Witt, A.; Oliveira, M.T.; Batista, H.; et al. Establishment of a cell line from the hematophagous Bat Desmodus rotundus susceptible to Lyssavirus rabies. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2025, 56, 1311–1320. [Google Scholar] [CrossRef]
- Brook, C.E.; Dobson, A.P. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015, 23, 172–180. [Google Scholar] [CrossRef]
- Rozwadowska, N.; Sikorska, M.; Bozyk, K.; Jarosinska, K.; Cieciuch, A.; Brodowska, S.; Andrzejczak, M.; Siemionow, M. Optimization of human myoblasts culture under different media conditions for application in the in vitro studies. Am. J. Stem Cells 2022, 11, 1–11. [Google Scholar] [PubMed]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017, 3, 17003. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Mamchaoui, K.; Trollet, C.; Bigot, A.; Negroni, E.; Chaouch, S.; Wolff, A.; Kandalla, P.K.; Marie, S.; Di Santo, J.; St Guily, J.L.; et al. Immortalized pathological human myoblasts: Towards a universal tool for the study of neuromuscular disorders. Skelet. Muscle 2011, 1, 34. [Google Scholar] [CrossRef]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef]
- Renault, V.; Thornell, L.E.; Eriksson, P.O.; Butler-Browne, G.; Mouly, V. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002, 1, 132–139. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Teixeira, M.Z. Telomere length: Biological marker of cellular vitality, aging, and health-disease process. Rev. Assoc. Med. Bras. 2021, 67, 173–177. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Vaziri, H.; Benchimol, S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 1998, 8, 279–282. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Zhu, C.H.; Mouly, V.; Cooper, R.N.; Mamchaoui, K.; Bigot, A.; Shay, J.W.; Di Santo, J.P.; Butler-Browne, G.S.; Wright, W.E. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: Consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 2007, 6, 515–523. [Google Scholar] [CrossRef]
- Robin, J.D.; Wright, W.E.; Zou, Y.; Cossette, S.C.; Lawlor, M.W.; Gussoni, E. Isolation and immortalization of patient-derived cell lines from muscle biopsy for disease modeling. J. Vis. Exp. 2015, e52307. [Google Scholar] [CrossRef]
- Saad, M.K.; Yuen, J.S.K., Jr.; Joyce, C.M.; Li, X.; Lim, T.; Wolfson, T.L.; Wu, J.; Laird, J.; Vissapragada, S.; Calkins, O.P.; et al. Continuous fish muscle cell line with capacity for myogenic and adipogenic-like phenotypes. Sci. Rep. 2023, 13, 5098. [Google Scholar] [CrossRef]
- Clare, E.L.; Adams, A.M.; Maya-Simoes, A.Z.; Eger, J.L.; Hebert, P.D.; Fenton, M.B. Diversification and reproductive isolation: Cryptic species in the only New World high-duty cycle bat, Pteronotus parnellii. BMC Evol. Biol. 2013, 13, 26. [Google Scholar] [CrossRef]
- Riskin, D.K.; Willis, D.J.; Iriarte-Diaz, J.; Hedrick, T.L.; Kostandov, M.; Chen, J.; Laidlaw, D.H.; Breuer, K.S.; Swartz, S.M. Quantifying the complexity of bat wing kinematics. J. Theor. Biol. 2008, 254, 604–615. [Google Scholar] [CrossRef]
- Ospina-Garcés, S.M.; Zamora-Gutierrez, V.; Lara-Delgado, J.M.; Morelos-Martínez, M.; Ávila-Flores, R.; Kurali, A.; Ortega, J.; Selem-Salas, C.I.; MacSwiney G, M.C. The relationship between wing morphology and foraging guilds: Exploring the evolution of wing ecomorphs in bats. Biol. J. Linn. Soc. 2023, 142, 481–498. [Google Scholar] [CrossRef]
- Smotherman, M.; Guillen-Servent, A. Doppler-shift compensation behavior by Wagner’s mustached bat, Pteronotus personatus. J. Acoust. Soc. Am. 2008, 123, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, H.U.; Denzinger, A. Auditory fovea and Doppler shift compensation: Adaptations for flutter detection in echolocating bats using CF-FM signals. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2011, 197, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef]
- Sikes, R.S.; The Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.; Bernal-Rivera, A.; Pena, V.; Morales-Sosa, P.; Robb, S.M.C.; Russell, J.; Yi, K.; Wang, Y.; Tsuchiya, D.; Murillo-Garcia, O.E.; et al. Sugar assimilation underlying dietary evolution of Neotropical bats. Nat. Ecol. Evol. 2024, 8, 1735–1750. [Google Scholar] [CrossRef]
- Elizarraras, J.M.; Liao, Y.; Shi, Z.; Zhu, Q.; Pico, A.R.; Zhang, B. WebGestalt 2024: Faster gene set analysis and new support for metabolomics and multi-omics. Nucleic Acids Res. 2024, 52, W415–W421. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Stephens, D.C.; Mungai, M.; Crabtree, A.; Beasley, H.K.; Garza-Lopez, E.; Vang, L.; Neikirk, K.; Vue, Z.; Vue, N.; Marshall, A.G.; et al. Protocol for isolating mice skeletal muscle myoblasts and myotubes via differential antibody validation. STAR Protoc. 2023, 4, 102591. [Google Scholar] [CrossRef]
- Zygmunt, K.; Otwinowska-Mindur, A.; Piorkowska, K.; Witarski, W. Influence of Media Composition on the Level of Bovine Satellite Cell Proliferation. Animals 2023, 13, 1855. [Google Scholar] [CrossRef]
- Anastasov, N.; Hofig, I.; Mall, S.; Krackhardt, A.M.; Thirion, C. Optimized Lentiviral Transduction Protocols by Use of a Poloxamer Enhancer, Spinoculation, and scFv-Antibody Fusions to VSV-G. Methods Mol. Biol. 2016, 1448, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kitajima, Y.; Okazaki, N.; Chiba, K.; Yonekura, A.; Ono, Y. A Modified Pre-plating Method for High-Yield and High-Purity Muscle Stem Cell Isolation From Human/Mouse Skeletal Muscle Tissues. Front. Cell Dev. Biol. 2020, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, C.J.; Lee, E.Y.; Son, Y.M.; Hwang, Y.H.; Joo, S.T. Optimal Pre-Plating Method of Chicken Satellite Cells for Cultured Meat Production. Food Sci. Anim. Resour. 2022, 42, 942–952. [Google Scholar] [CrossRef]
- Qu, Z.; Balkir, L.; van Deutekom, J.C.; Robbins, P.D.; Pruchnic, R.; Huard, J. Development of approaches to improve cell survival in myoblast transfer therapy. J. Cell Biol. 1998, 142, 1257–1267. [Google Scholar] [CrossRef]
- Gharaibeh, B.; Lu, A.; Tebbets, J.; Zheng, B.; Feduska, J.; Crisan, M.; Peault, B.; Cummins, J.; Huard, J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat. Protoc. 2008, 3, 1501–1509. [Google Scholar] [CrossRef]
- Simmons, N.B.; Conway, T.M. Phylogenetic Relationships Of Mormoopid Bats (Chiroptera: Mormoopidae) Based On Morphological Data. Bull. Am. Mus. Nat. Hist. 2001, 2001, 1–100. [Google Scholar] [CrossRef]
- Neyroud, N.; Nuss, H.B.; Leppo, M.K.; Marban, E.; Donahue, J.K. Gene delivery to cardiac muscle. Methods Enzymol. 2002, 346, 323–334. [Google Scholar] [CrossRef]
- Zhang, S.; Lou, H.; Lu, H.; Xu, E.; Liu, D.; Chen, Q. Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production. Fishes 2023, 8, 429. [Google Scholar] [CrossRef]
- Ricotti, L.; Polini, A.; Genchi, G.G.; Ciofani, G.; Iandolo, D.; Vazao, H.; Mattoli, V.; Ferreira, L.; Menciassi, A.; Pisignano, D. Proliferation and skeletal myotube formation capability of C2C12 and H9c2 cells on isotropic and anisotropic electrospun nanofibrous PHB scaffolds. Biomed. Mater. 2012, 7, 035010. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Chiu, C.P.; Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 1983, 32, 1171–1180. [Google Scholar] [CrossRef]
- Rando, T.A.; Blau, H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994, 125, 1275–1287. [Google Scholar] [CrossRef]
- Maffioletti, S.M.; Gerli, M.F.; Ragazzi, M.; Dastidar, S.; Benedetti, S.; Loperfido, M.; VandenDriessche, T.; Chuah, M.K.; Tedesco, F.S. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat. Protoc. 2015, 10, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Al Tanoury, Z.; Hestin, M.; Gobert, B.; Aivio, S.; Hick, A.; Cherrier, T.; Nesmith, A.P.; Parker, K.K.; Pourquie, O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 2016, 11, 1833–1850. [Google Scholar] [CrossRef]
- Krishnan, S.; Ulagesan, S.; Cadangin, J.; Lee, J.H.; Nam, T.J.; Choi, Y.H. Establishment and Characterization of Continuous Satellite Muscle Cells from Olive Flounder (Paralichthys olivaceus): Isolation, Culture Conditions, and Myogenic Protein Expression. Cells 2023, 12, 2325. [Google Scholar] [CrossRef] [PubMed]
- Doumit, M.E.; Cook, D.R.; Merkel, R.A. Fibroblast growth factor, epidermal growth factor, insulin-like growth factors, and platelet-derived growth factor-BB stimulate proliferation of clonally derived porcine myogenic satellite cells. J. Cell. Physiol. 1993, 157, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Milasincic, D.J.; Calera, M.R.; Farmer, S.R.; Pilch, P.F. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol. Cell. Biol. 1996, 16, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, H.F.; Hughes, D.C.; Allan, R.; Deane, C.S.; Coxon, C.R.; Morton, J.P.; Stewart, C.E.; Sharples, A.P. The role of resveratrol on skeletal muscle cell differentiation and myotube hypertrophy during glucose restriction. Mol. Cell. Biochem. 2018, 444, 109–123. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Li, F.; Wang, W.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Yin, Y. Effect of branched-chain amino acid ratio on the proliferation, differentiation, and expression levels of key regulators involved in protein metabolism of myocytes. Nutrition 2017, 36, 8–16. [Google Scholar] [CrossRef]
- Jin, C.L.; Ye, J.L.; Yang, J.; Gao, C.Q.; Yan, H.C.; Li, H.C.; Wang, X.Q. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef]
- Sinha, S.; Elbaz-Alon, Y.; Avinoam, O. Ca(2+) as a coordinator of skeletal muscle differentiation, fusion and contraction. FEBS J. 2022, 289, 6531–6542. [Google Scholar] [CrossRef]
- Ichio, 2nd; Kimura, I.; Ozawa, E. Promotion of Myoblast Proliferation by Hypoxanthine and RNA in Chick Embryo Extract: Hypoxanthine/RNA/myoblast proliferation/embryo extract). Dev. Growth Differ. 1985, 27, 101–110. [Google Scholar] [CrossRef]
- Ohashi, K.; Nagata, Y.; Wada, E.; Zammit, P.S.; Shiozuka, M.; Matsuda, R. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 2015, 333, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, J.D.; Zhao, X.G.; Chen, W.C.; Chen, W.X.; Hou, Y.R.; Ren, Y.H.; Xiao, Z.D.; Zhang, Q.; Diao, L.T.; et al. Simplifying the protocol for low-pollution-risk, efficient mouse myoblast isolation and differentiation. Adv. Biotechnol. 2025, 3, 8. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Dumont, N.A.; Rudnicki, M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013, 14, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.C.; Sampath, S.C.; Ho, A.T.V.; Corbel, S.Y.; Millstone, J.D.; Lamb, J.; Walker, J.; Kinzel, B.; Schmedt, C.; Blau, H.M. Induction of muscle stem cell quiescence by the secreted niche factor Oncostatin M. Nat. Commun. 2018, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Gabriel, B.M.; Dollet, L.; Smith, J.A.B.; Sardon Puig, L.; Botella, J.; Bishop, D.J.; Krook, A.; Zierath, J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020, 11, 470. [Google Scholar] [CrossRef]
- Rubenstein, A.B.; Hinkley, J.M.; Nair, V.D.; Nudelman, G.; Standley, R.A.; Yi, F.; Yu, G.; Trappe, T.A.; Bamman, M.M.; Trappe, S.W.; et al. Skeletal muscle transcriptome response to a bout of endurance exercise in physically active and sedentary older adults. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E260–E277. [Google Scholar] [CrossRef]
- Kokaji, T.; Eto, M.; Hatano, A.; Yugi, K.; Morita, K.; Ohno, S.; Fujii, M.; Hironaka, K.I.; Ito, Y.; Egami, R.; et al. In vivo transomic analyses of glucose-responsive metabolism in skeletal muscle reveal core differences between the healthy and obese states. Sci. Rep. 2022, 12, 13719. [Google Scholar] [CrossRef]




| Species/Source | Cell Line Name | Research Applications | References |
|---|---|---|---|
| Rat | L6 | Muscle metabolism, insulin signaling, hypertrophy | [4,5] |
| Mouse | C2C12 | Myogenesis, muscle regeneration, gene expression | [6] |
| Human (Myotonic Dystrophy) | Myotonic Dystrophy Cell Lines | Myotonic dystrophy disease modeling, therapy screening | [7,8] |
| Dog (Myok9) | Myok9 | Canine muscle disease and gene therapy studies | [9] |
| Dog (Dystrophic) | Dystrophic Myoblast Cell Lines | Muscular dystrophy modeling, therapy testing | [10] |
| Human (Healthy Muscle) | Human Myoblast Cell Line | Muscle aging, physiology, regenerative medicine | [11] |
| Grass Carp | CIM | Aquatic muscle growth, stress physiology | [12] |
| Crab-eating macaque | NHP iPAX7 | Myotube differentiation and muscle regeneration | [13] |
| Japanese Eel | JEM1129 | Fish muscle development, aquaculture research | [14] |
| Brown-Marbled Grouper | EfMS | Fish muscle stem cell and regenerative studies | [15] |
| Chicken | chTERT-Myoblasts/Primary Chicken Myoblasts | Avian muscle biology, myogenesis, gene studies | [16] |
| Cuvier’s beaked whale | pSV3neo myoblast cell line | Extreme hypoxia, fasting, and deep diving | [17] |
| Bat | iBatM-Pmeso-S1 iBatM-Pmeso-TC1 | Flight, muscle endurance, muscle stem cell and regeneration, metabolic resilience | This paper |
| Media | Base Medium | FBS | Additives | Antibiotics/Antifungals |
|---|---|---|---|---|
| GM1 | Ham’s F-10 Nutrient Mix (Gibco (Grand Island, NY, USA), Cat# 11550043) | 20% HyClone Characterized FBS (Cytiva (Marlborough, MA, USA), Cat# SH30071.03) | 5 ng/mL recombinant human epidermal growth factor (rh EGF) (ATCC (Manassas, VA, USA), Cat #: PCS-999-018), 10 µM dexamethasone (ATCC, Cat #: PCS-999-069), 25 µg/mL recombinant human insulin (ATCC, Cat #: PCS-999-068), 5 ng/mL recombinant human fibroblast growth factor basic protein (rh FGF-b) (ATCC, Cat #: PCS-999-020) | 2× Pen/Strep (Gibco, Cat# 15140122), 2 μg/mL Amphotericin B (R&D (Minneapolis, MN, USA), Cat# B23192) |
| GM2 | 50% Ham’s F-10 Nutrient Mix + 50% DMEM (ATCC, Cat# 30-2002) | 20% HyClone Characterized FBS | 5 ng/mL rh EGF, 10 µM dexamethasone, 25 µg/mL rh insulin, 5 ng/mL rh FGF-b | 2× Pen/Strep, 2 μg/mL Amphotericin B |
| GM3 | DMEM | 20% HyClone Characterized FBS | 5 ng/mL rh EGF, 10 µM dexamethasone, 25 µg/mL rh insulin, 5 ng/mL rh FGF-b | 2× Pen/Strep, 2 μg/mL Amphotericin B |
| Media | Base Medium | Horse Serum | Additives | Antibiotics/Antifungals |
|---|---|---|---|---|
| DM1 | DMEM | 2% | — | 2× Pen/Strep, 2 μg/mL Amphotericin B |
| DM2 | Ham’s F-10 Nutrient Mix | 2% | — | 2× Pen/Strep, 2 μg/mL Amphotericin B |
| DM3 | DMEM | 2% | 5 ng/mL rh EGF, 10 µM dexamethasone, 25 µg/mL rh insulin, 5 ng/mL rh FGF-b | 2× Pen/Strep, 2 μg/mL Amphotericin B |
| DM4 | Ham’s F-10 Nutrient Mix | 2% | 5 ng/mL rh EGF, 10 µM dexamethasone, 25 µg/mL rh insulin, 5 ng/mL rh FGF-b | 22× Pen/Strep, 2 μg/mL Amphotericin B |
| Cell Line | Doubling Time | Differentiation Timeline | Advantages/Disadvantages | References |
|---|---|---|---|---|
| C2C12 (mouse) | ~18–24 h | Myotubes by day 2–3; Contraction with induction after day 5–6 | Differentiation declines with passage; murine model; moderate NMJ relevance | [6,89] |
| L6 (rat) | ~22–30 h | Myotubes by day 5–6; Contraction by day 6 with induction | Reduced sarcomeric structure; lower nAChR expression; NMJ modeling limited | [4,5] |
| Primary Myoblasts | Variable (~24–36 h) | Myotubes by day 8–10; Contraction after day 10 with induction | Short culture lifespan; labor-intensive isolation; slower differentiation | [90] |
| iPSCs | Variable (~36–72 h) | Myotubes > 10 days with induction; variable contraction | Long, heterogeneous differentiation; genomic variability | [91,92] |
| iBatM-S1 (bat) | 26.33 h (P8), 28.96 h (P40) | Myotubes by day 2; Spontaneous contraction after day 2 | New line; long lifespan, no decline in function with passage; high NMJ relevance | This study |
| iBatM-TC (bat) | 23.42 h (P8), 25.46 h (P40) | Myotubes by day 2; Spontaneous contraction after day 2 | As above, immortalization effects under evaluation; high NMJ relevance | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, F.; Peña, V.; Morales-Sosa, P.; Bernal-Rivera, A.; Yang, B.; Huang, S.; Ghosh, S.; Katt, M.; Castellano, L.A.; Maddera, L.; et al. From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines. Cells 2025, 14, 1190. https://doi.org/10.3390/cells14151190
Deng F, Peña V, Morales-Sosa P, Bernal-Rivera A, Yang B, Huang S, Ghosh S, Katt M, Castellano LA, Maddera L, et al. From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines. Cells. 2025; 14(15):1190. https://doi.org/10.3390/cells14151190
Chicago/Turabian StyleDeng, Fengyan, Valentina Peña, Pedro Morales-Sosa, Andrea Bernal-Rivera, Bowen Yang, Shengping Huang, Sonia Ghosh, Maria Katt, Luciana Andrea Castellano, Lucinda Maddera, and et al. 2025. "From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines" Cells 14, no. 15: 1190. https://doi.org/10.3390/cells14151190
APA StyleDeng, F., Peña, V., Morales-Sosa, P., Bernal-Rivera, A., Yang, B., Huang, S., Ghosh, S., Katt, M., Castellano, L. A., Maddera, L., Yu, Z., Rohner, N., Zhao, C., & Camacho, J. (2025). From Development to Regeneration: Insights into Flight Muscle Adaptations from Bat Muscle Cell Lines. Cells, 14(15), 1190. https://doi.org/10.3390/cells14151190







