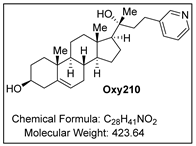
Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. RNA Isolation and Quantitative RT-PCR
2.3. Animal Studies
2.4. Statistical Analysis
3. Results
3.1. Gene Expression in Liver Tissue of the APOE*3-Leiden.CETP Mice During MASH Development
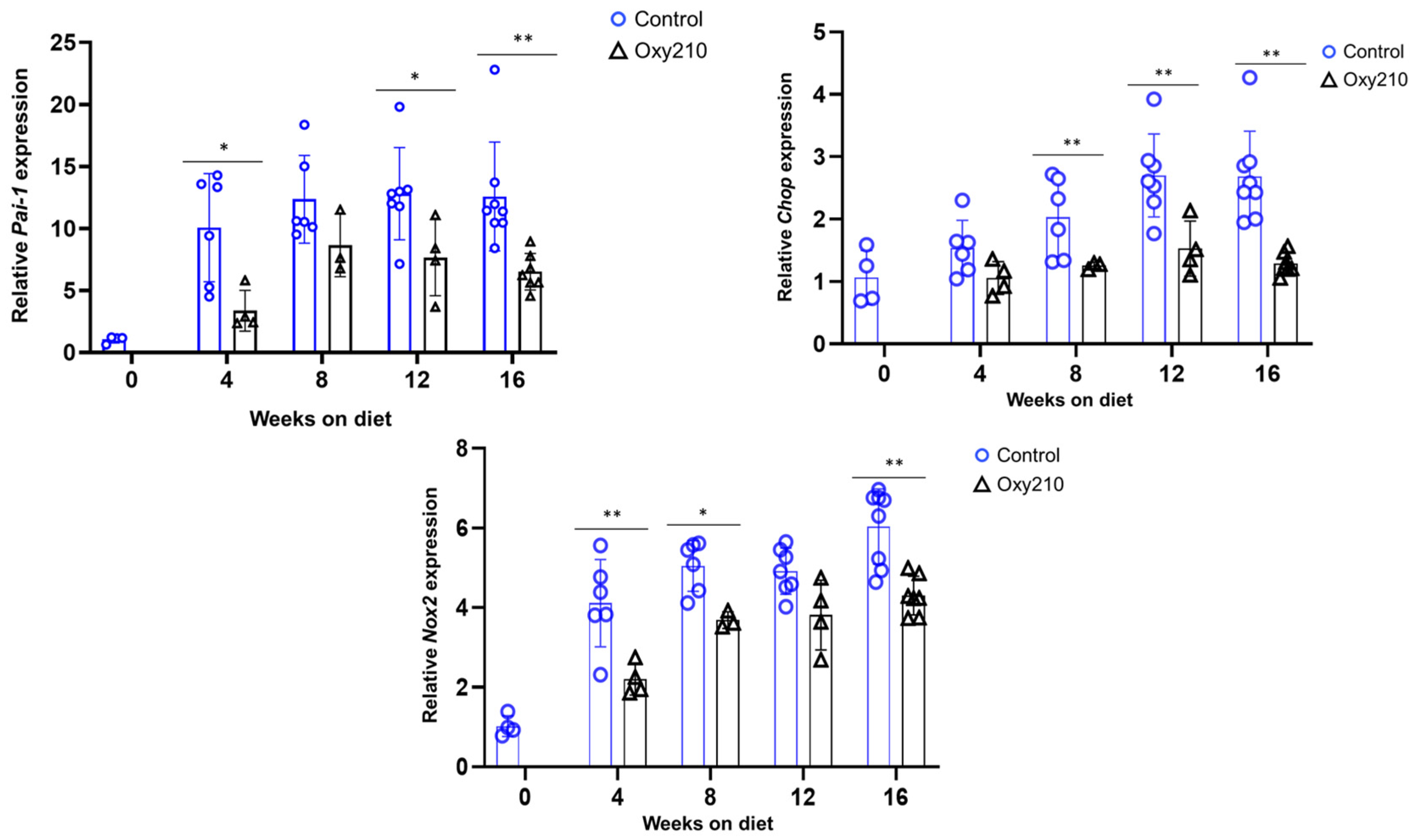
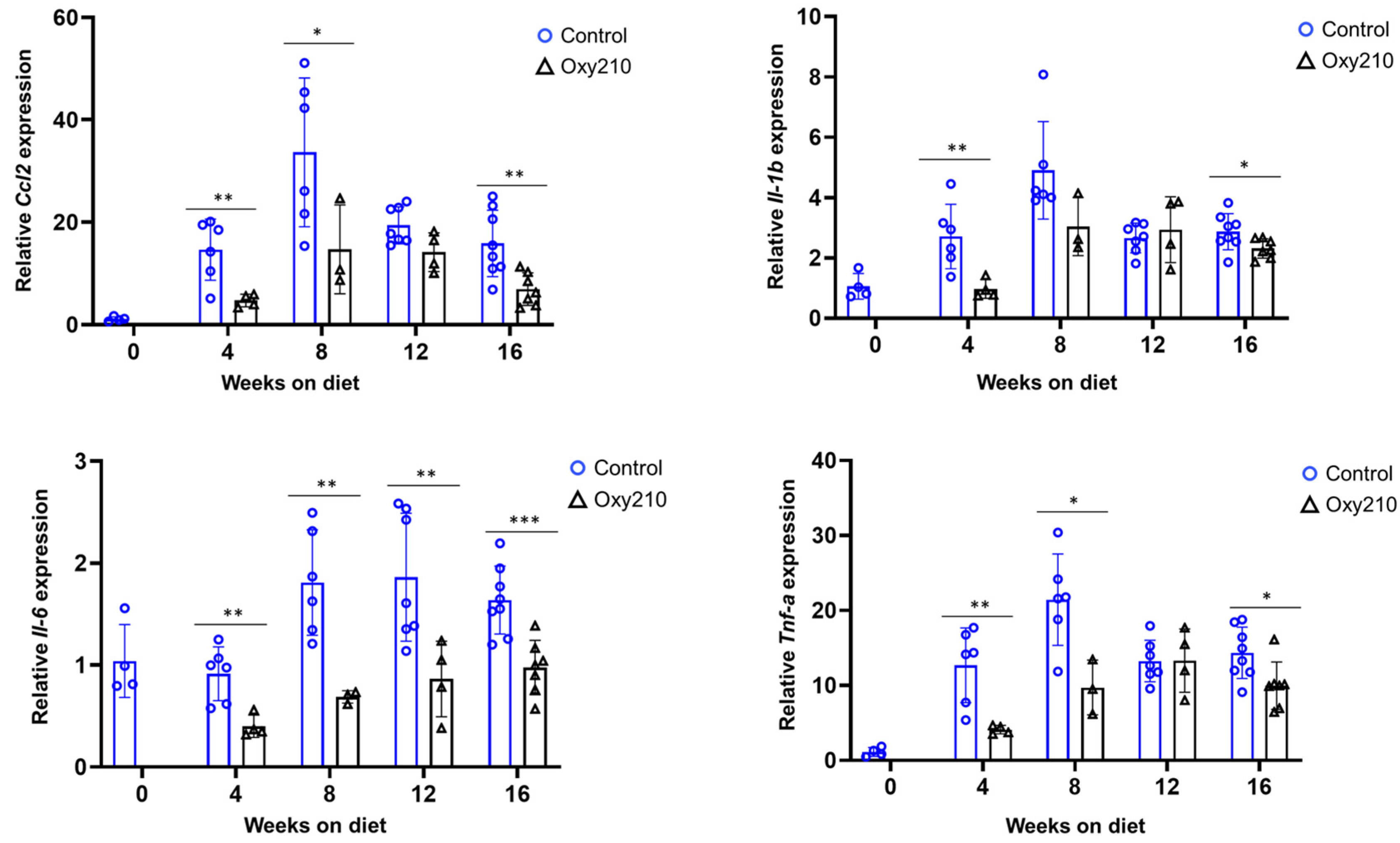
3.1.1. Senescence-Associated, SASP, and Pro-Inflammatory Genes (Figure 1, Figure 2 and Figure 3)

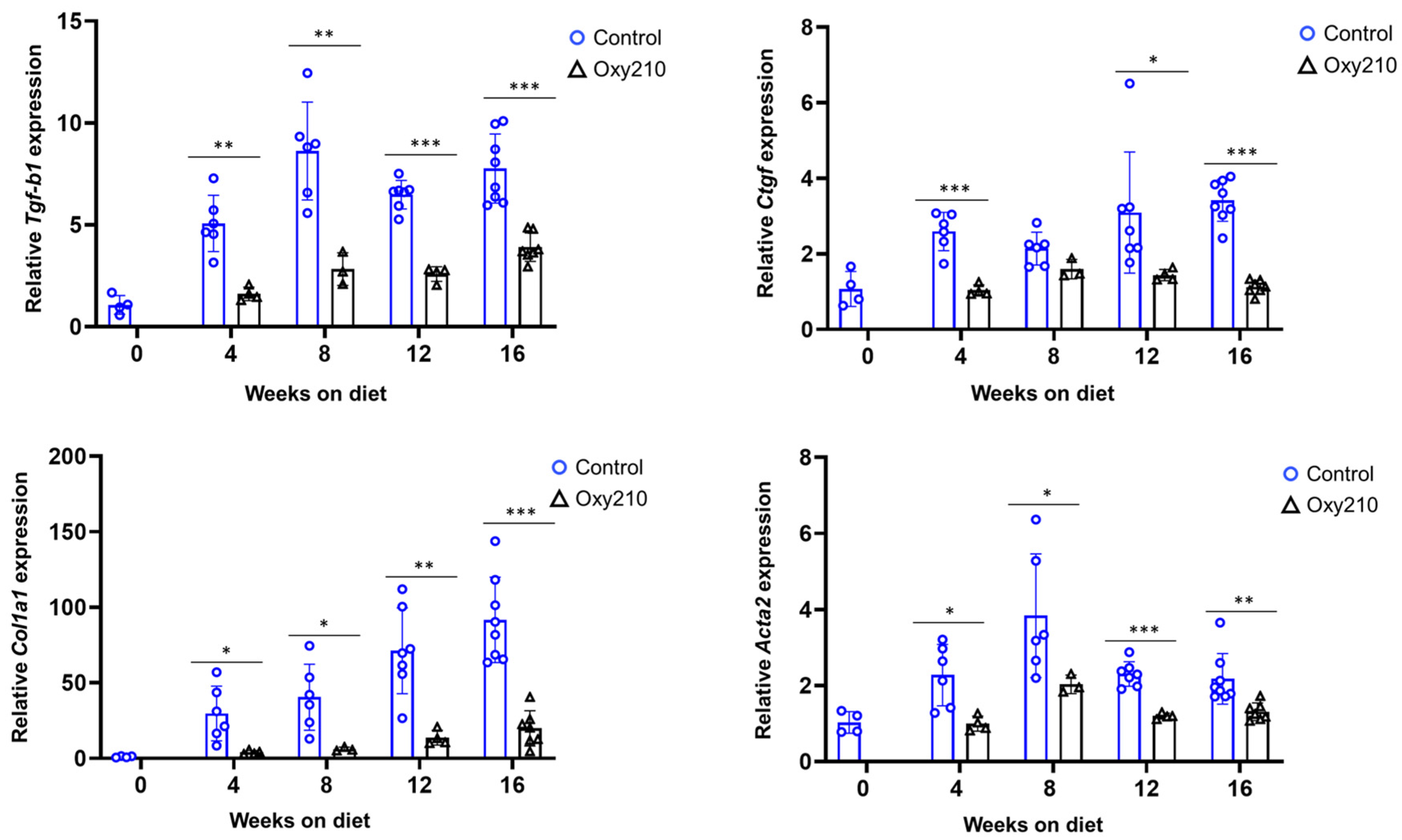
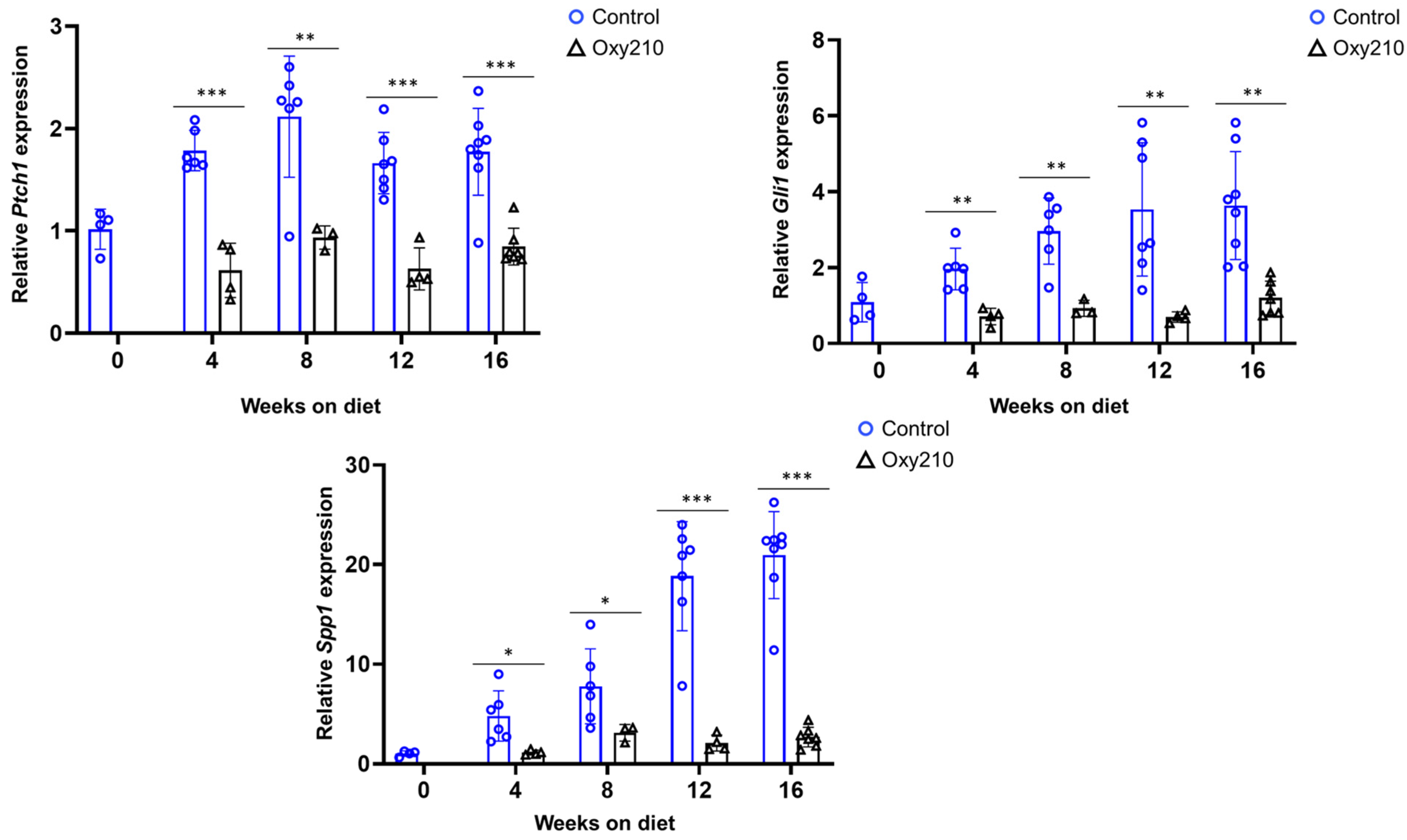
3.1.2. Pro-Fibrotic TGF-β and Hh Target Genes (Figure 4 and Figure 5)
3.2. Effect of Oxy210 on the Expression of Senescence-Associated and Secretory Phenotype (SASP) Genes in Human HepG2 Cells (Figure 6)

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, L.L.; Miao, H.; Wang, Y.N.; Liu, F.; Li, P.; Zhao, Y.Y. TGF-β as A Master Regulator of Aging-Associated Tissue Fibrosis. Aging Dis. 2023, 14, 1633–1650. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int. J. Mol. Sci. 2023, 24, 15473. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Amaya-Montoya, M.; Pérez-Londoño, A.; Guatibonza-García, V.; Vargas-Villanueva, A.; Mendivil, C.O. Cellular Senescence as a Therapeutic Target for Age-Related Diseases: A Review. Adv. Ther. 2020, 37, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, O.; Schosserer, M.; Grillari, J. Senopathies—Diseases Associated with Cellular Senescence. Biomolecules 2023, 13, 966. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Alexandersson, I.; Baboota, R.K.; Kroon, T.; Oscarsson, J.; Smith, U.; Boucher, J. Cellular Senescence in Hepatocytes Contributes to Metabolic Disturbances in NASH. Front. Endocrinol. 2022, 13, 957616. [Google Scholar] [CrossRef]
- Stahl, E.C.; Haschak, M.J.; Popovic, B.; Brown, B.N. Macrophages in the Aging Liver and Age-Related Liver Disease. Front. Immunol. 2018, 9, 2795. [Google Scholar] [CrossRef]
- Yashaswini, C.N.; Qin, T.; Bhattacharya, D.; Amor, C.; Lowe, S.; Lujambio, A.; Wang, S.; Friedman, S.L. Phenotypes and Ontogeny of Senescent Hepatic Stellate Cells in Metabolic Dysfunction-Associated Steatohepatitis. J. Hepatol. 2024, 81, 207–217. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Miwa, S.; Tchkonia, T.; Tiniakos, D.; Wilson, C.L.; Lahat, A.; Day, C.P.; Burt, A.; Palmer, A.; Anstee, Q.M.; et al. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017, 8, 15691. [Google Scholar] [CrossRef]
- Kim, H.Y.; Sakane, S.; Eguileor, A.; Yu, R.; Krizhanovsky, V.; Brenner, D.A.; Kisseleva, T. The Origin and Fate of Liver Myofibroblasts. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 93–106. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Maeda, H.; Noguchi, I.; Tanaka, M.; Wada, N.; Nagasaki, T.; Kobayashi, K.; Kanazawa, G.; Taguchi, K.; Chuang, V.T.G.; et al. Carbon Monoxide-Loaded Red Blood Cells Ameliorate Metabolic Dysfunction-Associated Steatohepatitis Progression via Enhancing AMP-Activated Protein Kinase Activity and Inhibiting Kupffer Cell Activation. Redox Biol. 2024, 76, 103314. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 Enhances TGF-β Signaling and Hepatic Fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Brenner, D.A. Recent Advancement of Molecular Mechanisms of Liver Fibrosis. J. Hepatobiliary Pancreat. Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.T.; Kurt, Z.; Tuominen, I.; Norheim, F.; Davis, R.C.; Pan, C.; Dirks, D.L.; Magyar, C.E.; French, S.W.; Chella Krishnan, K.; et al. The Genetic Architecture of Diet-Induced Hepatic Fibrosis in Mice. Hepatology 2018, 68, 2182–2196. [Google Scholar] [CrossRef]
- Hui, S.T.; Wang, F.; Stappenbeck, F.; French, S.W.; Magyar, C.E.; Parhami, F.; Lusis, A.J. Oxy210, a Novel Inhibitor of Hedgehog and TGF-β Signalling, Ameliorates Hepatic Fibrosis and Hypercholesterolemia in Mice. Endocrinol. Diabetes Metab. 2021, 4, e00296. [Google Scholar] [CrossRef]
- Stappenbeck, F.; Wang, F.; Sinha, S.K.; Hui, S.T.; Farahi, L.; Mukhamedova, N.; Fleetwood, A.; Murphy, A.J.; Sviridov, D.; Lusis, A.J.; et al. Anti-Inflammatory Oxysterol, Oxy210, Inhibits Atherosclerosis in Hyperlipidemic Mice and Inflammatory Responses of Vascular Cells. Cells 2024, 13, 1632. [Google Scholar] [CrossRef]
- Wang, F.; Stappenbeck, F.; Tang, L.Y.; Zhang, Y.E.; Hui, S.T.; Lusis, A.J.; Parhami, F. Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization. Int. J. Mol. Sci. 2022, 23, 5478. [Google Scholar] [CrossRef]
- Nair, B.; Kamath, A.J.; Pradeep, G.; Devan, A.R.; Sethi, G.; Nath, L.R. Unveiling the Role of the Hedgehog Signaling Pathway in Chronic Liver Disease: Therapeutic Insights and Strategies. Drug Discov. Today 2024, 29, 104064. [Google Scholar] [CrossRef]
- Yoshida, K.; Murata, M.; Yamaguchi, T.; Matsuzaki, K. TGF-β/Smad Signaling during Hepatic Fibro-carcinogenesis (Review). Int. J. Oncol. 2014, 45, 1363–1371. [Google Scholar] [CrossRef]
- Wang, F.; Stappenbeck, F.; Parhami, F. Oxy210, a Semi-Synthetic Oxysterol, Inhibits Profibrotic Signaling in Cellular Models of Lung and Kidney Fibrosis. Pharmaceuticals 2023, 16, 114. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Hari, P.; Millar, F.R.; Tarrats, N.; Rink, C.J.; Finch, A.J.; Brunton, V.G.; Passos, J.F.; Morton, J.P. The Innate Immune Sensor Toll-Like Receptor 2 Controls the Senescence-Associated Secretory Phenotype. Sci. Adv. 2019, 5, eaaw0254. [Google Scholar] [CrossRef]
- Yan, J.; Chen, S.; Yi, Z.; Shi, H.; Gao, C.; Zhang, Z. The Role of p21 in Cellular Senescence and Aging-Related Diseases. Mol. Cells 2024, 47, 100113. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular Senescence and Its Effector Programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef]
- Vilborg, A.; Wilhelm, M.T.; Wiman, K.G. Regulation of Tumor Suppressor p53 at the RNA Level. J. Mol. Med. 2010, 88, 645–652. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, X. Senescence Regulation by the p53 Protein Family. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 965, pp. 37–61. [Google Scholar]
- Wagner, K.D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Naik, I.; Braunstein, Z.; Zhong, J.; Ren, B. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front. Immunol. 2017, 8, 1612. [Google Scholar] [CrossRef]
- Salazar, G. NADPH Oxidases and Mitochondria in Vascular Senescence. Int. J. Mol. Sci. 2018, 19, 1327. [Google Scholar] [CrossRef]
- Lener, B.; Kozieł, R.; Pircher, H.; Kratochwil, M.; Hermann, M.; Dencher, N.A.; Jansen-Dürr, P. The NADPH Oxidase Nox4 Restricts the Replicative Lifespan of Human Endothelial Cells. Biochem. J. 2009, 423, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Tacke, F.; Sugimoto, A.; Friedman, S.L. Antifibrotic Therapies for Metabolic Dysfunction-associated Steatotic Liver Disease. JHEP Rep. 2025, 7, 101421. [Google Scholar] [CrossRef] [PubMed]
- Mladenić, K.; Lenartić, M.; Marinović, S.; Polić, B.; Wensveen, F.M. The “Domino effect” in MASLD: The inflammatory cascade of steatohepatitis. Eur. J. Immunol. 2024, 54, e2149641. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Transforming Growth Factor-β in Tissue Fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Dooley, S.; ten Dijke, P. TGF-β in Progression of Liver Disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L. Navigating the Complex Role of Senescence in Liver Disease. Chin. Med. J. 2024, 137, 3061–3072. [Google Scholar] [CrossRef]
- Philips, G.M.; Chan, I.S.; Swiderska, M.; Schroder, V.T.; Guy, C.; Karaca, G.F.; Moylan, C.; Venkatraman, T.; Feuerlein, S.; Syn, W.-K.; et al. Hedgehog Signaling Antagonist Promotes Regression of Both Liver Fibrosis and Hepatocellular Carcinoma in a Murine Model of Primary Liver Cancer. PLoS ONE 2011, 6, e23943. [Google Scholar] [CrossRef]
- Yang, L.; Roh, Y.S.; Song, J.; Li, S.; Seki, E.; Brenner, D.A.; Karin, M.; Karin, G.; Loomba, R.; Glaser, S.; et al. Transforming Growth Factor beta Signaling in Hepatocytes Participates in Steatohepatitis Through Regulation of Cell Death and Lipid Metabolism in Mice. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Herrema, H.; Scheithauer, T.P.M.; Kroon, J.; Nieuwdorp, M.; Groen, A.K. Evaluating Causality of Cellular Senescence in Non-alcoholic Fatty Liver Disease. JHEP Rep. 2021, 3, 100301. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular Senescence and Senolytics: The Path to the Clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of Activated Stellate Cells Limits Liver Fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Ferreira-Gonzalez, S.; Rodrigo-Torres, D.; Gadd, V.L.; Forbes, S.J. Cellular Senescence in Liver Disease and Regeneration. Semin. Liver Dis. 2021, 41, 50–66. [Google Scholar] [CrossRef]
- Chaudhary, R.; Weiskirchen, R.; Ehrlich, M.; Henis, Y.I. Dual Signaling Pathways of TGF-β Superfamily Cytokines in Hepatocytes: Balancing Liver Homeostasis and Disease Progression. Front. Pharmacol. 2025, 16, 1580500. [Google Scholar] [CrossRef]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.-I.; Hoshida, Y.; et al. A Simple Diet- and Chemical-Induced Murine NASH Model with Rapid Progression of Steatohepatitis, Fibrosis and Liver Cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Xia, L.; Wang, L.; Jin, Y.; Cao, Y.; Tang, Z.; Qin, W. Hepatocellular Carcinoma: Signaling Pathways and Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 35. [Google Scholar] [CrossRef]
- Giannelli, G.; Villa, E.; Lahn, M. Transforming Growth Factor-β as a Therapeutic Target in Hepatocellular Carcinoma. Cancer Res. 2014, 74, 1890–1894. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Zhang, Y.; Meng, B.; Ying, W.; Qian, X. Identification of Hedgehog Signaling as a Potential Oncogenic Driver in an Aggressive Subclass of Human Hepatocellular Carcinoma: A Reanalysis of the TCGA Cohort. Sci. China Life Sci. 2019, 62, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, H.Y.; Zhang, L.; Zhou, Y.; Wu, J. Hedgehog Signaling, a Critical Pathway Governing the Development and Progression of Hepatocellular Carcinoma. Cells 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, C.M.; Viscardi, G.; Papaccio, F.; D’Amodio, M.; Esposito, C.; Cennamo, G.; Ciardiello, F.; Martinelli, E. Implication of the Hedgehog Pathway in Hepatocellular Carcinoma. World J. Gastroenterol. 2017, 23, 4330–4340. [Google Scholar] [CrossRef]
- Jun, J.H.; Du, K.; Dutta, R.K.; Maeso-Diaz, R.; Oh, S.H.; Wang, L.; Gao, G.; Ferreira, A.; Hill, J.; Pullen, S.S.; et al. The senescence-associated secretome of Hedgehog-deficient hepatocytes drives MASLD progression. J. Clin. Investig. 2024, 134, e180310. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Found in Translation—Fibrosis in Metabolic Dysfunction-Associated Steatohepatitis (MASH). Sci. Transl. Med. 2023, 15, eadi0759. [Google Scholar] [CrossRef]
- Zeng, Q.; Gong, Y.; Zhu, N.; Shi, Y.; Zhang, C.; Qin, L. Lipids and Lipid Metabolism in Cellular Senescence: Emerging Targets for Age-Related Diseases. Ageing Res. Rev. 2024, 97, 102294. [Google Scholar] [CrossRef]
- Stappenbeck, F.; Wang, F.; Tang, L.Y.; Zhang, Y.E.; Parhami, F. Inhibition of Non-Small Cell Lung Cancer Cells by Oxy210, an Oxysterol-Derivative that Antagonizes TGFβ and Hedgehog Signaling. Cells 2019, 8, 1297. [Google Scholar] [CrossRef]
- Tang, L.Y.; Spezia, M.; Chen, T.; Stappenbeck, F.; Wang, F.; Parhami, F. Oxysterol Derivatives Oxy186 and Oxy210 Inhibit WNT Signaling in Non-Small Cell Lung Cancer. Cell Biosci. 2022, 12, 119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Hui, S.T.; Stappenbeck, F.; Kaminska, D.; Lusis, A.J.; Parhami, F. Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro. Cells 2025, 14, 1191. https://doi.org/10.3390/cells14151191
Wang F, Hui ST, Stappenbeck F, Kaminska D, Lusis AJ, Parhami F. Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro. Cells. 2025; 14(15):1191. https://doi.org/10.3390/cells14151191
Chicago/Turabian StyleWang, Feng, Simon T. Hui, Frank Stappenbeck, Dorota Kaminska, Aldons J. Lusis, and Farhad Parhami. 2025. "Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro" Cells 14, no. 15: 1191. https://doi.org/10.3390/cells14151191
APA StyleWang, F., Hui, S. T., Stappenbeck, F., Kaminska, D., Lusis, A. J., & Parhami, F. (2025). Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro. Cells, 14(15), 1191. https://doi.org/10.3390/cells14151191







