Regulation of Myogenesis by MechanomiR-200c/FoxO3 Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Isolation of Satellite Cells and Differentiation into Myoblasts
2.3. Stretch Protocol
2.4. miRNA Microarray Analysis
2.5. Reverse Transcription and Quantitative PCR (RT-qPCR)
2.6. Immunoblot Analysis
2.7. Transfection
2.8. Luciferase Assays
2.9. Chromatin Immunoprecipitation (ChIP) Assays
2.10. Immunocytochemistry
2.11. Cell Proliferation Assays
2.12. Statistical Analysis
3. Results
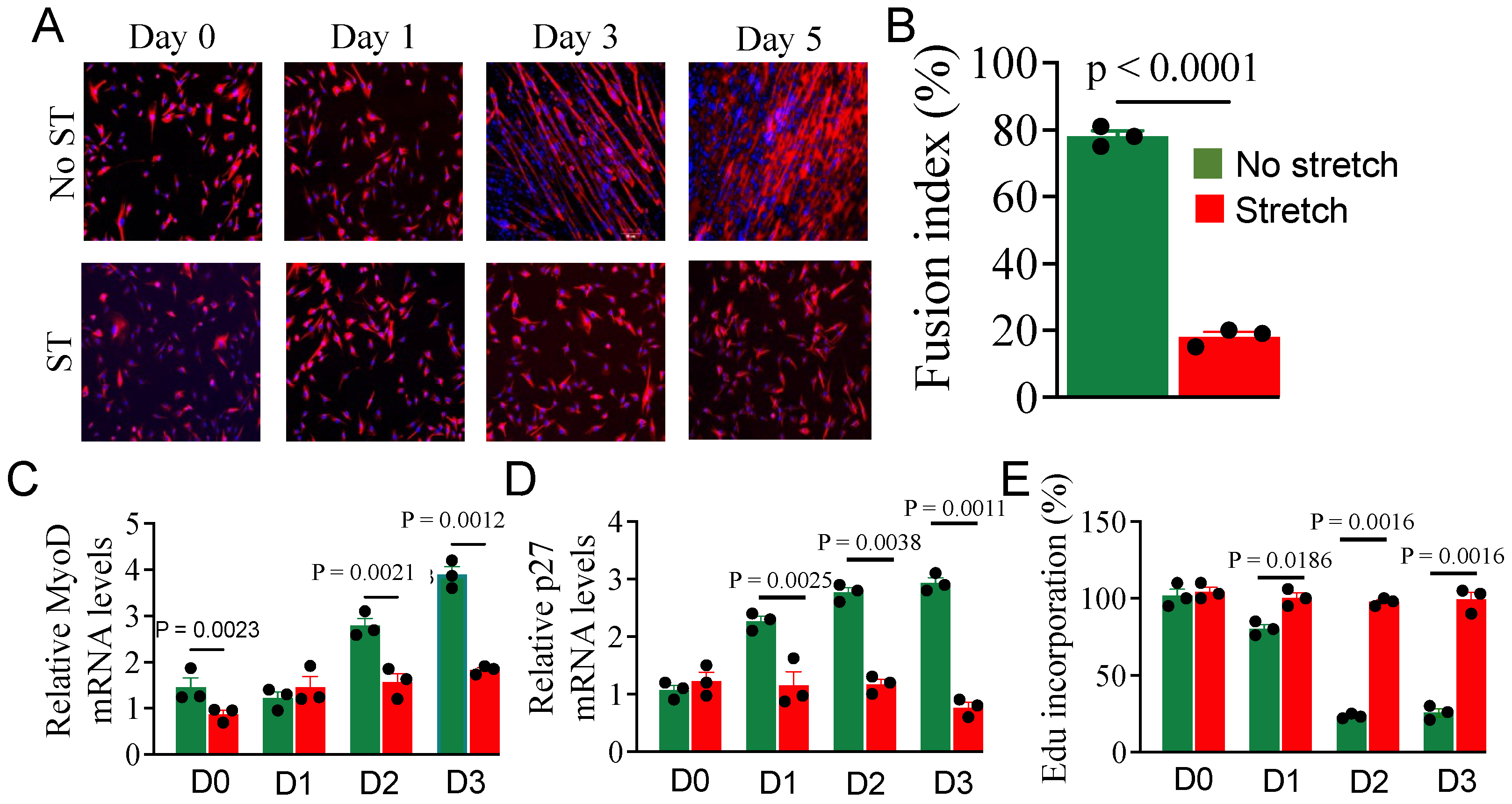
3.1. Effect of Stretch on Myoblast Differentiation
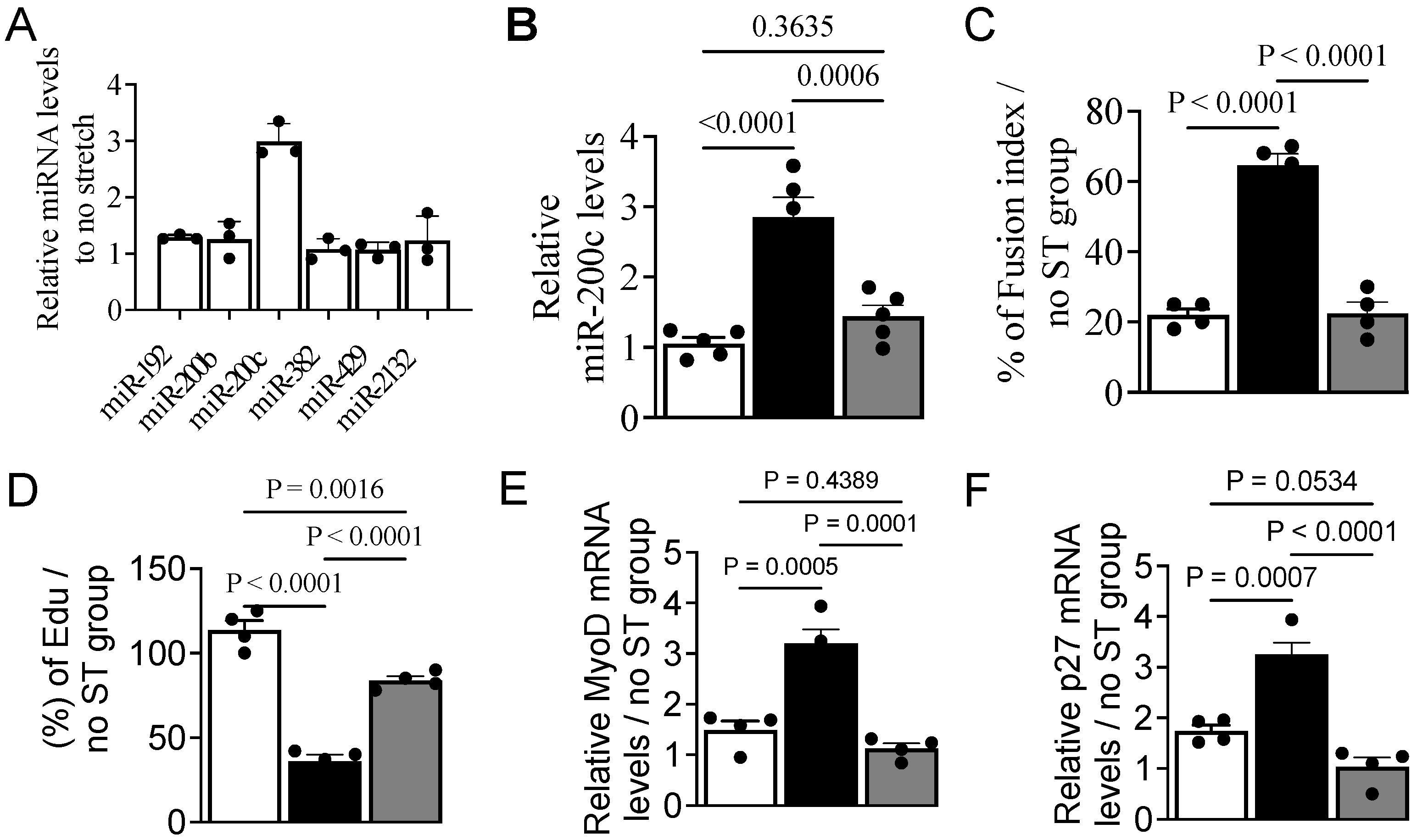
3.2. Mechanical Stretch Alters the miRNA Expression Profile
3.3. MechanomiR-200c Inhibits Myoblast Differentiation
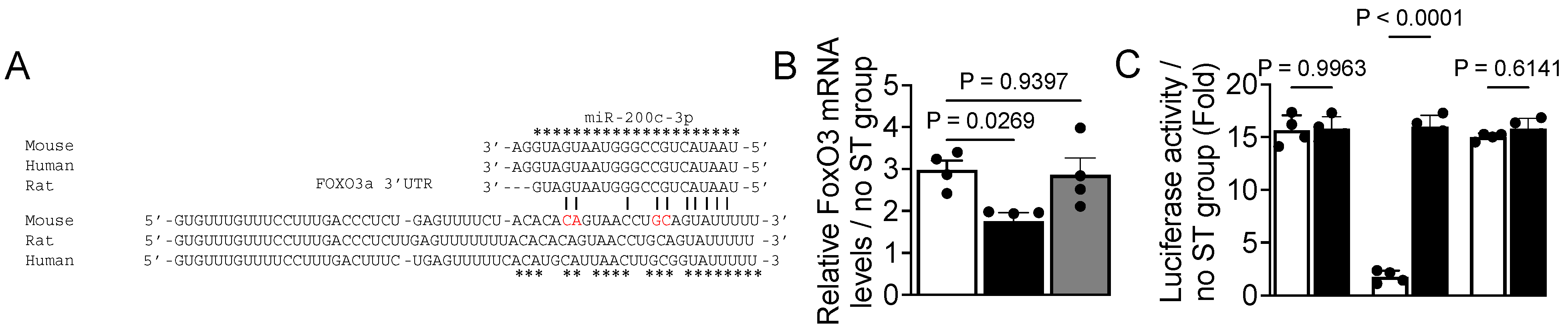
3.4. FoxO3 Is a Target Gene of miR-200c
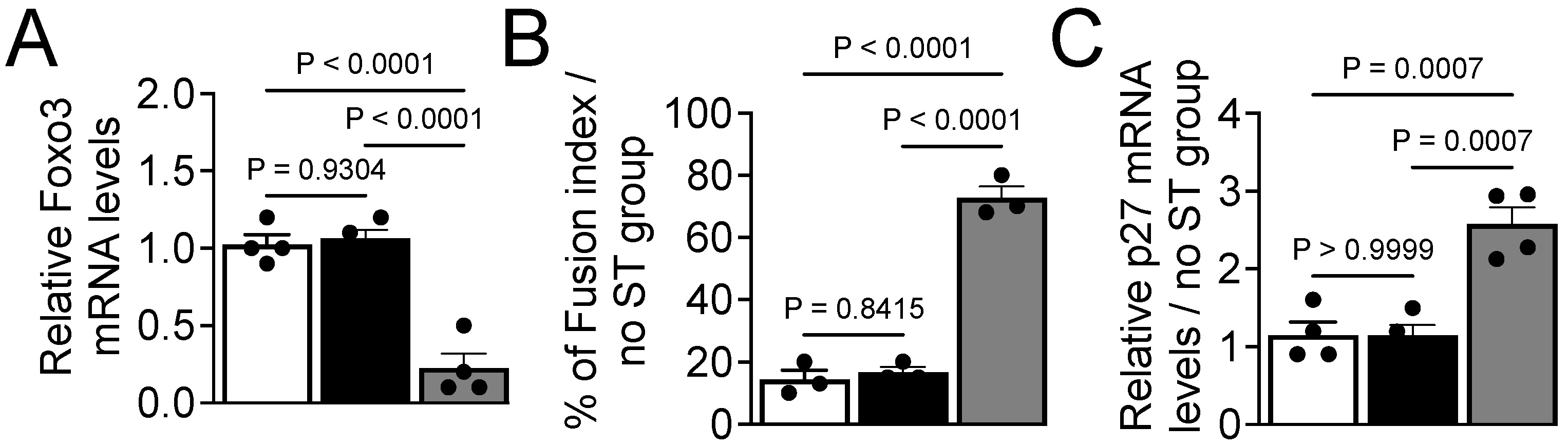
3.5. Expression of MiR-200c Is Under Transcriptional Control of MyoD
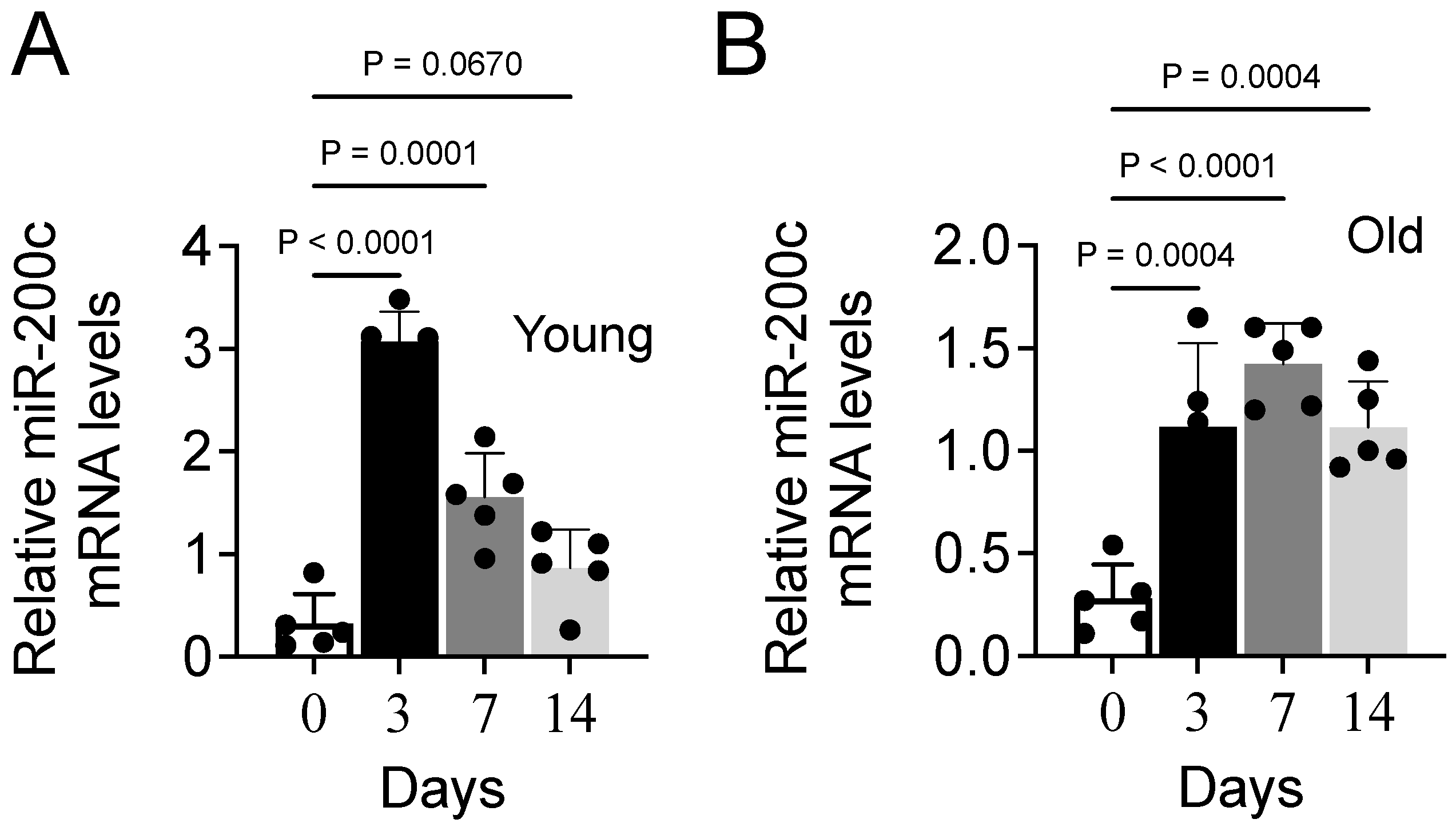
3.6. Aging Impairs Expression of MiR-200c During Muscle Repair in Mice
4. Discussion
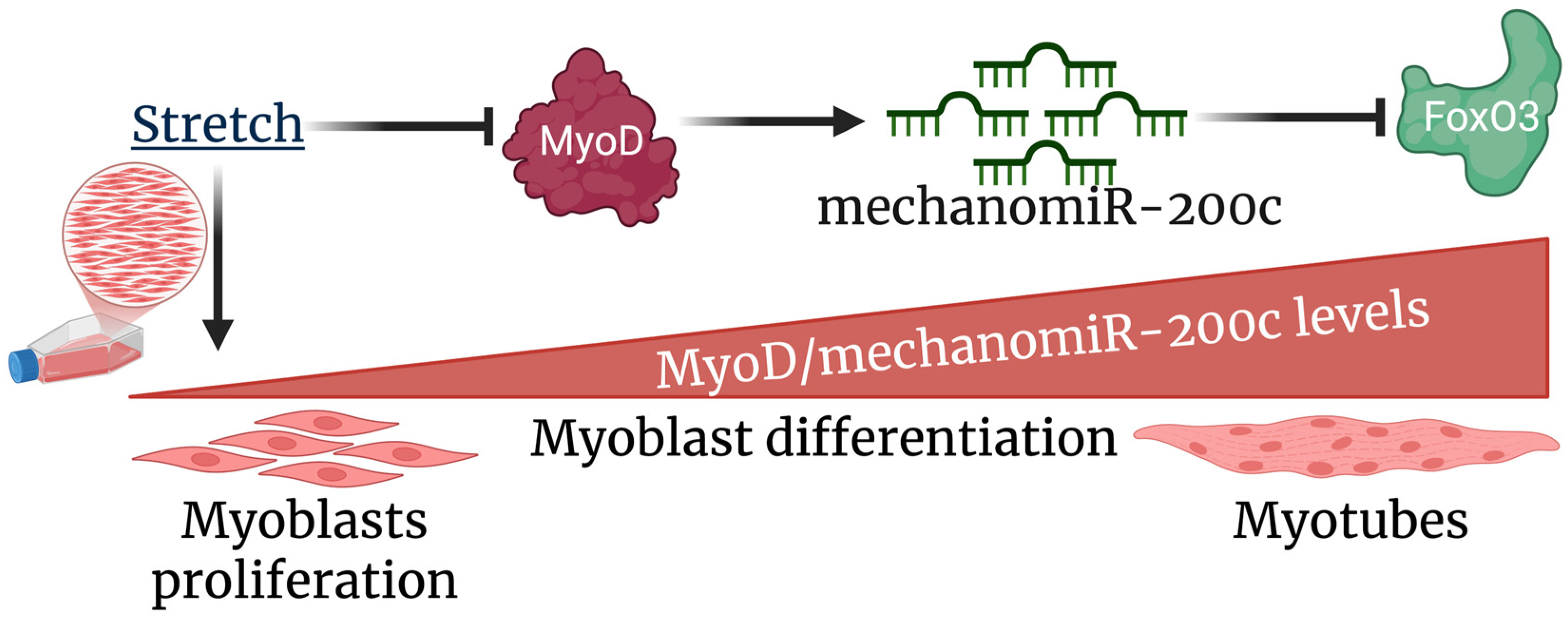
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Boriek, A.M. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: A possible role in Duchenne muscular dystrophy. FASEB J. 2003, 17, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhry, I.; Reid, M.B.; Boriek, A.M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J. Biol. Chem. 2002, 277, 46493–46503. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Murphy, R.; Robinson, P.; Wei, L.; Boriek, A.M. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 2004, 18, 1524–1535. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Hajira, A.; Lopez, M.A.; Boriek, A.M. Genome-wide Mechanosensitive MicroRNA (MechanomiR) Screen Uncovers Dysregulation of Their Regulatory Networks in the mdm Mouse Model of Muscular Dystrophy. J. Biol. Chem. 2015, 290, 24986–25011. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Cox, G.A.; Boriek, A.M. Anisotropic regulation of Ankrd2 gene expression in skeletal muscle by mechanical stretch. FASEB J. 2010, 24, 3330–3340. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Miyasaka, K.; Kida, Y.; Ogura, T. Mechanotransduction in cardiovascular and skeletal muscle. Seikagaku J. Jpn. Biochem. Soc. 2009, 81, 494–501. [Google Scholar]
- Wu, M.; Fannin, J.; Rice, K.M.; Wang, B.; Blough, E.R. Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 2011, 10, 1–15. [Google Scholar] [CrossRef]
- Patel, N.D.; Jannapureddy, S.R.; Hwang, W.; Chaudhry, I.; Boriek, A.M. Altered muscle force and stiffness of skeletal muscles in alpha-sarcoglycan-deficient mice. Am. J. Physiol. Cell Physiol. 2003, 284, C962–C968. [Google Scholar] [CrossRef]
- Kumar, A.; Khandelwal, N.; Malya, R.; Reid, M.B.; Boriek, A.M. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004, 18, 102–113. [Google Scholar] [CrossRef]
- Lopez, M.A.; Mayer, U.; Hwang, W.; Taylor, T.; Hashmi, M.A.; Jannapureddy, S.R.; Boriek, A.M. Force transmission, compliance, and viscoelasticity are altered in the alpha7-integrin-null mouse diaphragm. Am. J. Physiol. Cell Physiol. 2005, 288, C282–C289. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.S.; Lopez, M.A.; Mohamed, J.S.; Boriek, A.M. Anisotropic mechanosensitive pathways in the diaphragm and their implications in muscular dystrophies. Muscle Res. Cell Motil. 2017, 38, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Karalaki, M.; Fili, S.; Philippou, A.; Koutsilieris, M. Muscle regeneration: Cellular and molecular events. In Vivo 2009, 23, 779–796. [Google Scholar] [PubMed]
- Fukada, S.I. The roles of muscle stem cells in muscle injury, atrophy and hypertrophy. J. Biochem. 2018, 163, 353–358. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, M.; Wang, Y.; Yu, L.; Zhao, T.; Qiu, X.; Wang, L. Mechanical stretch regulates microRNA expression profile via NF-κB activation in C2C12 myoblasts. Mol. Med. Rep. 2016, 14, 5084–5092. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Lu, L.; Wang, X. Role of androgen receptor on cyclic mechanical stretch-regulated proliferation of C2C12 myoblasts and its upstream signals: IGF-1-mediated PI3K/Akt and MAPKs pathways. Mol. Cell. Endocrinol. 2017, 450, 83–93. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjostrom, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Akima, H.; Kano, Y.; Enomoto, Y.; Ishizu, M.; Okada, M.; Oishi, Y.; Katsuta, S.; Kuno, S. Muscle function in 164 men and women aged 20–84 yr. Med. Sci. Sports Exerc. 2001, 33, 220–226. [Google Scholar] [CrossRef]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef]
- Carlson, B.M.; Dedkov, E.I.; Borisov, A.B.; Faulkner, J.A. Skeletal muscle regeneration in very old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B224–B233. [Google Scholar] [CrossRef]
- Shefer, G.; Van de Mark, D.P.; Richardson, J.B.; Yablonka-Reuveni, Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006, 294, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Gutarra, S.; Garcia-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardi, M.; Ballestar, E.; Gonzalez, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- McCarthy, J.J. microRNA and skeletal muscle function: Novel potential roles in exercise, diseases, and aging. Front. Physiol. 2014, 5, 290. [Google Scholar] [CrossRef]
- Sharma, M.; Juvvuna, P.K.; Kukreti, H.; McFarlane, C. Mega roles of microRNAs in regulation of skeletal muscle health and disease. Front. Physiol. 2014, 5, 239. [Google Scholar] [CrossRef]
- Nie, M.; Deng, Z.L.; Liu, J.; Wang, D.Z. Noncoding RNAs, Emerging Regulators of Skeletal Muscle Development and Diseases. Biomed. Res. Int. 2015, 2015, 676575. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3β. J. Biol. Chem. 2010, 285, 29336–29347. [Google Scholar] [CrossRef]
- Guan, Y.-J.; Yang, X.; Wei, L.; Chen, Q. MiR-365: A mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 2011, 25, 4457–4466. [Google Scholar] [CrossRef]
- Huang, Y.; Crawford, M.; Higuita-Castro, N.; Nana-Sinkam, P.; Ghadiali, S.N. miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J. 2012, 26, 3351–3364. [Google Scholar] [CrossRef] [PubMed]
- Kobus, K.; Kopycinska, J.; Kozlowska-Wiechowska, A.; Urasinska, E.; Kempinska-Podhorodecka, A.; Haas, T.L.; Milkiewicz, P.; Milkiewicz, M. Angiogenesis within the duodenum of patients with cirrhosis is modulated by mechanosensitive Kruppel-like factor 2 and microRNA-126. Liver Int. 2012, 32, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: Mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y.J. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic. Biol. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Cox, G.A.; Boriek, A.M. Ankyrin repeat domain protein 2 and inhibitor of DNA binding 3 cooperatively inhibit myoblast differentiation by physical interaction. J. Biol. Chem. 2013, 288, 24560–24568. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Hajira, A.; Pardo, P.S.; Boriek, A.M. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1α network in skeletal muscle. Diabetes 2014, 63, 1546–1559. [Google Scholar] [CrossRef]
- Pardo, P.S.; Hajira, A.; Boriek, A.M.; Mohamed, J.S. MicroRNA-434-3p regulates age-related apoptosis through eIF5A1 in the skeletal muscle. Aging 2017, 9, 1012–1029. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Boriek, A.M. Stretch augments TGF-beta1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L413–L424. [Google Scholar] [CrossRef]
- Hinkle, E.R.; Essader, T.O.; Gentile, G.M.; Giudice, J. ViaFuse: Fiji macros to calculate skeletal muscle cell viability and fusion index. Skelet. Muscle 2021, 11, 28. [Google Scholar] [CrossRef]
- Fujio, Y.; Guo, K.; Mano, T.; Mitsuuchi, Y.; Testa, J.R.; Walsh, K. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell Biol. 1999, 19, 5073–5082. [Google Scholar] [CrossRef]
- Cheng, C.S.; El-Abd, Y.; Bui, K.; Hyun, Y.E.; Hughes, R.H.; Kraus, W.E.; Truskey, G.A. Conditions that promote primary human skeletal myoblast culture and muscle differentiation in vitro. Am. J. Physiol. Cell Physiol. 2014, 306, C385–C395. [Google Scholar] [CrossRef] [PubMed]
- Ballarino, M.; Morlando, M.; Fatica, A.; Bozzoni, I. Non-coding RNAs in muscle differentiation and musculoskeletal disease. J. Clin. Investig. 2016, 126, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Loher, P.; Rigoutsos, I. Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 2012, 28, 3322–3323. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Geles, K.G.; Paik, J.H.; DePinho, R.A.; Tjian, R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell 2008, 15, 534–546. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Ambrosio, R.; Guardiola, O.; Sibilio, A.; Paik, J.H.; Minchiotti, G.; DePinho, R.A.; Fenzi, G.; Larsen, P.R.; et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J. Clin. Investig. 2010, 120, 4021–4030. [Google Scholar] [CrossRef]
- Kassar-Duchossoy, L.; Gayraud-Morel, B.; Gomès, D.; Rocancourt, D.; Buckingham, M.; Shinin, V.; Tajbakhsh, S. Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 2004, 431, 466–471. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Schnegelsberg, P.N.; Stead, R.H.; Braun, T.; Arnold, H.H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef]
- Abe, S.; Rhee, S.; Iwanuma, O.; Hiroki, E.; Yanagisawa, N.; Sakiyama, K.; Ide, Y. Effect of mechanical stretching on expressions of muscle specific transcription factors MyoD, Myf-5, myogenin and MRF4 in proliferated myoblasts. Anat. Histol. Embryol. 2009, 38, 305–310. [Google Scholar] [CrossRef]
- Kook, S.H.; Lee, H.J.; Chung, W.T.; Hwang, I.H.; Lee, S.A.; Kim, B.S.; Lee, J.C. Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol. Cells 2008, 25, 479–486. [Google Scholar]
- Kaestner, K.H.; Knochel, W.; Martinez, D.E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000, 14, 142–146. [Google Scholar] [CrossRef]
- Furuyama, T.; Nakazawa, T.; Nakano, I.; Mori, N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 2000, 349, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Biggs, W.H., 3rd; Cavenee, W.K.; Arden, K.C. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm. Genome 2001, 12, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013, 288, 30515–30526. [Google Scholar] [CrossRef]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.W.; Kang, K.S.; Park, T.S. Forkhead box O3 promotes cell proliferation and inhibits myotube differentiation in chicken myoblast cells. Br. Poult. Sci. 2019, 60, 23–30. [Google Scholar] [CrossRef]
- Hribal, M.L.; Nakae, J.; Kitamura, T.; Shutter, J.R.; Accili, D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 2003, 162, 535–541. [Google Scholar] [CrossRef]
- Kitamura, T.; Kitamura, Y.I.; Funahashi, Y.; Shawber, C.J.; Castrillon, D.H.; Kollipara, R.; DePinho, R.A.; Kitajewski, J.; Accili, D. A FoxO/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Investig. 2007, 117, 2477–2485. [Google Scholar] [CrossRef]
- Gopinath, S.D.; Webb, A.E.; Brunet, A.; Rando, T.A. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Rep. 2014, 2, 414–426. [Google Scholar] [CrossRef]
- Wu, A.L.; Kim, J.H.; Zhang, C.; Unterman, T.G.; Chen, J. Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components. Endocrinology 2008, 149, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Calhabeu, F.; Hayashi, S.; Morgan, J.E.; Relaix, F.; Zammit, P.S. Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells. Oncogene 2013, 32, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Walters, Z.S.; Villarejo-Balcells, B.; Olmos, D.; Buist, T.W.; Missiaglia, E.; Allen, R.; Al-Lazikani, B.; Garrett, M.D.; Blagg, J.; Shipley, J. JARID2 is a direct target of the PAX3-FOXO1 fusion protein and inhibits myogenic differentiation of rhabdomyosarcoma cells. Oncogene 2014, 33, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Fang, Y.H.; Chi, H.C.; Chang, L.C.; Chung, S.Y.; Huang, W.C.; Wang, X.W.; Lee, K.W.; Chen, S.L. Insulin and LiCl Synergistically Rescue Myogenic Differentiation of FoxO1 Over-Expressed Myoblasts. PLoS ONE 2014, 9, e88450. [Google Scholar] [CrossRef]
- Pardo, P.S.; Lopez, M.A.; Boriek, A.M. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am. J. Physiol. Cell Physiol. 2008, 294, C1056–C1066. [Google Scholar] [CrossRef]
- Rawls, A.; Valdez, M.R.; Zhang, W.; Richardson, J.; Klein, W.H.; Olson, E.N. Overlapping functions of the myogenic bHLH genes MRF4 and MyoD revealed in double mutant mice. Development 1998, 125, 2349–2358. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999, 144, 631–643. [Google Scholar] [CrossRef]
- Hasty, P.; Bradley, A.; Morris, J.H.; Edmondson, D.G.; Venuti, J.M.; Olson, E.N.; Klein, W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993, 364, 501–506. [Google Scholar] [CrossRef]
- Nabeshima, Y.; Hanaoka, K.; Hayasaka, M.; Esumi, E.; Li, S.; Nonaka, I.; Nabeshima, Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 1993, 364, 532–535. [Google Scholar] [CrossRef]
- Yamamoto, M.; Legendre, N.P.; Biswas, A.A.; Lawton, A.; Yamamoto, S.; Tajbakhsh, S.; Kardon, G.; Goldhamer, D.J. Loss of MyoD and Myf5 in Skeletal Muscle Stem Cells Results in Altered Myogenic Programming and Failed Regeneration. Stem Cell Rep. 2018, 10, 956–969. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Z.; Sarkar, D.; Lawrence, M.; Sanchez, G.J.; Parker, M.H.; MacQuarrie, K.L.; Davison, J.; Morgan, M.T.; Ruzzo, W.L.; et al. Genome-wide MyoD binding in skeletal muscle cells: A potential for broad cellular reprogramming. Dev. Cell 2010, 18, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.; Vethantham, V.; Bowman, C.; Rudnicki, M.; Dynlacht, B.D. Genome-wide identification of enhancers in skeletal muscle: The role of MyoD1. Genes Dev. 2012, 26, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.I.; Georges, S.A.; Asawachaicharn, A.; Analau, E.; Tapscott, S.J. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J. Cell Biol. 2006, 175, 77–85. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Mastroyiannopoulos, N.P.; Furling, D.; Uney, J.B.; Phylactou, L.A. Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. BMC Dev. Biol. 2011, 11, 34. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, L.; Li, H.; Jiang, F.; Cao, L.; Hu, C.Y.; Wang, C. MiR-501-3p Forms a Feedback Loop with FOS, MDFI, and MyoD to Regulate C2C12 Myogenesis. Cells 2019, 8, 573. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef]
- Juan, A.H.; Kumar, R.M.; Marx, J.G.; Young, R.A.; Sartorelli, V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol. Cell 2009, 36, 61–74. [Google Scholar] [CrossRef]
- Li, G.; Luo, W.; Abdalla, B.A.; Ouyang, H.; Yu, J.; Hu, F.; Nie, Q.; Zhang, X. miRNA-223 upregulated by MYOD inhibits myoblast proliferation by repressing IGF2 and facilitates myoblast differentiation by inhibiting ZEB1. Cell Death Dis. 2017, 8, e3094. [Google Scholar] [CrossRef]
- Li, J.T.; Zhao, W.; Li, D.D.; Feng, J.; Ba, G.; Song, T.Z.; Zhang, H.P. miR-101a targeting EZH2 promotes the differentiation of goat skeletal muscle satellite cells. Yi Chuan 2017, 39, 828–836. [Google Scholar] [CrossRef]
- Rao, P.K.; Kumar, R.M.; Farkhondeh, M.; Baskerville, S.; Lodish, H.F. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc. Natl. Acad. Sci. USA 2006, 103, 8721–8726. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, D.; Goljanek, K.; Rathjen, T.; Oustanina, S.; Braun, T.; Dalmay, T.; Munsterberg, A. Specific requirements of MRFs for the expression of muscle specific microRNAs, miR-1, miR-206 and miR-133. Dev. Biol. 2008, 321, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Alway, S.E. Stretch-induced myogenin, MyoD, and MRF4 expression and acute hypertrophy in quail slow-tonic muscle are not dependent upon satellite cell proliferation. Cell Tissue Res. 1999, 296, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.; Booth, F.W. Effect of serum and mechanical stretch on skeletal α-actin gene regulation in cultured primary muscle cells. Am. J. Physiol. Cell Physiol. 1998, 275, C1438–C1448. [Google Scholar] [CrossRef]
- Baccam, A.; Benoni-Sviercovich, A.; Rocchi, M.; Moresi, V.; Seelaender, M.; Li, Z.; Adamo, S.; Xue, Z.; Coletti, D. The Mechanical Stimulation of Myotubes Counteracts the Effects of Tumor-Derived Factors Through the Modulation of the Activin/Follistatin Ratio. Front. Physiol. 2019, 10, 401. [Google Scholar] [CrossRef]
- Halle, J.L.; Counts-Franch, B.R.; Prince, R.M.; Carson, J.A. The Effect of Mechanical Stretch on Myotube Growth Suppression by Colon-26 Tumor-Derived Factors. Front. Cell Dev. Biol. 2021, 9, 690452. [Google Scholar] [CrossRef]
- Shcherbina, A.; Larouche, J.; Fraczek, P.; Yang, B.A.; Brown, L.A.; Markworth, J.F.; Chung, C.H.; Khaliq, M.; de Silva, K.; Choi, J.J.; et al. Dissecting Murine Muscle Stem Cell Aging through Regeneration Using Integrative Genomic Analysis. Cell Rep. 2020, 32, 107964. [Google Scholar] [CrossRef]
- Haroon, M.; Boers, H.E.; Bakker, A.D.; Bloks, N.G.C.; Hoogaars, W.M.H.; Giordani, L.; Musters, R.J.P.; Deldicque, L.; Koppo, K.; Le Grand, F.; et al. Reduced growth rate of aged muscle stem cells is associated with impaired mechanosensitivity. Aging 2022, 14, 28–53. [Google Scholar] [CrossRef]


| No | Primers | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Purpose |
|---|---|---|---|---|
| 1 | Foxo3 | CGGGCAGCCGAGGAAATGTT | TGTTGCTGTCGCCCTTATCCTT | qPCR |
| 2 | MyoD | CCTCTTTCGGTCCCTCTTTC | ATGGGTAGAGCGGCTGTAGA | qPCR |
| 3 | p27 | AAGGGCCAACAGAACAGAAG | GGATGTCCATTCAATGGAGTC | qPCR |
| 4 | Tubulin | ATATCGGTCCATGTGGGTCAA | TGAGTGCCAAAGGTTCCATCC | qPCR |
| 5 | Foxo3 | TGCTTGTGGTTTAGGTTCCC | ATCGGGGATGAGTAGGATAA | Mutagenesis |
| 6 | miR-200c | CTTCCGGTGCCCTTTCTCC | GGCGTCCAGCTAAGTCCTTCA | Promoter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, J.S.; Boriek, A.M. Regulation of Myogenesis by MechanomiR-200c/FoxO3 Axis. Cells 2025, 14, 868. https://doi.org/10.3390/cells14120868
Mohamed JS, Boriek AM. Regulation of Myogenesis by MechanomiR-200c/FoxO3 Axis. Cells. 2025; 14(12):868. https://doi.org/10.3390/cells14120868
Chicago/Turabian StyleMohamed, Junaith S., and Aladin M. Boriek. 2025. "Regulation of Myogenesis by MechanomiR-200c/FoxO3 Axis" Cells 14, no. 12: 868. https://doi.org/10.3390/cells14120868
APA StyleMohamed, J. S., & Boriek, A. M. (2025). Regulation of Myogenesis by MechanomiR-200c/FoxO3 Axis. Cells, 14(12), 868. https://doi.org/10.3390/cells14120868






