CTCF Represses CIB2 to Balance Proliferation and Differentiation of Goat Myogenic Satellite Cells via Integrin α7β1–PI3K/AKT Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sample Collection
2.2. Isolation, Purification, and Characterization of MuSCs
2.3. Cell Culture
2.4. Overexpression Plasmid Construction, siRNA Preparation, and Oligonucleotide Synthesis
2.5. Flow Cytometry
2.6. CCK-8 Cell Viability and EdU Proliferation Assays
2.7. Immunofluorescence
2.8. RNA Sequencing
2.9. Chromatin Immunoprecipitation–Quantitative PCR (ChIP–qPCR)
2.10. Isolation of Total RNA and Performance of RT–qPCR
2.11. Statistical Analysis
3. Results
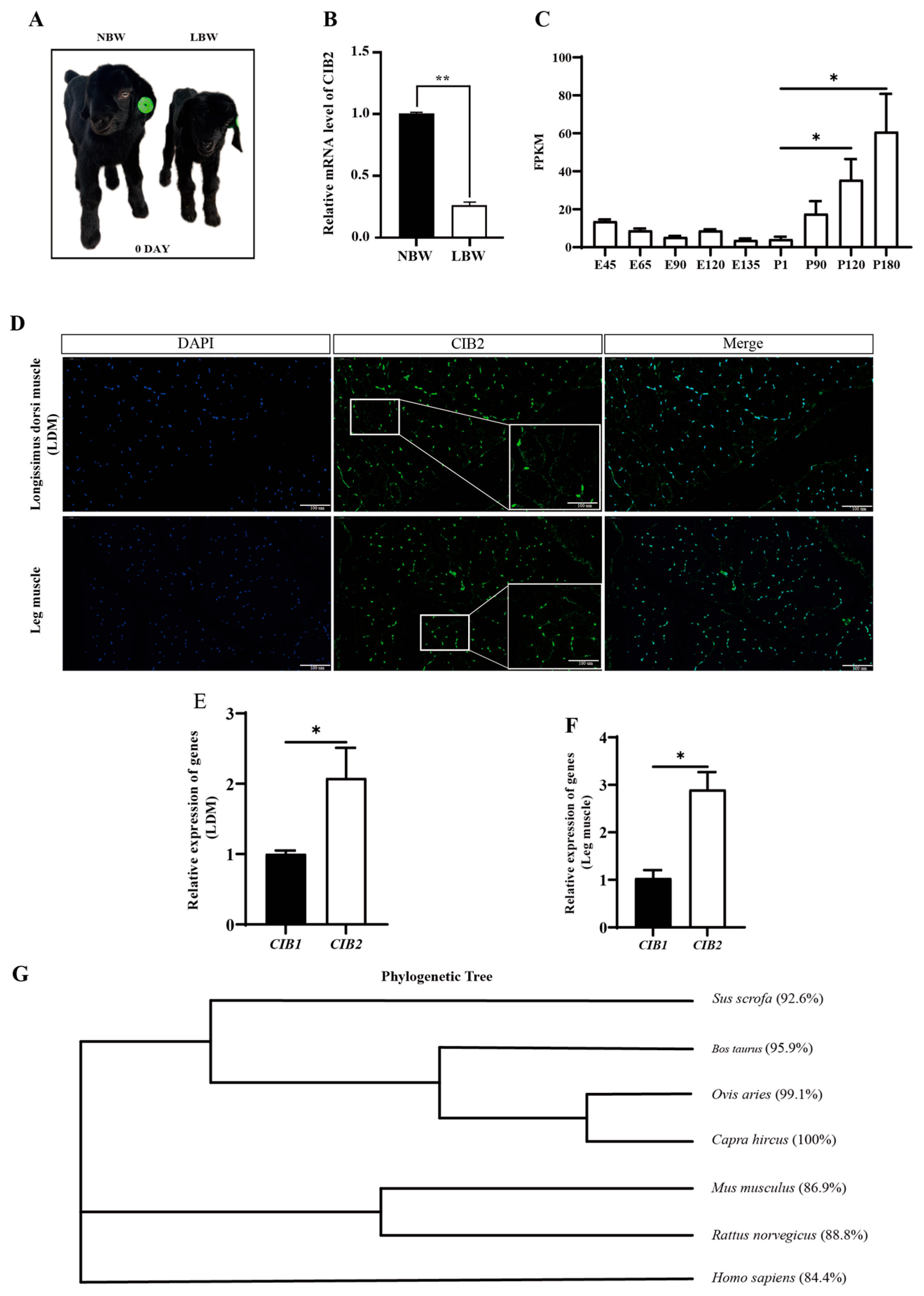
3.1. Developmental Function and Evolutionary Conservation of Caprine CIB2
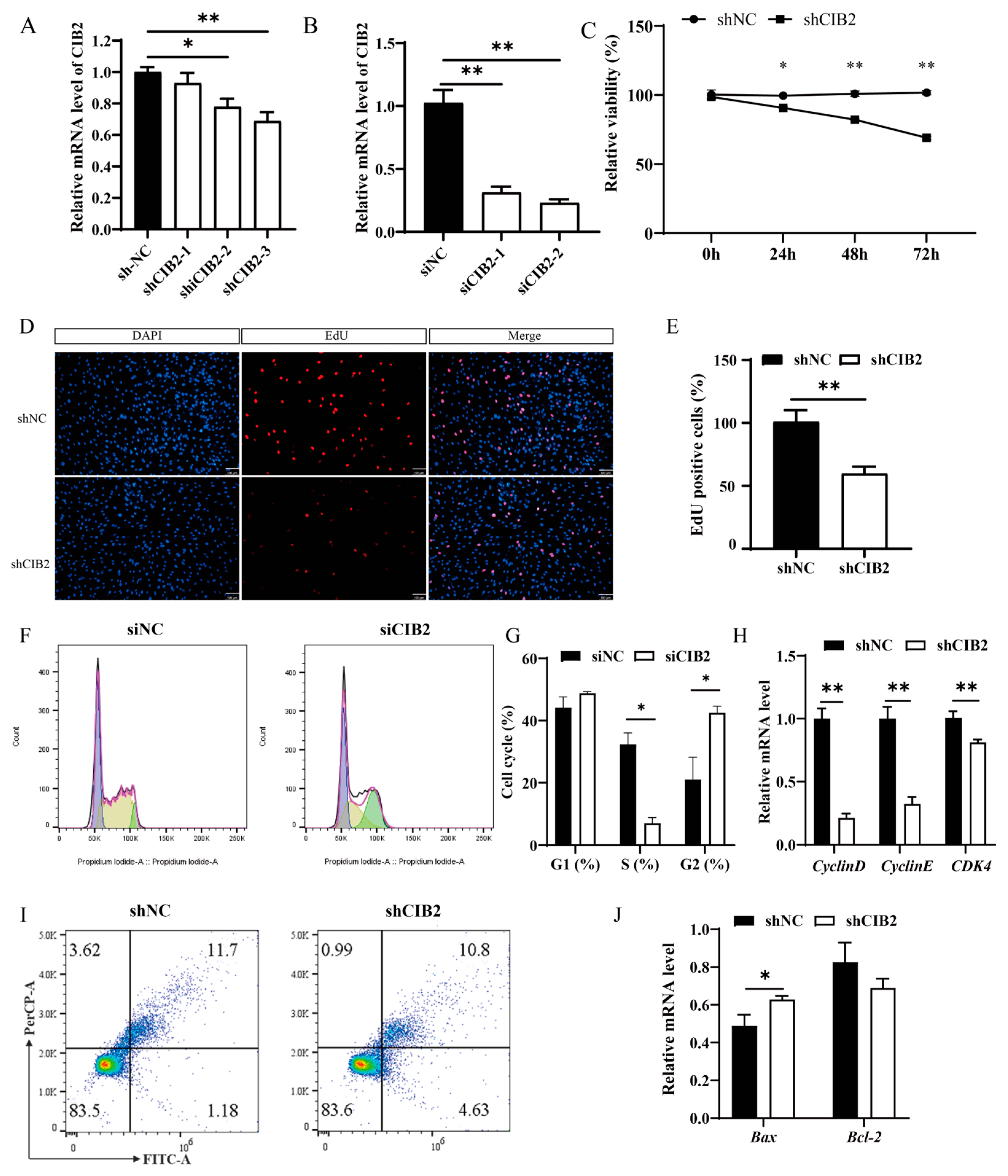
3.2. CIB2 Promotes Myoblast Proliferation Through Cell Cycle Regulation
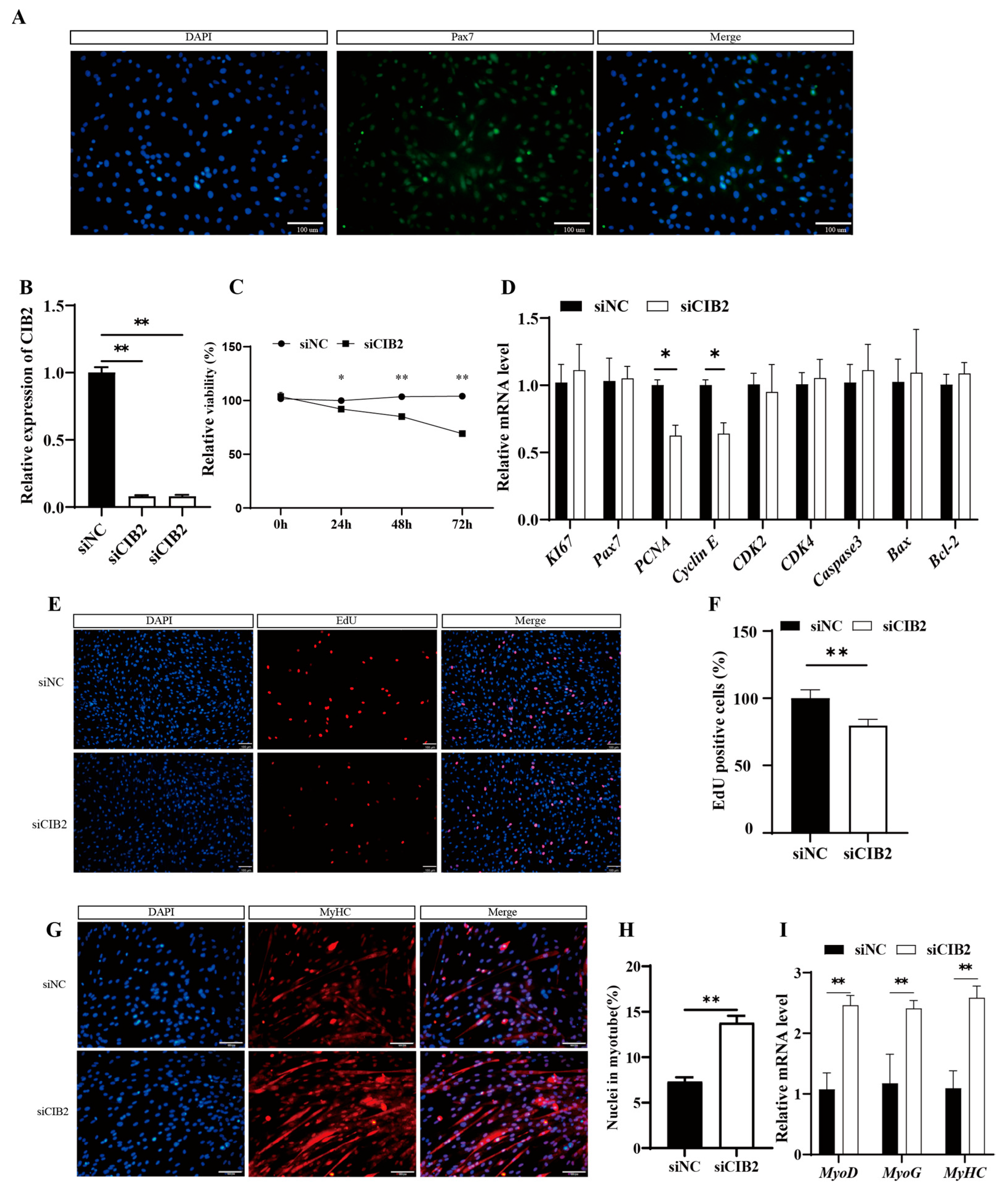
3.3. CIB2 Suppresses Proliferation While Enhancing Differentiation of Caprine MuSCs
3.3.1. Isolation and Validation of Caprine MuSCs
3.3.2. Interference with the CIB2 Gene Inhibits the Proliferation of Goat MuSCs
3.3.3. CIB2 Knockdown Promotes Myogenic Differentiation
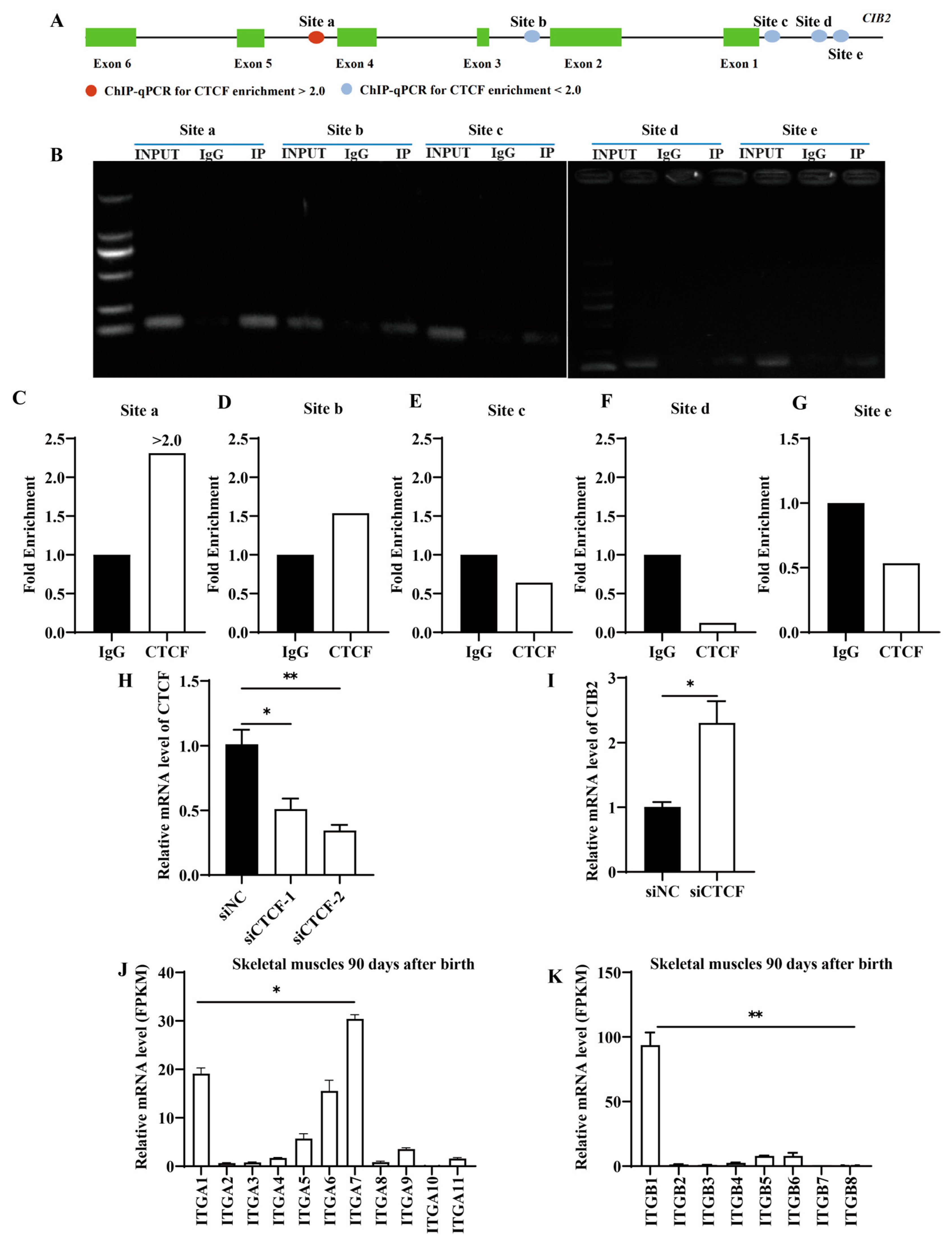
3.4. CTCF Negatively Regulates CIB2 mRNA Expression
3.4.1. Multi-Omics Analysis Identifies CTCF as a Putative CIB2 Regulator
3.4.2. ChIP-qPCR Validation of CTCF-Binding Sites
3.4.3. CTCF Represses CIB2 Transcription
3.5. Analysis of Interaction Between CIB2 and Integrins
3.6. Altered CIB2 Expression Disrupts Critical Regulatory Pathways in Caprine MuSCs
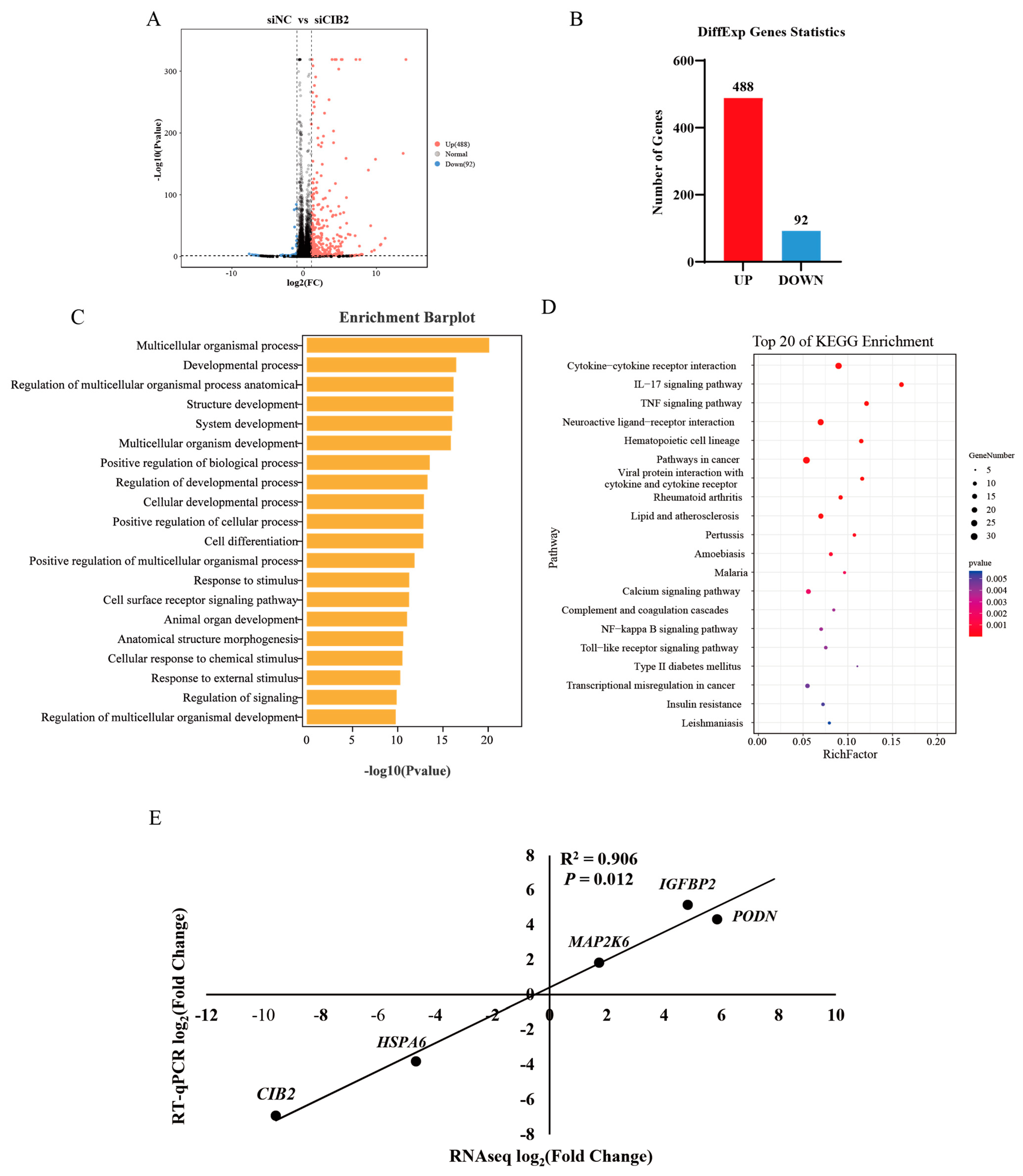
3.6.1. Differential Gene Expression Profiling
3.6.2. GO Enrichment Analysis
3.6.3. KEGG Enrichment Analysis
3.6.4. Transcriptomic Validation
4. Discussion
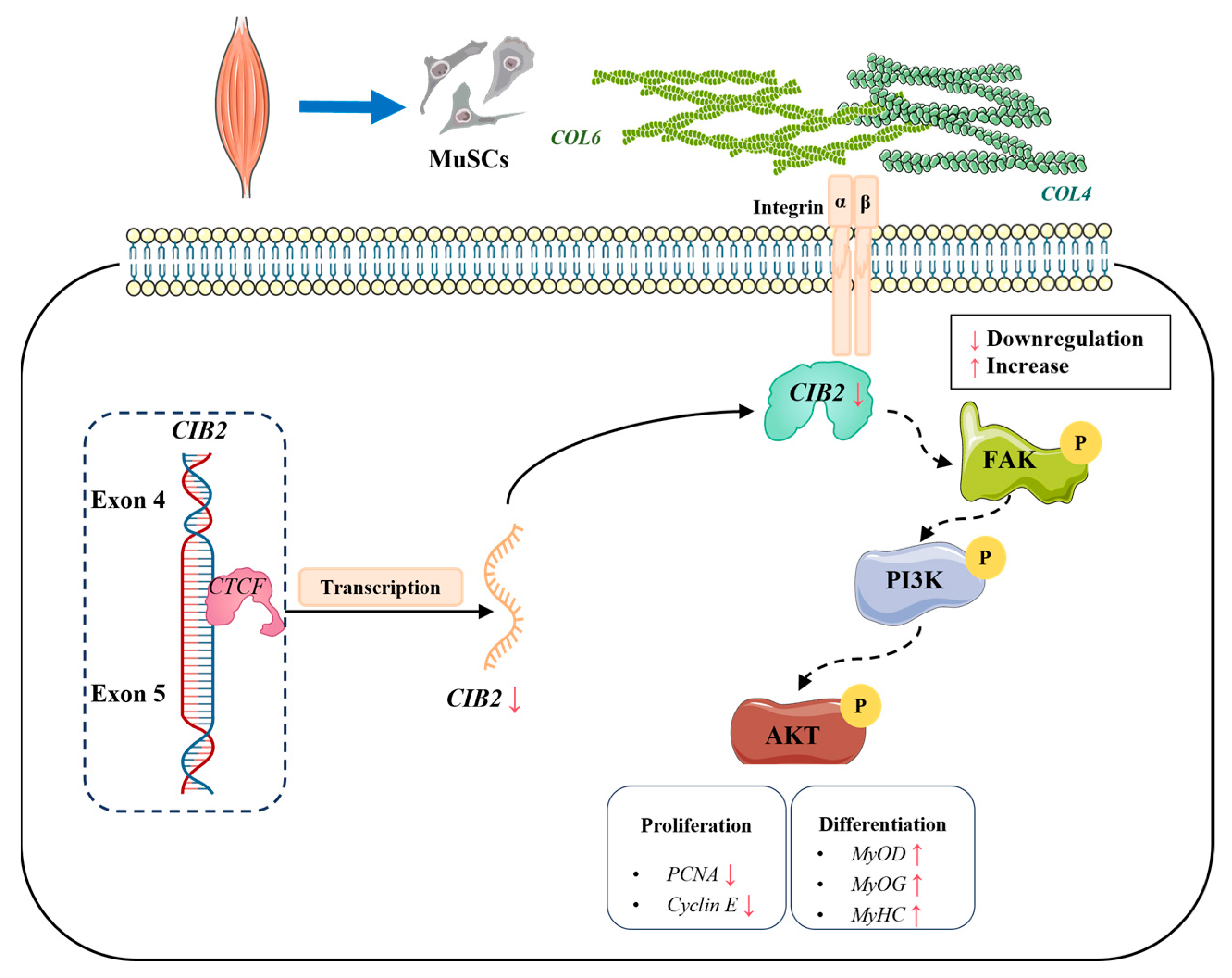
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIB2 | Calcium- and Integrin-Binding Family Member 2 |
| ECM | Extracellular Matrix |
| MuSCs | Muscle Satellite Cells |
| LBW | Low Birth Weight |
| DEGs | Differentially Expressed Genes |
| EdU | 5-Ethynyl-2′-Deoxyuridine |
| ChIP-qPCR | Chromatin Immunoprecipitation–Quantitative PCR |
| RNA-seq | RNA Sequencing |
| CTCF | CCCTC-Binding Factor (transcriptional repressor) |
| PI3K/AKT | Phosphoinositide 3-Kinase/Protein Kinase B Pathway |
| FAK | Focal Adhesion Kinase |
| CDK4 | Cyclin-Dependent Kinase 4 |
| MyHC | Myosin Heavy Chain |
References
- Luo, N.; Wang, J.; Hu, Y.; Zhao, Z.; Zhao, Y.; Chen, X. Cold and heat climatic variations reduce indigenous goat birth weight and enhance pre-weaning mortality in subtropical monsoon region of China. Trop. Anim. Health Prod. 2020, 52, 1385–1394. [Google Scholar] [CrossRef]
- Habibu, B.; Kawu, M.U.; Makun, H.J.; Aluwong, T.; Yaqub, L.S. Seasonal variation in body mass index, cardinal physiological variables and serum thyroid hormones profiles in relation to susceptibility to thermal stress in goat kids. Small Rumin. Res. 2016, 145, 20–27. [Google Scholar] [CrossRef]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix–What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Saher, G.; Fässler, R.; Bornemann, A.; Echtermeyer, F.; von der Mark, H.; Miosge, N.; Pöschl, E.; von der Mark, K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat. Genet. 1997, 17, 318–323. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhai, Y.Y.; Ding, R.R.; Huang, J.H.; Shi, X.C.; Liu, H.; Liu, X.P.; Zhang, J.F.; Lu, J.F.; Zhang, Z.; et al. FNDC1 is a myokine that promotes myogenesis and muscle regeneration. EMBO J. 2025, 44, 30–53. [Google Scholar] [CrossRef]
- Vallone, R.; Dal Cortivo, G.; D’Onofrio, M.; Dell’Orco, D. Preferential Binding of Mg(2+) Over Ca(2+) to CIB2 Triggers an Allosteric Switch Impaired in Usher Syndrome Type 1J. Front. Mol. Neurosci. 2018, 11, 274. [Google Scholar] [CrossRef]
- Jeong, H.; Clark, S.; Goehring, A.; Dehghani-Ghahnaviyeh, S.; Rasouli, A.; Tajkhorshid, E.; Gouaux, E. Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature 2022, 610, 796–803. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Shi, L.; Chen, X.; Li, D.; Cui, C.; Yang, K.; Lu, M.; Huang, J.; Zhang, L.; et al. CIB2 Is a Novel Endogenous Repressor of Atrial Remodeling. Circulation 2023, 147, 1758–1776. [Google Scholar] [CrossRef]
- Sethna, S.; Scott, P.A.; Giese, A.P.J.; Duncan, T.; Jian, X.; Riazuddin, S.; Randazzo, P.A.; Redmond, T.M.; Bernstein, S.L.; Riazuddin, S.; et al. CIB2 regulates mTORC1 signaling and is essential for autophagy and visual function. Nat. Commun. 2021, 12, 3906. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Yu, S.; Yu, D.; Liu, N.; Zhang, S.; Wu, A. Mycotoxin deoxynivalenol affects myoblast differentiation via downregulating cytoskeleton and ECM-integrin-FAK-RAC-PAK signaling pathway. Ecotoxicol. Environ. Saf. 2021, 226, 112850. [Google Scholar] [CrossRef] [PubMed]
- Seki, N.; Hattori, A.; Hayashi, A.; Kozuma, S.; Ohira, M.; Hori, T.; Saito, T. Structure, expression profile and chromosomal location of an isolog of DNA-PKcs interacting protein (KIP) gene. Biochim. Biophys. Acta 1999, 1444, 143–147. [Google Scholar] [CrossRef]
- Cushman, L.J.; Torres-Martinez, W.; Cherry, A.M.; Manning, M.A.; Abdul-Rahman, O.; Anderson, C.E.; Punnett, H.H.; Thurston, V.C.; Sweeney, D.; Vance, G.H. A report of three patients with an interstitial deletion of chromosome 15q24. Am. J. Med. Genet. A 2005, 137, 65–71. [Google Scholar] [CrossRef]
- Piórkowska, K.; Żukowski, K.; Ropka-Molik, K.; Tyra, M.; Gurgul, A. A comprehensive transcriptome analysis of skeletal muscles in two Polish pig breeds differing in fat and meat quality traits. Genet. Mol. Biol. 2018, 41, 125–136. [Google Scholar] [CrossRef]
- Häger, M.; Bigotti, M.G.; Meszaros, R.; Carmignac, V.; Holmberg, J.; Allamand, V.; Akerlund, M.; Kalamajski, S.; Brancaccio, A.; Mayer, U.; et al. Cib2 binds integrin alpha7Bbeta1D and is reduced in laminin alpha2 chain-deficient muscular dystrophy. J. Biol. Chem. 2008, 283, 24760–24769. [Google Scholar] [CrossRef]
- Wu, H.; Ren, Y.; Li, S.; Wang, W.; Yuan, J.; Guo, X.; Liu, D.; Cang, M. In vitro culture and induced differentiation of sheep skeletal muscle satellite cells. Cell Biol. Int. 2012, 36, 579–587. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Luo, N.; Lou, P.; Limesand, S.W.; Yang, Y.; Zhao, Y.; Chen, X. Hepatic Lipid Accumulation and Dysregulation Associate with Enhanced Reactive Oxygen Species and Pro-Inflammatory Cytokine in Low-Birth-Weight Goats. Animals 2022, 12, 766. [Google Scholar] [CrossRef]
- Ghasemi, M.; Zamani, P.; Vatankhah, M.; Abdoli, R. Genome-wide association study of birth weight in sheep. Animal 2019, 13, 1797–1803. [Google Scholar] [CrossRef]
- Gardner, D.S.; Buttery, P.J.; Daniel, Z.; Symonds, M.E. Factors affecting birth weight in sheep: Maternal environment. Reproduction 2007, 133, 297–307. [Google Scholar] [CrossRef]
- Afolayan, R.A.; Fogarty, N.M.; Gilmour, A.R.; Ingham, V.M.; Gaunt, G.M.; Cummins, L.J. Genetic correlations between reproduction of crossbred ewes and the growth and carcass performance of their progeny. Small Rumin. Res. 2008, 80, 73–79. [Google Scholar] [CrossRef]
- Endo, T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone 2015, 80, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sunadome, K.; Erickson, A.G.; Kah, D.; Fabry, B.; Adori, C.; Kameneva, P.; Faure, L.; Kanatani, S.; Kaucka, M.; Ellström, I.D.; et al. Directionality of developing skeletal muscles is set by mechanical forces. Nat. Commun. 2023, 14, 3060. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.M.; Pavlath, G.K. Molecular Control of Mammalian Myoblast Fusion. In Cell Fusion: Overviews and Methods; Chen, E.H., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 115–133. [Google Scholar]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Steyn, P.J.; Dzobo, K.; Smith, R.I.; Myburgh, K.H. Interleukin-6 Induces Myogenic Differentiation via JAK2-STAT3 Signaling in Mouse C2C12 Myoblast Cell Line and Primary Human Myoblasts. Int. J. Mol. Sci. 2019, 20, 5273. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Szeliga, A.; Moraczewski, J.; Czaplicka, I.; Brzóska, E. Comparison of satellite cell-derived myoblasts and C2C12 differentiation in two- and three-dimensional cultures: Changes in adhesion protein expression. Cell Biol. Int. 2011, 35, 125–133. [Google Scholar] [CrossRef]
- Alt, J.R.; Gladden, A.B.; Diehl, J.A. p21Cip1 Promotes Cyclin D1 Nuclear Accumulation via Direct Inhibition of Nuclear Export. J. Biol. Chem. 2002, 277, 8517–8523. [Google Scholar] [CrossRef]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef]
- Correia, C.; Lee, S.H.; Meng, X.W.; Vincelette, N.D.; Knorr, K.L.; Ding, H.; Nowakowski, G.S.; Dai, H.; Kaufmann, S.H. Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment. Biochim. Biophys. Acta 2015, 1853, 1658–1671. [Google Scholar] [CrossRef]
- Volkmann, N.; Marassi, F.M.; Newmeyer, D.D.; Hanein, D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014, 21, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Z.; Sarkar, D.; Lawrence, M.; Sanchez, G.J.; Parker, M.H.; MacQuarrie, K.L.; Davison, J.; Morgan, M.T.; Ruzzo, W.L.; et al. Genome-wide MyoD binding in skeletal muscle cells: A potential for broad cellular reprogramming. Dev. Cell 2010, 18, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Soyer, M.A.; Dey, B.K. MicroRNA-24-3p promotes skeletal muscle differentiation and regeneration by regulating HMGA1. Cell Mol. Life Sci. 2022, 79, 170. [Google Scholar] [CrossRef]
- Taylor, M.V.; Hughes, S.M. Mef2 and the skeletal muscle differentiation program. Semin. Cell Dev. Biol. 2017, 72, 33–44. [Google Scholar] [CrossRef]
- Gonzalez, M.L.; Busse, N.I.; Waits, C.M.; Johnson, S.E. Satellite cells and their regulation in livestock. J. Anim. Sci. 2020, 98, 5. [Google Scholar] [CrossRef]
- Lehka, L.; Rędowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Wang, R.R.; Qiu, X.; Pan, R.; Fu, H.; Zhang, Z.; Wang, Q.; Chen, H.; Wu, Q.Q.; Pan, X.; Zhou, Y.; et al. Dietary intervention preserves β cell function in mice through CTCF-mediated transcriptional reprogramming. J. Exp. Med. 2022, 219, e20211779. [Google Scholar] [CrossRef]
- Delgado-Olguín, P.; Brand-Arzamendi, K.; Scott, I.C.; Jungblut, B.; Stainier, D.Y.; Bruneau, B.G.; Recillas-Targa, F. CTCF Promotes Muscle Differentiation by Modulating the Activity of Myogenic Regulatory Factors*. J. Biol. Chem. 2011, 286, 12483–12494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xiang, Y.; Hu, M.; Xu, Y.; Hou, Y.; Qi, X.; Fu, L.; Luan, Y.; Wang, Z.; Li, X.; et al. Chromatin accessibility is associated with the changed expression of miRNAs that target members of the Hippo pathway during myoblast differentiation. Cell Death Dis. 2020, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, Q.; Gao, S.; Zhang, X.; Wang, Y.; Liu, J.; Fu, J.; Cao, M.; Wang, P. CTCF Mediates Replicative Senescence Through POLD1. Front. Cell Dev. Biol. 2021, 9, 618586. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, N.; Zhang, Y.; Wang, Y.; Su, Y.; Zhang, H.; An, Y. CTCF inhibits endoplasmic reticulum stress and apoptosis in cardiomyocytes by upregulating RYR2 via inhibiting S100A1. Life Sci. 2020, 242, 117158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, Y.; Pang, Y.; Tong, H.; Li, S.; Yan, Y. GRP94 promotes muscle differentiation by inhibiting the PI3K/AKT/mTOR signaling pathway. J. Cell Physiol. 2019, 234, 21211–21223. [Google Scholar] [CrossRef]
- Chen, W.-J.; Lin, I.-H.; Lee, C.-W.; Chen, Y.-F. Aged Skeletal Muscle Retains the Ability to Remodel Extracellular Matrix for Degradation of Collagen Deposition after Muscle Injury. Int. J. Mol. Sci. 2021, 22, 2123. [Google Scholar] [CrossRef]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes. Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Grönholm, M. How integrin phosphorylations regulate cell adhesion and signaling. Trends Biochem. Sci. 2022, 47, 265–278. [Google Scholar] [CrossRef]
- Mademtzoglou, D.; Relaix, F. From cyclins to CDKIs: Cell cycle regulation of skeletal muscle stem cell quiescence and activation. Exp. Cell Res. 2022, 420, 113275. [Google Scholar] [CrossRef]
- Weddell, J.C.; Chen, S.; Imoukhuede, P.I. VEGFR1 promotes cell migration and proliferation through PLCγ and PI3K pathways. npj Syst. Biol. Appl. 2017, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, S.; Tong, H.; Li, S.; Yan, Y. WDR13 promotes the differentiation of bovine skeletal muscle-derived satellite cells by affecting PI3K/AKT signaling. Cell Biol. Int. 2019, 43, 799–808. [Google Scholar] [CrossRef] [PubMed]
| Potential Transcription Factors | Cistrome DB (ID) | Peak Intensity | Putative Binding Regions |
|---|---|---|---|
| CTCF | 34432 | 4.20 | 54552400–54552700 |
| SMAD3 | 335 | 0.60 | 54554600–54555200 |
| SPI1 | 34422 | 0.58 | 54552200–54553100 |
| SRF | 34430 | 0.48 | 54553000–54553600 |
| YY1 | 57269 | 0.31 | 54554500–54555100 |
| TRIM28 | 51766 | 0.30 | 54554000–54554650 |
| TEAD4 | 74419 | 0.30 | 54552500–54553000 |
| CEBPB | 34428 | 0.28 | 54552900–54553600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Song, H.; Hao, Z.; Zhang, Z.; Luo, N.; Chen, X. CTCF Represses CIB2 to Balance Proliferation and Differentiation of Goat Myogenic Satellite Cells via Integrin α7β1–PI3K/AKT Axis. Cells 2025, 14, 1199. https://doi.org/10.3390/cells14151199
Gong C, Song H, Hao Z, Zhang Z, Luo N, Chen X. CTCF Represses CIB2 to Balance Proliferation and Differentiation of Goat Myogenic Satellite Cells via Integrin α7β1–PI3K/AKT Axis. Cells. 2025; 14(15):1199. https://doi.org/10.3390/cells14151199
Chicago/Turabian StyleGong, Changliang, Huihui Song, Zhuohang Hao, Zhengyi Zhang, Nanjian Luo, and Xiaochuan Chen. 2025. "CTCF Represses CIB2 to Balance Proliferation and Differentiation of Goat Myogenic Satellite Cells via Integrin α7β1–PI3K/AKT Axis" Cells 14, no. 15: 1199. https://doi.org/10.3390/cells14151199
APA StyleGong, C., Song, H., Hao, Z., Zhang, Z., Luo, N., & Chen, X. (2025). CTCF Represses CIB2 to Balance Proliferation and Differentiation of Goat Myogenic Satellite Cells via Integrin α7β1–PI3K/AKT Axis. Cells, 14(15), 1199. https://doi.org/10.3390/cells14151199







