Spotlight on Proteases: Roles in Ovarian Health and Disease
Abstract
1. Introduction
2. Materials and Methods
3. Roles of Proteases in Ovarian Follicle Development
3.1. Metalloproteinases, Plasminogen Activators, and Their Inhibitors
| Protease | Mechanism of Regulation | Specific Stage | Function | Species | Reference |
|---|---|---|---|---|---|
| MMP1 | Increased expression following hCG administration | Preovulatory | Degradation of collagenous ECM | Rhesus monkey | [18,76] |
| MMP2 and MMP9 | Increased in the granulosa and thecal cells of atretic follicles during proestrus and in corpus luteum during metestrus | Preovulatory follicles | ECM remodeling | Guinea pigs | [45] |
| MMP2, and MMP9 | Localized to the oogonium/oocyte cytoplasm and surface epithelium | Folliculogenesis | ECM remodeling during gonadal development and cell–matrix interactions | Human | [77] |
| MMP1 and MMP13 | Expression increased in response to LH surge | Preovulatory | Degradation of collagenous ECM | Bovine | [78] |
| MMP1, MMP2, MMP3, MMP9, MMP13 | Increased in mRNA expression by gonadotropins | Prehierarchical white (WFs), yellowish (YFs), and preovulatory follicles | Involved in the atresia of the early stage of follicle while not participating in the regulation of advanced stage atresia | Chicken | [24,79] |
| MMP1, MMP3, and MMP9 | Increased MMP1 and MMP3 expression levels in granulosa | Folliculogenesis | MMP9 induced by TGFB1; MMP1and MMP3 stimulated by FSH, LH, P4, and E2 | Chicken | [80] |
| MMP10 and MMP11 | Expression patterns changes following hCG administration | Ovulation and luteogenesis | Mmp10 mRNA was increased and MMP11 decreased in granulosa and theca cells during Ovulation | Human and Rats | [81] |
| MMP13, MMP14, MMP16, ADAMT1 | Increased expression in cumulus cells following hCG administration | Ovulation and luteogenesis | Migratory phenotype of the cumulus–oocyte complex at the time of ovulation | Rat | [82] |
| MMP19 | Localized to granulosa and theca-interstitial cells with temporal increases following hCG administration | Preovulatory follicles | ECM remodeling and tissue degradation | Mouse, Rat, Bovineand Human | [76,82,83] |
| MMP2, MMP9, TIMP-1, and TIMP-2 | The ratio of MMP-2/TIMP-2 decreased in small antral follicles; the MMP-9/TIMP-1 ratio increased in large-preovulatory follicles | Preovulatory follicles | Tissue reorganization during ovulation | Equine | [29] |
| TIMP-2 and TIMP-3 | Increased transcript abundance of TIMP-2 in yellow atretic follicles; decreased mRNA expression of TIMP-3 | Prehierarchical white (WFs), yellowish (YFs), and preovulatory follicles | Involved in the atresia | Chicken | [24,79] |
| TIMP4 | Increased significantly during the luteinization process of granulosa cells | Localized to the theca of antral and preovulatory follicles and adjacent ovarian stroma | Maintenance of luteal function | Mice, Rat | [84,85] |
| tPA and uPA | Activity increased during the periovulatory period | Granulosa and theca cells | Conversion of plasminogen to plasmin during ovulation | Rat | [66,86,87] |
| tPA and uPA | TNFα suppressed FSH-stimulated tPA activity but potentiated FSH-induced uPA activity in undifferentiated granulosa cells | Undifferentiated granulosa cells of preantral and antral follicles | Follicular wall remodeling during ovarian follicular development | Rat | [67,88] |
| PAI-1 and PAI-2 | mRNAs upregulated after the gonadotrophin surge | PAI-1 localized to the thecal layer of preovulatory follicles. PAI-2 localized to the granulosa cell | Control plasminogen activator activity associated with ovulation and early corpus luteum formation. | Bovine | [89] |
| CTSL | Expression increased following hCG administration | Oocyte meiosis, Preovulatory to ovulation | Degradation of the follicular wall | Rat, Rhesus monkey, Bovine | [54,76] |
| CTSB | Expression increased following hCG administration, Autophagy induction | Preovulatory to ovulation | Regulation of follicular development | Mice, Bovine | [53,90] |
| CTSB, K, L, and H | Expressed in germinal epithelium throughout the estrous cycle | Oocytes and granulosa cells of primordial, primary follicles and corpus luteum | Degradation of extracellular matrix | Mice | [91,92] |
| Kallikreins | Response to steroid hormones (androgens and estrogens); various expression patterns with eCG/hCG stimulation | Primordial to ovulation | Proteolytic processing of growth factors and hormones; angiogenesis | Rat | [93,94] |
3.2. Cathepsins
- Cathepsin B (CTSB): CTSB can function both as endo- and exo-(carboxy) peptidase [97,98]. CTSB has been identified as a critical regulator of ovarian reserve maintenance in mice [99]. The inhibition of Ctsb by myricetin significantly increased the number of primordial and primary follicles, suggesting a role in follicle activation. This effect seems mediated by the inhibition of autophagy and upregulation of the IGF1R and AKT-mTOR pathways [99]. Similarly, Liang et al. reported that the inhibition of CTSB activity preserved oocyte quality and enhanced developmental competence by mitigating age-related mitochondrial dysfunction and oxidative stress [100]. Chen et al. reported that the silencing of Ctsb in mouse granulosa cells decreased apoptosis by downregulating TNF-α, Casp8, and Casp3 while upregulating Bcl2 expression [53]. Ctsb knockdown also increased granulosa cell proliferation by activating the p-Akt and p-ERK pathways [53]. Komatsu et al. reported that Stefin A, an inhibitor of CTSB, blocked the activation of primordial follicles in mouse newborn ovaries in vitro [101]. In the follicle fluid of pregnant women undergoing ICSI, Bastu et al. found higher levels of CTSB compared to non-pregnant patients [102].
- Cathepsin L (CTSL): Ctsl is involved in the activation of primordial follicles adjacent to ovulatory follicles, and its inhibition results in a significant reduction in growing follicle numbers [92]. Ctsl expression was detected in large cuboidal cells of small, developing corpora lutea, suggesting possible roles in corpus luteum function [91,103]. Ezz et al. showed that CTSL regulates oocyte meiosis, and its supplementation improves oocyte quality and early embryo development in the bovine [54] (Table 2).
- Cathepsin S (CTSS): Song et al. reported that Ctss overexpression significantly increased progesterone (P4) and estrogen (E2) production by upregulating Star and Cyp19a1 in rabbit granulosa cells [104,105]. The overexpression of Ctss also increased granulosa cell proliferation while decreasing apoptosis by enhancing the expression of Pcna and Bcl2. Conversely, Ctss knockdown significantly decreased the secretion of P4 and E2 while increasing apoptosis [104].
| Protease | KO/Inhibitor | Effect on Follicular Development | Specific Stage | Molecular Mechanism | Localization | Species | Reference |
|---|---|---|---|---|---|---|---|
| MMP1, MMP9, MMP10, and MMP19 | Inhibitor (GM6001) | Reduced ovulation rate | Preovulatory to ovulation | Degradation of the follicular wall | Granulosa and theca cells | Rhesus monkey | [76] |
| MMP2 | Inhibitor (ZK158252) | Inhibited hCG-induced ovulation and MMP-2 activation | Preovulatory to ovulation | Leukotriene B4-receptor antagonism | Ovarian follicles | Rat | [106] |
| MMP1, MMP2, and MMP3 | Inhibitor (GM6001) | Reduction in CL and E2 with GM6001 | Preovulatory to ovulation | Inhibits MMP activity in photostimulated ovaries | - | Hamster | [107,108] |
| MMP10 | Inhibitor AG1478 | Up-regulation of Mmp10 by LH. | Ovulation and luteinization. | Suppressed the induction of Mmp10 mRNA | Granulosa cells | Rat | [81] |
| TIMP-1 | KO | Increased number of primary and preantral follicles | Primordial to primary/preantral | Regulation of MMP activity | - | Rodent | [38,109] |
| CTSB | Inhibitor (Myricetin) | Increased oocyte reserve | Primordial to primary | Inhibition of autophagy and upregulation of the IGF1R and AKT-mTOR pathways | Oocytes | Mouse | [99] |
| CTSL | siRNA | Enhanced fertilization capability and blastocyst formation | Oocytes | Increasing mitochondrial function, reducing accumulated ROS, lowering apoptosis, and recovering lysosome capacity | Oocytes | Mouse | [91] |
| CTSL | KO | Reduced ovulation rate | Preovulatory to ovulation | Degradation of the follicular wall | Granulosa and theca cells | Mice | [48] |
| CTSL | rCTSL supplementation | Regulated oocyte meiosis during maturation and early embryo development | Oocyte maturation | Meiotic regulation | Oocytes | Bovine | [54,76] |
| CTSB | Stefin A | Blocked activation of primordial follicles | Primordial follicles | 17β-estradiol increased Stefin A mRNA expression and inhibited follicle development | - | Mouse | [101] |
| ADAMTS1 | KO | Lower numbers of mature follicles and impaired ovulation | Antral to ovulation | Maintenance of follicular basement membrane integrity | Granulosa cells | Mouse | [110,111] |
| ADAMTS1 | KO | Failure of ovulation and fertilization | Preovulatory to ovulation | Expansion of cumulus–oocyte complexes (COCs) | COCs | Mouse | [49,52,112] |
| ADAMTS9 | KO | Ovarian malformation and inability to ovulate | Primordial to ovulation | - | - | Zebrafish | [113] |
| LONP1 | Oocyte-specific KO | Impaired follicular development and progressive oocyte death | Primordial to antral | Regulation of mitochondrial function | Oocytes | Mouse | [69] |
| FURIN | Oocyte-specific KO | Arrested oogenesis at early secondary follicles | Primary to secondary | - | Oocytes | Mouse | [70] |
| PAPPA | KO | Decreased litter size and ovulatory capacity | Antral to ovulation | Regulation of IGF bioavailability | - | Mouse | [71,72,73] |
| TMPRSS6 | KO | Retardation in ovarian maturation | Primordial to antral | Regulation of iron homeostasis | - | Mouse | [74] |
| tPA and uPA | Inhibitor (PAI-1) | Significantly reduced ovulation rate | Preovulatory to ovulation | ECM degradation | - | Rat, Human | [87,114,115,116,117] |
| PA | Protease nexin-1 (SerpinE2) | tPA activity higher in cells from small follicles; SerpinE2 levels higher in large follicles | Antral and basal granulosa cells | SerpinE2 secretion regulated at the transcriptional level | Granulosa cells | Bovine | [41] |
4. Role of Proteases in Antral Follicle Development and Ovulation
4.1. Metalloproteinases, Plasminogen Activators, and Their Inhibitors
4.2. Cathepsins
5. Role of Proteases in Corpus Luteum Formation and Function
6. Role of Proteases in Ovarian Disease
6.1. Ovarian Cancer
- MMPs and TIMPs: MMPs participate in several processes that are involved in ovarian cancer progression, including the degradation of the ECM, the promotion of angiogenesis, and the induction of epithelial–mesenchymal transition (EMT) [6,35,46,157,158,159]. Several studies have shown the upregulation of MMPs, such as MMP2 and MMP9, in ovarian cancer tissues compared to normal or benign ovarian tissues, and their expression levels correlate with clinical stage, tumor invasiveness, and metastatic potential [155,157,160,161]. Tumor-derived MMP2 and MMP9 expression has been identified as a negative prognostic indicator in ovarian cancer patients, predicting lower overall survival rates [162,163,164,165,166]. Ovarian cancer cells (Ovcar3) treated with an activator of the PKC pathway, phorbol-12-myristate 13-acetate (PMA), increased MMP7 and MMP10 mRNA [167,168]. MMP14 was shown to activate pro-MMP2 to MMP2, playing a role in the development of vasculogenic-like networks and matrix remodeling by aggressive ovarian cancer cells [168,169,170]. MMP1 activates PAR1, inducing the secretion of angiogenic factors in ovarian carcinoma cells [171]. MMP3 is involved in the estradiol-induced migration and invasion of SKOV3 ovarian cancer cells via the PI3K/Akt/FOXO3 pathway [172]. MMP7 promotes the invasion and metastasis of ovarian cancer cells by activating gelatinases and through the MAPK/ERK and JNK pathways [173,174]. MMP8 upregulates IL-1β, whose expression levels correlate with tumor grade and poor prognosis [175]. The MMP12 82A/G polymorphism has been associated with increased susceptibility to ovarian cancer [176,177], and MMP13 in ascitic fluids of ovarian cancer patients has been identified as a potential marker for disease risk and survival outcomes [178]. Taken together, these studies underline the association between the dysregulation of MMP expression and activity and ovarian cancer.In addition to MMPs, several TIMPs, including TIMP1 and TIMP3, have been found upregulated in ovarian cancer [179,180]. However, Davidson et al. found decreased TIMP levels alongside increased MMP2 in ovarian cancer [181]. These seemingly contradictory results highlight the complex mechanisms involved in ovarian cancer and show how dysregulation of the MMP/TIMP balance may have a more significant impact than the overexpression of a single class of proteins [157,182]. In addition, there are several possible explanations for the conflicting findings of increased levels of both MMPs and their inhibitors: (1) TIMPs regulate processes independent of their protease inhibitory activity, including cell growth, migration, and angiogenesis [183]; (2) the stoichiometric balance between MMPs and TIMPs may be more critical than absolute levels, and elevated TIMP levels may sometimes be insufficient to counteract excessive MMP activity in aggressive cancers [184]; (3) TIMPs have been found to activate MMPs in certain instances [19]; and (4) different tissue compartments may have varying MMP ratios, allowing MMPs to remain active in specific microenvironments despite elevated TIMP levels [185].
- The PA and PAI system: In vitro analyses have shown that uPA is highly expressed in several types of cancer cells, including ovarian cancer [186,187,188,189,190,191,192,193]. The overexpression of uPA and PAI-1 was found in more than 75% of primary ovarian carcinomas, and in most metastatic epithelial ovarian cancer (EOC) [194]. Further, Kenny et al. reported that in vitro and in vivo treatments with a uPA receptor (uPAR) antibody inhibited ovarian cancer cell invasion, migration, and adhesion by inhibiting α5-integrin and decreasing the expression of urokinase, uPAR, β3-integrin, and fibroblast growth factor receptor-1 [195]. High levels of PAI-1 have been associated with poor clinical outcomes in ovarian serous carcinoma [187,196]. In ovarian cancer cells, PAI-1 inhibition resulted in cell cycle arrest and decreased proliferation, and, in xenograft models, significantly reduced peritoneal dissemination [196]. Similarly, PAI-1 silencing in SKOV3 cells disrupted the platelet-induced upregulation of the genes involved in proliferation and ECM remodeling [197]. At the molecular level, some reports suggest that PAI-1 inhibits cell adhesion and migration by blocking vitronectin (VN) binding to integrins or by displacing uPAR from VN in the extracellular matrix [198,199]. However, other studies have shown that PAI-1 can enhance cancer cell adhesion [200,201].As for MMPs/TIMPs, it is unclear why the upregulation of both uPA and PAIs correlates with cancer progression and poor clinical outcomes. Several mechanisms may explain this apparent contradiction: (1) PAI have additional functions beyond uPA inhibition, including activation of pathways that promote tumor growth, angiogenesis, and cell detachment [202,203,204]; (2) the PAI-1/uPA/uPAR complex can be internalized and recycled, potentially leading to increased uPAR on the cell surface and enhanced invasiveness [205]; (3) PAI-1 can elicit inflammatory responses and immune cell recruitment in the tumor microenvironment, potentially promoting a pro-tumorigenic milieu [206]. Overall, these findings suggest that PAI function is context-dependent and highlight the complex regulation of the PA/PAI system in ovarian cancer.
- Cathepsins: Cathepsins and their inhibitors cystatins have also been associated with ovarian cancer. Liu et al. showed that CTSB and its binding proteins AMBP and TSRC1 modulated TNF-induced apoptosis in ovarian cancer cells [207]. Additionally, Ctsl knockdown inhibited proliferation, invasion, and tumor growth both in vitro and in vivo, while Ctsl overexpression had the opposite effects [208,209]. In malignant serous tumors, cystic fluid levels of CTSB, CTSL, and their inhibitor Cystatin C (Cst3) were significantly elevated compared to benign serous tumors [210]. Gashenko et al. found significantly increased levels of procathepsin B, cystatin B (CstB), and Cst3 in serum and ascite fluids of ovarian cancer patients compared to controls, suggesting their possible use as disease biomarkers [211]. Nishikawa et al. found significantly elevated levels of Cst3, but not CTSB, in ovarian cancer compared to benign samples and healthy controls [212]. Interestingly, invasion assays showed that the inhibition of Cst3 or CTSB suppressed cancer cell invasion in a dose-dependent manner [212]. Once again, some of these findings appear contradictory. Elevated CysC in cancer may represent a compensatory mechanism to control excessive cathepsin activity [213]. In addition, similar to other protease inhibitors, Cst3 may have additional functions including the regulation of immune response and cell signaling [214]. Furthermore, changes in the balance between cathepsins and cystatins may be more important than absolute expression levels of either protein [215]. Finally. Cst3 primarily regulates extracellular cathepsin activity, while pro-tumorigenic effects of CTSB may be, at least in part, intracellular [216].
| Protease | Finding in Ovarian Cancer | Localization | Species | Prognostic Value | Role | Reference |
|---|---|---|---|---|---|---|
| MMP1 | Activates PAR1 | Ovarian carcinoma cells | Human, Epithelial ovary cell lines | Not reported | Induces the secretion of angiogenic factors | [231,232,233,234] |
| MMP2 | Upregulated in ovarian cancer tissues compared to normal/benign ovarian tissues | Ovarian cancer tissue, epithilial, stroma | Human | Negative prognostic indicator with lower overall survival rates | Degrades ECM, promotes angiogenesis, and induces EMT | [161,162,163,182,235,236,237] |
| MMP3 | Involved in estradiol-induced migration and invasion | SKOV3 ovarian cancer cells | Human cell line | Not reported | Mediates estrogen-induced cancer progression via PI3K/Akt/FOXO3 pathway | [172] |
| MMP7 | Promotes invasion and metastasis | Ovarian cancer cells | Human cell line | Not reported | Acts through MAPK/ERK and JNK pathways; activates gelatin enzymes | [174] |
| MMP8 | Upregulates IL-1β | Ovarian cancer tissue | Human | Correlates with tumor grade and poor prognosis | Promotes inflammatory microenvironment | [175] |
| MMP9 | Upregulated in ovarian cancer tissues | Ovarian cancer tissue | Human | Correlates with clinical stage, tumor invasiveness, and metastatic potential | Degrades ECM, promotes angiogenesis, and induces EMT; cleaves fibronectin and type IV collagen | [23,165,165,238,239] |
| MMP10 | Increased expression with PKC pathway activation | Ovarian cancer cells (Ovcar3, EOC) | Human cell line | Not reported | Regulated by PKC pathway, Wnt signaling | [167,240] |
| MMP11 | Overexpression in stromal cells | Ovarian carcinomas | Human | Overexpression not correlates with survival. | Tumor progression | [241] |
| MMP12 | 82 A/G polymorphism associated with increased susceptibility | Genetic study | Human | Not reported | Genetic predisposition factor | [176,177] |
| MMP13 | Elevated in ascitic fluids | Ascitic fluid | Human | Potential marker for disease risk and survival outcomes | Not reported | [178,179] |
| MMP14 | Activates pro-MMP2 to MMP2 | Ovarian cancer cells | Human | Associated with vasculogenic-like networks | Matrix remodeling; activates pro-MMP2 | [82,168,242] |
| TIMP1 | Upregulated in ovarian cancer | Ovarian cancer tissue | Human | Not reported | Complex: May have MMP-independent roles in cell growth, migration, and angiogenesis | [180,181] |
| TIMP3 | Upregulated in ovarian cancer | Ovarian cancer tissue | Human | Not reported | Complex regulatory roles beyond MMP inhibition | [243,244,245] |
| uPA | Highly expressed in cancer cells; overexpressed in >75% of primary ovarian carcinomas and metastatic EOC samples | Ovarian cancer cells | Human and cell lines | Associated with invasion and metastasis | Promotes invasion, migration, and adhesion | [187,188,189,190,191] |
| PAI-1 | High levels in ovarian serous carcinoma | Ovarian cancer tissue | Human, cell lines, and xenograft models | Associated with poor clinical outcomes | Complex: Inhibits cell adhesion by blocking vitronectin; disrupts platelet-induced gene upregulation | [189,197,198] |
| CTSB | Modulates TNF-induced apoptosis; elevated in cystic fluid and serum | Cystic fluid, serum, and cancer cells | Human | Serum procathepsin B significantly elevated compared to healthy controls | Binding proteins AMBP and TSRC1 involved in TNF-induced apoptosis | [210,211,212] |
| CTSL | Overexpressed; knockdown inhibits proliferation, invasion, and tumor growth | Cancer cells | Human, cell lines, and mouse models | Associated with paclitaxel resistance | Promotes proliferation and migration; confers chemoresistance | [208,209] |
| CTSS | Inhibition stimulates TRAIL-induced apoptosis | Cancer cells | Human | Not reported | Downregulation of Bcl-2 and Cbl-mediated c-FLIP by ROS-mediated p53 expression | [222] |
| Cst3 | Elevated in malignant tissues, serum, and cystic fluid | Malignant tissue, Serum, Cystic fluid | Human | Elevated in ovarian cancer compared to benign samples | Complex: May represent failed compensatory mechanism; has additional immune and signaling functions | [210,212,213,214,215] |
| CTSK | Overexpressed in peritoneal metastatic ovarian carcinomas; elevated serum levels | Peritoneal metastases, Serum | Human | Potential biomarker | Associated with peritoneal metastasis | [217,246] |
| CTSD | Expression more common (65.1%) in tumors with low malignant potential vs. invasive tumors (43.7%); promotes the proliferation and migration of endothelial cells | Epithelial cells, Stromal cells | Human and cell lines | Independent prognostic factor for disease-free survival in invasive ovarian cancer | Pro-angiogenic and pro-metastatic role via ERK1/2 and AKT activation; correlates with microvessel density | [218,219,220,221] |
| Hepsin | Overexpressed in ovarian cancer | Desmosomal junctions | Human and mouse model | Not reported | Cleaves HGF and pro-uPA; localizes with substrate HGF; disrupts epithelial barriers | [223,224,225,227,229] |
6.2. Polycystic Ovary Syndrome (PCOS)
- MMPs and TIMPs: MMPs and TIMPs have been associated with the pathogenesis of PCOS (Table 4) [252]. It has been reported that MMP2 and MMP9 concentrations are elevated in the follicular fluid of patients with PCOS compared to healthy controls [252,253]. The increased MMP activity was associated with higher levels of androgens, insulin resistance, disrupted follicular development, and ovulatory dysfunction [6,253,254]. Consistent with these observations, it was found that the granulosa cells of women with PCOS express fewer MMP inhibitors TIMP-1 and TIMP-2 compared to healthy controls [252,255]. Recently, Butler et al. reported that women with PCOS showed significantly elevated MMP9 [254]. Interestingly, the ratios of MMP9 to all TIMPs were significantly higher in the PCOS group, while MMP17/TIMP-1 and MMP17/TIMP-2 were lower. Higher expression of Mmp2/9 was also observed in antral follicles compared to the preantral follicle and primordial follicle of a Letrozole-induced PCOS rat model [256].
- The PA and PAI system: Elevated PAI-1 levels in plasma have been reported in patients with PCOS compared to controls (Table 4) [257,258,259,260,261,262]. However, findings regarding PAI-1 distribution within ovarian tissue have been inconsistent. Devin et al. reported increased PAI-1 in granulosa cells of cystic and atretic follicles in mouse models of PCOS [263]. Atiomo et al. detected PAI-1 in granulosa and theca cells without significant differences between PCOS and control ovaries, whereas other authors reported increased PAI-1 expression in follicular fluid from patients with PCOS [257,264,265]. Genetic predisposition seems to contribute to PAI-1 dysregulation in PCOS, which has been reported associated with the 4G/4G and 4G/5G genotypic subtypes in the PAI-1 promoter region, leading to increased protein levels [266].Kelly et al. observed increased tPA antigen levels inversely correlating with insulin resistance, whereas Tarkun et al. found a direct correlation of PAI-1 levels, even in lean PCOS women [197,259]. Orio et al. reported elevated PAI-1 activity independent of obesity, while Sahay et al. found a correlation with both insulin resistance and obesity [260,267]. Ma et al. provided mechanistic insight through a mouse model demonstrating that PAI-1 deficiency prevented diet-induced obesity and insulin resistance [268,269]. Finally, Ibrahim et al. reported the presence of KLK2 in the serum of women with PCOS in association with hirsutism, but the nature of this relationship remains unclear [270].
- Cathepsins: The downregulation of CTSD has been reported in the ovaries of patients with PCOS [271]. CTSD downregulation may contribute to the abnormal follicle development associated with PCOS, leading to ovulatory dysfunction and infertility. Dawood et al. found significantly increased levels of CTSS, among patients with PCOS compared to healthy females [272]. Additionally, genetics may also play a role as CTSB polymorphisms have recently been associated with PCOS risks [273].
| Protease | Finding in PCOS | Localization | Species | Proposed Pathogenic Role | Reference |
|---|---|---|---|---|---|
| MMP2 And MMP9 | Elevated in follicular fluid of patients with PCOS compared to healthy controls | Follicular fluid and serum | Human | Associated with higher levels of androgens, insulin resistance, disrupted follicular development, and ovulatory dysfunction. Associated with higher MMP9/TIMP ratios, ECM remodeling, and follicular development | [252,253,256] |
| MMP2/9 | Higher expression in antral follicles compared to preantral and primordial follicles | Ovarian follicles | Rat (Letrozole-induced PCOS model) | ECM remodeling in PCOS ovaries | [256] |
| MMP17 | Lower MMP17/TIMP-1 and MMP17/TIMP-2 ratios in PCOS | Serum | Human | ECM remodeling | [254] |
| TIMP-1 and TIMP-2 | Decreased expression in granulosa cells of women with PCOS | Granulosa cells | Human | Excessive ECM degradation | [255,274] |
| PAI-1 | Elevated in plasma, granulosa cells, and follicular fluid; homogeneous distribution throughout PCOS ovaries | Plasma, granulosa cells, follicular fluid, and theca cells | Human and mouse (PCOS model) | Associated with insulin resistance; higher expression in 4G/4G and 4G/5G genotypes | [260,262,263,264,266,267,268,269] |
| Plasminogen | Uniquely present in small follicles of PCOS ovaries | Small follicles | Human | Altered proteolytic activity in early follicular development | [258,259,261] |
| tPA | Increased antigen levels inversely correlating with insulin resistance | Plasma | Human | Associated with insulin resistance | [275] |
| KLK2/3 | Present in the serum of women with PCOS in association with hirsutism | Serum | Human | Associated with androgsen excess and hirsutism | [270] |
| CTSB | CTSB polymorphisms contribute to PCOS pathogenesis | Blood | Human | rs12898, rs8898, and rs3779659 variants associated with PCOS risk | [273] |
| CTSD | Downregulated in ovaries of patients with PCOS | Cytoplasm and cell membrane of stromal and granulosa cells | Human | Abnormal follicle development | [271] |
| CTSS | Significantly increased levels in patients with PCOS | Serum | Human | Inflammation associated with PCOS | [272] |
6.3. Primary Ovarian Insufficiency (POI)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin-like Motifs |
| AIFM1 | Apoptosis-Inducing Factor Mitochondrion-associated 1 |
| AKT | Protein Kinase B |
| AMBP | Alpha-1-Microglobulin/Bikunin Precursor |
| ATG5 | Autophagy Related 5 |
| BCL2 | B-Cell Lymphoma 2 |
| CASP3/8 | Caspase 3/8 |
| CL | Corpus Luteum |
| COC | Cumulus–Oocyte Complex |
| CTSB | Cathepsin B |
| CTSD | Cathepsin D |
| CTSE | Cathepsin E |
| CTSG | Cathepsin G |
| CTSK | Cathepsin K |
| CTSL | Cathepsin L |
| CTSS | Cathepsin S |
| CstB | Cystatin B |
| Cst3 | Cystatin C |
| CYP17A1 | Cytochrome P450 Family 17 Subfamily A Member 1 |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| DNA | Deoxyribonucleic Acid |
| Dpc | Days Post Coitus |
| Dpp | Days Postpartum |
| E2 | Estradiol |
| ECM | Extracellular Matrix |
| EOC | Epithelial Ovarian Cancer |
| ERK | Extracellular Signal-Regulated Kinase |
| FAK | Focal Adhesion Kinase |
| FF | Follicular Fluid |
| FGF1 | Fibroblast Growth Factor 1 |
| FSH | Follicle-Stimulating Hormone |
| FURIN | Paired Basic Amino Acid Cleaving Enzyme |
| GCNA | Germ Cell Nuclear Antigen |
| GDF9 | Growth Differentiation Factor 9 |
| hCG | Human Chorionic Gonadotropin |
| HGF | Hepatocyte Growth Factor |
| ICSI | Intracytoplasmic Sperm Injection |
| IGF | Insulin-like Growth Factor |
| IGF1R | Insulin-like Growth Factor 1 Receptor |
| IL-1β | Interleukin 1 Beta |
| JNK | c-Jun N-terminal Kinase |
| KLK | Kallikrein |
| LC3-I | Microtubule-associated Protein 1A/1B-Light Chain 3 |
| LH | Luteinizing Hormone |
| LONP1 | Lon Peptidase 1 |
| LMP | Low Malignant Potential |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| mRNA | Messenger Ribonucleic Acid |
| mTOR | Mammalian Target of Rapamycin |
| MYC | Myelocytomatosis Oncogene |
| NFkB | Nuclear Factor Kappa B |
| P4 | Progesterone |
| PA | Plasminogen Activator |
| PAI | Plasminogen Activator Inhibitor |
| PAPPA | Pregnancy-Associated Plasma Protein A |
| PAR1 | Protease-Activated Receptor 1 |
| PCNA | Proliferating Cell Nuclear Antigen |
| PCOS | Polycystic Ovary Syndrome |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PMA | Phorbol-12-myristate 13-acetate |
| PMSG | Pregnant Mare Serum Gonadotropin |
| POF/POI | Premature Ovarian Failure/Primary Ovarian Insufficiency |
| PR | Progesterone Receptor |
| RGD | Arg-Gly-Asp (Arginine–Glycine–Aspartic acid) |
| ROS | Reactive Oxygen Species |
| SEC | Securities and Exchange Commission |
| siRNA | Small Interfering RNA |
| SMAD | Small Mothers Against Decapentaplegic |
| SPINK1 | Serine Protease Inhibitor Kazal Type 1 |
| STAR | Steroidogenic Acute Regulatory Protein |
| TGFα | Transforming Growth Factor Alpha |
| TGF-β | Transforming Growth Factor Beta |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TMPRSS6 | Transmembrane Serine Protease 6 (Matriptase-2) |
| TNF-α | Tumor Necrosis Factor Alpha |
| tPA | Tissue-type Plasminogen Activator |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TSRC1 | Thrombospondin and Calcium-binding domains 1 |
| uPA | Urokinase-type Plasminogen Activator |
| uPAR | Urokinase-type Plasminogen Activator Receptor |
| VEGF | Vascular Endothelial Growth Factor |
| VN | Vitronectin |
| ZMP-2 | Zinc Metalloproteinase-2 |
| ZP3 | Zona Pellucida Glycoprotein 3 |
References
- Curry, T.E., Jr.; Osteen, K.G. Cyclic Changes in the Matrix Metalloproteinase System in the Ovary and Uterus. Biol. Reprod. 2001, 64, 1285–1296. [Google Scholar] [CrossRef]
- Curry, T.E., Jr.; Osteen, K.G. The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F.; Russell, D.L. Extracellular matrix of the developing ovarian follicle. Reproduction 2003, 126, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X. Plasminogen activator/plasminogen activator inhibitors in ovarian physiology. Front. Biosci. J. Virtual Libr. 2004, 9, 3356–3373. [Google Scholar] [CrossRef]
- Levene, P.A. The Cleavage Products of Proteoses. J. Biol. Chem. 1905, 1, 45–58. [Google Scholar] [CrossRef]
- Goldman, S. MMPS and TIMPS in ovarian physiology and pathophysiology. Front. Biosci. 2004, 9, 2474. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Hey, S.; Linder, S. Matrix metalloproteinases at a glance. J. Cell Sci. 2024, 137, jcs261898. [Google Scholar] [CrossRef]
- Page, M.J.; Di Cera, E. Serine peptidases: Classification, structure and function. Cell. Mol. Life Sci. 2008, 65, 1220–1236. [Google Scholar] [CrossRef]
- Levin, G.; Coelho, T.M.; Nóbrega, N.G.; Trombetta-Lima, M.; Sogayar, M.C.; Carreira, A.C.O. Spatio-temporal expression profile of matrix metalloproteinase (Mmp) modulators Reck and Sparc during the rat ovarian dynamics. Reprod. Biol. Endocrinol. RBE 2018, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.L.L.; Sznajder, N.A.; Riley, S.C.; Anderson, R.A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human fetal testis and ovary. Mol. Hum. Reprod. 2001, 7, 641–648. [Google Scholar] [CrossRef]
- Miyakoshi, K.; Murphy, M.J.; Yeoman, R.R.; Mitra, S.; Dubay, C.J.; Hennebold, J.D. The Identification of Novel Ovarian Proteases Through the Use of Genomic and Bioinformatic Methodologies. Biol. Reprod. 2006, 75, 823–835. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Donepudi, M.; Grütter, M.G. Structure and zymogen activation of caspases. Biophys. Chem. 2002, 101–102, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, C.L.; Stouffer, R.L. Expression of Matrix Metalloproteinases and Their Tissue Inhibitor Messenger Ribonucleic Acids in Macaque Periovulatory Granulosa Cells: Time Course and Steroid Regulation1. Biol. Reprod. 1999, 61, 14–21. [Google Scholar] [CrossRef]
- Lambert, E.; Dassé, E.; Haye, B.; Petitfrère, E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol. 2004, 49, 187–198. [Google Scholar] [CrossRef]
- Moracho, N.; Learte, A.I.R.; Muñoz-Sáez, E.; Marchena, M.A.; Cid, M.A.; Arroyo, A.G.; Sánchez-Camacho, C. Emerging roles of MT-MMPs in embryonic development. Dev. Dyn. 2022, 251, 240–275. [Google Scholar] [CrossRef]
- Moore, C.S.; Crocker, S.J. An Alternate Perspective on the Roles of TIMPs and MMPs in Pathology. Am. J. Pathol. 2012, 180, 12–16. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Belotti, D.; Paganoni, P.; Manenti, L.; Garofalo, A.; Marchini, S.; Taraboletti, G.; Giavazzi, R. Matrix Metalloproteinases (MMP9 and MMP2) Induce the Release of Vascular Endothelial Growth Factor (VEGF) by Ovarian Carcinoma Cells: Implications for Ascites Formation1. Cancer Res. 2003, 63, 5224–5229. [Google Scholar] [PubMed]
- Wolak, D.; Hrabia, A. Alternations in the expression of selected matrix metalloproteinases (MMP-2, -9, -10, and -13) and their tissue inhibitors (TIMP-2 and -3) and MMP-2 and -9 activity in the chicken ovary during pause in laying induced by fasting. Theriogenology 2021, 161, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Skinner, M.K. Cellular Interactions That Control Primordial Follicle Development and Folliculogenesis. J. Soc. Gynecol. Investig. JSGI 2001, 8, S17–S20. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Pepling, M.E. From primordial germ cell to primordial follicle: Mammalian female germ cell development. Genesis 2006, 44, 622–632. [Google Scholar] [CrossRef]
- Mazaud, S.; Guyot, R.; Guigon, C.J.; Coudouel, N.; Le Magueresse-Battistoni, B.; Magre, S. Basal membrane remodeling during follicle histogenesis in the rat ovary: Contribution of proteinases of the MMP and PA families. Dev. Biol. 2005, 277, 403–416. [Google Scholar] [CrossRef]
- Sessions, D.R.; Vick, M.M.; Fitzgerald, B.P. Characterization of matrix metalloproteinase-2 and matrix metalloproteinase-9 and their inhibitors in equine granulosa cells in vivo and in vitro. J. Anim. Sci. 2009, 87, 3955–3966. [Google Scholar] [CrossRef]
- Duncan, W.C.; McNeilly, A.S.; Illingworth, P.J. The Effect of Luteal “Rescue” on the Expression and Localization of Matrix Metalloproteinases and Their Tissue Inhibitors in the Human Corpus Luteum. J. Clin. Endocrinol. Metab. 1998, 83, 2470–2478. [Google Scholar] [CrossRef][Green Version]
- Bagavandoss, P. Differential distribution of gelatinases and tissue inhibitor of metalloproteinase-1 in the rat ovary. J. Endocrinol. 1998, 158, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Downs, L.S., Jr.; Lima, P.H.; Bliss, R.L.; Blomquist, C.H. Cathepsins B and D Activity and Activity Ratios in Normal Ovaries, Benign Ovarian Neoplasms, and Epithelial Ovarian Cancer. J. Soc. Gynecol. Investig. 2005, 12, 539–544. [Google Scholar] [CrossRef]
- Smith, M.F.; McIntush, E.W.; Ricke, W.A.; Kojima, F.N.; Smith, G.W. Regulation of ovarian extracellular matrix remodelling by metalloproteinases and their tissue inhibitors: Effects on follicular development, ovulation and luteal function. J. Reprod. Fertil. Suppl. 1999, 54, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.F.; Ricke, W.A.; Bakke, L.J.; Dow, M.P.D.; Smith, G.W. Ovarian tissue remodeling: Role of matrix metalloproteinases and their inhibitors. Mol. Cell. Endocrinol. 2002, 191, 45–56. [Google Scholar] [CrossRef]
- Nothnick, W.B. Reduction in reproductive lifespan of tissue inhibitor of metalloproteinase 1 (TIMP-1)-deficient female mice. Reproduction 2001, 122, 923–927. [Google Scholar] [CrossRef]
- Nothnick, W.B. Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Deficient Mice Display Reduced Serum Progesterone Levels during Corpus Luteum Development. Endocrinology 2003, 144, 5–8. [Google Scholar] [CrossRef]
- Stilley, J.A.W.; Birt, J.A.; Nagel, S.C.; Sutovsky, M.; Sutovsky, P.; Sharpe-Timms, K.L. Neutralizing TIMP1 Restores Fecundity in a Rat Model of Endometriosis and Treating Control Rats with TIMP1 Causes Anomalies in Ovarian Function and Embryo Development1. Biol. Reprod. 2010, 83, 185–194. [Google Scholar] [CrossRef]
- Curry, T.E.; Dean, D.D.; Sanders, S.L.; Pedigo, N.G.; Jones, P.B.C. The role of ovarian proteases and their inhibitors in ovulation. Steroids 1989, 54, 501–521. [Google Scholar] [CrossRef]
- Bédard, J.; Brûlé, S.; Price, C.A.; Silversides, D.W.; Lussier, J.G. Serine protease inhibitor-E2 (SERPINE2) is differentially expressed in granulosa cells of dominant follicle in cattle. Mol. Reprod. Dev. 2003, 64, 152–165. [Google Scholar] [CrossRef]
- Cao, M.; Sahmi, M.; Lussier, J.G.; Price, C.A. Plasminogen activator and serine protease inhibitor-E2 (protease nexin-1) expression by bovine granulosa cells in vitro. Biol. Reprod. 2004, 71, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Kim, T.; Gu, D.-H.; Lee, T.S.; Kim, T.H.; Shin, S.; Shin, B.S. Pharmacokinetics of Nafamostat, a Potent Serine Protease Inhibitor, by a Novel LC-MS/MS Analysis. Molecules 2022, 27, 1881. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, W.J.; Gottsch, M.L. Proteolytic Mechanisms in the Ovulatory Folliculo-Luteal Transformation. Connect. Tissue Res. 2003, 44, 50–57. [Google Scholar] [CrossRef]
- Eykelbosh, A.J.; Van Der Kraak, G. A role for the lysosomal protease cathepsin B in zebrafish follicular apoptosis. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2010, 156, 218–223. [Google Scholar] [CrossRef]
- Li, J.R.; Shen, T. Expression characteristics of MMP-2 and MMP-9 in guinea pig ovaries during the estrous cycle. Genet. Mol. Res. 2015, 14, 17329–17340. [Google Scholar] [CrossRef]
- Vos, M.C.; van der Wurff, A.A.; Last, J.T.; de Boed, E.A.; Smeenk, J.M.; van Kuppevelt, T.H.; Massuger, L.F. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod. Biol. Endocrinol. 2014, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Motoyama, T. Relation Between Laminin-5 γ2 Chain and Cell Surface Metalloproteinase MT1-MMP in Clear Cell Carcinoma of the Ovary. Int. J. Gynecol. Pathol. 2009, 28, 49. [Google Scholar] [CrossRef]
- Robker, R.L.; Russell, D.L.; Espey, L.L.; Lydon, J.P.; O’Malley, B.W.; Richards, J.S. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc. Natl. Acad. Sci. USA 2000, 97, 4689–4694. [Google Scholar] [CrossRef]
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Pritchard, M.; Russell, D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev. Biol. 2006, 300, 699–709. [Google Scholar] [CrossRef]
- Russell, D.L.; Doyle, K.M.H.; Ochsner, S.A.; Sandy, J.D.; Richards, J.S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003, 278, 42330–42339. [Google Scholar] [CrossRef]
- Russell, D.L.; Brown, H.M.; Dunning, K.R. ADAMTS proteases in fertility. Matrix Biol. 2015, 44–46, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Dunning, K.R.; Robker, R.L.; Boerboom, D.; Pritchard, M.; Lane, M.; Russell, D.L. ADAMTS1 Cleavage of Versican Mediates Essential Structural Remodeling of the Ovarian Follicle and Cumulus-Oocyte Matrix During Ovulation in Mice1. Biol. Reprod. 2010, 83, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ahmad, M.J.; Ye, T.; Du, C.; Zhang, X.; Liang, A.; Yang, L. Cathepsin B Regulates Mice Granulosa Cells’ Apoptosis and Proliferation In Vitro. Int. J. Mol. Sci. 2021, 22, 11827. [Google Scholar] [CrossRef]
- Ezz, M.A.; Takahashi, M.; Rivera, R.M.; Balboula, A.Z. Cathepsin L regulates oocyte meiosis and preimplantation embryo development. Cell Prolif. 2023, 57, e13526. [Google Scholar] [CrossRef]
- Nothnick, W.B.; Soloway, P.; Curry, T.E., Jr. Assessment of the Role of Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) during the Periovulatory Period in Female Mice Lacking a Functional TIMP-1 Gene1. Biol. Reprod. 1997, 56, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Ho, A.T.-V.; Leco, K.J.; Moorehead, R.A.; Khokha*, R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: Functions of metalloproteinases and their inhibitors. Cell. Mol. Life Sci. CMLS 2000, 57, 77–95. [Google Scholar] [CrossRef]
- Ny, T.; Wahlberg, P.; Brändström, I.J.M. Matrix remodeling in the ovary: Regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol. Cell. Endocrinol. 2002, 187, 29–38. [Google Scholar] [CrossRef]
- Bodén, I. The Roles of the Plasminogen Activator and Matrix Metalloproteinase Systems in Ovulation and Corpus Luteum Formation. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2004. [Google Scholar]
- Epifano, O.; Riminucci, M.; Manna, C.; Apa, R.; Greco, E.; Lanzone, A.; Stefanini, M.; Canipari, R. In vitro production of plasminogen activator by human granulosa cells. J. Clin. Endocrinol. Metab. 1994, 78, 174–179. [Google Scholar] [CrossRef]
- Zhu, C.; Frederick Woessner, J., Jr. A Tissue Inhibitor of Metalloproteinases and α-Macroglobulins in the Ovulating Rat Ovary: Possible Regulators of Collagen Matrix Breakdown1. Biol. Reprod. 1991, 45, 334–342. [Google Scholar] [CrossRef]
- Peng, X.-R.; Hsueh, A.J.W.; Ny, T. Transient and cell-specific expression of tissue-type plasminogen activator and plasminogen-activator-inhibitor type 1 results in controlled and directed proteolysis during gonadotropin-induced ovulation. Eur. J. Biochem. 1993, 214, 147–156. [Google Scholar] [CrossRef]
- Hägglund, A.-C.; Ny, A.; Leonardsson, G.; Ny, T. Regulation and Localization of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in the Mouse Ovary during Gonadotropin-Induced Ovulation. Endocrinology 1999, 140, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, A.-C.; Basset, P.; Ny, T. Stromelysin-3 Is Induced in Mouse Ovarian Follicles Undergoing Hormonally Controlled Apoptosis, but This Metalloproteinase Is Not Required for Follicular Atresia. Biol. Reprod. 2001, 64, 457–463. [Google Scholar] [CrossRef]
- Ogiwara, K.; Hagiwara, A.; Rajapakse, S.; Takahashi, T. The Role of Urokinase Plasminogen Activator and Plasminogen Activator Inhibitor-1 in Follicle Rupture During Ovulation in the Teleost Medaka. Biol. Reprod. 2015, 92, 1–17. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.L.; Canipari, R.; Strickland, S. Hormonal regulation of tissue plasminogen activator secretion and mRNA levels in rat granulosa cells. J. Biol. Chem. 1987, 262, 2339–2344. [Google Scholar] [CrossRef]
- Li, M.; Karakji, E.G.; Xing, R.; Fryer, J.N.; Carnegie, J.A.; Rabbani, S.A.; Tsang, B.K. Expression of Urokinase-Type Plasminogen Activator and Its Receptor during Ovarian Follicular Development. Endocrinology 1997, 138, 2790–2799. [Google Scholar] [CrossRef]
- Karakji, E.G.; Tsang, B.K. Regulation of Rat Granulosa Cell Plasminogen Activator System: Influence of Interleukin-1β and Ovarian Follicular Development. Biol. Reprod. 1995, 53, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.; Finci-Yeheskel, Z.; Dushnik, M.; Milwidsky, A.; Ben-Chetrit, A.; Yagel, S.; Adashi, E.Y.; Mayer, M. Cytokine-mediated regulation of rat ovarian function: Interleukin-1 inhibits plasminogen activator activity through the induction of plasminogen activator inhibitor-1 (PAI-1). Mol. Cell. Endocrinol. 1994, 101, 307–314. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, C.; Yan, G.; Li, G.; Liu, J.; Yang, Y.; Li, S.; Li, Z.; Zhou, J.; Zhen, X.; et al. The mitochondrial protease LONP1 maintains oocyte development and survival by suppressing nuclear translocation of AIFM1 in mammals. eBioMedicine 2022, 75, 103790. [Google Scholar] [CrossRef]
- Meng, T.-G.; Hu, M.-W.; Ma, X.-S.; Huang, L.; Liang, Q.-X.; Yuan, Y.; Hou, Y.; Wang, H.; Schatten, H.; Wang, Z.-B.; et al. Oocyte-specific deletion of furin leads to female infertility by causing early secondary follicle arrest in mice. Cell Death Dis. 2017, 8, e2846. [Google Scholar] [CrossRef]
- Nyegaard, M.; Overgaard, M.T.; Su, Y.-Q.; Hamilton, A.E.; Kwintkiewicz, J.; Hsieh, M.; Nayak, N.R.; Conti, M.; Conover, C.A.; Giudice, L.C. Lack of Functional Pregnancy-Associated Plasma Protein-A (PAPPA) Compromises Mouse Ovarian Steroidogenesis and Female Fertility. Biol. Reprod. 2010, 82, 1129–1138. [Google Scholar] [CrossRef]
- Oxvig, C. The role of PAPP-A in the IGF system: Location, location, location. J. Cell Commun. Signal. 2015, 9, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.J.; Grell, J.A.; Wan, J.; Cohen, P.; Conover, C.A. Insulin-like growth factor (IGF)-I and IGF-II contribute differentially to the phenotype of pregnancy associated plasma protein-A knock-out mice. Growth Horm. IGF Res. 2011, 21, 243–247. [Google Scholar] [CrossRef]
- Folgueras, A.R.; de Lara, F.M.; Pendás, A.M.; Garabaya, C.; Rodríguez, F.; Astudillo, A.; Bernal, T.; Cabanillas, R.; López-Otín, C.; Velasco, G. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood 2008, 112, 2539–2545. [Google Scholar] [CrossRef]
- Tonai, S.; Kawabata, A.; Nakanishi, T.; Lee, J.Y.; Okamoto, A.; Shimada, M.; Yamashita, Y. Iron deficiency induces female infertile in order to failure of follicular development in mice. J. Reprod. Dev. 2020, 66, 475–483. [Google Scholar] [CrossRef]
- Turk, V.; Turk, B.; Turk, D. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 2001, 20, 4629–4633. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Oksjoki, S.; Söderström, M.; Vuorio, E.; Anttila, L. Differential expression patterns of cathepsins B, H, K, L and S in the mouse ovary. Mol. Hum. Reprod. 2001, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Peluffo, M.C.; Murphy, M.J.; Talcott Baughman, S.; Stouffer, R.L.; Hennebold, J.D. Systematic Analysis of Protease Gene Expression in the Rhesus Macaque Ovulatory Follicle: Metalloproteinase Involvement in Follicle Rupture. Endocrinology 2011, 152, 3963–3974. [Google Scholar] [CrossRef]
- Lind, A.-K.; Dahm-Kähler, P.; Weijdegård, B.; Sundfeldt, K.; Brännström, M. Gelatinases and their tissue inhibitors during human ovulation: Increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol. Hum. Reprod. 2006, 12, 725–736. [Google Scholar] [CrossRef]
- Bakke, L.J.; Li, Q.; Cassar, C.A.; Dow, M.P.D.; Pursley, J.R.; Smith, G.W. Gonadotropin Surge-Induced Differential Upregulation of Collagenase-1 (MMP-1) and Collagenase-3 (MMP-13) mRNA and Protein in Bovine Preovulatory Follicles. Biol. Reprod. 2004, 71, 605–612. [Google Scholar] [CrossRef]
- Hrabia, A.; Wolak, D.; Kwaśniewska, M.; Kieronska, A.; Socha, J.K.; Sechman, A. Expression of gelatinases (MMP-2 and MMP-9) and tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary in relation to follicle development and atresia. Theriogenology 2019, 125, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kang, L.; Wei, Q.; Cui, X.; Wang, S.; Chen, Y.; Jiang, Y. Expression and Regulation of MMP1, MMP3, and MMP9 in the Chicken Ovary in Response to Gonadotropins, Sex Hormones, and TGFB1. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef]
- McCord, L.A.; Li, F.; Rosewell, K.L.; Brännström, M.; Curry, T.E. Ovarian Expression and Regulation of the Stromelysins During the Periovulatory Period in the Human and the Rat. Biol. Reprod. 2012, 86, 78. [Google Scholar] [CrossRef]
- Shrestha, K.; Puttabyatappa, M.; Wynn, M.A.; Hannon, P.R.; Al-Alem, L.F.; Rosewell, K.L.; Akin, J.; Curry, T.E., Jr. Protease expression in the human and rat cumulus–oocyte complex during the periovulatory period: A role in cumulus–oocyte complex migration†. Biol. Reprod. 2024, 111, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Rosewell, K.L.; Al-Alem, L.; Zakerkish, F.; McCord, L.; Akin, J.W.; Chaffin, C.L.; Brännström, M.; Curry, T.E. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil. Steril. 2015, 103, 826–833. [Google Scholar] [CrossRef]
- Bu, S.; Cao, C.; Yang, Y.; Miao, C.; Hu, Z.; Cao, Y.; Sang, Q.A.; Duan, E. Localization and temporal regulation of tissue inhibitor of metalloproteinases-4 in mouse ovary. Reproduction 2006, 131, 1099–1107. [Google Scholar] [CrossRef]
- Simpson, K.S.; Komar, C.M.; Curry, T.E., Jr. Localization and Expression of Tissue Inhibitor of Metalloproteinase-4 in the Immature Gonadotropin-Stimulated and Adult Rat Ovary. Biol. Reprod. 2003, 68, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Cajander, S.B.; Ny, T.; Kristensen, P.; Hsueh, A.J.W. Gonadotropin regulation of tissue-type and urokinase-type plasminogen activators in rat granulosa and theca-interstitial cells during the periovulatory period. Mol. Cell. Endocrinol. 1987, 54, 221–229. [Google Scholar] [CrossRef]
- Macchione, E.; Epifano, O.; Stefanini, M.; Belin, D.; Canipari, R. Urokinase Redistribution from the Secreted to the Cell-Bound Fraction in Granulosa Cells of Rat Preovulatory Follicles1. Biol. Reprod. 2000, 62, 895–903. [Google Scholar] [CrossRef]
- Karakji, E.G.; Tsang, B.K. Tumor Necrosis Factor Alpha Inhibits Rat Granulosa Cell Plasminogen Activator Activity in Vitro during Follicular Development. Biol. Reprod. 1995, 52, 745–752. [Google Scholar] [CrossRef]
- Dow, M.P.D.; Bakke, L.J.; Cassar, C.A.; Peters, M.W.; Pursley, J.R.; Smith, G.W. Gonadotropin Surge-Induced Up-Regulation of the Plasminogen Activators (Tissue Plasminogen Activator and Urokinase Plasminogen Activator) and the Urokinase Plasminogen Activator Receptor Within Bovine Periovulatory Follicular and Luteal Tissue. Biol. Reprod. 2002, 66, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Balboula, A.Z.; Aboelenain, M.; Fujii, T.; Moriyasu, S.; Bai, H.; Kawahara, M.; Takahashi, M. Effect of autophagy induction and cathepsin B inhibition on developmental competence of poor quality bovine oocytes. J. Reprod. Dev. 2020, 66, 83–91. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, R.; Zheng, L.; Zhang, H.; Qian, Z.; Li, C.; Xue, M.; He, Z.; Ma, J.; Li, Z.; et al. Elevated N-glycosylated cathepsin L impairs oocyte function and contributes to oocyte senescence during reproductive aging. Aging Cell 2025, 24, e14397. [Google Scholar] [CrossRef]
- Holland, A.; Findlay, J.; Clements, J. Kallikrein gene expression in the gonadotrophin-stimulated rat ovary. J. Endocrinol. 2001, 170, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.A.; Mukhtar, A.; Holland, A.M.; Ehrlich, A.R.; Fuller, P.J. Kallikrein gene family expression in the rat ovary: Localization to the granulosa cell. Endocrinology 1995, 136, 1137–1144. [Google Scholar] [CrossRef]
- Musil, D.; Zucic, D.; Turk, D.; Engh, R.A.; Mayr, I.; Huber, R.; Popovic, T.; Turk, V.; Towatari, T.; Katunuma, N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: The structural basis for its specificity. EMBO J. 1991, 10, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Cavallo-Medved, D.; Moin, K.; Sloane, B. Cathepsin B: Basis sequence: Mouse. AFCS-Nat. Mol. Pages 2011, 2011, A000508. [Google Scholar]
- Mohanty, A.; Kumari, A.; Kumar, S.L.; Kumar, A.; Birajdar, P.; Beniwal, R.; Athar, M.; Kumar P, K.; Rao, H.B.D.P. Cathepsin B regulates ovarian reserve quality and quantity via mitophagy by modulating IGF1R turnover. Aging Cell 2024, e70066. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, H.; Shen, X.-H.; Zhang, J.-B.; Kim, N.-H. Inhibition of cathepsin B activity prevents deterioration in the quality of in vitro aged porcine oocytes. Theriogenology 2018, 116, 103–111. [Google Scholar] [CrossRef]
- Komatsu, K.; Wei, W.; Murase, T.; Masubuchi, S. 17β-Estradiol and cathepsins control primordial follicle growth in mouse ovaries. Reproduction 2021, 162, 277–287. [Google Scholar] [CrossRef]
- Bastu, E.; Gokulu, S.G.; Dural, O.; Yasa, C.; Bulgurcuoglu, S.; Karamustafaoglu Balci, B.; Celik, C.; Buyru, F. The association between follicular fluid levels of cathepsin B, relaxin or AMH with clinical pregnancy rates in infertile patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 187, 30–34. [Google Scholar] [CrossRef]
- Kondo, Y.; Rajapakse, S.; Ogiwara, K. Involvement of cathepsin L in the degradation and degeneration of postovulatory follicle of the medaka ovary†. Biol. Reprod. 2023, 109, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Matousek, M.; Mitsube, K.; Mikuni, M.; Brännström, M. Inhibition of ovulation in the rat by a leukotriene B4 receptor antagonist. Mol. Hum. Reprod. 2001, 7, 35–42. [Google Scholar] [CrossRef]
- Shahed, A.; Simmons, J.J.; Featherstone, S.L.; Young, K.A. Matrix metalloproteinase inhibition influences aspects of photoperiod stimulated ovarian recrudescence in Siberian hamsters. Gen. Comp. Endocrinol. 2015, 216, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Whited, J.; Shahed, A.; McMichael, C.F.; Young, K.A. Inhibition of Matrix Metalloproteinases (MMPs) in Siberian Hamsters Impedes Photostimulated Recrudescence of Ovaries. Reproduction 2010, 140, 875–883. [Google Scholar] [CrossRef]
- Oksjoki, S.; Sallinen, S.; Vuorio, E.; Anttila, L. Cyclic expression of mRNA transcripts for connective tissue components in the mouse ovary. Mol. Hum. Reprod. 1999, 5, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Shindo, T.; Kurihara, H.; Kuno, K.; Yokoyama, H.; Wada, T.; Kurihara, Y.; Imai, T.; Wang, Y.; Ogata, M.; Nishimatsu, H.; et al. ADAMTS-1: A metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Investig. 2000, 105, 1345–1352. [Google Scholar] [CrossRef]
- Mittaz, L.; Russell, D.L.; Wilson, T.; Brasted, M.; Tkalcevic, J.; Salamonsen, L.A.; Hertzog, P.J.; Pritchard, M.A. Adamts-1 Is Essential for the Development and Function of the Urogenital System. Biol. Reprod. 2004, 70, 1096–1105. [Google Scholar] [CrossRef]
- Shozu, M.; Minami, N.; Yokoyama, H.; Inoue, M.; Kurihara, H.; Matsushima, K.; Kuno, K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J. Mol. Endocrinol. 2005, 35, 343–355. [Google Scholar] [CrossRef]
- Carter, N.J.; Roach, Z.A.; Byrnes, M.M.; Zhu, Y. Adamts9 is necessary for ovarian development in zebrafish. Gen. Comp. Endocrinol. 2019, 277, 130–140. [Google Scholar] [CrossRef]
- Liu, K.; Brändström, A.; Liu, Y.X.; Ny, T.; Selstam, G. Coordinated expression of tissue-type plasminogen activator and plasminogen activator inhibitor type 1 during corpus luteum formation and luteolysis in the adult pseudopregnant rat. Endocrinology 1996, 137, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, E.; Itin, A.; Keshet, E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc. Natl. Acad. Sci. USA 1992, 89, 10686–10690. [Google Scholar] [CrossRef]
- Jones, P.B.; Muse, K.N.; Wilson, E.A.; Curry, T.E. Expression of plasminogen activator (PA) and a PA inhibitor in human granulosa cells from preovulatory follicles. J. Clin. Endocrinol. Metab. 1988, 67, 857–860. [Google Scholar] [CrossRef]
- Chen, B.; Chang, H.-M.; Zhang, Z.; Cao, Y.; Leung, P.C.K. ALK4-SMAD3/4 mediates the effects of activin A on the upregulation of PAI-1 in human granulosa lutein cells. Mol. Cell. Endocrinol. 2020, 505, 110731. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Jiang, Y.; Wang, Y.; Song, M.; Niu, X.; Xu, H.; Li, M. Modulation of Cathepsin S (CTSS) Regulates the Secretion of Progesterone and Estradiol, Proliferation, and Apoptosis of Ovarian Granulosa Cells in Rabbits. Animals 2021, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Qin, Q.; Yuan, C.; Li, H.; Zhang, F.; Fan, L. Metabolomic Profiling of Poor Ovarian Response Identifies Potential Predictive Biomarkers. Front. Endocrinol. 2021, 12, 774667. [Google Scholar] [CrossRef]
- Espey, L.L. Ovarian Proteolytic Enzymes and Ovulation. Biol. Reprod. 1974, 10, 216–235. [Google Scholar] [CrossRef]
- Espey, L.L.; Richards, J.; Neill, J. Physiology of Reproduction; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2006, pp. 425–474. [Google Scholar]
- Basini, G.; Bussolati, S.; Baioni, L.; Grasselli, F. Gelatinases (MMP2 and MMP9) in swine antral follicle. BioFactors 2011, 37, 117–120. [Google Scholar] [CrossRef]
- Deady, L.D.; Shen, W.; Mosure, S.A.; Spradling, A.C.; Sun, J. Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in Drosophila. PLoS Genet. 2015, 11, e1004989. [Google Scholar] [CrossRef]
- Fujihara, M.; Yamamizu, K.; Wildt, D.; Songsasen, N. Expression pattern of matrix metalloproteinases changes during folliculogenesis in the cat ovary. Reprod. Domest. Anim. 2016, 51, 717–725. [Google Scholar] [CrossRef]
- Jo, M.; Curry, T.E., Jr. Regulation of Matrix Metalloproteinase-19 Messenger RNA Expression in the Rat Ovary. Biol. Reprod. 2004, 71, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Politis, I.; Srikandakumar, A.; Turner, J.D.; Tsang, B.K.; Ainsworth, L.; Downey, B.R. Changes in and Partial Identification of the Plasminogen Activator and Plasminogen Activator Inhibitor Systems During Ovarian Follicular Maturation in the Pig. Biol. Reprod. 1990, 43, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Zupanič, N.; Počič, J.; Leonardi, A.; Šribar, J.; Kordiš, D.; Križaj, I. Serine pseudoproteases in physiology and disease. FEBS J. 2023, 290, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R.; Strickland, S. Plasminogen activator in the rat ovary. Production and gonadotropin regulation of the enzyme in granulosa and thecal cells. J. Biol. Chem. 1985, 260, 5121–5125. [Google Scholar] [CrossRef]
- Canipari, R.; O’Connell, M.L.; Meyer, G.; Strickland, S. Mouse ovarian granulosa cells produce urokinase-type plasminogen activator, whereas the corresponding rat cells produce tissue-type plasminogen activator. J. Cell Biol. 1987, 105, 977–981. [Google Scholar] [CrossRef]
- Shen, X.; Minoura, H.; Yoshida, T.; Toyoda, N. Changes in Ovarian Expression of Tissue-Type Plasminogen Activator and Plasminogen Activator Inhibitor Type-1 Messenger Ribonucleic Acids during Ovulation in Rat. Endocr. J. 1997, 44, 341–348. [Google Scholar] [CrossRef]
- Liu, Y.-X. Regulation of the Plasminogen Activator System in the Ovary. Biol. Signals Recept. 1999, 8, 160–177. [Google Scholar] [CrossRef]
- Plendl, J.; Snyman, C.; Naidoo, S.; Sawant, S.; Mahabeer, R.; Bhoola, K.D. Expression of Tissue Kallikrein and Kinin Receptors in Angiogenic Microvascular Endothelial Cells. Biol. Chem. 2000, 381, 1103–1115. [Google Scholar] [CrossRef]
- Hazzard, T.M.; Stouffer, R.L. Angiogenesis in ovarian follicular and luteal development. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 883–900. Available online: https://www.sciencedirect.com/science/article/pii/S1521693400901330 (accessed on 21 April 2025). [CrossRef]
- Tamanini, C.; De Ambrogi, M. Angiogenesis in Developing Follicle and Corpus Luteum. Reprod. Domest. Anim. 2004, 39, 206–216. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshino, O.; Nakashima, A.; Ito, M.; Nishio, K.; Ono, Y.; Kusabiraki, T.; Kunitomi, C.; Takahashi, N.; Harada, M.; et al. Inhibition of autophagy in theca cells induces CYP17A1 and PAI-1 expression via ROS/p38 and JNK signalling during the development of polycystic ovary syndrome. Mol. Cell. Endocrinol. 2020, 508, 110792. [Google Scholar] [CrossRef] [PubMed]
- Paliouras, M.; Diamandis, E.P. Intracellular signaling pathways regulate hormone-dependent kallikrein gene expression. Tumour Biol. 2008, 29, 63–75. [Google Scholar] [CrossRef]
- Iwasaki, T.; Tokumori, M.; Matsubara, M.; Ojima, F.; Kamigochi, K.; Aizawa, S.; Ogoshi, M.; Kimura, A.P.; Takeuchi, S.; Takahashi, S. A regulatory mechanism of mouse kallikrein 1 gene expression by estrogen. Mol. Cell. Endocrinol. 2023, 577, 112044. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, S.; Marcoccia, D.; Narciso, L.; Mantovani, A. Cell viability and PSA secretion assays in LNCaP cells: A tiered in vitro approach to screen chemicals with a prostate-mediated effect on male reproduction within the ReProTect project. Reprod. Toxicol. 2010, 30, 25–35. [Google Scholar] [CrossRef]
- Raimondo, S.; Gentile, M.; Esposito, G.; Gentile, T.; Ferrara, I.; Crescenzo, C.; Palmieri, M.; Cuomo, F.; De Filippo, S.; Lettieri, G.; et al. Could Kallikrein-Related Serine Peptidase 3 Be an Early Biomarker of Environmental Exposure in Young Women? Int. J. Environ. Res. Public Health 2021, 18, 8833. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Lai, J.; Clements, J.A. Kallikreins on Steroids: Structure, Function, and Hormonal Regulation of Prostate-Specific Antigen and the Extended Kallikrein Locus. Endocr. Rev. 2010, 31, 407–446. [Google Scholar] [CrossRef] [PubMed]
- Borgoño, C.A.; Diamandis, E.P. The emerging roles of human tissue kallikreins in cancer. Nat. Rev. Cancer 2004, 4, 876–890. [Google Scholar] [CrossRef]
- Kurlender, L.; Yousef, G.M.; Memari, N.; Robb, J.-D.; Michael, I.P.; Borgoño, C.; Katsaros, D.; Stephan, C.; Jung, K.; Diamandis, E.P. Differential Expression of a Human Kallikrein 5 (KLK5) Splice Variant in Ovarian and Prostate Cancer. Tumor Biol. 2004, 25, 149–156. [Google Scholar] [CrossRef]
- Ahmed, N.; Dorn, J.; Napieralski, R.; Drecoll, E.; Kotzsch, M.; Goettig, P.; Zein, E.; Avril, S.; Kiechle, M.; Diamandis, E.P.; et al. Clinical relevance of kallikrein-related peptidase 6 (KLK6) and 8 (KLK8) mRNA expression in advanced serous ovarian cancer. Biol. Chem. 2016, 397, 1265–1276. [Google Scholar] [CrossRef]
- Sriraman, V.; Richards, J.S. Cathepsin L Gene Expression and Promoter Activation in Rodent Granulosa Cells. Endocrinology 2004, 145, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Cionna, C.; Tosti, L.; Lubzens, E.; Maradonna, F. Role of cathepsins in ovarian follicle growth and maturation. Gen. Comp. Endocrinol. 2006, 146, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Balboula, A.Z.; Yamanaka, K.; Sakatani, M.; Hegab, A.O.; Zaabel, S.M.; Takahashi, M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol. Reprod. Dev. 2010, 77, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Balboula, A.Z.; Yamanaka, K.; Sakatani, M.; Kawahara, M.; Hegab, A.O.; Zaabel, S.M.; Takahashi, M. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus–oocyte complexes exposed to heat shock during in vitro maturation. Reproduction 2013, 146, 407–417. [Google Scholar] [CrossRef]
- García, V.; Kohen, P.; Maldonado, C.; Sierralta, W.; Muñoz, A.; Villarroel, C.; Strauss, J.F.; Devoto, L. Transient expression of progesterone receptor and cathepsin-l in human granulosa cells during the periovulatory period. Fertil. Steril. 2012, 97, 707–713.e1. [Google Scholar] [CrossRef]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants1. J. Dairy Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Wiltbank, M.C.; Mezera, M.A.; Toledo, M.Z.; Drum, J.N.; Baez, G.M.; García-Guerra, A.; Sartori, R. Physiological mechanisms involved in maintaining the corpus luteum during the first two months of pregnancy. Anim. Reprod. 2018, 15 (Suppl. 1), 805–821. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.-P.; Nguyen, T.-N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef]
- Wahlberg, P.; Nylander, Å.; Ahlskog, N.; Liu, K.; Ny, T. Expression and Localization of the Serine Proteases High-Temperature Requirement Factor A1, Serine Protease 23, and Serine Protease 35 in the Mouse Ovary. Endocrinology 2008, 149, 5070–5077. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Elias, K.M.; Guo, J.; Bast, R.C. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Tingulstad, S.; Skjeldestad, F.E.; Halvorsen, T.B.; Hagen, B. jørn Survival and prognostic factors in patients with ovarian cancer. Obstet. Gynecol. 2003, 101, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403. [Google Scholar] [CrossRef] [PubMed]
- Scorilas, A.; Borgoño, C.A.; Harbeck, N.; Dorn, J.; Schmalfeldt, B.; Schmitt, M.; Diamandis, E.P. Human Kallikrein 13 Protein in Ovarian Cancer Cytosols: A New Favorable Prognostic Marker. J. Clin. Oncol. 2004, 22, 678–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q. Relationship between matrix metalloproteinases and the occurrence and development of ovarian cancer. Braz. J. Med. Biol. Res. 2017, 50, e6104. [Google Scholar] [CrossRef]
- Goldman, S.; Shalev, E. The role of the matrix metalloproteinases in human endometrial and ovarian cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 111, 109–121. [Google Scholar] [CrossRef]
- Irving-Rodgers, H.F.; Rodgers, R.J. Extracellular matrix in ovarian follicular development and disease. Cell Tissue Res. 2005, 322, 89–98. [Google Scholar] [CrossRef]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Sakata, K.; Shigemasa, K.; Nagai, N.; Ohama, K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol. 2000, 17, 673–754. [Google Scholar] [CrossRef]
- Davidson, B.; Goldberg, I.; Gotlieb, W.H.; Kopolovic, J.; Ben-Baruch, G.; Nesland, J.M.; Berner, A.; Bryne, M.; Reich, R. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin. Exp. Metastasis 1999, 17, 799–808. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, S.; Xu, Y.; Ma, J.; Li, J.; Xu, P. The Expression of Tumor-Derived and Stromal-Derived Matrix Metalloproteinase 2 Predicted Prognosis of Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Goldberg, I.; Gotlieb, W.H.; Kopolovic, J.; Ben-Baruch, G.; Nesland, J.M.; Reich, R. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol. Cell. Endocrinol. 2002, 187, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, S.; Anttila, M.; Voutilainen, K.; Ropponen, K.; Turpeenniemi-Hujanen, T.; Puistola, U.; Tammi, R.; Tammi, M.; Sironen, R.; Saarikoski, S.; et al. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Liu, Y.; Diamandis, E.P.; Kiechle, M.; Bronger, H.; Dorn, J.; Dreyer, T.; Magdolen, V. Prognostic value of kallikrein-related peptidase 7 (KLK7) mRNA expression in advanced high-grade serous ovarian cancer. J. Ovarian Res. 2020, 13, 125. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; McCord, L.A.; Southard, R.C.; Kilgore, M.W.; Curry, T.E., Jr. Activation of the PKC Pathway Stimulates Ovarian Cancer Cell Proliferation, Migration, and Expression of MMP7 and MMP101. Biol. Reprod. 2013, 89, 1–7. [Google Scholar] [CrossRef]
- Al-Alem, L.; Curry, T.E. Ovarian cancer: Involvement of the matrix metalloproteinases. Reproduction 2015, 150, R55–R64. [Google Scholar] [CrossRef]
- Kamat, A.A.; Fletcher, M.; Gruman, L.M.; Mueller, P.; Lopez, A.; Landen, C.N., Jr.; Han, L.; Gershenson, D.M.; Sood, A.K. The Clinical Relevance of Stromal Matrix Metalloproteinase Expression in Ovarian Cancer. Clin. Cancer Res. 2006, 12, 1707–1714. [Google Scholar] [CrossRef]
- Sood, A.K.; Fletcher, M.S.; Coffin, J.E.; Yang, M.; Seftor, E.A.; Gruman, L.M.; Gershenson, D.M.; Hendrix, M.J.C. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am. J. Obstet. Gynecol. 2004, 190, 899–909. [Google Scholar] [CrossRef]
- Agrawal, A.; Ambad, R.; Lahoti, R.; Muley, P.; Pande, P.S. Role of Artificial Intelligence in PCOS Detection. J. Datta Meghe Inst. Med. Sci. Univ. 2022, 17, 491. [Google Scholar] [CrossRef]
- Gao, X.-W.; Su, X.-T.; Lu, Z.-H.; Ou, J. 17β-Estradiol Prevents Extracellular Matrix Degradation by Downregulating MMP3 Expression via PI3K/Akt/FOXO3 Pathway. Spine 2020, 45, 292. [Google Scholar] [CrossRef]
- Wang, F.-Q.; So, J.; Reierstad, S.; Fishman, D.A. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int. J. Cancer 2005, 114, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, C.-A.; Chen, P.-J.; Chiang, Y.-C.; Chen, Y.-L.; Mao, T.-L.; Lin, H.-W.; Lin Chiang, W.-H.; Cheng, W.-F. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 2012, 442, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Pollheimer, J.; Moser, P.L.; Raggi, A.; Amberger, A.; Margreiter, R.; Offner, F.A.; Mikuz, G.; Dirnhofer, S.; Moch, H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 2003, 39, 2499–2505. [Google Scholar] [CrossRef]
- Chen, S.-S.; Song, J.; Tu, X.-Y.; Zhao, J.-H.; Ye, X.-Q. The association between MMP-12 82 A/G polymorphism and susceptibility to various malignant tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10845–10854. [Google Scholar]
- Liu, L.; Sun, J.; Li, G.; Gu, B.; Wang, X.; Chi, H.; Guo, F. Association between MMP-12-82A/G polymorphism and cancer risk: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 11896–11904. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4612787/ (accessed on 21 April 2025).
- Hantke, B.; Harbeck, N.; Schmalfeldt, B.; Claes, I.; Hiller, O.; Luther, M.-O.; Welk, A.; Kuhn, W.; Schmitt, M.; Tschesche, H.; et al. Clinical Relevance of Matrix Metalloproteinase-13 Determined with a New Highly Specific and Sensitive ELISA in Ascitic Fluid of Advanced Ovarian Carcinoma Patients. Biol. Chem. 2003, 384, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Escalona, R.M.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. Expression of TIMPs and MMPs in Ovarian Tumors, Ascites, Ascites-Derived Cells, and Cancer Cell Lines: Characteristic Modulatory Response Before and After Chemotherapy Treatment. Front. Oncol. 2022, 11, 796588. [Google Scholar] [CrossRef]
- Escalona, R.M.; Bilandzic, M.; Western, P.; Kadife, E.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. TIMP-2 regulates proliferation, invasion and STAT3-mediated cancer stem cell-dependent chemoresistance in ovarian cancer cells. BMC Cancer 2020, 20, 960. [Google Scholar] [CrossRef]
- Davidson, B.; Reich, R.; Berner, A.; Givant-Horwitz, V.; Goldberg, I.; Risberg, B.; Kristensen, G.B.; Trope, C.G.; Bryne, M.; Kopolovic, J.; et al. Ovarian carcinoma cells in serous effusions show altered MMP-2 and TIMP-2 mRNA levels. Eur. J. Cancer 2001, 37, 2040–2049. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Piskór, B.; Gacuta, E.; Zajkowska, M.; Osada, J.; Szmitkowski, M.; Dąbrowska, M.; Ławicki, S. Diagnostic Power of Selected Cytokines, MMPs and TIMPs in Ovarian Cancer Patients–ROC Analysis. Anticancer Res. 2019, 39, 2575–2582. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. Tissue Inhibitors of Metalloproteinases in Cell Signaling: Metalloproteinase-Independent Biological Activities. Sci. Signal. 2008, 1, re6. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Cruz-Munoz, W.; Khokha, R. The Role of Tissue Inhibitors of Metalloproteinases in Tumorigenesis and Metastasis. Crit. Rev. Clin. Lab. Sci. 2008, 45, 291–338. [Google Scholar] [CrossRef] [PubMed]
- ASTEDT, B. Immunological identity of urokinase and ovarian carcinoma plasminogen activator released in tissue culture. Eur. J Cancer 1981, 17, 239–241. [Google Scholar] [CrossRef]
- Kuhn, W.; Schmalfeldt, B.; Reuning, U.; Pache, L.; Berger, U.; Ulm, K.; Harbeck, N.; Späthe, K.; Dettmar, P.; Höfler, H.; et al. Prognostic significance of urokinase (uPA) and its inhibitor PAI-1 for survival in advanced ovarian carcinoma stage FIGO IIIc. Br. J. Cancer 1999, 79, 1746–1751. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, P.; Dong, S.; Li, L.; Cai, J.; Xu, M. Downregulation of SPINK13 Promotes Metastasis by Regulating uPA in Ovarian Cancer Cells. Cell. Physiol. Biochem. 2018, 45, 1061–1071. [Google Scholar] [CrossRef]
- Chambers, S.K.; Ivins, C.M.; Carcangiu, M.L. Plasminogen activator inhibitor-1 is an independent poor prognostic factor for survival in advanced stage epithelial ovarian cancer patients. Int. J. Cancer 1998, 79, 449–454. [Google Scholar] [CrossRef]
- Alberti, C.; Pinciroli, P.; Valeri, B.; Ferri, R.; Ditto, A.; Umezawa, K.; Sensi, M.; Canevari, S.; Tomassetti, A. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene 2012, 31, 4139–4149. [Google Scholar] [CrossRef] [PubMed]
- Dorn, J.; Harbeck, N.; Kates, R.; Gkazepis, A.; Scorilas, A.; Soosaipillai, A.; Diamandis, E.; Kiechle, M.; Schmalfeldt, B.; Schmitt, M. Impact of expression differences of kallikrein-related peptidases and of uPA and PAI-1 between primary tumor and omentum metastasis in advanced ovarian cancer. Ann. Oncol. 2011, 22, 877–883. [Google Scholar] [CrossRef]
- Wang, L.; Madigan, M.C.; Chen, H.; Liu, F.; Patterson, K.I.; Beretov, J.; O’Brien, P.M.; Li, Y. Expression of urokinase plasminogen activator and its receptor in advanced epithelial ovarian cancer patients. Gynecol. Oncol. 2009, 114, 265–272. [Google Scholar] [CrossRef]
- Harbeck, N.; Krüger, A.; Sinz, S.; Kates, R.E.; Thomssen, C.; Schmitt, M.; Jänicke, F. Clinical Relevance of the Plasminogen Activator Inhibitor Type1—A Multifaceted Proteolytic Factor. Onkologie 2001, 24, 238–244. [Google Scholar] [CrossRef]
- van Dam, P.A.; Coelho, A.; Rolfo, C. Is there a role for urokinase-type plasminogen activator inhibitors as maintenance therapy in patients with ovarian cancer? Eur. J. Surg. Oncol. EJSO 2017, 43, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Leonhardt, P.; Ladanyi, A.; Yamada, S.D.; Montag, A.; Im, H.K.; Jagadeeswaran, S.; Shaw, D.E.; Mazar, A.P.; Lengyel, E. Targeting the Urokinase Plasminogen Activator Receptor Inhibits Ovarian Cancer Metastasis. Clin. Cancer Res. 2011, 17, 459–471. [Google Scholar] [CrossRef]
- Nakatsuka, E.; Sawada, K.; Nakamura, K.; Yoshimura, A.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Mabuchi, S.; Makino, H.; Morii, E.; et al. Plasminogen activator inhibitor-1 is an independent prognostic factor of ovarian cancer and IMD-4482, a novel plasminogen activator inhibitor-1 inhibitor, inhibits ovarian cancer peritoneal dissemination. Oncotarget 2017, 8, 89887–89902. [Google Scholar] [CrossRef]
- Kelly, T.E.; Spillane, C.L.; Ward, M.P.; Hokamp, K.; Huang, Y.; Tewari, P.; Martin, C.M.; Norris, L.A.; Mohamed, B.M.; Bates, M.; et al. Plasminogen activator inhibitor 1 is associated with high-grade serous ovarian cancer metastasis and is reduced in patients who have received neoadjuvant chemotherapy. Front. Cell Dev. Biol. 2023, 11, 1150991. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, D.; Loskutoff, D.J. Type 1 plasminogen activator inhibitor induces multimerization of plasma vitronectin: A suggested mechanism for the generation of the tissue form of vitronectin in vivo. J. Biol. Chem. 1996, 271, 29644–29651. [Google Scholar] [CrossRef]
- Seiffert, D.; Ciambrone, G.; Wagner, N.V.; Binder, B.R.; Loskutoff, D.J. The somatomedin B domain of vitronectin. Structural requirements for the binding and stabilization of active type 1 plasminogen activator inhibitor. J. Biol. Chem. 1994, 269, 2659–2666. [Google Scholar] [CrossRef]
- Planus, E.; Barlovatz-Meimon, G.; Rogers, R.A.; Bonavaud, S.; Ingber, D.E.; Wang, N. Binding of urokinase to plasminogen activator inhibitor type-1 mediates cell adhesion and spreading. J. Cell Sci. 1997, 110, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, V.; Sarra Ferraris, G.M.; Madsen, J.B.; Lupia, M.; Andreasen, P.A.; Sidenius, N. Urokinase links plasminogen activation and cell adhesion by cleavage of the RGD motif in vitronectin. EMBO Rep. 2016, 17, 982–998. [Google Scholar] [CrossRef]
- Czekay, R.-P.; Wilkins-Port, C.E.; Higgins, S.P.; Freytag, J.; Overstreet, J.M.; Klein, R.M.; Higgins, C.E.; Samarakoon, R.; Higgins, P.J. PAI-1: An Integrator of Cell Signaling and Migration. Int. J. Cell Biol. 2011, 2011, 562481. [Google Scholar] [CrossRef]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Wong, M.K.K.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen Activator Inhibitor-1 Regulates Tumor Growth and Angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. CMLS 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Cortese, K.; Sahores, M.; Madsen, C.D.; Tacchetti, C.; Blasi, F. Clathrin and LRP-1-Independent Constitutive Endocytosis and Recycling of uPAR. PLoS ONE 2008, 3, e3730. [Google Scholar] [CrossRef]
- Placencio, V.R.; DeClerck, Y.A. Plasminogen Activator Inhibitor-1 in Cancer: Rationale and Insight for Future Therapeutic Testing. Cancer Res. 2015, 75, 2969–2974. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Q.; Chen, B.; Yu, Y.; Lu, H.; Li, Y.-Y. Cathepsin B and its interacting proteins, bikunin and TSRC1, correlate with TNF-induced apoptosis of ovarian cancer cells OV-90. FEBS Lett. 2006, 580, 245–250. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, L.; Shen, G.; He, B.; Gong, W.; Min, N.; Zhang, L.; Duan, Y.; Xie, J.; Luo, H.; et al. Cathepsin L is involved in proliferation and invasion of ovarian cancer cells. Mol. Med. Rep. 2015, 11, 468–474. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Wang, Q.; Yang, Z.; Pan, Z.; Li, L. Overexpression of cysteine cathepsin L is a marker of invasion and metastasis in ovarian cancer. Oncol. Rep. 2014, 31, 1334–1342. [Google Scholar] [CrossRef]
- Kolwijck, E.; Massuger, L.F.A.G.; Thomas, C.M.G.; Span, P.N.; Krasovec, M.; Kos, J.; Sweep, F.C.G.J. Cathepsins B, L and cystatin C in cyst fluid of ovarian tumors. J. Cancer Res. Clin. Oncol. 2010, 136, 771–778. [Google Scholar] [CrossRef]
- Gashenko, E.A.; Lebedeva, V.A.; Brak, I.V.; Tsykalenko, E.A.; Vinokurova, G.V.; Korolenko, T.A. Evaluation of serum procathepsin B, cystatin B and cystatin C as possible biomarkers of ovarian cancer. Int. J. Circumpolar Health 2013, 72, 21215. [Google Scholar] [CrossRef]
- Nishikawa, H.; Ozaki, Y.; Nakanishi, T.; Blomgren, K.; Tada, T.; Arakawa, A.; Suzumori, K. The role of cathepsin B and cystatin C in the mechanisms of invasion by ovarian cancer. Gynecol. Oncol. 2004, 92, 881–886. [Google Scholar] [CrossRef]
- Kos, J.; Werle, B.; Lah, T.; Brunner, N. Cysteine proteinases and their inhibitors in extracellular fluids: Markers for diagnosis and prognosis in cancer. Int. J. Biol. Markers 2000, 15, 84–89. [Google Scholar] [CrossRef]
- Magister, S.; Kos, J. Cystatins in immune system. J. Cancer 2013, 4, 45–56. [Google Scholar] [CrossRef]
- Jedeszko, C.; Sloane, B.F. Cysteine cathepsins in human cancer. Biol. Chem. 2004, 385, 1017–1027. [Google Scholar] [CrossRef]
- Wallin, H.; Bjarnadottir, M.; Vogel, L.K.; Wassélius, J.; Ekström, U.; Abrahamson, M. Cystatins–Extra- and intracellular cysteine protease inhibitors: High-level secretion and uptake of cystatin C in human neuroblastoma cells. Biochimie 2010, 92, 1625–1634. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Y.; Zhang, Y.; Pan, Z.; Lu, Y.; Liu, P.; Lu, B. Identification of Cathepsin K in the Peritoneal Metastasis of Ovarian Carcinoma Using In-silico, Gene Expression Analysis. J. Cancer 2016, 7, 722–729. [Google Scholar] [CrossRef]
- Lösch, A.; Schindl, M.; Kohlberger, P.; Lahodny, J.; Breitenecker, G.; Horvat, R.; Birner, P. Cathepsin D in ovarian cancer: Prognostic value and correlation with p53 expression and microvessel density. Gynecol. Oncol. 2004, 92, 545–552. [Google Scholar] [CrossRef]
- Scambia, G.; Benedetti, P.; Ferrandina, G.; Battaglia, F.; Baiocchi, G.; Mancuso, S. Cathepsin D assay in ovarian cancer: Correlation with pathological features and receptors for oestrogen, progesterone and epidermal growth factor. Br. J. Cancer 1991, 64, 182–184. [Google Scholar] [CrossRef]
- Pranjol, M.Z.I.; Gutowski, N.; Hannemann, M.; Whatmore, J. The Potential Role of the Proteases Cathepsin D and Cathepsin L in the Progression and Metastasis of Epithelial Ovarian Cancer. Biomolecules 2015, 5, 3260–3279. [Google Scholar] [CrossRef]
- Pranjol, M.Z.I.; Gutowski, N.J.; Hannemann, M.; Whatmore, J.L. Cathepsin D non-proteolytically induces proliferation and migration in human omental microvascular endothelial cells via activation of the ERK1/2 and PI3K/AKT pathways. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2018, 1865, 25–33. [Google Scholar] [CrossRef]
- Seo, B.R.; Min, K.; Woo, S.M.; Choe, M.; Choi, K.S.; Lee, Y.-K.; Yoon, G.; Kwon, T.K. Inhibition of Cathepsin S Induces Mitochondrial ROS That Sensitizes TRAIL-Mediated Apoptosis Through p53-Mediated Downregulation of Bcl-2 and c-FLIP. Antioxid. Redox Signal. 2017, 27, 215–233. [Google Scholar] [CrossRef]
- Tripathi, M.; Nandana, S.; Yamashita, H.; Ganesan, R.; Kirchhofer, D.; Quaranta, V. Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression. J. Biol. Chem. 2008, 283, 30576–30584. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, H.; Yan, Y.; Clarke, J.; Korourian, S.; Shigemasa, K.; Parmley, T.H.; Parham, G.P.; O’Brien, T.J. Hepsin, a Cell Surface Serine Protease Identified in Hepatoma Cells, Is Overexpressed in Ovarian Cancer. Cancer Res. 1997, 57, 2884–2887. [Google Scholar] [PubMed]
- Rosewell, K.; Al-Alem, L.; Li, F.; Kelty, B.; Curry, T.E., Jr. Identification of Hepsin and Protein Disulfide Isomerase A3 as Targets of Gelatinolytic Action in Rat Ovarian Granulosa Cells During the Periovulatory Period1. Biol. Reprod. 2011, 85, 858–866. [Google Scholar] [CrossRef]
- Duru, N.; Pawar, N.R.; Martin, E.W.; Buzza, M.S.; Conway, G.D.; Lapidus, R.G.; Liu, S.; Reader, J.; Rao, G.G.; Roque, D.M.; et al. Selective targeting of metastatic ovarian cancer using an engineered anthrax prodrug activated by membrane-anchored serine proteases. Proc. Natl. Acad. Sci. USA 2022, 119, e2201423119. [Google Scholar] [CrossRef]
- Miao, J.; Mu, D.; Ergel, B.; Singavarapu, R.; Duan, Z.; Powers, S.; Oliva, E.; Orsulic, S. Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int. J. Cancer J. Int. Cancer 2008, 123, 2041–2047. [Google Scholar] [CrossRef]
- Lu, L.; Cole, A.; Huang, D.; Wang, Q.; Guo, Z.; Yang, W.; Lu, J. Clinical Significance of Hepsin and Underlying Signaling Pathways in Prostate Cancer. Biomolecules 2022, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Herter, S.; Piper, D.E.; Aaron, W.; Gabriele, T.; Cutler, G.; Cao, P.; Bhatt, A.S.; Choe, Y.; Craik, C.S.; Walker, N.; et al. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem. J. 2005, 390, 125–136. [Google Scholar] [CrossRef]
- Willbold, R.; Wirth, K.; Martini, T.; Sültmann, H.; Bolenz, C.; Wittig, R. Correction: Excess hepsin proteolytic activity limits oncogenic signaling and induces ER stress and autophagy in prostate cancer cells. Cell Death Dis. 2020, 11, 120. [Google Scholar] [CrossRef]
- Kenny, H.A.; Lengyel, E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 2009, 8, 683–688. [Google Scholar] [CrossRef]
- Morales-Vásquez, F.; Castillo-Sánchez, R.; Gómora, M.J.; Almaraz, M.Á.; Pedernera, E.; Pérez-Montiel, D.; Rendón, E.; López-Basave, H.N.; Román-Basaure, E.; Cuevas-Covarrubias, S.; et al. Expression of metalloproteinases MMP-2 and MMP-9 is associated to the presence of androgen receptor in epithelial ovarian tumors. J. Ovarian Res. 2020, 13, 86. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Kang, L.; Li, L.; Wang, W.; Shan, B. Activated Matrix Metalloproteinase-2—A Potential Marker of Prognosis for Epithelial Ovarian Cancer. Gynecol. Oncol. 2002, 84, 126–134. [Google Scholar] [CrossRef]
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The prognostic values of matrix metalloproteinases in ovarian cancer. J. Int. Med. Res. 2020, 48, 0300060519825983. [Google Scholar] [CrossRef]
- Li, L.-N.; Zhou, X.; Gu, Y.; Yan, J. Prognostic Value of MMP-9 in Ovarian Cancer: A Meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 4107–4113. [Google Scholar] [CrossRef]
- Vos, M.C.; van der Wurff, A.A.M.; van Kuppevelt, T.H.; Massuger, L.F.A.G. The role of MMP-14 in ovarian cancer: A systematic review. J. Ovarian Res. 2021, 14, 101. [Google Scholar] [CrossRef]
- Agarwal, A.; Tressel, S.L.; Kaimal, R.; Balla, M.; Lam, F.H.; Covic, L.; Kuliopulos, A. Identification of a Metalloprotease-Chemokine Signaling System in the Ovarian Cancer Microenvironment: Implications for Antiangiogenic Therapy. Cancer Res. 2010, 70, 5880–5890. [Google Scholar] [CrossRef]
- Wang, F.; Fisher, J.; Fishman, D.A. MMP-1-PAR1 axis mediates LPA-induced epithelial ovarian cancer (EOC) invasion. Gynecol. Oncol. 2011, 120, 247–255. [Google Scholar] [CrossRef]
- Wang, L.; Kong, B. Analysis of the Association of Matrix Metalloproteinase-1 Gene Promoter (rs1799750) Polymorphism and Risk of Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 961–967. [Google Scholar] [CrossRef]
- Hobbs, C.; Huang, Z.; Murphy, S.; Berchuck, A. The function of matrix metalloproteinase 1 in ovarian cancer. Gynecol. Oncol. 2019, 154, 80. [Google Scholar] [CrossRef]
- Mariya, T.; Hirohashi, Y.; Torigoe, T.; Tabuchi, Y.; Asano, T.; Saijo, H.; Kuroda, T.; Yasuda, K.; Mizuuchi, M.; Saito, T.; et al. Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget 2016, 7, 26806–26822. [Google Scholar] [CrossRef]
- Périgny, M.; Bairati, I.; Harvey, I.; Beauchemin, M.; Harel, F.; Plante, M.; Têtu, B. Role of Immunohistochemical Overexpression of Matrix Metalloproteinases MMP-2 and MMP-11 in the Prognosis of Death by Ovarian Cancer. Am. J. Clin. Pathol. 2008, 129, 226–231. [Google Scholar] [CrossRef]
- Hakamy, S.; Assidi, M.; Jafri, M.A.; Nedjadi, T.; Alkhatabi, H.; Al-Qahtani, A.; Al-Maghrabi, J.; Sait, K.; Al-Qahtani, M.; Buhmeida, A.; et al. Assessment of prognostic value of tissue inhibitors of metalloproteinase 3 (TIMP3) protein in ovarian cancer. Libyan J. Med. 2021, 16, 1937866. [Google Scholar] [CrossRef]
- Liu, M.C.P.; Choong, D.Y.H.; Hooi, C.S.F.; Williams, L.H.; Campbell, I.G. Genetic and epigenetic analysis of the TIMP-3 gene in ovarian cancer. Cancer Lett. 2007, 247, 91–97. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Machaliński, B.; Menkiszak, J.; Sompolska-Rzechuła, A. Suitability assessment of baseline concentration of MMP3, TIMP3, HE4 and CA125 in the serum of patients with ovarian cancer. J. Ovarian Res. 2018, 11, 1. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C.; Song, X.; Liu, H.; Li, X.; Zhang, Y. Elevated Cathepsin K potentiates metastasis of epithelial ovarian cancer. Histol. Histopathol. 2018, 33, 673–680. [Google Scholar] [CrossRef]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef]
- Pasquali, R.; Casimirri, F.; Vicennati, V. Weight control and its beneficial effect on fertility in women with obesity and polycystic ovary syndrome. Hum. Reprod. 1997, 12 (Suppl. 1), 82–87. [Google Scholar] [CrossRef]
- Carmassi, F.; De Negri, F.; Fioriti, R.; De Giorgi, A.; Giannarelli, C.; Fruzzetti, F.; Pedrinelli, R.; Dell’Omo, G.; Bersi, C. Insulin resistance causes impaired vasodilation and hypofibrinolysis in young women with polycystic ovary syndrome. Thromb. Res. 2005, 116, 207–214. [Google Scholar] [CrossRef]
- Mannerås-Holm, L.; Baghaei, F.; Holm, G.; Janson, P.O.; Ohlsson, C.; Lönn, M.; Stener-Victorin, E. Coagulation and fibrinolytic disturbances in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 1068–1076. [Google Scholar] [CrossRef]
- Burchall, G.F.; Piva, T.J.; Linden, M.D.; Gibson-Helm, M.E.; Ranasinha, S.; Teede, H.J. Comprehensive Assessment of the Hemostatic System in Polycystic Ovarian Syndrome. Semin. Thromb. Hemost. 2015, 42, 55–62. [Google Scholar] [CrossRef]
- Ranjbaran, J.; Farimani, M.; Tavilani, H.; Ghorbani, M.; Karimi, J.; Poormonsefi, F.; Khodadadi, I. Matrix metalloproteinases 2 and 9 and MMP9/NGAL complex activity in women with PCOS. Reproduction 2016, 151, 305–311. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Komorowski, J.; O’Callaghan, C.J.; Tan, B.K.; Chen, J.; Prelevic, G.M.; Randeva, H.S. Increased Circulating Levels of Matrix Metalloproteinase-2 and -9 in Women with the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Nandakumar, M.; Sathyapalan, T.; Brennan, E.; Atkin, S.L. Matrix Metalloproteinases, Tissue Inhibitors of Metalloproteinases, and Their Ratios in Women with Polycystic Ovary Syndrome and Healthy Controls. Int. J. Mol. Sci. 2025, 26, 321. [Google Scholar] [CrossRef] [PubMed]
- Lahav-Baratz, S.; Kraiem, Z.; Shiloh, H.; Koifman, M.; Ishai, D.; Dirnfeld, M. Decreased expression of tissue inhibitor of matrix metalloproteinases in follicular fluid from women with polycystic ovaries compared with normally ovulating patients undergoing in vitro fertilization. Fertil. Steril. 2003, 79, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Li, F.; Shi, Y.; Wang, Y. Expression of Vascular Endothelial Growth Factor, Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 in Polycystic Ovarian Syndrome Rats and Its Implication. J. Biomater. Tissue Eng. 2021, 11, 841–846. Available online: https://www.ingentaconnect.com/contentone/asp/jbte/2021/00000011/00000005/art00006 (accessed on 22 April 2025). [CrossRef]
- Atiomo, W.U.; Hilton, D.; Fox, R.; Lee, D.; Shaw, S.; Friend, J.; Wilkin, T.J.; Prentice, A.G. Immunohistochemical detection of plasminogen activator inhibitor-1 in polycystic ovaries. Gynecol. Endocrinol. 2000, 14, 162–168. [Google Scholar] [CrossRef]
- Atiomo, W.U.; Bates, S.A.; Condon, J.E.; Shaw, S.; West, J.H.; Prentice, A.G. The plasminogen activator system in women with polycystic ovary syndrome. Fertil. Steril. 1998, 69, 236–241. [Google Scholar] [CrossRef]
- Tarkun, I.; Cantürk, Z.; Arslan, B.C.; Türemen, E.; Tarkun, P. The plasminogen activator system in young and lean women with polycystic ovary syndrome. Endocr. J. 2004, 51, 467–472. [Google Scholar] [CrossRef]
- Sahay, S.; Jain, M.; Dash, D.; Choubey, L.; Jain, S.; Singh, T.B. Role of plasminogen activator inhibitor type 1 (PAI-1) in PCOS patient. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 4052–4058. [Google Scholar] [CrossRef]
- Burchall, G.F.; Pouniotis, D.S.; Teede, H.J.; Ranasinha, S.; Walters, K.A.; Piva, T.J. Expression of the plasminogen system in the physiological mouse ovary and in the pathological polycystic ovary syndrome (PCOS) state. Reprod. Biol. Endocrinol. RBE 2019, 17, 33. [Google Scholar] [CrossRef]
- Ibáñez, L.; Aulesa, C.; Potau, N.; Ong, K.; Dunger, D.B.; De Zegher, F. Plasminogen Activator Inhibitor-1 in Girls with Precocious Pubarche: A Premenarcheal Marker for Polycystic Ovary Syndrome? Pediatr. Res. 2002, 51, 244–248. [Google Scholar] [CrossRef]
- Devin, J.K.; Johnson, J.E.; Eren, M.; Gleaves, L.A.; Bradham, W.S.; Bloodworth, J.R.; Vaughan, D.E. Transgenic overexpression of plasminogen activator inhibitor-1 promotes the development of polycystic ovarian changes in female mice. J. Mol. Endocrinol. 2007, 39, 9–16. [Google Scholar] [CrossRef]
- Shah, A.K.; Yadav, B.K.; Shah, A.K.; Suri, A.; Deo, S.K. Cardiovascular Risk Predictors High Sensitivity C-Reactive Protein and Plasminogen Activator Inhibitor-1 in Women with Lean Phenotype of Polycystic Ovarian Syndrome: A Prospective Case-Control Study. J. Lab. Physicians 2022, 15, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, A.S.; Kelkar, D.S.; Pinto, S.M.; Sharma, R.; Hinduja, I.; Zaveri, K.; Pandey, A.; Prasad, T.S.K.; Gowda, H.; Mukherjee, S. Proteomics of Follicular Fluid From Women With Polycystic Ovary Syndrome Suggests Molecular Defects in Follicular Development. J. Clin. Endocrinol. Metab. 2015, 100, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Palioniko, G.; Alexandraki, K.; Bergiele, A.; Koutsouba, T.; Bartzis, M. The prevalence of 4G5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in polycystic ovarian syndrome and its association with plasma PAI-1 levels. Eur. J. Endocrinol. 2004, 150, 793–798. [Google Scholar] [CrossRef][Green Version]
- Orio, F.; Palomba, S.; Cascella, T.; Tauchmanová, L.; Nardo, L.G.; Di Biase, S.; Labella, D.; Russo, T.; Tolino, A.; Zullo, F.; et al. Is plasminogen activator inhibitor-1 a cardiovascular risk factor in young women with polycystic ovary syndrome? Reprod. Biomed. Online 2004, 9, 505–510. [Google Scholar] [CrossRef]
- Ma, L.-J.; Mao, S.-L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; Wasserman, D.H. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 2004, 53, 336–346. Available online: https://diabetesjournals.org/diabetes/article-abstract/53/2/336/11495 (accessed on 22 April 2025). [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef]
- Obiezu, C.V.; Scorilas, A.; Magklara, A.; Thornton, M.H.; Wang, C.Y.; Stanczyk, F.Z.; Diamandis, E.P. Prostate-Specific Antigen and Human Glandular Kallikrein 2 Are Markedly Elevated in Urine of Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 1558–1561. [Google Scholar] [CrossRef]
- Jin, M.; Cai, J.; Hu, Y.; Lu, X.; Huang, H. Cathepsin D expression in ovaries from polycystic ovarian syndrome patients. Zhejiang Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2007, 36, 429–432. [Google Scholar] [CrossRef]
- Dawood Salman, Z.; Ismaeel Jassim, W. Role of Cathepsin S and Insulin Resistance in Iraqi Women with Polycystic Ovary Syndrome. J. Obstet. Gynecol. Cancer Res. 2024, 9, 379–384. [Google Scholar] [CrossRef]
- Sabeti Akbar-Abad, M.; Majidpour, M.; Sargazi, S.; Ghasemi, M.; Saravani, R. Unraveling the Role of Cathepsin B Variants in Polycystic Ovary Syndrome: Insights from a Case-Control Study and Computational Analyses. Reprod. Sci. Thousand Oaks Calif 2025, 32, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Shalev, E.; Goldman, S.; Ben-Shlomo, I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: Comparison between women with PCOS and normal ovulatory women. Mol. Hum. Reprod. 2001, 7, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.G.; Lyall, H.; Petrie, J.R.; Gould, G.W.; Connell, J.M.C.; Rumley, A.; Lowe, G.D.O.; Sattar, N. A Specific Elevation in Tissue Plasminogen Activator Antigen in Women with Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 3287–3290. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Simonsick, E.; Forabosco, A.; Garcia-Ortiz, J.E.; Schlessinger, D. Dynamics of the Ovarian Reserve and Impact of Genetic and Epidemiological Factors on Age of Menopause. Biol. Reprod. 2015, 92, 130. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L. Premature ovarian failure. Orphanet J. Rare Dis. 2006, 1, 9. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, C.; Gen, A.; Li, Y.; Yang, H.; Tian, X.; Chi, Y. Propofol affects the biological behavior of ovarian cancer SKOV3 cells via ERK1/2-MMP-2/9 signaling pathway. Trop. J. Pharm. Res. 2020, 19, 233–238. [Google Scholar] [CrossRef]
- An, H.J.; Ahn, E.H.; Kim, J.O.; Park, H.S.; Ryu, C.S.; Cho, S.H.; Kim, J.H.; Lee, W.S.; Kim, N.K. Association between tissue inhibitor of metalloproteinase (TIMP) genetic polymorphisms and primary ovarian insufficiency (POI). Maturitas 2019, 120, 77–82. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Billig, H.; Tsafriri, A. Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocr. Rev. 1994, 15, 707–724. [Google Scholar] [CrossRef]
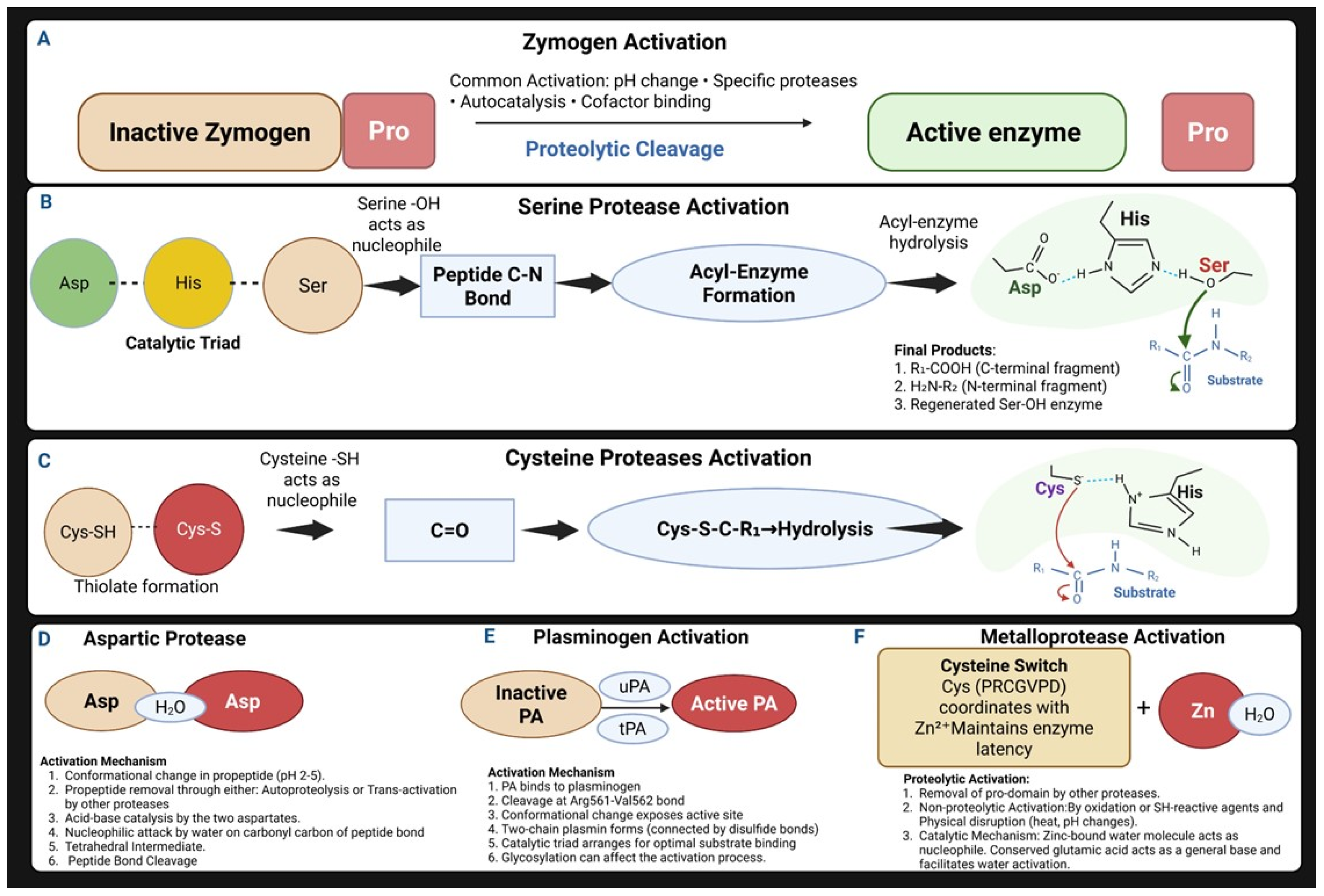
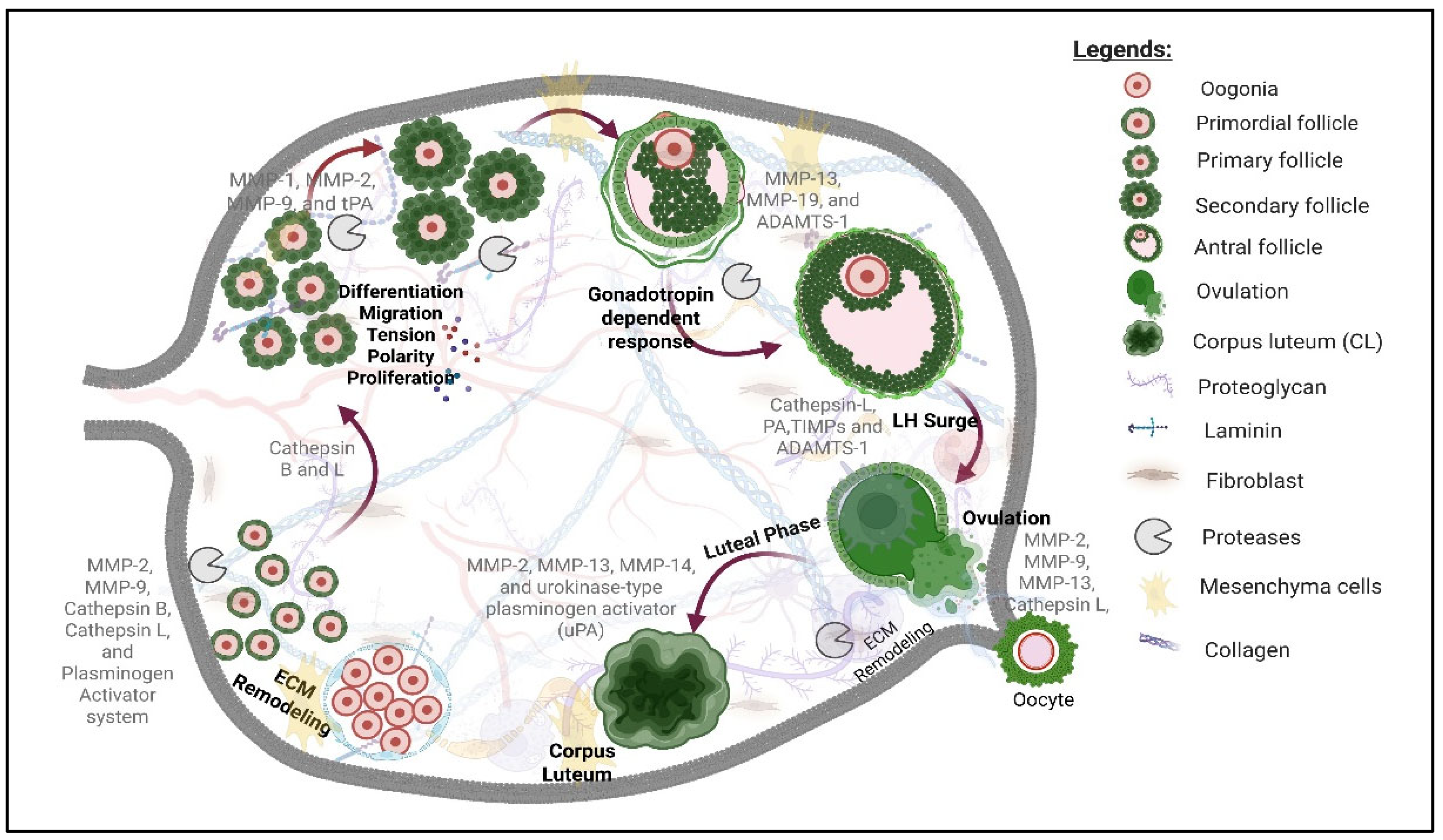
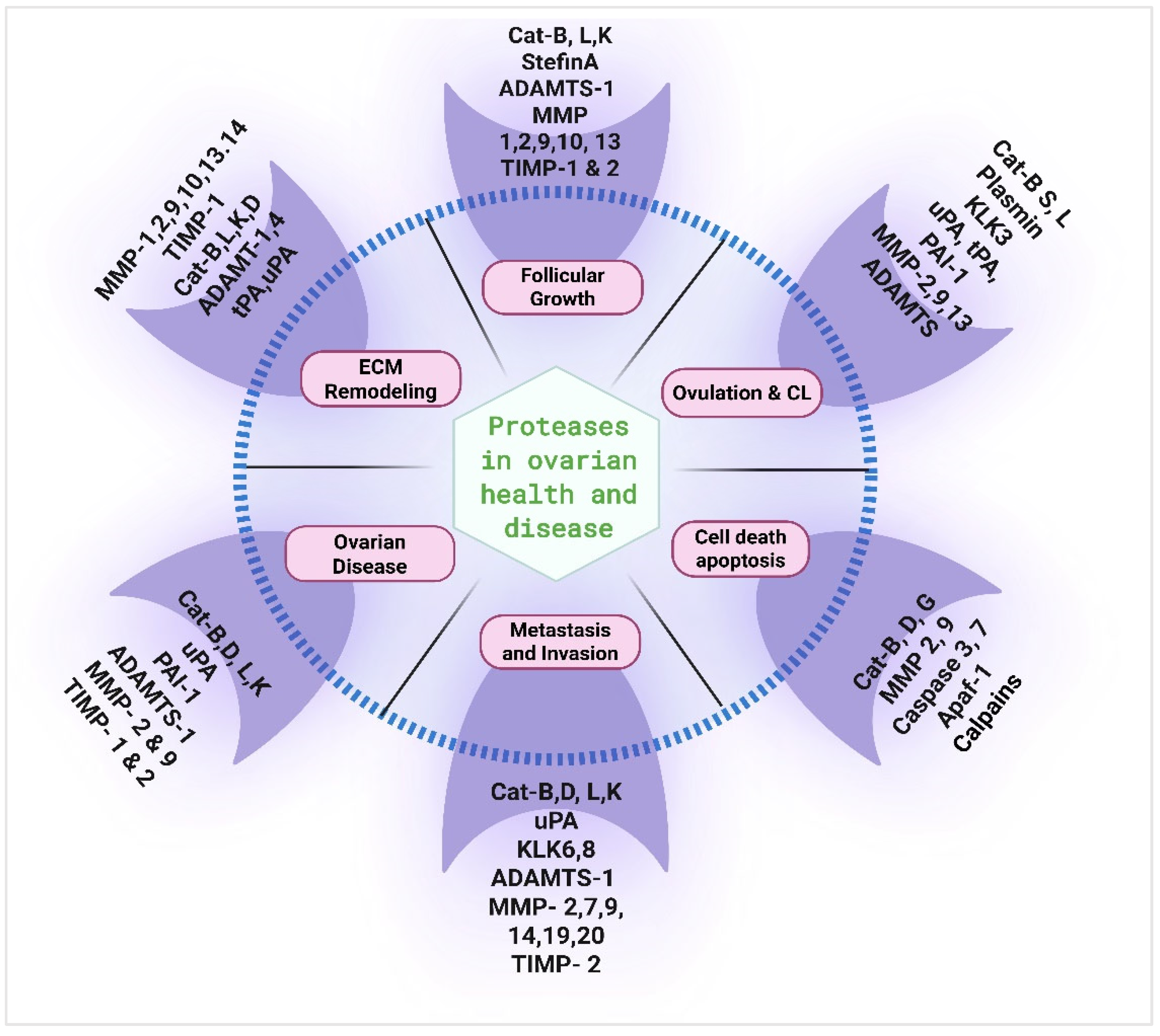
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushawaha, B.; Pelosi, E. Spotlight on Proteases: Roles in Ovarian Health and Disease. Cells 2025, 14, 921. https://doi.org/10.3390/cells14120921
Kushawaha B, Pelosi E. Spotlight on Proteases: Roles in Ovarian Health and Disease. Cells. 2025; 14(12):921. https://doi.org/10.3390/cells14120921
Chicago/Turabian StyleKushawaha, Bhawna, and Emanuele Pelosi. 2025. "Spotlight on Proteases: Roles in Ovarian Health and Disease" Cells 14, no. 12: 921. https://doi.org/10.3390/cells14120921
APA StyleKushawaha, B., & Pelosi, E. (2025). Spotlight on Proteases: Roles in Ovarian Health and Disease. Cells, 14(12), 921. https://doi.org/10.3390/cells14120921






