Biological Aging and Uterine Fibrosis in Cattle: Reproductive Trade-Offs from Enhanced Productivity
Abstract
1. Introduction
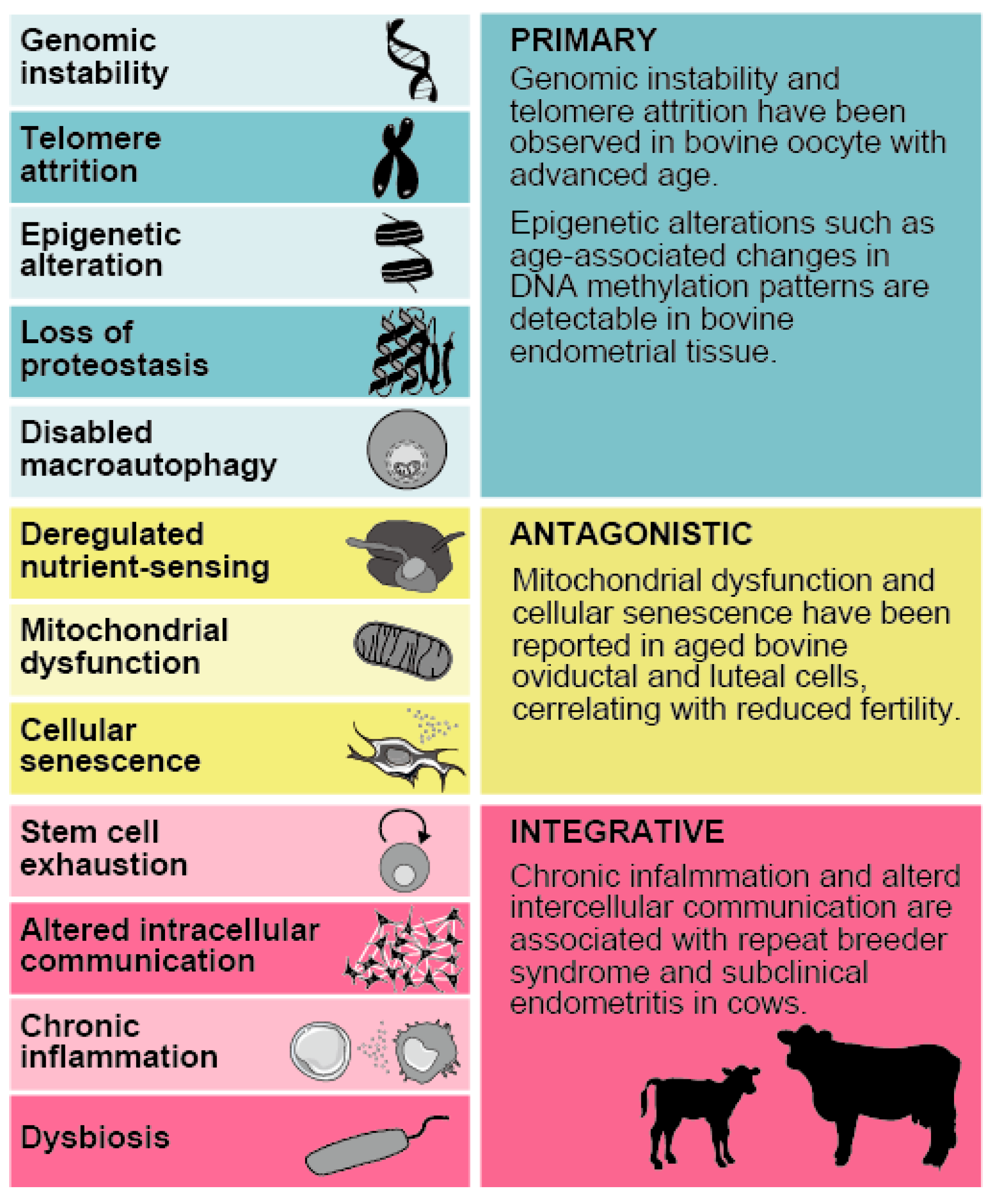
2. Biological Aging
2.1. Biological Aging and Uterine Function and Infertility
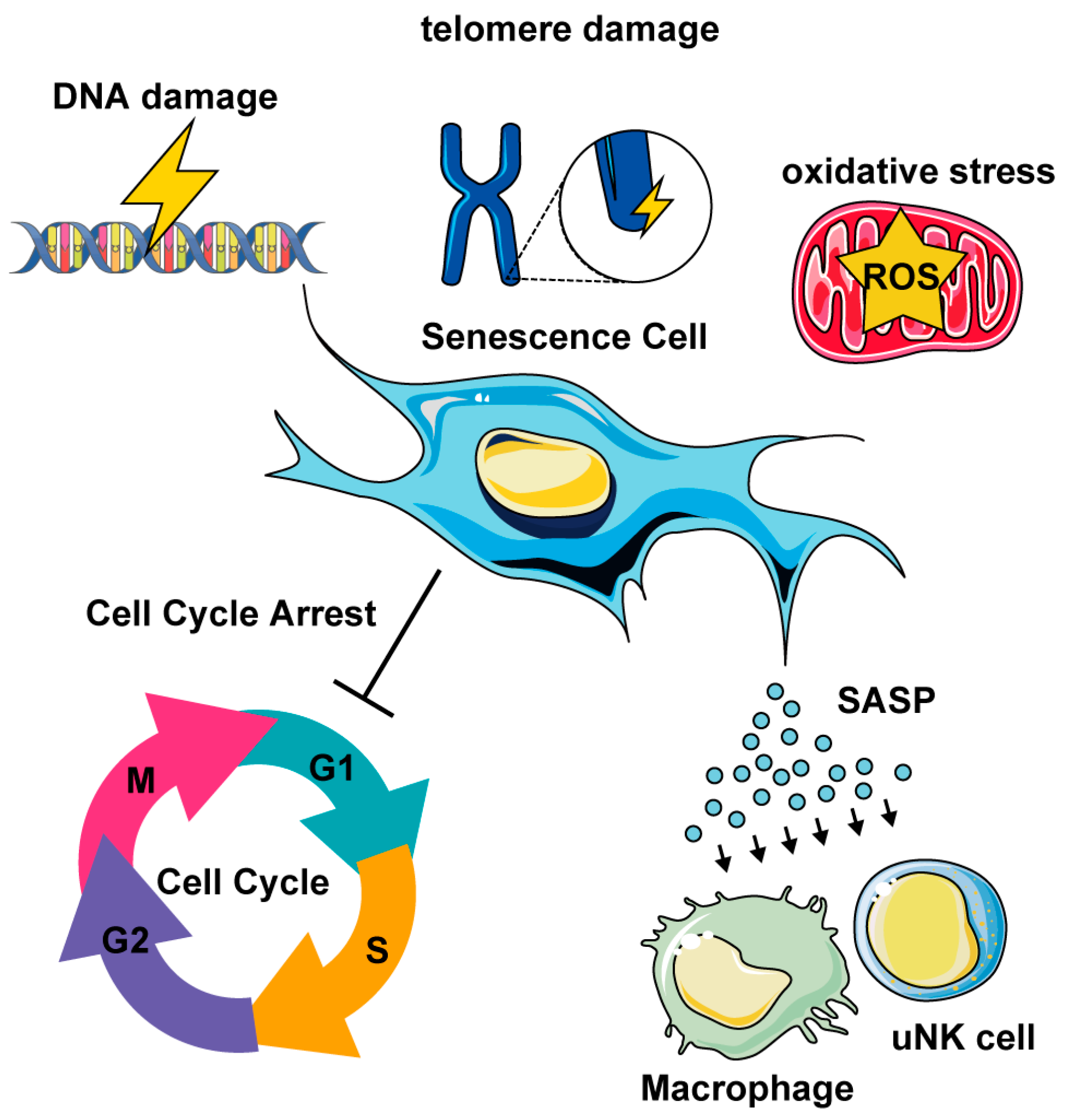
2.2. Cellular Senescence in Uterine Biological Aging
2.3. Molecular Drivers of Biological Aging in the Uterus
2.4. Epigenetic Aging
2.5. Epigenetic Aging in Pregnancy
3. Fibrosis
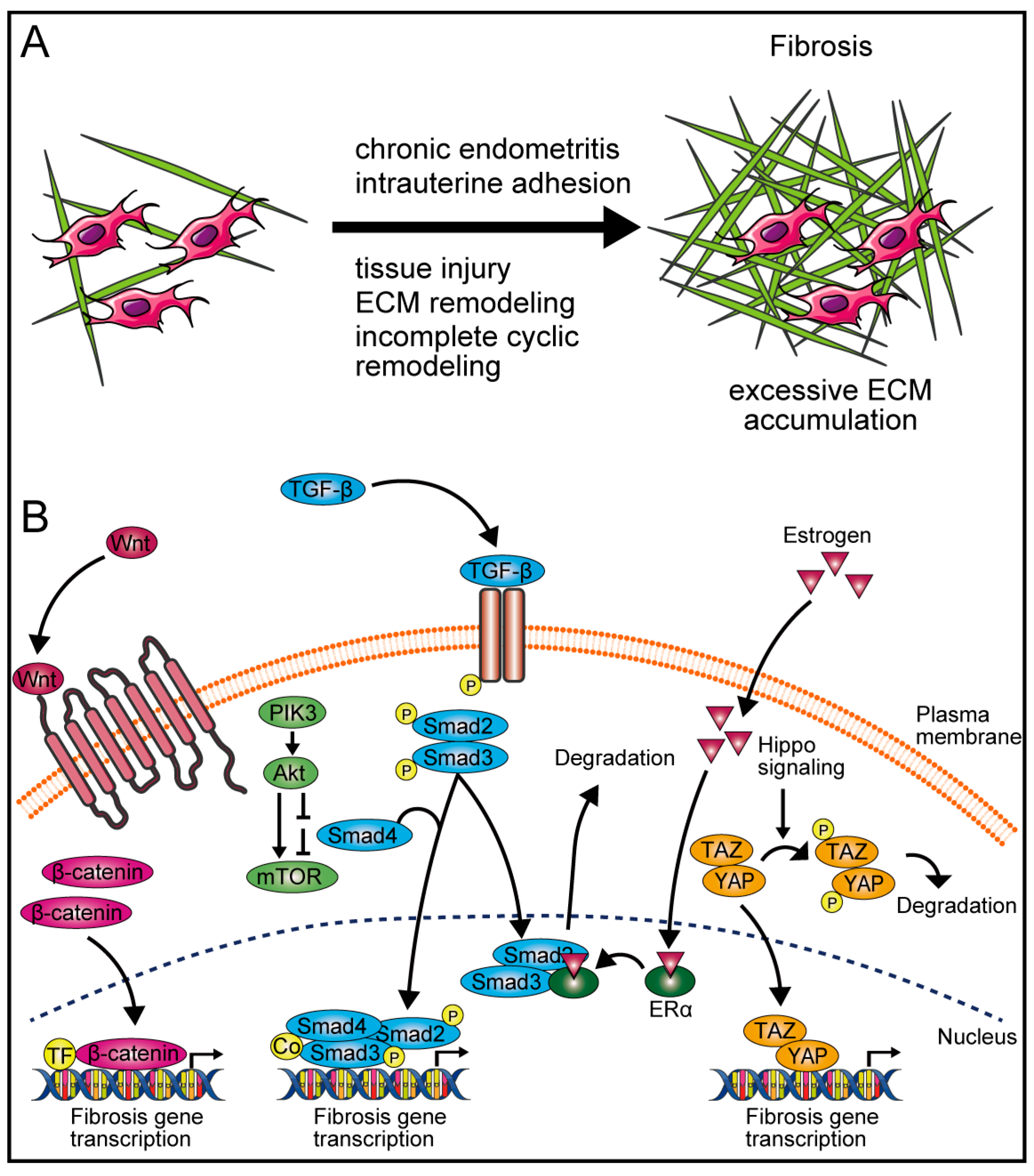
3.1. Fibrosis in Female Reproductive Organs
3.2. Mechanisms of Fibrosis
4. Future Direction
4.1. Targeting Senescent Cells in the Bovine Uterus: Promise and Limitations
4.2. Preventing Fibrosis Development in Female Reproductive Tissues: From Rodents to Ruminants
4.3. Microbiome-Based Strategies to Modulate Bovine Uterine Immunity
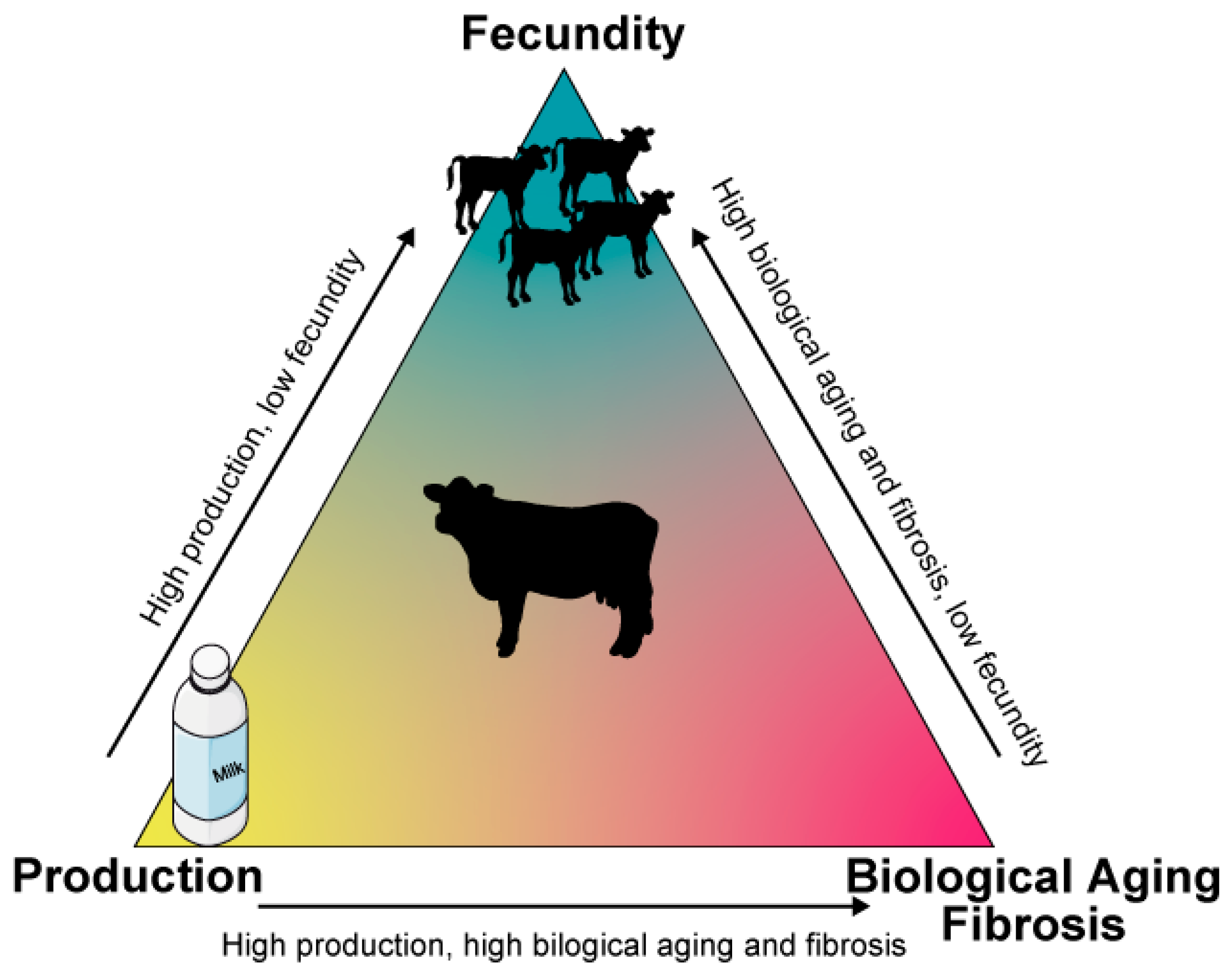
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extra-cellular matrix |
| FSH | Follicle-stimulating hormone |
| SASP | Senescence-associated secretory phenotype |
| LMNB1 | Lamin B1 |
| SIRT1 | Sirtuin 1 |
| NAD | Nicotinamide adenine dinucleotide |
| ROS | Reactive oxygen species |
| mTOR | Mammalian target of rapamycin |
| AGEs | Advanced glycation end products |
| ncRNA | Non-coding RNA |
| cfDNA | Cell-free DNA |
| AMH | Anti-mullerian hormone |
| TGF-β | Transforming growth factor beta |
| TGFBR3 | Transforming growth factor beta receptor 3 |
| Smad | Suppressor of mother against decapentaplegic |
| GPER1 | G protein-coupled estrogen receptor 1 |
| HSP47 | Heat shock protein 47 |
| WNT | Wingless-type MMTV integration site family |
| MST1/2 | Mammalian Ste20-like kinase 1 and 2 |
| LATS1/2 | Large tumor suppressor 1 and 2 |
| YAP | Yes-associated protein |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TEAD | TEA domain family member |
| DUSP4 | Dual specificity protein phosphatase 4 |
| GSK3β | Glycogen synthase kinase-3 beta |
| SNAI1 | Snail family transcriptional repressor 1 |
| MAPK | Mitogen-activated protein kinase |
| PIK3 | Phosphatidylinositol-3 kinase |
| ERα | Estrogen receptor alpha |
| Notch1 | Neurogenic locus notch homolog protein 1 |
| IL6 | Interleukin 6 |
| VEGF | Vascular endothelial growth factor |
| IgG | Immunoglobulin G |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAR | Protease-activated receptor |
| DHEA | Dehydroepiandrosterone |
| RB1 | RB transcriptional corepressor 1 |
| D+Q | Dasatinib and Quercetin combination |
| NKG2-CAR | Natural killer group 2 member D chimeric antigen receptor |
| MitoTam | Mitochondria-targeted tamoxifen |
| BCL-W | B-cell lymphoma-w |
| BCL-XL | B-cell lymphoma-extra-large |
| SGLT2 | Sodium–glucose transport protein 2 |
| PD-L1 | Programmed cell death ligand 1 |
| NFE2L2 | Nuclear factor, erythroid derived 2, like 2 |
| HSC | Hepatic stellate cell |
| NLRP3 | NLR family pyrin domain containing 3 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| LAB | Lactic acid bacteria |
| uNK | uterine natural killer |
References
- Diskin, M.G.; Waters, S.M.; Parr, M.H.; Kenny, D.A. Pregnancy Losses in Cattle: Potential for Improvement. Reprod. Fertil. Dev. 2016, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Kidder, H.E.; Black, W.G.; Wiltbank, J.N.; Ulberg, L.C.; Casida, L.E. Fertilization Rates and Embryonic Death Rates in Cows Bred to Bulls of Different Levels of Fertility. J. Dairy Sci. 1954, 37, 691–697. [Google Scholar] [CrossRef]
- Artificial Insemination Association of Japan. Survey of Bovine Conception Rates in Japan (2023–2024). Available online: https://aiaj.lin.gr.jp/3/tyosa.html (accessed on 31 March 2025). (In Japanese).
- Mann, G.; Lamming, G. The Influence of Progesterone During Early Pregnancy in Cattle. Reprod. Domest. Anim. 1999, 34, 269–274. [Google Scholar] [CrossRef]
- Reese, S.T.; Franco, G.A.; Poole, R.K.; Hood, R.; Fernadez Montero, L.; Oliveira Filho, R.V.; Cooke, R.F.; Pohler, K.G. Pregnancy Loss in Beef Cattle: A Meta-Analysis. Anim. Reprod. Sci. 2020, 212, 106251. [Google Scholar] [CrossRef]
- Velarde, M.C.; Menon, R. Positive and Negative Effects of Cellular Senescence during Female Reproductive Aging and Pregnancy. J. Endocrinol. 2016, 230, R59–R76. [Google Scholar] [CrossRef]
- Zavatta, A.; Parisi, F.; Mandò, C.; Scaccabarozzi, C.; Savasi, V.M.; Cetin, I. Role of Inflammaging on the Reproductive Function and Pregnancy. Clin. Rev. Allergy Immunol. 2022, 64, 145–160. [Google Scholar] [CrossRef]
- Hurwitz, J.M.; Santoro, N. Inhibins, Activins, and Follistatin in the Aging Female and Male. Semin. Reprod. Med. 2004, 22, 209–217. [Google Scholar] [CrossRef]
- Yaffe, H.; Ron, M.; Polishuk, W.Z. Amenorrhea, Hypomenorrhea, and Uterine Fibrosis. Am. J. Obstet. Gynecol. 1978, 130, 599–601. [Google Scholar] [CrossRef] [PubMed]
- de Ziegler, D.; Pirtea, P.; Ayoubi, J.M. Inflammation and Uterine Fibrosis: The Possible Role of Chronic Endometritis. Fertil. Steril. 2019, 111, 890–891. [Google Scholar] [CrossRef]
- Hu, J.; Zeng, B.; Jiang, X.; Hu, L.; Meng, Y.; Zhu, Y.; Mao, M. The Expression of Marker for Endometrial Stem Cell and Fibrosis Was Increased in Intrauterine Adhesious. Int. J. Clin. Exp. Pathol. 2015, 8, 1525. [Google Scholar]
- Winkler, I.; Tolkachov, A.; Lammers, F.; Lacour, P.; Daugelaite, K.; Schneider, N.; Koch, M.-L.; Panten, J.; Grünschläger, F.; Poth, T.; et al. The Cycling and Aging Mouse Female Reproductive Tract at Single-Cell Resolution. Cell 2024, 187, 981–998.e25. [Google Scholar] [CrossRef]
- Epel, E.S. Psychological and Metabolic Stress: A Recipe for Accelerated Cellular Aging? Hormones 2009, 8, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakou, I.P.; Katsilambros, N.L.; Benetos, A.; Mikhailidis, D.P.; Perrea, D.N. Is Obesity Linked to Aging? Ageing Res. Rev. 2012, 11, 220–229. [Google Scholar] [CrossRef]
- Ratan, P.; Rubbi, L.; Thompson, M.; Naresh, K.; Waddell, J.; Jones, B.; Pellegrini, M. Epigenetic Aging in Cows Is Accelerated by Milk Production. Epigenetics 2023, 18, 2240188. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.D.; Santos, V.G.; Giordano, J.O.; Wiltbank, M.C.; Fricke, P.M. Development of Fertility Programs to Achieve High 21-Day Pregnancy Rates in High-Producing Dairy Cows. Theriogenology 2018, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; VanRaden, P.M. Symposium Review: Possibilities in an Age of Genomics: The Future of Selection Indices. J. Dairy Sci. 2018, 101, 3686–3701. [Google Scholar] [CrossRef]
- Lucy, M.C. Symposium Review: Selection for Fertility in the Modern Dairy Cow—Current Status and Future Direction for Genetic Selection. J. Dairy Sci. 2019, 102, 3706–3721. [Google Scholar] [CrossRef]
- Guinan, F.L.; Wiggans, G.R.; Norman, H.D.; Dürr, J.W.; Cole, J.B.; Van Tassell, C.P.; Misztal, I.; Lourenco, D. Changes in Genetic Trends in US Dairy Cattle since the Implementation of Genomic Selection. J. Dairy Sci. 2023, 106, 1110–1129. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.J.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.P.; Sartori, R. Pivotal Periods for Pregnancy Loss during the First Trimester of Gestation in Lactating Dairy Cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and Physiological Manifestations and Measurement of Aging in Humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Zhang, J.; Wang, S. Unveiling Uterine Aging: Much More to Learn. Ageing Res. Rev. 2023, 86, 101879. [Google Scholar] [CrossRef]
- Tinelli, A.; Andjić, M.; Morciano, A.; Pecorella, G.; Malvasi, A.; D’Amato, A.; Sparić, R. Uterine Aging and Reproduction: Dealing with a Puzzle Biologic Topic. Int. J. Mol. Sci. 2023, 25, 322. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Balough, J.L.; Dipali, S.S.; Velez, K.; Kumar, T.R.; Duncan, F.E. Hallmarks of Female Reproductive Aging in Physiologic Aging Mice. Nat. Aging 2024, 4, 1711–1730. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, S.; Chen, Y.; Wang, W.; Wang, B.; Chen, Q.; Duan, E.; Wang, H. Determinants of Uterine Aging: Lessons from Rodent Models. Sci. China Life Sci. 2012, 55, 687–693. [Google Scholar] [CrossRef]
- Ananda, H.M.; Wurlina, W.; Hidajati, N.; Hariadi, M.; Samik, A.; Restiadi, T.I. Hubungan antara umur dengan calving interval, days open, dan service per conseption sapifriesian holstein (FH). Ovozoa J. Anim. Reprod. 2020, 8, 94. [Google Scholar] [CrossRef]
- Osoro, K.; Wright, I.A. The Effect of Body Condition, Live Weight, Breed, Age, Calf Performance, and Calving Date on Reproductive Performance of Spring-Calving Beef Cows. J. Anim. Sci. 1992, 70, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.R. Nutritional Interactions with Reproductive Performance in Dairy Cattle. Anim. Reprod. Sci. 2000, 60–61, 449–457. [Google Scholar] [CrossRef]
- Malhi, P.S.; Adams, G.P.; Mapletoft, R.J.; Singh, J. Oocyte Developmental Competence in a Bovine Model of Reproductive Aging. Reproduction 2007, 134, 233–239. [Google Scholar] [CrossRef]
- Malhi, P.S.; Adams, G.P.; Singh, J. Bovine Model for the Study of Reproductive Aging in Women: Follicular, Luteal, and Endocrine Characteristics1. Biol. Reprod. 2005, 73, 45–53. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ji, S. Cellular Senescence: Molecular Mechanisms and Pathogenicity. J. Cell Physiol. 2018, 233, 9121–9135. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.M.D.; Ibana, J.A.; Velarde, M.C. The Female Reproduction and Senescence Nexus. Am. J. Reprod. Immunol. 2017, 77, e12646. [Google Scholar] [CrossRef]
- Gong, G.-S.; Muyayalo, K.P.; Zhang, Y.-J.; Lin, X.-X.; Liao, A.-H. Flip a Coin: Cell Senescence at the Maternal–Fetal Interface. Biol. Reprod. 2023, 109, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Perdiguero, E.; Muñoz-Cánoves, P.; de Keizer, P.L. Musculoskeletal Senescence: A Moving Target Ready to Be Eliminated. Curr. Opin. Pharmacol. 2018, 40, 147–155. [Google Scholar] [CrossRef]
- Tomari, H.; Kawamura, T.; Asanoma, K.; Egashira, K.; Kawamura, K.; Honjo, K.; Nagata, Y.; Kato, K. Contribution of Senescence in Human Endometrial Stromal Cells during Proliferative Phase to Embryo Receptivity. Biol. Reprod. 2020, 103, 104–113. [Google Scholar] [CrossRef]
- Garcia, D.N.; Hense, J.D.; Zanini, B.M.; Isola, J.V.; Prosczek, J.B.; Ashiqueali, S.; Oliveira, T.L.; Mason, J.B.; Schadock, I.C.; Barros, C.C.; et al. Senolytic Treatment Fails to Improve Ovarian Reserve or Fertility in Female Mice. Geroscience 2024, 46, 3445–3455. [Google Scholar] [CrossRef]
- Malvezzi, H.; Dobo, C.; Filippi, R.Z.; Mendes do Nascimento, H.; Palmieri da Silva e Sousa, L.; Meola, J.; Piccinato, C.A.; Podgaec, S. Altered P16Ink4a, IL-1β, and Lamin B1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. Int. J. Mol. Sci. 2022, 23, 2476. [Google Scholar] [CrossRef]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated P16ink4a and β-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive Oxidative Stress in Cumulus Granulosa Cells Induced Cell Senescence Contributes to Endometriosis-Associated Infertility. Redox Biol. 2020, 30, 101431. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Hernandes, C.; de Azevedo, B.C.; Piccinato, C.A.; Meola, J.; Podgaec, S. Increased cellular senescence in deep infiltrating endometriosis. Fertil. Steril. 2020, 114, e79. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; He, Y.; Liu, J.; Chen, Y.; Huang, J.; Qi, G.; Li, P. SIRT1 Upregulation Promotes Epithelial-Mesenchymal Transition by Inducing Senescence Escape in Endometriosis. Sci. Rep. 2022, 12, 12302. [Google Scholar] [CrossRef]
- Vassiliadis, S. Premature Immunosenescence Impairs Immune Surveillance Allowing the Endometriotic Stem Cell to Migrate: The Cytokine Profile as a Common Denominator. J. Endometr. 2010, 2, 7–18. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.-S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent Pregnancy Loss Is Associated with a Pro-Senescent Decidual Response during the Peri-Implantation Window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Borodkina, A. V Stromal Cell Senescence Contributes to Impaired Endometrial Decidualization and Defective Interaction with Trophoblast Cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iwata, H.; Kuwayama, T.; Shirasuna, K. S100A8, Which Increases with Age, Induces Cellular Senescence-like Changes in Bovine Oviduct Epithelial Cells. Am. J. Reprod. Immunol. 2019, 82, e13163. [Google Scholar] [CrossRef]
- Nakamura, Y.; Aihara, R.; Iwata, H.; Kuwayama, T.; Shirasuna, K. IL1B Triggers Inflammatory Cytokine Production in Bovine Oviduct Epithelial Cells and Induces Neutrophil Accumulation via CCL2. Am. J. Reprod. Immunol. 2021, 85, e13365. [Google Scholar] [CrossRef]
- Tanaka, H.; Ohtsu, A.; Shiratsuki, S.; Kawahara-Miki, R.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Age-Dependent Changes in Inflammation and Extracellular Matrix in Bovine Oviduct Epithelial Cells during the Post-Ovulatory Phase. Mol. Reprod. Dev. 2016, 83, 815–826. [Google Scholar] [CrossRef]
- Tanikawa, N.; Ohtsu, A.; Kawahara-Miki, R.; Kimura, K.; Matsuyama, S.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Age-Associated MRNA Expression Changes in Bovine Endometrial Cells in Vitro. Reprod. Biol. Endocrinol. 2017, 15, 63. [Google Scholar] [CrossRef]
- Hori, K.; Matsuyama, S.; Nakamura, S.; Iwata, H.; Kuwayama, T.; Miyamoto, A.; Shirasuna, K. Age-related Changes in the Bovine Corpus Luteum Function and Progesterone Secretion. Reprod. Domest. Anim. 2019, 54, 23–30. [Google Scholar] [CrossRef]
- Jin, W.; Zheng, J.; Xiao, Y.; Ju, L.; Chen, F.; Fu, J.; Jiang, H.; Zhang, Y. A Universal Molecular Mechanism Driving Aging. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lenart, P.; Krejci, L. DNA, the Central Molecule of Aging. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2016, 786, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M. V Aging: ROS or TOR. Cell Cycle 2008, 7, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V.; Hall, M.N. Growth and Aging: A Common Molecular Mechanism. Aging 2009, 1, 357–362. [Google Scholar] [CrossRef]
- Golubev, A.; Hanson, A.D.; Gladyshev, V.N. Non-Enzymatic Molecular Damage as a Prototypic Driver of Aging. J. Biol. Chem. 2017, 292, 6029–6038. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Bürkle, A. Mechanisms of Ageing. Eye 2001, 15, 371–375. [Google Scholar] [CrossRef]
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef]
- Cao, X.; Yang, G.; Li, X.; Fu, J.; Mohedaner, M.; Danzengzhuoga; Høj Jørgensen, T.S.; Agogo, G.O.; Wang, L.; Zhang, X.; et al. Weight Change across Adulthood and Accelerated Biological Aging in Middle-Aged and Older Adults. Am. J. Clin. Nutr. 2023, 117, 1–11. [Google Scholar] [CrossRef]
- Zheng, Y.; Manson, J.E.; Yuan, C.; Liang, M.H.; Grodstein, F.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA 2017, 318, 255. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Parks, C.G.; DeRoo, L.A.; Chen, H.; Taylor, J.A.; Cawthon, R.M.; Sandler, D.P. Obesity and Weight Gain in Adulthood and Telomere Length. Cancer Epidemiol. Biomark. Prev. 2009, 18, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef]
- Shirasuna, K.; Iwata, H. Effect of Aging on the Female Reproductive Function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P.; Wall, E.; Pryce, J.E. Genetics and Genomics of Reproductive Performance in Dairy and Beef Cattle. Animal 2014, 8, 105–121. [Google Scholar] [CrossRef]
- Oltenacu, P.A.; Algers, B. Selection for Increased Production and the Welfare of Dairy Cows: Are New Breeding Goals Needed? AMBIO A J. Hum. Environ. 2005, 34, 311–315. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The Nexus between Nutrient Metabolism, Oxidative Stress and Inflammation in Transition Cows. Anim. Prod. Sci. 2014, 54, 1204. [Google Scholar] [CrossRef]
- Ward, W.R.; Parker, C.S. Field Evidence of Metabolic Stress in Dairy Cows? BSAP Occas. Publ. 1999, 24, 21–26. [Google Scholar] [CrossRef]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nürnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.H.D.; Wiedemann, S.; Gronwald, W. Nuclear Magnetic Resonance and Mass Spectrometry-Based Milk Metabolomics in Dairy Cows during Early and Late Lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Pospiech, M.; Turner, M.L. Symposium Review: Mechanisms Linking Metabolic Stress with Innate Immunity in the Endometrium. J. Dairy Sci. 2018, 101, 3655–3664. [Google Scholar] [CrossRef]
- Yonekura, S. The Role of Endoplasmic Reticulum Stress in Metabolic Diseases and Mammary Epithelial Cell Homeostasis in Dairy Cows. Anim. Sci. J. 2024, 95, e13935. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.P. Genetics—A Tool to Improve Productivity and Profitability. Int. J. Dairy. Technol. 2008, 61, 30–35. [Google Scholar] [CrossRef]
- Gonzalo, S. Epigenetic Alterations in Aging. J. Appl. Physiol. 2010, 109, 586–597. [Google Scholar] [CrossRef]
- Brunet, A.; Berger, S.L. Epigenetics of Aging and Aging-Related Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S17–S20. [Google Scholar] [CrossRef]
- Yu, M.; Hazelton, W.D.; Luebeck, G.E.; Grady, W.M. Epigenetic Aging: More Than Just a Clock When It Comes to Cancer. Cancer Res. 2020, 80, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef]
- Liang, X.; Ma, J.; Schatten, H.; Sun, Q. Epigenetic Changes Associated with Oocyte Aging. Sci. China Life Sci. 2012, 55, 670–676. [Google Scholar] [CrossRef]
- Klutstein, M.; Gonen, N. Epigenetic Aging of Mammalian Gametes. Mol. Reprod. Dev. 2023, 90, 785–803. [Google Scholar] [CrossRef]
- Kordowitzki, P.; Haghani, A.; Zoller, J.A.; Li, C.Z.; Raj, K.; Spangler, M.L.; Horvath, S. Epigenetic Clock and Methylation Study of Oocytes from a Bovine Model of Reproductive Aging. Aging Cell 2021, 20, e13349. [Google Scholar] [CrossRef]
- Knight, A.K.; Spencer, J.B.; Smith, A.K. DNA Methylation as a Window into Female Reproductive Aging. Epigenomics 2024, 16, 175–188. [Google Scholar] [CrossRef]
- Ryan, C.P. “Epigenetic Clocks”: Theory and Applications in Human Biology. Am. J. Hum. Biol. 2021, 33, e23488. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.G.; Lowe, R.; Adams, P.D.; Baccarelli, A.A.; Beck, S.; Bell, J.T.; Christensen, B.C.; Gladyshev, V.N.; Heijmans, B.T.; Horvath, S.; et al. DNA Methylation Aging Clocks: Challenges and Recommendations. Genome Biol. 2019, 20, 249. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic Clock: A Promising Biomarker and Practical Tool in Aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- Caulton, A.; Dodds, K.G.; McRae, K.M.; Couldrey, C.; Horvath, S.; Clarke, S.M. Development of Epigenetic Clocks for Key Ruminant Species. Genes 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Higgins-Chen, A.T.; Thrush, K.L.; Wang, Y.; Minteer, C.J.; Kuo, P.-L.; Wang, M.; Niimi, P.; Sturm, G.; Lin, J.; Moore, A.Z.; et al. A Computational Solution for Bolstering Reliability of Epigenetic Clocks: Implications for Clinical Trials and Longitudinal Tracking. Nat. Aging 2022, 2, 644–661. [Google Scholar] [CrossRef]
- Kabacik, S.; Lowe, D.; Fransen, L.; Leonard, M.; Ang, S.-L.; Whiteman, C.; Corsi, S.; Cohen, H.; Felton, S.; Bali, R.; et al. The Relationship between Epigenetic Age and the Hallmarks of Aging in Human Cells. Nat. Aging 2022, 2, 484–493. [Google Scholar] [CrossRef]
- Liu, Z.; Leung, D.; Thrush, K.; Zhao, W.; Ratliff, S.; Tanaka, T.; Schmitz, L.L.; Smith, J.A.; Ferrucci, L.; Levine, M.E. Underlying Features of Epigenetic Aging Clocks in Vivo and in Vitro. Aging Cell 2020, 19, e13229. [Google Scholar] [CrossRef]
- Wagner, W. The Link Between Epigenetic Clocks for Aging and Senescence. Front. Genet. 2019, 10, 303. [Google Scholar] [CrossRef]
- Teo, Y.V.; Capri, M.; Morsiani, C.; Pizza, G.; Faria, A.M.C.; Franceschi, C.; Neretti, N. Cell-free DNA as a Biomarker of Aging. Aging Cell 2019, 18, e12890. [Google Scholar] [CrossRef]
- Ryan, C.P.; Hayes, M.G.; Lee, N.R.; McDade, T.W.; Jones, M.J.; Kobor, M.S.; Kuzawa, C.W.; Eisenberg, D.T.A. Reproduction Predicts Shorter Telomeres and Epigenetic Age Acceleration among Young Adult Women. Sci. Rep. 2018, 8, 11100. [Google Scholar] [CrossRef]
- Ryan, C.P.; Lee, N.R.; Carba, D.B.; MacIsaac, J.L.; Lin, D.T.S.; Atashzay, P.; Belsky, D.W.; Kobor, M.S.; Kuzawa, C.W. Pregnancy Is Linked to Faster Epigenetic Aging in Young Women. Proc. Natl. Acad. Sci. USA 2024, 121, e2317290121. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Harmon, Q.E.; Xu, Z.; Nichols, H.B.; Sandler, D.P.; Taylor, J.A. Reproduction, DNA Methylation, and Biological Age. Obstet. Gynecol. Surv. 2020, 75, 106–107. [Google Scholar] [CrossRef]
- Suvakov, S.; Ghamrawi, R.; Cubro, H.; Tu, H.; White, W.M.; Tobah, Y.S.B.; Milic, N.M.; Grande, J.P.; Cunningham, J.M.; Chebib, F.T.; et al. Epigenetic and Senescence Markers Indicate an Accelerated Ageing-like State in Women with Preeclamptic Pregnancies. EBioMedicine 2021, 70, 103536. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.M.; Carroll, J.E.; Horvath, S.; Hobel, C.J.; Coussons-Read, M.E.; Dunkel Schetter, C. Epigenetic Age and Pregnancy Outcomes: GrimAge Acceleration Is Associated with Shorter Gestational Length and Lower Birthweight. Clin. Epigenet. 2020, 12, 120. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Tholen, E.; Held-Hoelker, E.; Shellander, K.; Blaschka, C.; Drillich, M.; Iwersen, M.; Suess, D.; Gebremedhn, S.; Tesfaye, D.; et al. Endometrial DNA Methylation Signatures during the Time of Breeding in Relation to the Pregnancy Outcome in Postpartum Dairy Cows Fed a Control Diet or Supplemented with Rumen-Protected Methionine. Front. Genet. 2024, 14, 1267053. [Google Scholar] [CrossRef]
- Walker, C.G.; Littlejohn, M.D.; Meier, S.; Roche, J.R.; Mitchell, M.D. DNA Methylation Is Correlated with Gene Expression during Early Pregnancy in Bos Taurus. Physiol. Genom. 2013, 45, 276–286. [Google Scholar] [CrossRef]
- Landry, D.A.; Vaishnav, H.T.; Vanderhyden, B.C. The Significance of Ovarian Fibrosis. Oncotarget 2020, 11, 4366–4370. [Google Scholar] [CrossRef] [PubMed]
- Umehara, T.; Winstanley, Y.E.; Andreas, E.; Morimoto, A.; Williams, E.J.; Smith, K.M.; Carroll, J.; Febbraio, M.A.; Shimada, M.; Russell, D.L.; et al. Female Reproductive Life Span Is Extended by Targeted Removal of Fibrotic Collagen from the Mouse Ovary. Sci. Adv. 2022, 8, eabn4564. [Google Scholar] [CrossRef]
- Briley, S.M.; Jasti, S.; McCracken, J.M.; Hornick, J.E.; Fegley, B.; Pritchard, M.T.; Duncan, F.E. Reproductive Age-Associated Fibrosis in the Stroma of the Mammalian Ovary. Reproduction 2016, 152, 245–260. [Google Scholar] [CrossRef]
- Landry, D.A.; Yakubovich, E.; Cook, D.P.; Fasih, S.; Upham, J.; Vanderhyden, B.C. Metformin Prevents Age-Associated Ovarian Fibrosis by Modulating the Immune Landscape in Female Mice. Sci. Adv. 2022, 8, eabq1475. [Google Scholar] [CrossRef]
- Mara, J.N.; Zhou, L.T.; Larmore, M.; Johnson, B.; Ayiku, R.; Amargant, F.; Pritchard, M.T.; Duncan, F.E. Ovulation and Ovarian Wound Healing Are Impaired with Advanced Reproductive Age. Aging 2020, 12, 9686–9713. [Google Scholar] [CrossRef]
- Machlin, J.H.; Barishansky, S.J.; Kelsh, J.; Larmore, M.J.; Johnson, B.W.; Pritchard, M.T.; Pavone, M.E.; Duncan, F.E. Fibroinflammatory Signatures Increase with Age in the Human Ovary and Follicular Fluid. Int. J. Mol. Sci. 2021, 22, 4902. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The Extracellular Matrix Contributes to Mechanotransduction in Uterine Fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular Matrix in Uterine Leiomyoma Pathogenesis: A Potential Target for Future Therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-F.; Fang, Z.; Chen, X.; Qiao, L.; Jiang, Y.; Wang, X.-G.; Chang, D.; Song, L.; Yao, H. Melatonin Alleviates Endometrial Fibrosis in Bovine Endometritis by Regulating Tgf-Β/Smad and Mapk Signaling Pathways Via Mt2. SSRN Electron. J. 2025, 169, 104519. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Li, X.; Xiao, L. Ginsenoside Rg1 Alleviates Lipopolysaccharide-Induced Fibrosis of Endometrial Epithelial Cells in Dairy Cows by Inhibiting Reactive Oxygen Species-Activated NLRP3. Animals 2023, 13, 3723. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, Q.; Duan, H.; Sheng, X.; Qi, X.; Xing, K.; Liu, B.; Chang, D.; Guo, Y.; Wang, X.; et al. 17β-Estradiol Mediates TGFBR3/Smad2/3 Signaling to Attenuate the Fibrosis of TGF-β1-induced Bovine Endometrial Epithelial Cells via GPER. J. Cell Physiol. 2024, 239, 166–179. [Google Scholar] [CrossRef]
- Ramos, I.S.; Caldeira, M.O.; Poock, S.E.; Moraes, J.G.N.; Lucy, M.C.; Patterson, A.L. Adenomyosis and Fibrosis Define the Morphological Memory of the Postpartum Uterus of Dairy Cows Previously Exposed to Metritis. JDS Commun. 2025, 6, 250–255. [Google Scholar] [CrossRef]
- Wanjiru, D.K.; Niyonzima, Y.B.; Kadokawa, H. Lower Expression of Colony-Stimulating Factor 2, an Embryokine, in the Endometrial Epithelium of Old Cows. Reprod. Fertil. Dev. 2025, 37, RD24163. [Google Scholar] [CrossRef]
- Ferdousy, R.N.; Kadokawa, H. Specific Locations and Amounts of Denatured Collagen and Collagen-Specific Chaperone HSP47 in the Oviducts and Uteri of Old Cows as Compared with Those of Heifers. Reprod. Fertil. Dev. 2022, 34, 619–632. [Google Scholar] [CrossRef]
- Ferdousy, R.N.; Suong, N.T.; Kadokawa, H. Specific Locations and Amounts of Denatured Collagen and Collagen-Specific Chaperone HSP47 in the Uterine Cervices of Old Cows Compared with Those of Heifers. Theriogenology 2023, 196, 10–17. [Google Scholar] [CrossRef]
- Ferdousy, R.N.; Kereilwe, O.; Kadokawa, H. Anti-Müllerian Hormone Receptor Type 2 (AMHR2) Expression in Bovine Oviducts and Endometria: Comparison of AMHR2 MRNA and Protein Abundance between Old Holstein and Young and Old Wagyu Females. Reprod. Fertil. Dev. 2020, 32, 738. [Google Scholar] [CrossRef]
- Ferdousy, R.N.; Kadokawa, H. Anti-Müllerian Hormone Stimulates Expression of the Collagen-specific Chaperone 47-kDa Heat Shock Protein in Bovine Uterine Epithelial Cells. Anim. Sci. J. 2022, 93, e13787. [Google Scholar] [CrossRef] [PubMed]
- Bogado Pascottini, O.; Hostens, M.; Dini, P.; Vandepitte, J.; Ducatelle, R.; Opsomer, G. Distribution of Inflammation and Association between Active and Chronic Alterations within the Endometrium of Dairy Cows. Reprod. Domest. Anim. 2016, 51, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Massoud Talebkhan, G.; Farhang, S.; Parviz, H. The Histopathological Survey of Uterine Tissue in Holstein Dairy Cows with or without Recorded Reproductive Disorders. Iran. J. Vet. Sci. Technol. 2010, 2, 100–108. [Google Scholar]
- Palomares, R.A.; Gutiérrez-Añez, J.C.; Zambrano, S.; Boscan-Ocando, J.C.; Montero, M.; Camacho, J.; Perea-Ganchou, F.P.; Ferrer, M.S.; Rodríguez-Márquez, J.M.; Portillo-Martinez, G.; et al. Chronic Inflammatory and Degenerative Endometrial Lesions in Subfertile Criollo Limonero Cattle; a B. Taurus Latin-American Breed Threatened with Extinction; A Case-Control Study. Anim. Reprod. Sci. 2018, 197, 22–32. [Google Scholar] [CrossRef]
- Doğan, İ.; Sönmez, G.; Sağırkaya, H. Histopathological Investigation of Endometrium in Repeat Breeder Cows. Indian J. Anim. Sci. 2002, 72, 223–226. [Google Scholar]
- Bonnett, B.N.; Miller, R.B.; Martin, S.W.; Etherington, W.G.; Buckrell, B.C. Correlations between Histological Criteria. Can. J. Vet. Res. 1991, 2, 162. [Google Scholar]
- Hanada, M.; Maeda, Y.; Oikawa, M. Histopathological Characteristics of Endometrosis in Thoroughbred Mares in Japan: Results from 50 Necropsy Cases. J. Equine Sci. 2014, 25, 45–52. [Google Scholar] [CrossRef]
- Walter, I.; Handler, J.; Reifinger, M.; Aurich, C. Association of Endometriosis in Horses with Differentiation of Periglandular Myofibroblasts and Changes of Extracellular Matrix Proteins. Reproduction 2001, 121, 581–586. [Google Scholar] [CrossRef]
- Rebordão, M.; Galvão, A.; Szóstek, A.; Amaral, A.; Mateus, L.; Skarzynski, D.; Ferreira-Dias, G. Physiopathologic Mechanisms Involved in Mare Endometrosis. Reprod. Domest. Anim. 2014, 49, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, J.; Cao, S.; Wang, L. A Mechanisms of Endometrial Fibrosis and the Potential Application of Stem Cell Therapy. Discov. Med. 2019, 27, 267–279. [Google Scholar]
- Zhu, H.-Y.; Ge, T.-X.; Pan, Y.-B.; Zhang, S.-Y. Advanced Role of Hippo Signaling in Endometrial Fibrosis. Chin. Med. J. 2017, 130, 2732–2737. [Google Scholar] [CrossRef]
- Fujisawa, C.; Castellot, J.J. Matrix Production and Remodeling as Therapeutic Targets for Uterine Leiomyoma. J. Cell Commun. Signal 2014, 8, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Moustakas, A. Signaling Receptors for TGF-β Family Members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.-L.; Luo, T.; Luo, M. The Hippo Signalling Pathway and Its Implications in Human Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.I.; Polenov, N.I.; Kunitsa, V.V. Uterine Fibroids: The Role of Signaling Pathways in the Pathogenesis. A Literature Review. J. Obstet. Women’s Dis. 2020, 69, 113–124. [Google Scholar] [CrossRef]
- El Sabeh, M.; Afrin, S.; Miyashita-Ishiwata, M.; Borahay, M.A. TGF-Β signaling regulates wnt/β-catenin pathway in uterine leiomyoma cells. Fertil. Steril. 2021, 116, e414. [Google Scholar] [CrossRef]
- Zhao, G.; Li, R.; Cao, Y.; Song, M.; Jiang, P.; Wu, Q.; Zhou, Z.; Zhu, H.; Wang, H.; Dai, C.; et al. ΔNp63α-Induced DUSP4/GSK3β/SNAI1 Pathway in Epithelial Cells Drives Endometrial Fibrosis. Cell Death Dis. 2020, 11, 449. [Google Scholar] [CrossRef]
- Swanepoel, A.C.; Lindeque, B.G.; Swart, P.J.; Abdool, Z.; Pretorius, E. Estrogen Causes Ultrastructural Changes of Fibrin Networks during the Menstrual Cycle: A Qualitative Investigation. Microsc. Res. Tech. 2014, 77, 594–601. [Google Scholar] [CrossRef]
- Coleman, J.R.; Moore, E.E.; Schmitt, L.; Hansen, K.; Dow, N.; Freeman, K.; Cohen, M.J.; Silliman, C.C. Estradiol Provokes Hypercoagulability and Affects Fibrin Biology: A Mechanistic Exploration of Sex Dimorphisms in Coagulation. J. Trauma. Acute Care Surg. 2023, 94, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Ito, I.; Hanyu, A.; Wayama, M.; Goto, N.; Katsuno, Y.; Kawasaki, S.; Nakajima, Y.; Kajiro, M.; Komatsu, Y.; Fujimura, A.; et al. Estrogen Inhibits Transforming Growth Factor β Signaling by Promoting Smad2/3 Degradation. J. Biol. Chem. 2010, 285, 14747–14755. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, W.; Liu, X.; Yan, W.; Lu, L.; Song, S.; Wei, S.; Liu, Y.; Kang, J.; Su, R. Notch1 Signaling Enhances Collagen Expression and Fibrosis in Mouse Uterus. BioFactors 2021, 47, 852–864. [Google Scholar] [CrossRef]
- Protic, O.; Islam, M.; Greco, S.; Giannubilo, S.; Lamanna, P.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; Hinz, B.; Ciarmela, P. Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth. Semin. Reprod. Med. 2017, 35, 499–509. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in Fibrosis: Novel Roles and Mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwenhoven, F.A.; Turner, N.A. The Role of Cardiac Fibroblasts in the Transition from Inflammation to Fibrosis Following Myocardial Infarction. Vasc. Pharmacol. 2013, 58, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.D.; Naylor, A.J.; Buckley, C.; Filer, A.; Tak, P.-P. Fibroblasts and Osteoblasts in Inflammation and Bone Damage. In Stromal Immunology. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1060, pp. 37–54. [Google Scholar]
- Zhu, S.; Wang, A.; Xu, W.; Hu, L.; Sun, J.; Wang, X. The Heterogeneity of Fibrosis and Angiogenesis in Endometriosis Revealed by Single-Cell RNA-Sequencing. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166602. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jayes, F.; Johnson, L.; Schomberg, D.; Leppert, P. Biochemical Pathways and Myometrial Cell Differentiation Leading to Nodule Formation Containing Collagen and Fibronectin. Curr. Protein Pept. Sci. 2016, 18, 155–166. [Google Scholar] [CrossRef]
- Barragan, F.; Irwin, J.C.; Balayan, S.; Erikson, D.W.; Chen, J.C.; Houshdaran, S.; Piltonen, T.T.; Spitzer, T.L.B.; George, A.; Rabban, J.T.; et al. Human Endometrial Fibroblasts Derived from Mesenchymal Progenitors Inherit Progesterone Resistance and Acquire an Inflammatory Phenotype in the Endometrial Niche in Endometriosis1. Biol. Reprod. 2016, 94, 118. [Google Scholar] [CrossRef]
- Ma, S.; Ji, Z.; Zhang, B.; Geng, L.; Cai, Y.; Nie, C.; Li, J.; Zuo, Y.; Sun, Y.; Xu, G.; et al. Spatial Transcriptomic Landscape Unveils Immunoglobin-Associated Senescence as a Hallmark of Aging. Cell 2024, 187, 7025–7044.e34. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wan, Q.; Liu, Q.; Fan, Y.; Zhou, Q.; Skowronski, A.A.; Wang, S.; Shao, Z.; Liao, C.-Y.; Ding, L.; et al. IgG Is an Aging Factor That Drives Adipose Tissue Fibrosis and Metabolic Decline. Cell Metab. 2024, 36, 793–807.e5. [Google Scholar] [CrossRef]
- Levi, M.; Dik, K. Fibrophilia: A New Disease Entity? J. Thromb. Haemost. 2008, 6, 1334–1335. [Google Scholar] [CrossRef]
- Hattori, N.; Degen, J.L.; Sisson, T.H.; Liu, H.; Moore, B.B.; Pandrangi, R.G.; Simon, R.H.; Drew, A.F. Bleomycin-Induced Pulmonary Fibrosis in Fibrinogen-Null Mice. J. Clin. Investig. 2000, 106, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Wilberding, J.A.; Ploplis, V.A.; McLennan, L.; Liang, Z.; Cornelissen, I.; Feldman, M.; Deford, M.E.; Rosen, E.D.; Castellino, F.J. Development of Pulmonary Fibrosis in Fibrinogen-Deficient Mice. Ann. N. Y. Acad. Sci. 2001, 936, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.S.; Probst, C.K.; Brazee, P.L.; Rotile, N.J.; Blasi, F.; Weinreb, P.H.; Black, K.E.; Sosnovik, D.E.; Van Cott, E.M.; Violette, S.M.; et al. Uncoupling of the Profibrotic and Hemostatic Effects of Thrombin in Lung Fibrosis. JCI Insight 2017, 2, e86608. [Google Scholar] [CrossRef]
- Loskutoff, D.J.; Quigley, J.P. PAI-1, Fibrosis, and the Elusive Provisional Fibrin Matrix. J. Clin. Investig. 2000, 106, 1441–1443. [Google Scholar] [CrossRef]
- Ruppert, C.; Markart, P.; Wygrecka, M.; Preissner, K.T.; Günther, A. Role of Coagulation and Fibrinolysis in Lung and Renal Fibrosis. Hamostaseologie 2008, 28, 30–36. [Google Scholar] [CrossRef]
- Schuliga, M.; Grainge, C.; Westall, G.; Knight, D. The Fibrogenic Actions of the Coagulant and Plasminogen Activation Systems in Pulmonary Fibrosis. Int. J. Biochem. Cell Biol. 2018, 97, 108–117. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Shen, S.; Xia, Y.J.; Yi, L.; Gao, Q.; Wang, Y. Dehydroepiandrosterone Induces Ovarian and Uterine Hyperfibrosis in Female Rats. Hum. Reprod. 2013, 28, 3074–3085. [Google Scholar] [CrossRef]
- Matsuno, Y.; Amin, Y.A.; Kusama, K.; Imakawa, K. Formation of Fibrin at Sites of Conceptus Adhesion in the Ewe. Reproduction 2021, 161, 709–720. [Google Scholar] [CrossRef]
- Yamada, O.; Todoroki, J.; Takahashi, T.; Hashizume, K. The Dynamic Expression of Extracellular Matrix in the Bovine Endometrium at Implantation. J. Vet. Med. Sci. 2002, 64, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Spencer, T.E.; Bazer, F.W. Cathepsins in the Ovine Uterus: Regulation by Pregnancy, Progesterone, and Interferon Tau. Endocrinology 2005, 146, 4825–4833. [Google Scholar] [CrossRef]
- Salamonsen, L.; Nagase, H.; Woolley, D. Matrix Metalloproteinases and Their Tissue Inhibitors at the Ovine Trophoblast-Uterine Interface. Biosci. Proc. 2019, 3, 29–37. [Google Scholar] [CrossRef]
- Egashira, M.; Hirota, Y.; Shimizu-Hirota, R.; Saito-Fujita, T.; Haraguchi, H.; Matsumoto, L.; Matsuo, M.; Hiraoka, T.; Tanaka, T.; Akaeda, S.; et al. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology 2017, 158, 2344–2353. [Google Scholar] [CrossRef]
- Secomandi, L.; Borghesan, M.; Velarde, M.; Demaria, M. The Role of Cellular Senescence in Female Reproductive Aging and the Potential for Senotherapeutic Interventions. Hum. Reprod. Update 2022, 28, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sun, B.; Li, S.; Wei, W.; Liu, X.; Cui, X.; Zhang, X.; Liu, N.; Yan, L.; Deng, Y.; et al. NKG2D-CAR T Cells Eliminate Senescent Cells in Aged Mice and Nonhuman Primates. Sci. Transl. Med. 2023, 15, eadd1951. [Google Scholar] [CrossRef] [PubMed]
- Hubackova, S.; Davidova, E.; Rohlenova, K.; Stursa, J.; Werner, L.; Andera, L.; Dong, L.; Terp, M.G.; Hodny, Z.; Ditzel, H.J.; et al. Selective Elimination of Senescent Cells by Mitochondrial Targeting Is Regulated by ANT2. Cell Death Differ. 2019, 26, 276–290. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed Elimination of Senescent Cells by Inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Suda, M.; Yoshida, Y.; Furihata, T.; Joki, Y.; Hsiao, C.-L.; Jiaqi, L.; Fujiki, S.; Abe, M.; et al. SGLT2 Inhibition Eliminates Senescent Cells and Alleviates Pathological Aging. Nat. Aging 2024, 4, 926–938. [Google Scholar] [CrossRef]
- Katsuumi, G.; Suda, M.; Shimizu, I.; Yoshida, Y.; Furihata, T.; Joki, Y.; Hsiao, C.L.L.; Minamino, T. Abstract 14285: Sodium Glucose Co-Transporter 2 Inhibition Eliminates Senescent Cells Through Downregulation of PD-L1 and Improves Pathological Aging. Circulation 2023, 148, A14285. [Google Scholar] [CrossRef]
- Guo, G.; Amor, C. SGLT2 Regulates Immune-Mediated Senolysis. Nat. Aging 2024, 4, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Moon, J.H.; Cho, Y.M. Sodium-glucose Cotransporter-2 Inhibition Reduces Cellular Senescence in the Diabetic Kidney by Promoting Ketone Body-induced NRF2 Activation. Diabetes Obes. Metab. 2021, 23, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, Y.; Feng, D.; Yao, J.; Cao, Y.; Deng, L. Hepatic Gluconeogenesis and Regulatory Mechanisms in Lactating Ruminants: A Literature Review. Anim. Res. One Health 2024, 1–10. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Saccon, T.D.; Nunes, A.D.C.; Kirkland, J.L.; Tchkonia, T.; Schneider, A.; Masternak, M.M. Dasatinib plus Quercetin Prevents Uterine Age-Related Dysfunction and Fibrosis in Mice. Aging 2020, 12, 2711–2722. [Google Scholar] [CrossRef]
- Kusama, K.; Yamauchi, N.; Yoshida, K.; Azumi, M.; Yoshie, M.; Tamura, K. Senolytic Treatment Modulates Decidualization in Human Endometrial Stromal Cells. Biochem. Biophys. Res. Commun. 2021, 571, 174–180. [Google Scholar] [CrossRef]
- Luo, M.; Cai, X.; Yan, D.; Liu, X.; Guo, S.-W. Sodium Tanshinone IIA Sulfonate Restrains Fibrogenesis through Induction of Senescence in Mice with Induced Deep Endometriosis. Reprod. Biomed. Online 2020, 41, 373–384. [Google Scholar] [CrossRef]
- Gasek, N.S.; Yan, P.; Zhu, J.; Purushothaman, K.-R.; Kim, T.; Wang, L.; Wang, B.; Flynn, W.F.; Sun, M.; Guo, C.; et al. Clearance of P21 Highly Expressing Senescent Cells Accelerates Cutaneous Wound Healing. Nat. Aging 2024, 5, 21–27. [Google Scholar] [CrossRef]
- Cohn, R.L.; Gasek, N.S.; Kuchel, G.A.; Xu, M. The Heterogeneity of Cellular Senescence: Insights at the Single-Cell Level. Trends Cell Biol. 2023, 33, 9–17. [Google Scholar] [CrossRef]
- Glasser, S.W.; Hagood, J.S.; Wong, S.; Taype, C.A.; Madala, S.K.; Hardie, W.D. Mechanisms of Lung Fibrosis Resolution. Am. J. Pathol. 2016, 186, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gentilini, A.; Pastore, M.; Gitto, S.; Marra, F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells 2021, 10, 2759. [Google Scholar] [CrossRef]
- Iredale, J.P.; Benyon, R.C.; Pickering, J.; McCullen, M.; Northrop, M.; Pawley, S.; Hovell, C.; Arthur, M.J. Mechanisms of Spontaneous Resolution of Rat Liver Fibrosis. Hepatic Stellate Cell Apoptosis and Reduced Hepatic Expression of Metalloproteinase Inhibitors. J. Clin. Investig. 1998, 102, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Iredale, J.; Fallowfield, J. Resolution of Liver Fibrosis: Basic Mechanisms and Clinical Relevance. Semin. Liver Dis. 2015, 35, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Yi, Q.; Tang, L. Liver Fibrosis Resolution: From Molecular Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 9671. [Google Scholar] [CrossRef]
- Ward-Caviness, C.K.; Huffman, J.E.; Everett, K.; Germain, M.; van Dongen, J.; Hill, W.D.; Jhun, M.A.; Brody, J.A.; Ghanbari, M.; Du, L.; et al. DNA Methylation Age Is Associated with an Altered Hemostatic Profile in a Multiethnic Meta-Analysis. Blood 2018, 132, 1842–1850. [Google Scholar] [CrossRef]
- Chamani, I.J.; Keefe, D.L. Epigenetics and Female Reproductive Aging. Front. Endocrinol. 2019, 10, 473. [Google Scholar] [CrossRef]
- Li Piani, L.; Vigano’, P.; Somigliana, E. Epigenetic Clocks and Female Fertility Timeline: A New Approach to an Old Issue? Front. Cell Dev. Biol. 2023, 11, 1121231. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino Acid Metabolism in Intestinal Bacteria and Its Potential Implications for Mammalian Reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef]
- Van Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated Fatty Acids in Male Ruminant Reproduction—A Review. Asian-Australas. J. Anim. Sci. 2016, 30, 622–637. [Google Scholar] [CrossRef]
- Genís, S.; Bach, À.; Fàbregas, F.; Arís, A. Potential of Lactic Acid Bacteria at Regulating Escherichia Coli Infection and Inflammation of Bovine Endometrium. Theriogenology 2016, 85, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Whiston, R.; Tasara, T.; Bleul, U.; Chapwanya, A. Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals 2024, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Gärtner, M.A.; Michel, G.; Ibrahim, M.; Klopfleisch, R.; Lübke-Becker, A.; Jung, M.; Einspanier, R.; Gabler, C. Influence of Intrauterine Administration of Lactobacillus Buchneri on Reproductive Performance and Pro-Inflammatory Endometrial MRNA Expression of Cows with Subclinical Endometritis. Sci. Rep. 2018, 8, 5473. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal Lactic Acid Bacteria Modulated Local and Systemic Immune Responses and Lowered the Incidence of Uterine Infections in Periparturient Dairy Cows. PLoS ONE 2015, 10, e0124167. [Google Scholar] [CrossRef]
- Li, Z.; Teng, Y.; Feng, S.; Hu, Z.; Zhao, J.; Ding, H.; Fang, Y.; Liu, H.; Ma, X.; Guo, J.; et al. Microbial Responses and Changes in Metabolic Products in Bovine Uteri Infected with Staphylococcus Aureus. Int. J. Biol. Macromol. 2024, 262, 130039. [Google Scholar] [CrossRef]
- Nørstebø, S.F.; Rodriguez-Campos, S.; Umu, Ö.C.O.; Abril-Parreño, L.; Dalland, M.; Gilfillan, G.D.; Fair, S.; Krogenaes, A. The Cervical Microbiome of Ewe Breeds with Known Divergent Fertility Following Artificial Insemination with Frozen-Thawed Semen. Sci. Rep. 2025, 15, 14614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuno, Y.; Imakawa, K. Biological Aging and Uterine Fibrosis in Cattle: Reproductive Trade-Offs from Enhanced Productivity. Cells 2025, 14, 955. https://doi.org/10.3390/cells14130955
Matsuno Y, Imakawa K. Biological Aging and Uterine Fibrosis in Cattle: Reproductive Trade-Offs from Enhanced Productivity. Cells. 2025; 14(13):955. https://doi.org/10.3390/cells14130955
Chicago/Turabian StyleMatsuno, Yuta, and Kazuhiko Imakawa. 2025. "Biological Aging and Uterine Fibrosis in Cattle: Reproductive Trade-Offs from Enhanced Productivity" Cells 14, no. 13: 955. https://doi.org/10.3390/cells14130955
APA StyleMatsuno, Y., & Imakawa, K. (2025). Biological Aging and Uterine Fibrosis in Cattle: Reproductive Trade-Offs from Enhanced Productivity. Cells, 14(13), 955. https://doi.org/10.3390/cells14130955





