Lower Zinc but Higher Calcium Content in Rodent Spinal Cord Compared to Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Preparation
2.2. Inductively Coupled Plasma Optic Emission Spectroscopy
2.3. Statistical Analysis of the ICP-OES Results
2.4. ZnT3-HA Imaging by Confocal Microscopy in Brain and Spinal Cord Sections
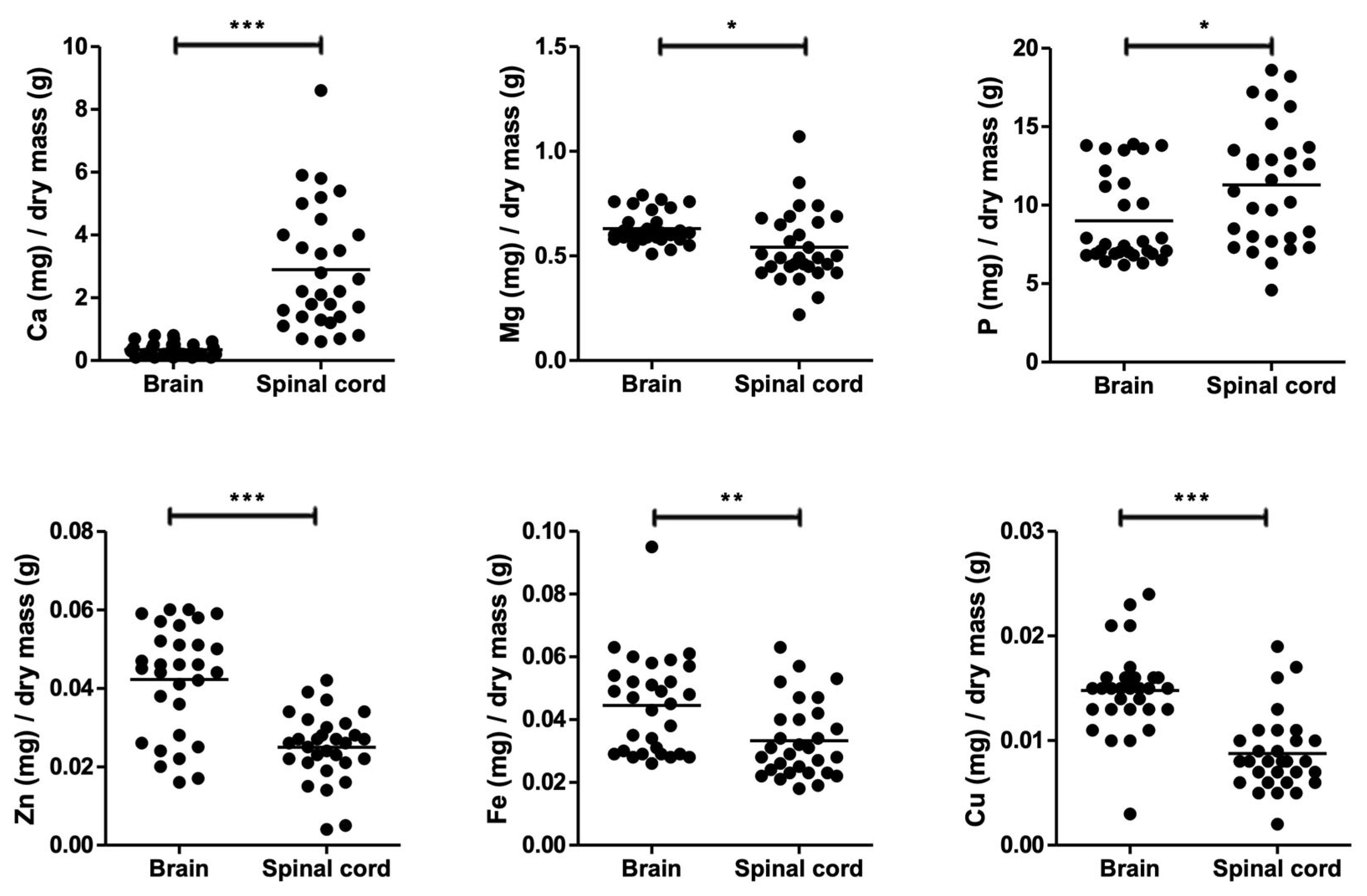
3. Results
| Experiment | Organ | Ca | Mg | P | Zn | Fe | Cu |
|---|---|---|---|---|---|---|---|
| #1 male untreated (n = 11) | Brain | 0.4 ± 0.2 | 0.64 ± 0.08 | 9.4 ± 3.1 | 0.041 ± 0.015 | 0.047 ± 0.014 | 0.016 ± 0.005 |
| Spinal cord | 4.2 ± 2.3 | 0.68 ± 0.18 | 13.7 ± 3.2 | 0.030 ± 0.010 | 0.040 ± 0.013 | 0.008 ± 0.003 | |
| #2 male mock inj.* (n = 11) | Brain | 0.2 ± 0.1 | 0.67 ± 0.08 | 10.1 ± 2.9 | 0.040 ± 0.017 | 0.046 ± 0.010 | 0.013 ± 0.004 |
| Spinal cord | 2.4 ± 1.4 | 0.48 ± 0.14 | 11.5 ± 3.9 | 0.020 ± 0.007 | 0.033 ± 0.011 | 0.010 ± 0.005 | |
| #3 female untreated (n = 8) | Brain | 0.4 ± 0.3 | 0.57 ± 0.03 | 7.0 ± 0.3 | 0.047 ± 0.005 | 0.039 ± 0.023 | 0.015 ± 0.001 |
| Spinal cord | 1.8 ± 1.2 | 0.44 ± 0.04 | 7.7 ± 0.7 | 0.025 ± 0.003 | 0.024 ± 0.005 | 0.007 ± 0.001 | |
| Mean (n = 3) | Brain | 0.3 ± 0.1 | 0.63 ± 0.05 | 8.8 ± 1.7 | 0.043 ± 0.004 | 0.044 ± 0.004 | 0.015 ± 0.001 |
| Spinal cord | 2.8 ± 1.1 | 0.53 ± 0.13 | 11.0 ± 3.1 | 0.025 ± 0.005 | 0.032 ± 0.008 | 0.009 ± 0.001 | |
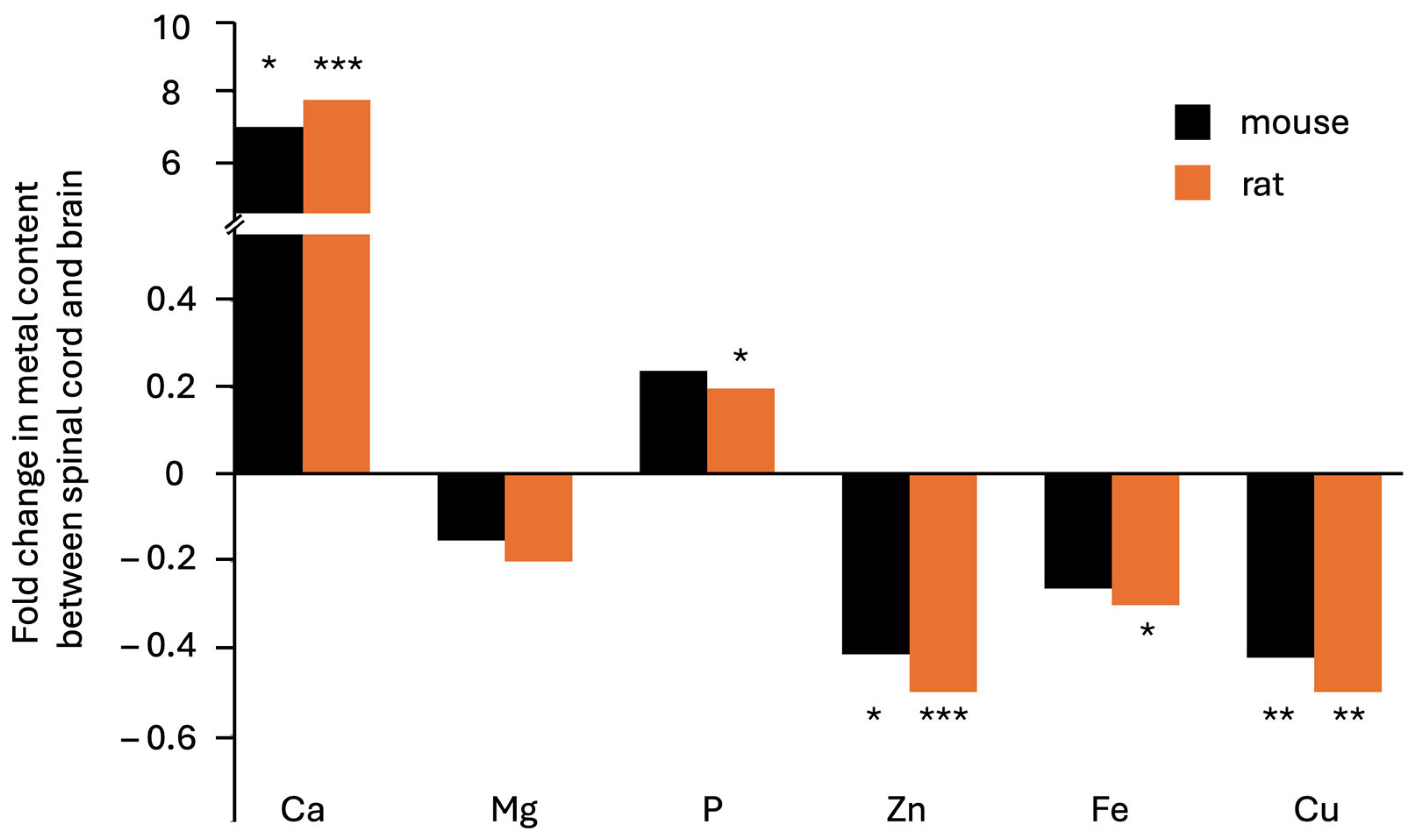
| Fold difference | 7.1 | −0.2 | 0.2 | −0.4 | −0.3 | −0.4 | |
| T-test (p-value) | 0.03 | 0.30 | 0.35 | 0.01 | 0.09 | 0.004 |
| Experiment | Organ | Ca | Mg | P | Zn | Fe | Cu |
|---|---|---|---|---|---|---|---|
| #1 male ND (n = 11) | Brain | 0.3 ± 0.2 | 0.75 ± 0.11 | 14.7 ± 3.4 | 0.063 ± 0.015 | 0.086 ± 0.017 | 0.011 ± 0.003 |
| Spinal cord | 4.1 ± 2.0 | 0.73 ± 0.08 | 20.2 ± 4.1 | 0.035 ± 0.011 | 0.073 ± 0.037 | 0.009 ± 0.008 | |
| #2 male RD (n = 11) | Brain | 0.3 ± 0.2 | 0.80 ± 0.07 | 16.2 ± 1.7 | 0.067 ± 0.015 | 0.100 ± 0.046 | 0.011 ± 0.002 |
| Spinal cord | 2.5 ± 2.2 | 0.63 ± 0.08 | 17.8 ± 4.1 | 0.033 ± 0.005 | 0.057 ± 0.022 | 0.006 ± 0.003 | |
| #3 female ND (n = 10) | Brain | 0.6 ± 0.6 | 0.73 ± 0.08 | 15.0 ± 1.8 | 0.065 ± 0.010 | 0.078 ± 0.014 | 0.011 ± 0.002 |
| Spinal cord | 4.3 ± 3.9 | 0.58 ± 0.08 | 16.9 ± 3.0 | 0.028 ± 0.004 | 0.049 ± 0.014 | 0.004 ± 0.002 | |
| #4 female RD (n = 9) | Brain | 0.4 ± 0.2 | 0.79 ± 0.06 | 15.6 ± 0.9 | 0.071 ± 0.006 | 0.077 ± 0.012 | 0.012 ± 0.002 |
| Spinal cord | 2.8 ± 4.3 | 0.57 ± 0.07 | 15.7 ± 2.9 | 0.035 ± 0.016 | 0.051 ± 0.016 | 0.004 ± 0.002 | |
| Mean (n = 4) | Brain | 0.4 ± 0.1 | 0.77 ± 0.04 | 15.4 ± 0.6 | 0.066 ± 0.003 | 0.085 ± 0.011 | 0.012 ± 0.001 |
| Spinal cord | 3.4 ± 0.9 | 0.63 ± 0.08 | 17.7 ± 1.9 | 0.033 ± 0.000 | 0.057 ± 0.011 | 0.006 ± 0.002 | |
| Fold difference | 7.8 | −0.2 | 0.2 | −0.5 | −0.3 | −0.5 | |
| T-test (p-value) | 0.0006 | 0.02 | 0.06 | 0.000006 | 0.01 | 0.003 |
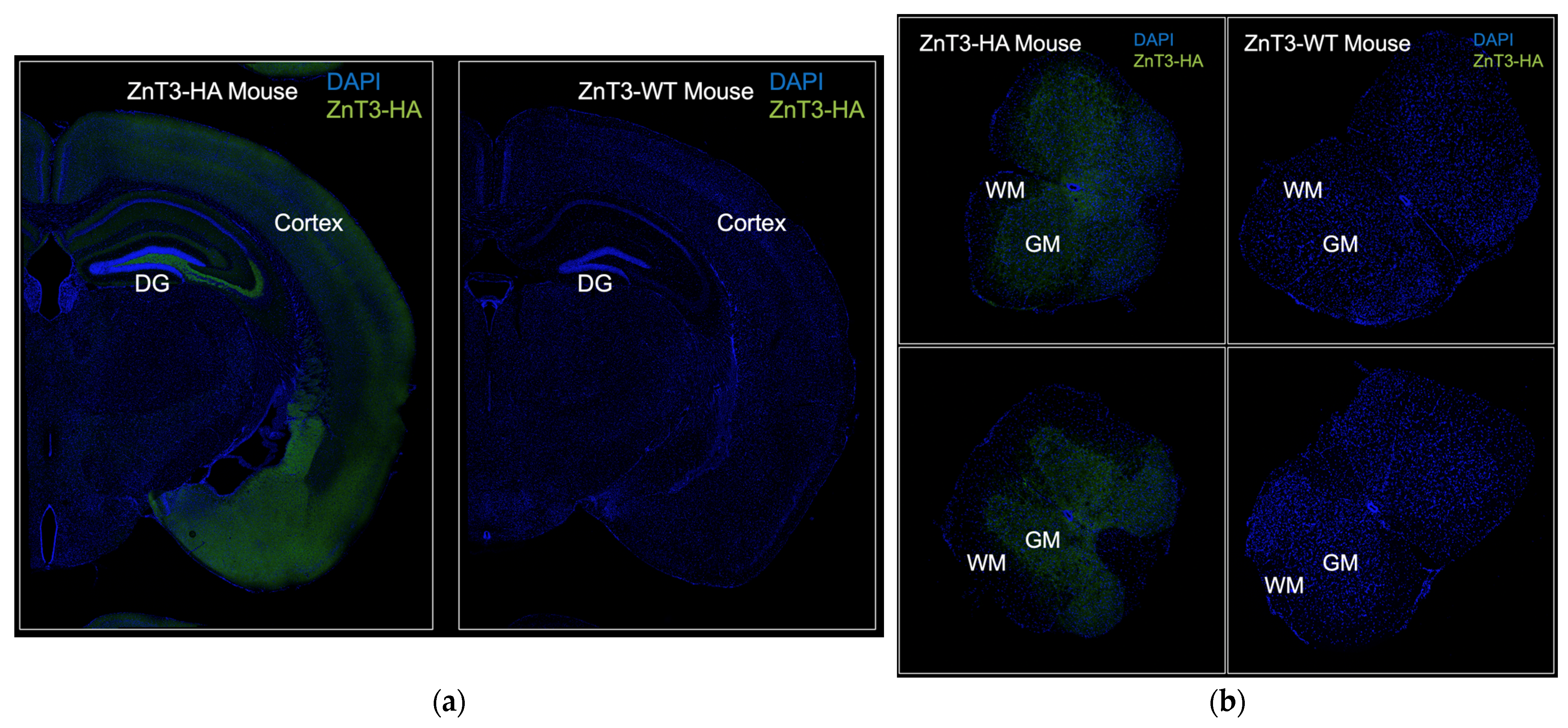
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krall, R.F.; Tzounopoulos, T.; Aizenman, E. The Function and Regulation of Zinc in the Brain. Neuroscience 2021, 457, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Hollings, A.L.; Ellison, G.C.; Willans, M.; Lam, V.; Munyard, T.; Remy, A.R.; Takechi, R.; Mamo, J.C.L.; Webb, S.; New, E.J.; et al. Subventricular Accumulation of Cu in the Aging Mouse Brain Does Not Associate with Anticipated Increases in Markers of Oxidative Stress. ACS Chem. Neurosci. 2025, 16, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.E.; Bossoni, L.; Connor, J.R.; Crichton, R.R.; Does, M.D.; Ward, R.J.; Zecca, L.; Zucca, F.A.; Ronen, I. Iron, Myelin, and the Brain: Neuroimaging Meets Neurobiology. Trends Neurosci. 2019, 42, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Posadas, Y.; Lopez-Guerrero, V.E.; Arcos-Lopez, T.; Sayler, R.I.; Sanchez-Lopez, C.; Perez-Cruz, C.; Quintanar, L. The role of d-block metal ions in neurodegenerative diseases. In Comprehensive Inorganic Chemistry III, 3rd ed.; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 575–628. [Google Scholar]
- Yoo, J.; Han, J.; Lim, M.H. Transition metal ions and neurotransmitters: Coordination chemistry and implications for neurodegeneration. RSC Chem. Biol. 2023, 4, 548–563. [Google Scholar] [CrossRef]
- Kenkhuis, B.; Bush, A.I.; Ayton, S. How iron can drive neurodegeneration. Trends Neurosci. 2023, 46, 333–335. [Google Scholar] [CrossRef]
- Popescu, B.F.; Robinson, C.A.; Rajput, A.; Rajput, A.H.; Harder, S.L.; Nichol, H. Iron, copper, and zinc distribution of the cerebellum. Cerebellum 2009, 8, 74–79. [Google Scholar] [CrossRef]
- Ellison, G.; Hollings, A.L.; Hackett, M.J. A review of the “metallome” within neurons and glia, as revealed by elemental mapping of brain tissue. BBA Adv. 2021, 2, 100038. [Google Scholar] [CrossRef]
- Pushie, M.J.; Sylvain, N.J.; Hou, H.; Pendleton, N.; Wang, R.; Zimmermann, L.; Pally, M.; Cayabyab, F.S.; Peeling, L.; Kelly, M.E. X-Ray fluorescence mapping of brain tissue reveals the profound extent of trace element dysregulation in stroke pathophysiology. Metallomics 2024, 16, mfae054. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 465–479. [Google Scholar] [CrossRef]
- Muccilli, A.; Seyman, E.; Oh, J. Spinal Cord MRI in Multiple Sclerosis. Neurol. Clin. 2018, 36, 35–57. [Google Scholar] [CrossRef]
- Calabro, F.J.; Perez, M.A. Bilateral reach-to-grasp movement asymmetries after human spinal cord injury. J. Neurophysiol. 2016, 115, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sirabella, R.; Valsecchi, V.; Anzilotti, S.; Cuomo, O.; Vinciguerra, A.; Cepparulo, P.; Brancaccio, P.; Guida, N.; Blondeau, N.; Canzoniero, L.M.T.; et al. Ionic Homeostasis Maintenance in ALS: Focus on New Therapeutic Targets. Front. Neurosci. 2018, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Elliot, J.L. Metallothionein expression is altered in a transgenic murine model of familial amyotrophic lateral sclerosis. Exp. Neurol. 2000, 162, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.J.; Zhou, Q.S.; Huang, H.B.; Wan, Y.L.; Tian, S.F.; Duan, D.M. Effects of ketamine on the balance of ions Ca2+, Mg2+, Cu2+ and Zn2+ in the ischemia-reperfusion affected spinal cord tissues in rabbits. Neurochem. Res. 2009, 34, 2192–2196. [Google Scholar] [CrossRef]
- Tomik, B.; Chwiej, J.; Szczerbowska-Boruchowska, M.; Lankosz, M.; Wójcik, S.; Adamek, D.; Falkenberg, G.; Bohic, S.; Simionovici, A.; Stegowski, Z.; et al. Implementation of X-Ray fluorescence microscopy for investigation of elemental abnormalities in amyotrophic lateral sclerosis. Neurochem. Res. 2006, 31, 321–331. [Google Scholar] [CrossRef]
- Kinebuchi, M.; Matsuura, A.; Kiyono, T.; Nomura, Y.; Kimura, S. Diagnostic copper imaging of Menkes disease by synchrotron radiation-generated X-Ray fluorescence analysis. Sci. Rep. 2016, 6, 33247. [Google Scholar] [CrossRef]
- Santos-Díaz, A.I.; Solís-López, J.; Díaz-Torres, E.; Guadarrama-Olmos, J.C.; Osorio, B.; Kroll, T.; Webb, S.M.; Hiriart, M.; Jiménez-Estrada, I.; Missirlis, F. Metal ion content of internal organs in the calorically restricted Wistar rat. J. Trace Elem. Med. Biol. 2023, 78, 127182. [Google Scholar] [CrossRef]
- Weed, L.H. The Cells of the Arachnoid. Bull. Johns Hopkins Hosp. 1920, 31, 343. [Google Scholar]
- Herren, Y.R. Occurrence and distribution of calcified plaques in the spinal arachnoid in man. Arch. Neurol. Psych. 1939, 41, 1180–1186. [Google Scholar] [CrossRef]
- Weed, L.H. Studies on Cerebro-Spinal Fluid. No. III: The pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. J. Med. Res. 1914, 31, 51–91. [Google Scholar]
- Tanaka, K.; Nishiura, I.; Koyama, T. Arachnoiditis ossificans after repeated myelographies and spinal operations–A case report and review of the literature. Neurol. Surg. 1987, 15, 89–93. [Google Scholar]
- Young, W. Role of calcium in central nervous system injuries. J. Neurotrauma 1992, 9, S9–S25. [Google Scholar]
- Frizzell, B.; Kaplan, P.; Dussault, R.; Sevick, R. Arachnoiditis ossificans: MR imaging features in five patients. AJR Am. J. Roentgenol. 2001, 177, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Khalfallah, M.; Perrouin-Verbe, B.; Caillon, F.; Deschamps, C.; Bord, E.; Mathe, J.F.; Robert, R. Arachnoiditis ossificans of the cauda equina. Case report and review of the literature. J. Neurosurg. 2002, 97, 239–243. [Google Scholar] [PubMed]
- Wright, M.H.; Denney, L.C. A comprehensive review of spinal arachnoiditis. Orthop. Nurs. 2003, 22, 215–219. [Google Scholar] [CrossRef]
- Slavin, K.V.; Nixon, R.R.; Nesbit, G.M.; Burchiel, K.J. Extensive arachnoid ossification with associated syringomyelia presenting as thoracic myelopathy. Case report and review of the literature. J. Neurosurg. 1999, 91, 223–229. [Google Scholar]
- Haug, F.M. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie 1967, 8, 355–368. [Google Scholar] [CrossRef]
- Crawford, J.D.; Connor, J.D. Zinc in maturing rat brain: Hippocampal concentration and localization. J. Neurochem. 1972, 19, 1451–1458. [Google Scholar] [CrossRef]
- Fjerdingstad, E.; Danscher, G.; Fjerdingstad, E.J. Zinc content in hippocampus and whole brain of normal rats. Brain Res. 1974, 79, 338–342. [Google Scholar] [CrossRef]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Kantheti, P.; Qiao, X.; Diaz, M.E.; Peden, A.A.; Meyer, G.E.; Carskadon, S.L.; Kapfhamer, D.; Sufalko, D.; Robinson, M.S.; Noebels, J.L.; et al. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 1998, 21, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Guzmán, C.; Rosas-Arellano, A.; Kroll, T.; Webb, S.M.; Barajas-Aceves, M.; Osorio, B.; Missirlis, F. Biogenesis of zinc storage granules in Drosophila melanogaster. J. Exp. Biol. 2018, 221, jeb168419. [Google Scholar]
- Howell, G.A.; Welch, M.G.; Frederickson, C.J. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 1984, 308, 736–738. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.Y.; Dahlström, A.; Danscher, G. Zinc-enriched GABAergic terminals in mouse spinal cord. Brain Res. 2001, 921, 165–172. [Google Scholar] [CrossRef]
- Qian, J.; Noebels, J.L. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 2005, 566, 747–758. [Google Scholar] [CrossRef]
- Brown, C.E.; Dyck, R.H. Modulation of synaptic zinc in barrel cortex by whisker stimulation. Neuroscience 2005, 134, 355–359. [Google Scholar] [CrossRef]
- Nakashima, A.S.; Dyck, R.H. Enhanced plasticity in zincergic, cortical circuits after exposure to enriched environments. J. Neurosci. 2008, 28, 13995–13999. [Google Scholar] [CrossRef]
- Nakashima, A.S.; Dyck, R.H. Dynamic, experience-dependent modulation of synaptic zinc within the excitatory synapses of the mouse barrel cortex. Neuroscience 2010, 170, 1015–1019. [Google Scholar] [CrossRef]
- Sindreu, C.; Palmiter, R.D.; Storm, D.R. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3366–3370. [Google Scholar] [CrossRef]
- Amico-Ruvio, S.A.; Murthy, S.E.; Smith, T.P.; Popescu, G.K. Zinc effects on NMDA receptor gating kinetics. Biophys. J. 2011, 100, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rosello, T.; Anderson, C.T.; Schopfer, F.J.; Zhao, Y.; Gilad, D.; Salvatore, S.R.; Freeman, B.A.; Hershfinkel, M.; Aizenman, E.; Tzounopoulos, T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. 2013, 33, 9259–9272. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T.; Radford, R.J.; Zastrow, M.L.; Zhang, D.Y.; Apfel, U.P.; Lippard, S.J.; Tzounopoulos, T. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc. Natl. Acad. Sci. USA 2015, 112, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Kalappa, B.I.; Anderson, C.T.; Goldberg, J.M.; Lippard, S.J.; Tzounopoulos, T. AMPA receptor inhibition by synaptically released zinc. Proc. Natl. Acad. Sci. USA 2015, 112, 15749–15754. [Google Scholar] [CrossRef]
- Perez-Rosello, T.; Anderson, C.T.; Ling, C.; Lippard, S.J.; Tzounopoulos, T. Tonic zinc inhibits spontaneous firing in dorsal cochlear nucleus principal neurons by enhancing glycinergic neurotransmission. Neurobiol. Dis. 2015, 81, 14–19. [Google Scholar] [CrossRef]
- Patrick-Wu, H.P.; Dyck, R.H. Signaling by Synaptic Zinc is Required for Whisker-Mediated, Fine Texture Discrimination. Neuroscience 2018, 369, 242–247. [Google Scholar] [CrossRef]
- McAllister, B.B.; Wright, D.K.; Wortman, R.C.; Shultz, S.R.; Dyck, R.H. Elimination of vesicular zinc alters the behavioural and neuroanatomical effects of social defeat stress in mice. Neurobiol. Stress 2018, 9, 199–213. [Google Scholar] [CrossRef]
- Kouvaros, S.; Kumar, M.; Tzounopoulos, T. Synaptic Zinc Enhances Inhibition Mediated by Somatostatin, but not Parvalbumin, Cells in Mouse Auditory Cortex. Cereb. Cortex 2020, 30, 3895–3909. [Google Scholar] [CrossRef]
- Kouvaros, S.; Bizup, B.; Solis, O.; Kumar, M.; Ventriglia, E.; Curry, F.P.; Michaelides, M.; Tzounopoulos, T. A CRE/DRE dual recombinase transgenic mouse reveals synaptic zinc-mediated thalamocortical neuromodulation. Sci. Adv. 2023, 9, eadf3525. [Google Scholar] [CrossRef]
- Bender, P.T.R.; McCollum, M.; Boyd-Pratt, H.; Mendelson, B.Z.; Anderson, C.T. Synaptic zinc potentiates AMPA receptor function in mouse auditory cortex. Cell Rep. 2023, 42, 112932. [Google Scholar] [CrossRef]
- Chrusch, M.J.; Fu, S.; Spanswick, S.C.; Vecchiarelli, H.A.; Patel, P.P.; Hill, M.N.; Dyck, R.H. Environmental enrichment engages vesicular zinc signaling to enhance hippocampal neurogenesis. Cells 2023, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Bizup, B.; Tzounopoulos, T. On the genesis and unique functions of zinc neuromodulation. J. Neurophysiol. 2024, 132, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Linkous, D.H.; Flinn, J.M.; Koh, J.Y.; Lanzirotti, A.; Bertsch, P.M.; Jones, B.F.; Giblin, L.J.; Frederickson, C.J. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J. Histochem. Cytochem. 2008, 56, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, N.; Jin, J.; Emde, H.V.; Ganzella, M.; Bösche, L.; Malviya, V.N.; Zhuleku, E.; Politi, A.Z.; Ninov, M.; Silbern, I.; et al. Colocalization of different neurotransmitter transporters on synaptic vesicles is sparse except for VGLUT1 and ZnT3. Neuron 2022, 110, 1483–1497. [Google Scholar] [CrossRef]
- Bizup, B.; Brutsaert, S.; Cunningham, C.L.; Thathiah, A.; Tzounopoulos, T. Cochlear zinc signaling dysregulation is associated with noise-induced hearing loss, and zinc chelation enhances cochlear recovery. Proc. Natl. Acad. Sci. USA 2024, 121, e2310561121. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef]
- Brown, C.E.; Dyck, R.H. Distribution of zincergic neurons in the mouse forebrain. J. Comp. Neurol. 2004, 479, 156–167. [Google Scholar] [CrossRef]
- Chi, T.; Kim, M.S.; Lang, S.; Bose, N.; Kahn, A.; Flechner, L.; Blaschko, S.D.; Zee, T.; Muteliefu, G.; Bond, N.; et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE 2015, 10, e0124150. [Google Scholar] [CrossRef]
- Ustriyana, P.; Hennefarth, M.R.; Srirangapatanam, S.; Jung, H.; Wang, Y.; Chen, L.; Lue, T.F.; Lin, G.; Kang, M.; Stoller, M.L.; et al. Mineralized Peyronie’s plaque has a phenotypic resemblance to bone. Acta Biomater. 2022, 140, 457–466. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, D.K.; Cho, Y.E.; Kwun, I.S. Zinc Action in Vascular Calcification. Prev. Nutr. Food Sci. 2024, 29, 118–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Díaz, A.I.; Bizup, B.; Pantaleón-Gómez, A.K.; Osorio, B.; Barbier, O.C.; Tzounopoulos, T.; Missirlis, F. Lower Zinc but Higher Calcium Content in Rodent Spinal Cord Compared to Brain. Cells 2025, 14, 922. https://doi.org/10.3390/cells14120922
Santos-Díaz AI, Bizup B, Pantaleón-Gómez AK, Osorio B, Barbier OC, Tzounopoulos T, Missirlis F. Lower Zinc but Higher Calcium Content in Rodent Spinal Cord Compared to Brain. Cells. 2025; 14(12):922. https://doi.org/10.3390/cells14120922
Chicago/Turabian StyleSantos-Díaz, Alma I., Brandon Bizup, Ana Karen Pantaleón-Gómez, Beatriz Osorio, Olivier Christophe Barbier, Thanos Tzounopoulos, and Fanis Missirlis. 2025. "Lower Zinc but Higher Calcium Content in Rodent Spinal Cord Compared to Brain" Cells 14, no. 12: 922. https://doi.org/10.3390/cells14120922
APA StyleSantos-Díaz, A. I., Bizup, B., Pantaleón-Gómez, A. K., Osorio, B., Barbier, O. C., Tzounopoulos, T., & Missirlis, F. (2025). Lower Zinc but Higher Calcium Content in Rodent Spinal Cord Compared to Brain. Cells, 14(12), 922. https://doi.org/10.3390/cells14120922








