Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. DSS-Induced Colitis
2.3. House Dust Mite Induced Airway Inflammation (HDM-AI)
2.4. Immunoproteasome Inhibition by ONX 0914
2.5. Organ Preparation
2.6. Flow Cytometric Analysis
2.7. Intracellular Cytokine Staining
2.8. In Vitro T Cell Activation
2.9. In Vitro Th17 Differentiation
2.10. Statistics
3. Results
3.1. Impaired Induction of Th17 Response upon Immunoproteasome Inhibition in DSS-Induced Colitis
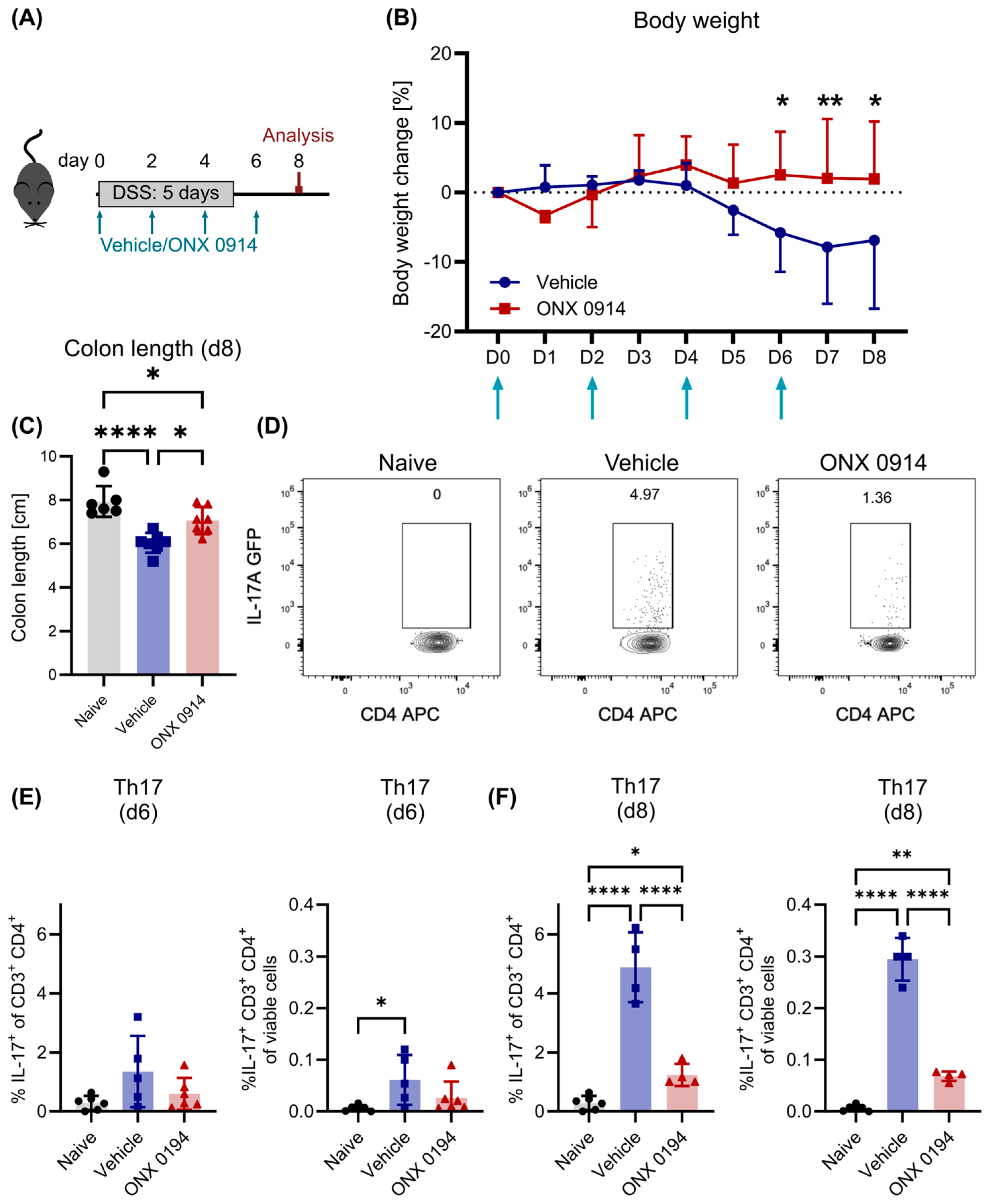
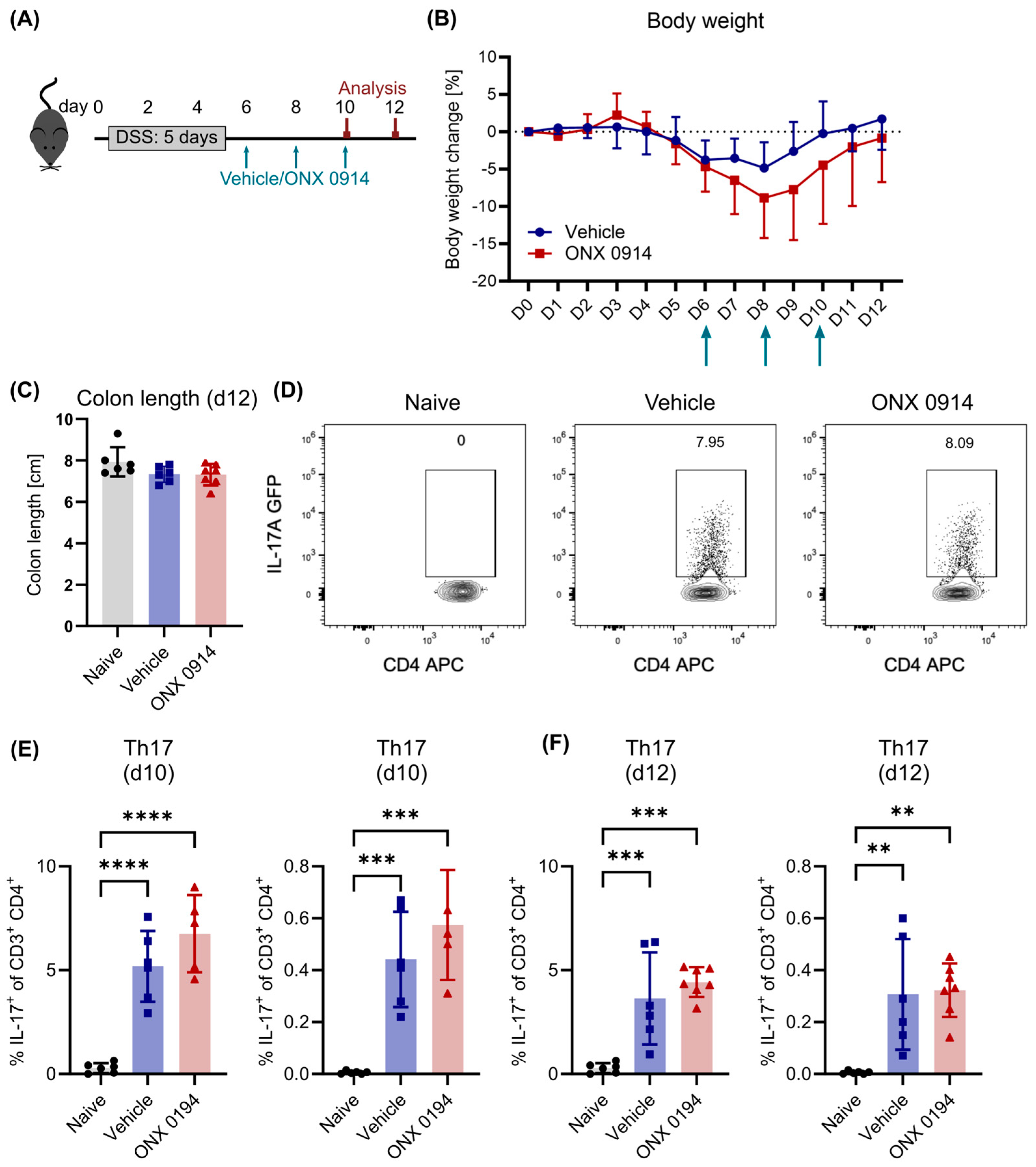
3.2. Immunoproteasome Inhibition Does Not Reduce Differentiated Th17 Cells in DSS-Induced Colitis
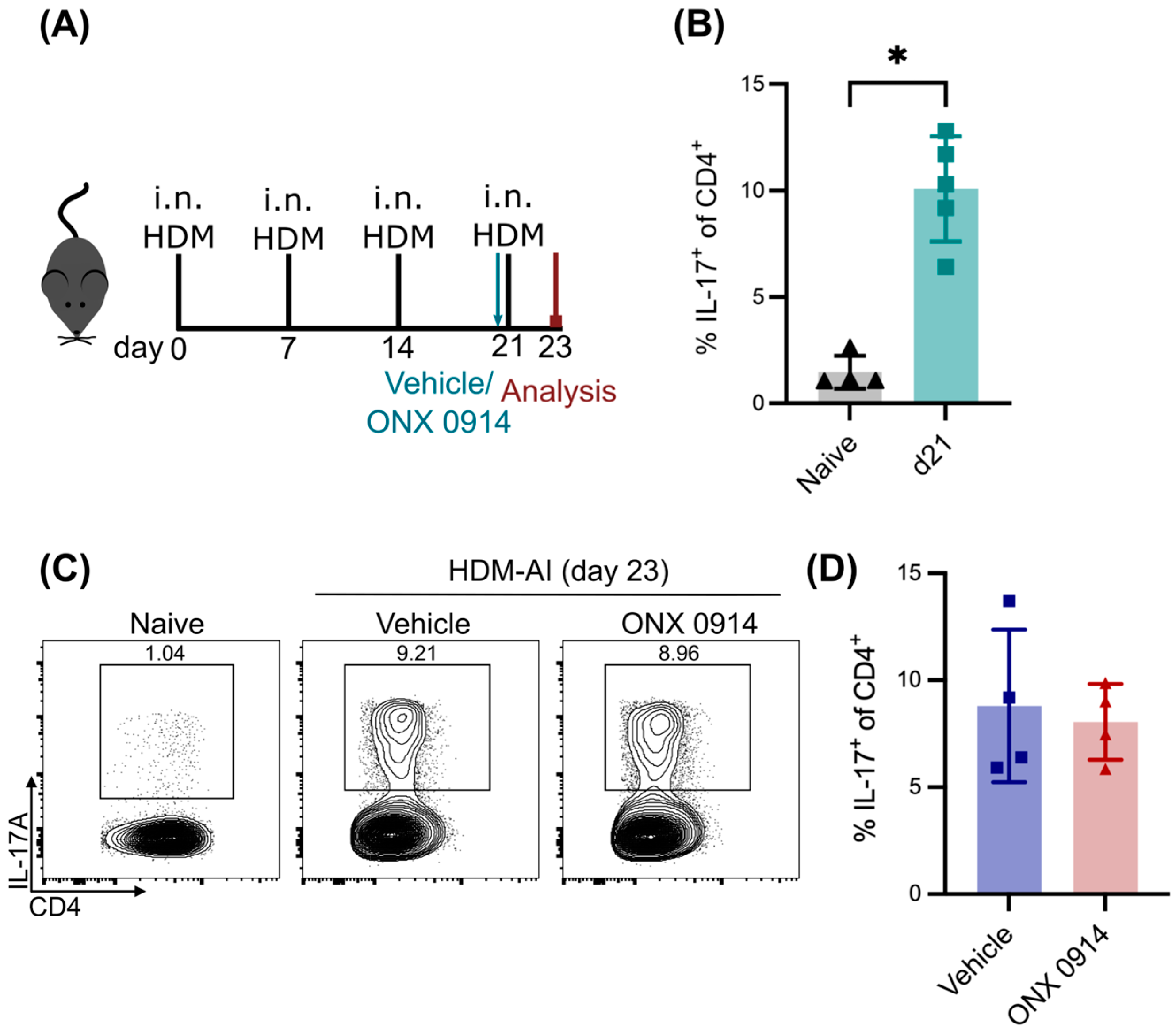
3.3. No Effect of Immunoproteasome Inhibition on Differentiated Th17 Cells in HDM-AI
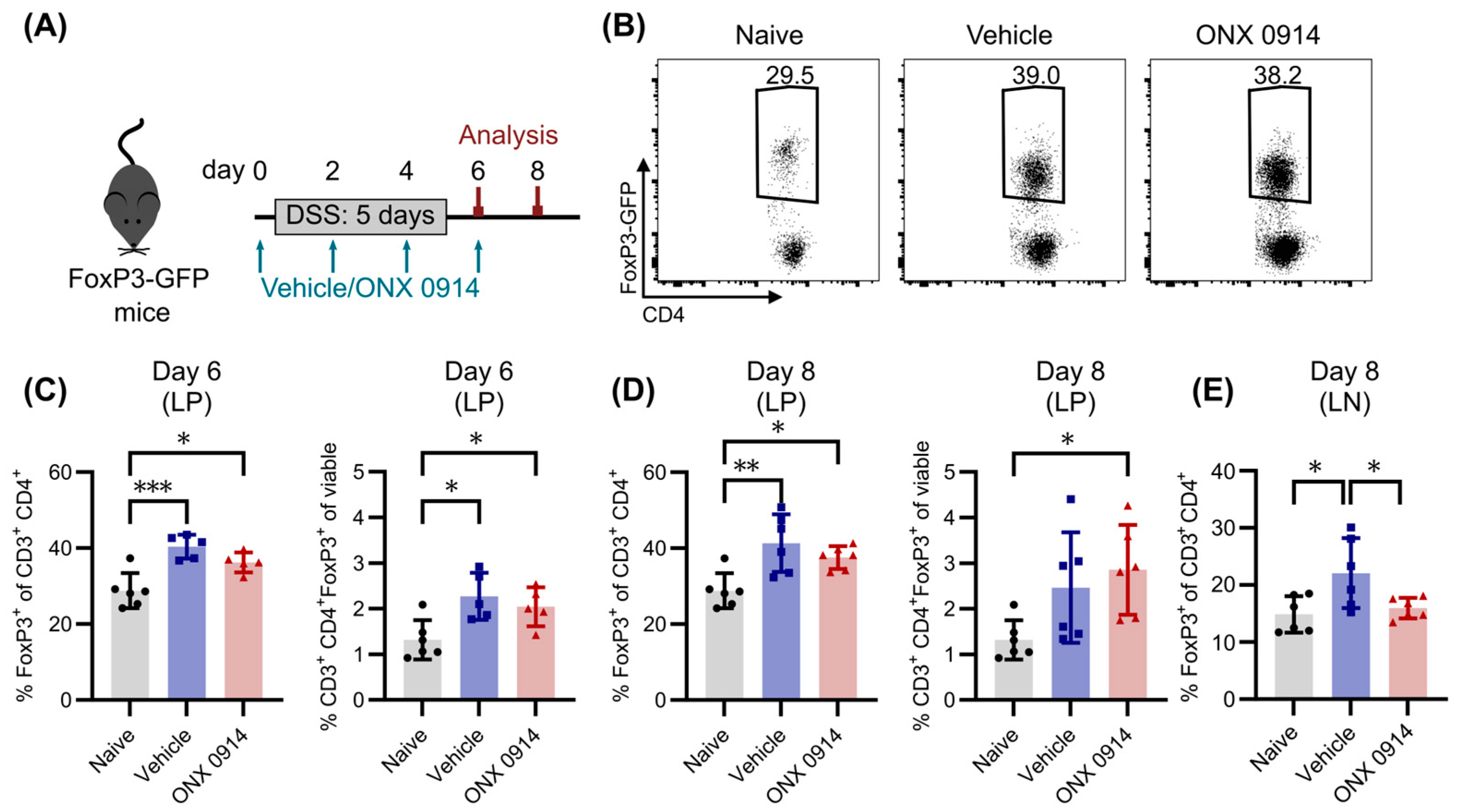
3.4. Immunoproteasome Inhibition Does Not Enhance the Induction of Tregs in DSS-Induced Colitis
3.5. Immunoproteasome Inhibition Slightly Reduces Dendritic Cells in Mesenteric Lymph Nodes
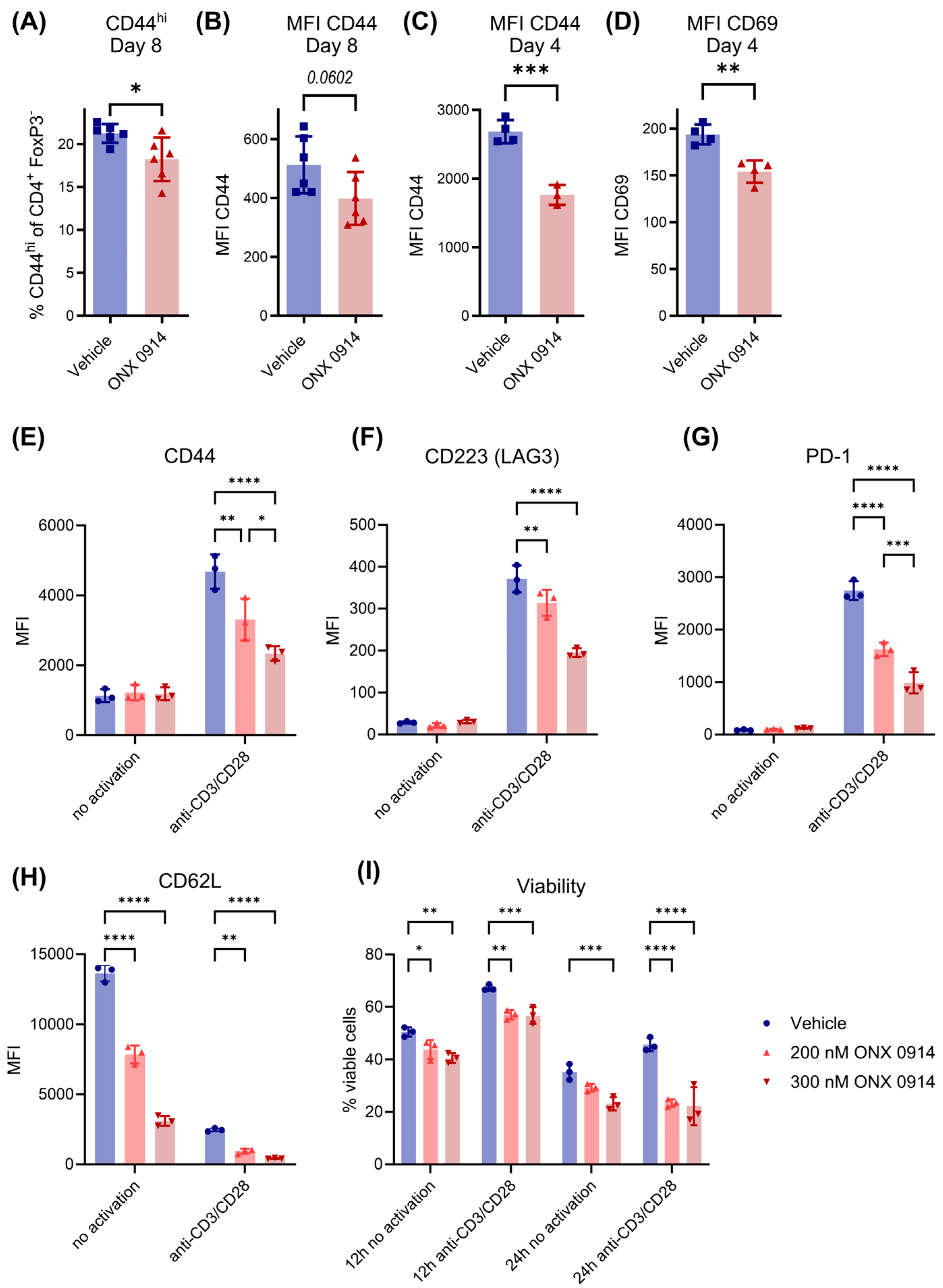
3.6. Immunoproteasome Inhibition Reduces T Cell Activation In Vivo and In Vitro
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | airway inflammation |
| APC | antigen presenting cell |
| DC | dendritic cell |
| DMSO | dimethyl sulfoxide |
| DSS | dextran sulfate sodium |
| EAE | experimental autoimmune encephalomyelitis |
| FCS | fetal calf serum |
| GFP | green fluorescent protein |
| HDM | house dust mite |
| IBD | inflammatory bowel disease |
| IFN-γ | interferon-γ |
| LMP | low molecular weight polypeptide |
| LP(L) | lamina propria (lymphocytes) |
| MECL | multicatalytic endopeptidase complex-like |
| MS | multiple sclerosis |
| PBS | phosphate-buffered saline |
| SD | standard deviation |
| SLE | systemic lupus erythematosus |
| Th | T helper cell |
| Treg | regulatory T cell |
References
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015, 43, 1040–1051. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J., 3rd. Th17 Cells in Immunity and Autoimmunity. Clin. Dev. Immunol. 2013, 2013, 986789. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Schmidt, C.; Berger, T.; Groettrup, M.; Basler, M. Immunoproteasome Inhibition Impairs T and B Cell Activation by Restraining ERK Signaling and Proteostasis. Front. Immunol. 2018, 9, 02386. [Google Scholar] [CrossRef] [PubMed]
- Kalim, K.W.; Basler, M.; Kirk, C.J.; Groettrup, M. Immunoproteasome Subunit LMP7 Deficiency and Inhibition Suppresses Th1 and Th17 but Enhances Regulatory T Cell Differentiation. J. Immunol. 2012, 189, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A Selective Inhibitor of the Immunoproteasome Subunit LMP7 Blocks Cytokine Production and Attenuates Progression of Experimental Arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar] [CrossRef]
- Basler, M.; Dajee, M.; Moll, C.; Groettrup, M.; Kirk, C.J. Prevention of Experimental Colitis by a Selective Inhibitor of the Immunoproteasome. J. Immunol. 2010, 185, 634–641. [Google Scholar] [CrossRef]
- Basler, M.; Mundt, S.; Muchamuel, T.; Moll, C.; Jiang, J.; Groettrup, M.; Kirk, C.J. Inhibition of the Immunoproteasome Ameliorates Experimental Autoimmune Encephalomyelitis. EMBO Mol. Med. 2014, 6, 226–238. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, X.; Tian, J.; Wang, X.X.; Chen, Q.; Rui, K.; Ma, J.; Wang, S.; Wang, Q.; Wang, X.X.; et al. Proteasome Inhibition Suppresses Th17 Cell Generation and Ameliorates Autoimmune Development in Experimental Sjögren’s Syndrome. Cell Mol. Immunol. 2017, 14, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Li, D.; Liu, H.; Ding, Y.; Han, R.; Shi, Y.; Ma, X. PR-957 Mediates Neuroprotection by Inhibiting Th17 Differentiation and Modulating Cytokine Production in a Mouse Model of Ischaemic Stroke. Clin. Exp. Immunol. 2018, 193, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Vachharajani, N.; Pautz, S.; Visekruna, A.; Luu, M.; Steinhoff, U.; Hartmann, S.; Joeris, T.; Jenike, E.; Pantazis, G.; Hofer, M.J.; et al. Prevention of Colitis-Associated Cancer by Selective Targeting of Immunoproteasome Subunit LMP7. Oncotarget 2017, 8, 50447–50459. [Google Scholar] [CrossRef]
- Inholz, K.; Anderl, J.L.; Klawitter, M.; Goebel, H.; Maurits, E.; Kirk, C.J.; Fan, R.A.; Basler, M. Proteasome Composition in Immune Cells Implies Special Immune-Cell-Specific Immunoproteasome Function. Eur. J. Immunol. 2024, 54, 2350613. [Google Scholar] [CrossRef]
- Griffin, B.T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Colbert, R.A. Immunoproteasome Assembly: Cooperative Incorporation of Interferon γ (IFN-γ)–inducible Subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Aki, M.; Shimbara, N.; Takashina, M.; Akiyama, K.; Kagawa, S.; Tamura, T.; Tanahashi, N.; Yoshimura, T.; Tanaka, K.; Ichihara, A. Interferon-γ Induces Different Subunit Organizations and Functional Diversity of Proteasomes1. J. Biochem. 1994, 115, 257–269. [Google Scholar] [CrossRef]
- Boes, B.; Hengel, H.; Ruppert, T.; Multhaup, G.; Koszinowski, U.H.; Kloetzel, P.M. Interferon Gamma Stimulation Modulates the Proteolytic Activity and Cleavage Site Preference of 20S Mouse Proteasomes. J. Exp. Med. 1994, 179, 901–909. [Google Scholar] [CrossRef]
- Akiyama, K.; Kagawa, S.; Tamura, T.; Shimbara, N.; Takashina, M.; Kristensen, P.; Hendil, K.B.; Tanaka, K.; Ichihara, A. Replacement of Proteasome Subunits X and Y by LMP7 and LMP2 Induced by Interferon-γ for Acquirement of the Functional Diversity Responsible for Antigen Processing. FEBS Lett. 2002, 343, 85–88. [Google Scholar] [CrossRef]
- Oliveri, F.; Basler, M.; Rao, T.N.; Fehling, H.J.; Groettrup, M. Immunoproteasome Inhibition Reduces the T Helper 2 Response in Mouse Models of Allergic Airway Inflammation. Front. Immunol. 2022, 13, 870720. [Google Scholar] [CrossRef]
- Liu, H.; Wan, C.; Ding, Y.; Han, R.; He, Y.; Xiao, J.; Hao, J. PR-957, a Selective Inhibitor of Immunoproteasome Subunit Low-MW Polypeptide 7, Attenuates Experimental Autoimmune Neuritis by Suppressing Th17-Cell Differentiation and Regulating Cytokine Production. FASEB J. 2017, 31, 1756–1766. [Google Scholar] [CrossRef]
- Nagayama, Y.; Nakahara, M.; Shimamura, M.; Horie, I.; Arima, K.; Abiru, N. Prophylactic and Therapeutic Efficacies of a Selective Inhibitor of the Immunoproteasome for Hashimoto’s Thyroiditis, but Not for Graves’ Hyperthyroidism, in Mice. Clin. Exp. Immunol. 2012, 168, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.T.; Conley, T.; Muchamuel, T.; Jiang, J.; Lee, S.; Owen, T.; Barnard, J.; Nevarez, S.; Goldman, B.I.; Kirk, C.J.; et al. Beneficial Effect of Novel Proteasome Inhibitors in Murine Lupus via Dual Inhibition of Type I Interferon and Autoantibody-Secreting Cells. Arthritis Rheum. 2012, 64, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-T.; Zhang, P.; Yang, C.-L.; Pang, Y.; Zhang, M.; Zhang, N.; Yue, L.-T.; Li, X.-L.; Li, H.; Duan, R.-S. ONX-0914, a Selective Inhibitor of Immunoproteasome, Ameliorates Experimental Autoimmune Myasthenia Gravis by Modulating Humoral Response. J. Neuroimmunol. 2017, 311, 71–78. [Google Scholar] [CrossRef]
- del Rio Oliva, M.; Kirk, C.J.; Groettrup, M.; Basler, M. Effective Therapy of Polymyositis in Mice via Selective Inhibition of the Immunoproteasome. Eur. J. Immunol. 2022, 52, 1510–1522. [Google Scholar] [CrossRef]
- del Rio Oliva, M.; Mellett, M.; Basler, M. Immunoproteasome Inhibition Attenuates Experimental Psoriasis. Front. Immunol. 2022, 13, 1075615. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Rao, T.N.; Kumar, S.; Pulikkottil, A.J.; Oliveri, F.; Hendriks, R.W.; Beckel, F.; Fehling, H.J. Novel, Non–Gene-Destructive Knock-In Reporter Mice Refute the Concept of Monoallelic GATA-3 Expression. J. Immunol. 2020, 204, 2600–2611. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies (Second Edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Ramachandran, K.V.; Margolis, S.S. A Mammalian Nervous-System-Specific Plasma Membrane Proteasome Complex That Modulates Neuronal Function. Nat. Struct. Mol. Biol. 2017, 24, 419–430. [Google Scholar] [CrossRef]
- Norimoto, A.; Hirose, K.; Iwata, A.; Tamachi, T.; Yokota, M.; Takahashi, K.; Saijo, S.; Iwakura, Y.; Nakajima, H. Dectin-2 Promotes House Dust Mite–Induced T Helper Type 2 and Type 17 Cell Differentiation and Allergic Airway Inflammation in Mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 201–209. [Google Scholar] [CrossRef]
- McGee, H.S.; Stallworth, A.L.; Agrawal, T.; Shao, Z.; Lorence, L.; Agrawal, D.K. Fms-Like Tyrosine Kinase 3 Ligand Decreases T Helper Type 17 Cells and Suppressors of Cytokine Signaling Proteins in the Lung of House Dust Mite–Sensitized and –Challenged Mice. Am. J. Respir. Cell Mol. Biol. 2010, 43, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Gu, J.; Zhou, J.; Wang, Q.; Li, X.; Deng, Z.; Lu, L. Tissue Tregs and Maintenance of Tissue Homeostasis. Front. Cell Dev. Biol. 2021, 9, 717903. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Wang, Y.; Patel, S.R.; Williams, I.R.; Pulendran, B. Lamina Propria Macrophages and Dendritic Cells Differentially Induce Regulatory and Interleukin 17–Producing T Cell Responses. Nat. Immunol. 2007, 8, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Zanker, D.; Dolan, B.P.; David, A.; Hickman, H.D.; Embry, A.C.; Skon, C.N.; Grebe, K.M.; Griffin, T.A.; Chen, W.; et al. Unexpected Role for the Immunoproteasome Subunit LMP2 in Antiviral Humoral and Innate Immune Responses. J. Immunol. 2010, 184, 4115–4122. [Google Scholar] [CrossRef]
- Chapatte, L.; Ayyoub, M.; Morel, S.; Peitrequin, A.-L.; Lévy, N.; Servis, C.; Van den Eynde, B.J.; Valmori, D.; Lévy, F. Processing of Tumor-Associated Antigen by the Proteasomes of Dendritic Cells Controls in Vivo T-Cell Responses. Cancer Res. 2006, 66, 5461–5468. [Google Scholar] [CrossRef]
- French, T.; Israel, N.; Düsedau, H.P.; Tersteegen, A.; Steffen, J.; Cammann, C.; Topfstedt, E.; Dieterich, D.; Schüler, T.; Seifert, U.; et al. The Immunoproteasome Subunits LMP2, LMP7 and MECL-1 Are Crucial Along the Induction of Cerebral Toxoplasmosis. Front. Immunol. 2021, 12, 1047. [Google Scholar] [CrossRef]
- de Verteuil, D.A.; Rouette, A.; Hardy, M.-P.; Lavallée, S.; Trofimov, A.; Gaucher, É.; Perreault, C. Immunoproteasomes Shape the Transcriptome and Regulate the Function of Dendritic Cells. J. Immunol. 2014, 193, 1121–1132. [Google Scholar] [CrossRef]
- Mann, E.R.; Li, X. Intestinal Antigen-Presenting Cells in Mucosal Immune Homeostasis: Crosstalk between Dendritic Cells, Macrophages and B-Cells. World J. Gastroenterol. 2014, 20, 9353–9664. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Schmidt, N.; Gonzalez, E.; Visekruna, A.; Kühl, A.A.; Loddenkemper, C.; Mollenkopf, H.; Kaufmann, S.H.E.; Steinhoff, U.; Joeris, T. Targeting the Proteasome: Partial Inhibition of the Proteasome by Bortezomib or Deletion of the Immunosubunit LMP7 Attenuates Experimental Colitis. Gut 2010, 59, 896–906. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. TTregs, PTregs, and ITregs: Similarities and Differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.U.N.; He, S.; Lv, C.; Sun, X.X.U.N.; Wang, J.; Zheng, W.; Wang, D. Analysis of Murine and Human Treg Subsets in Inflammatory Bowel Disease. Mol. Med. Rep. 2017, 16, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Sprent, J. The Role of Interleukin-2 during Homeostasis and Activation of the Immune System. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Ridwan, B.U.; Tennyson, G.S.; Beagley, K.W.; Bucy, R.P.; Elson, C.O. Dextran Sulfate Sodium-Induced Colitis Occurs in Severe Combined Immunodeficient Mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Basler, M.; Buerger, S.; Engler, H.; Groettrup, M. Inhibiting the Immunoproteasome Exacerbates the Pathogenesis of Systemic Candida Albicans Infection in Mice. Sci. Rep. 2016, 6, 19434. [Google Scholar] [CrossRef]
- Berndt, B.E.; Zhang, M.; Chen, G.-H.; Huffnagle, G.B.; Kao, J.Y. The Role of Dendritic Cells in the Development of Acute Dextran Sulfate Sodium Colitis1. J. Immunol. 2007, 179, 6255–6262. [Google Scholar] [CrossRef]
- Qualls, J.E.; Tuna, H.; Kaplan, A.M.; Cohen, D.A. Suppression of Experimental Colitis in Mice by CD11c+ Dendritic Cells. Inflamm. Bowel Dis. 2009, 15, 236–247. [Google Scholar] [CrossRef]
- Zhou, J.; Lai, W.; Yang, W.; Pan, J.; Shen, H.; Cai, Y.; Yang, C.; Ma, N.; Zhang, Y.; Zhang, R.; et al. BLT1 in Dendritic Cells Promotes Th1/Th17 Differentiation and Its Deficiency Ameliorates TNBS-Induced Colitis. Cell Mol. Immunol. 2018, 15, 1047–1056. [Google Scholar] [CrossRef]
- Flück, K.; Breves, G.; Fandrey, J.; Winning, S. Hypoxia-Inducible Factor 1 in Dendritic Cells Is Crucial for the Activation of Protective Regulatory T Cells in Murine Colitis. Mucosal Immunol. 2016, 9, 379–390. [Google Scholar] [CrossRef]
- Abe, K.; Nguyen, K.P.; Fine, S.D.; Mo, J.-H.; Shen, C.; Shenouda, S.; Corr, M.; Jung, S.; Lee, J.; Eckmann, L.; et al. Conventional Dendritic Cells Regulate the Outcome of Colonic Inflammation Independently of T Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 17022–17027. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Mu, S.; Han, Y.; Chen, Y.; Kuang, Z.; Wu, X.; Luo, Y.; Tong, C.; Zhang, Y.; Yang, Y.; et al. Gpr174 Knockout Alleviates DSS-Induced Colitis via Regulating the Immune Function of Dendritic Cells. Front. Immunol. 2022, 13, 841254. [Google Scholar] [CrossRef] [PubMed]
- Kuttke, M.; Hromadová, D.; Yildirim, C.; Brunner, J.S.; Vogel, A.; Paar, H.; Peters, S.; Weber, M.; Hofmann, M.; Kerndl, M.; et al. PI3K Signaling in Dendritic Cells Aggravates DSS-Induced Colitis. Front. Immunol. 2022, 13, 695576. [Google Scholar] [CrossRef]
- Sula Karreci, E.; Fan, H.; Uehara, M.; Mihali, A.B.; Singh, P.K.; Kurdi, A.T.; Solhjou, Z.; Riella, L.V.; Ghobrial, I.; Laragione, T.; et al. Brief Treatment with a Highly Selective Immunoproteasome Inhibitor Promotes Long-Term Cardiac Allograft Acceptance in Mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8425–E8432. [Google Scholar] [CrossRef] [PubMed]
- Bockstahler, M.; Fischer, A.; Goetzke, C.C.; Neumaier, H.L.; Sauter, M.; Kespohl, M.; Müller, A.M.; Meckes, C.; Salbach, C.; Schenk, M.; et al. Heart-Specific Immune Responses in an Animal Model of Autoimmune-Related Myocarditis Mitigated by an Immunoproteasome Inhibitor and Genetic Ablation. Circulation 2020, 141, 1885–1902. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Wang, J.; Xia, J.; Yin, J.; Cheng, G.; Qian, X.; Jiang, Y.; Ge, X.; Wang, Q. PR-957 Retards Rheumatoid Arthritis Progression and Inflammation by Inhibiting LMP7-Mediated CD4+ T Cell Imbalance. Int. Immunopharmacol. 2023, 124, 110860. [Google Scholar] [CrossRef]
- Basler, M.; Maurits, E.; de Bruin, G.; Koerner, J.; Overkleeft, H.S.; Groettrup, M. Amelioration of Autoimmunity with an Inhibitor Selectively Targeting All Active Centres of the Immunoproteasome. Br. J. Pharmacol. 2018, 175, 38–52. [Google Scholar] [CrossRef]
- Basler, M.; Lindstrom, M.M.; LaStant, J.J.; Bradshaw, J.M.; Owens, T.D.; Schmidt, C.; Maurits, E.; Tsu, C.; Overkleeft, H.S.; Kirk, C.J.; et al. Co-Inhibition of Immunoproteasome Subunits LMP2 and LMP7 Is Required to Block Autoimmunity. EMBO Rep. 2018, 19, e46512. [Google Scholar] [CrossRef]
- Johnson, H.W.B.; Lowe, E.; Anderl, J.L.; Fan, A.; Muchamuel, T.; Bowers, S.; Moebius, D.C.; Kirk, C.; McMinn, D.L. Required Immunoproteasome Subunit Inhibition Profile for Anti-Inflammatory Efficacy and Clinical Candidate KZR-616 ((2S,3R)-N-((S)-3-(Cyclopent-1-En-1-Yl)-1-((R)-2-Methyloxiran-2-Yl)-1-Oxopropan-2-Yl)-3-Hydroxy-3-(4-Methoxyphenyl)-2-((S)-2-(2-Morpholinoac. J. Med. Chem. 2018, 61, 11127–11143. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveri, F.; Mink, D.; Muchamuel, T.; Basler, M. Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells. Cells 2025, 14, 689. https://doi.org/10.3390/cells14100689
Oliveri F, Mink D, Muchamuel T, Basler M. Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells. Cells. 2025; 14(10):689. https://doi.org/10.3390/cells14100689
Chicago/Turabian StyleOliveri, Franziska, Dennis Mink, Tony Muchamuel, and Michael Basler. 2025. "Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells" Cells 14, no. 10: 689. https://doi.org/10.3390/cells14100689
APA StyleOliveri, F., Mink, D., Muchamuel, T., & Basler, M. (2025). Immunoproteasome Inhibition Impairs Differentiation but Not Survival of T Helper 17 Cells. Cells, 14(10), 689. https://doi.org/10.3390/cells14100689






