The Contribution of Innate Immunity in Large-Vessel Vasculitis: Detangling New Pathomechanisms beyond the Onset of Vascular Inflammation
Abstract
1. Introduction
2. Dendritic Cells Drive the Dysregulated Immune Response within the Arterial Wall
3. Macrophages Are Critically Involved across All Phases of Vascular Inflammation
4. Polymorphonuclear Leukocytes: An Heterogenous Group of Cells Deeply Involved in LVV Pathogenesis
5. Mast Cells Display Pleiotropic Functions in LVV with a Possible Dichotomic Behavior in Boosting Inflammation
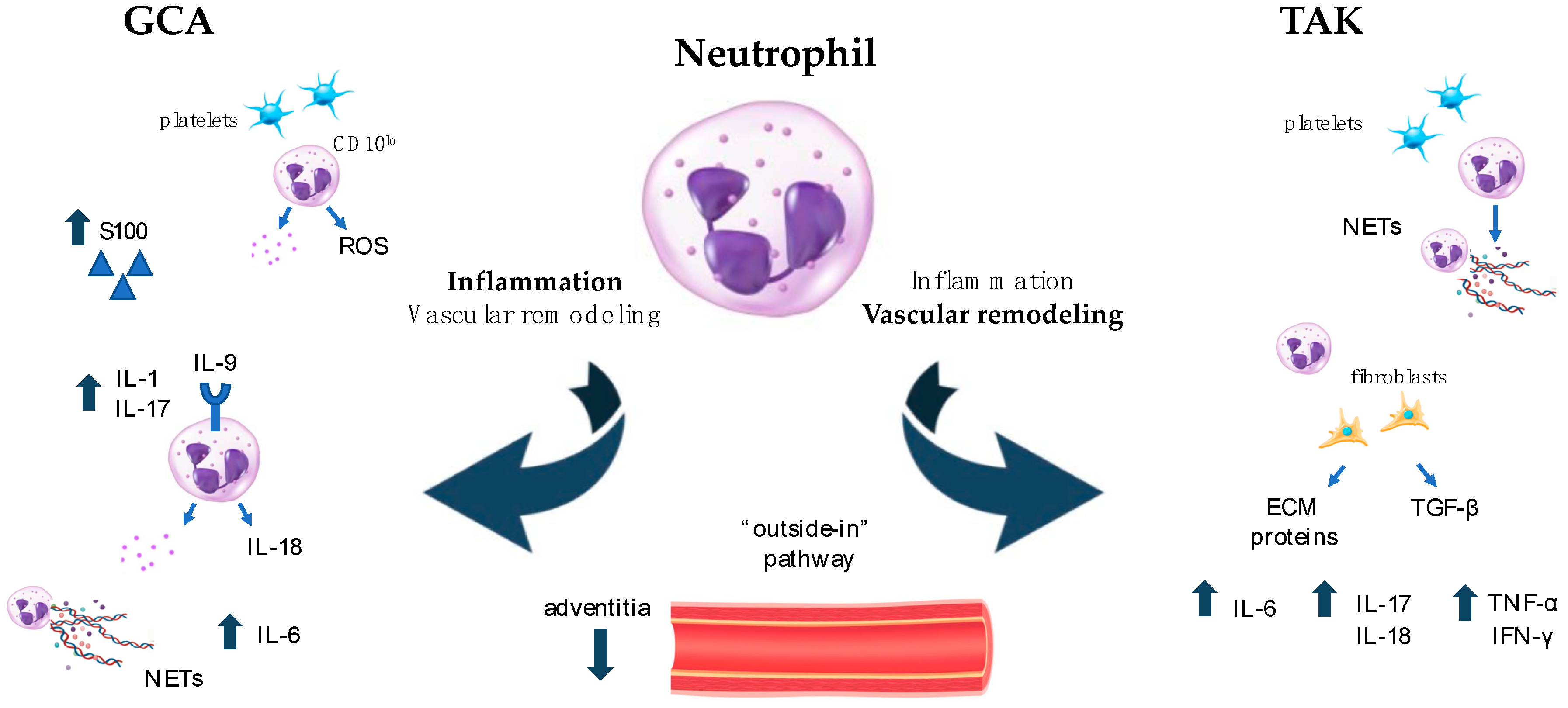
6. Neutrophils: Old Cells with Newly Described Functions and Plasticity in Driving Both Inflammation and Fibrosis in LVV
7. Other Immune Pathways Involved in Innate Immunity: Focus on Complement System and Inflammasome Activation in LVV
7.1. Complement System
7.2. Inflammasome
8. Conclusions and Future Therapeutic Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Pugh, D.; Karabayas, M.; Basu, N.; Cid, M.C.; Goel, R.; Goodyear, C.S.; Grayson, P.C.; McAdoo, S.P.; Mason, J.C.; Owen, C.; et al. Large-Vessel Vasculitis. Nat. Rev. Dis. Primers 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Svensson, L.G.; Arafat, A.; Roselli, E.E.; Idrees, J.; Clifford, A.; Tan, C.; Hoffman, G.; Eng, C.; Langford, C.; Rodriguez, E.R.; et al. Inflammatory Disease of the Aorta: Patterns and Classification of Giant Cell Aortitis, Takayasu Arteritis, and Nonsyndromic Aortitis. J. Thorac. Cardiovasc. Surg. 2015, 149, S170–S175. [Google Scholar] [CrossRef]
- Salvarani, C.; Macchioni, P.; Rossi, F.; Castri, C.; Capozzoli, N.; Baricchi, R.; Boiardi, L.; Chiaravalloti, F.; Portioli, I.; Zizzi, F.; et al. Epidemiologic and Immunogenetic Aspects of Polymyalgia Rheumatica and Giant Cell Arteritis in Northern Italy. Arthritis Rheum. 1991, 34, 351–356. [Google Scholar] [CrossRef]
- Zaldivar Villon, M.L.F.; De La Rocha, J.A.L.; Espinoza, L.R. Takayasu Arteritis: Recent Developments. Curr. Rheumatol. Rep. 2019, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Liao, Y.J.; Kim, J.W.; Goronzy, J.J.; Weyand, C.M. Giant Cell Arteritis: Immune and Vascular Aging as Disease Risk Factors. Arthritis Res. Ther. 2011, 13, 231. [Google Scholar] [CrossRef]
- Gruver, A.; Hudson, L.; Sempowski, G. Immunosenescence of Ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Brandes, J.C.; Schmidt, D.; Fulbright, J.W.; Goronzy, J.J. Functional Properties of CD4+CD28− T Cells in the Aging Immune System. Mech. Ageing Dev. 1998, 102, 131–147. [Google Scholar] [CrossRef]
- Adrover, J.M.; Nicolás-Ávila, J.A.; Hidalgo, A. Aging: A Temporal Dimension for Neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Akiyama, M.; Ohtsuki, S.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Innate and Adaptive Immunity in Giant Cell Arteritis. Front. Immunol. 2021, 11, 621098. [Google Scholar] [CrossRef]
- Galli, E.; Muratore, F.; Boiardi, L.; Restuccia, G.; Cavazza, A.; Catanoso, M.; Macchioni, P.; Spaggiari, L.; Casali, M.; Pipitone, N.; et al. Significance of Inflammation Restricted to Adventitial/Periadventitial Tissue on Temporal Artery Biopsy. Semin. Arthritis Rheum. 2020, 50, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Immune Mechanisms in Medium and Large-Vessel Vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Kermani, T.A. Takayasu Arteritis and Giant Cell Arteritis: Are They a Spectrum of the Same Disease? Int. J. Rheum. Dis. 2019, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Younge, B.R.; Olshen, R.A.; Goronzy, J.J.; Weyand, C.M. Th17 and Th1 T-Cell Responses in Giant Cell Arteritis. Circulation 2010, 121, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, D.; Garrido, M.; Comarmond, C.; Desbois, A.C.; Domont, F.; Savey, L.; Terrier, B.; Geri, G.; Rosenzwajg, M.; Klatzmann, D.; et al. Th1 and Th17 Cytokines Drive Inflammation in Takayasu Arteritis. Arthritis Rheumatol. 2015, 67, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Watanabe, R.; Zhang, H.; Akiyama, M.; Berry, G.J.; Goronzy, J.J. Cytokines, Growth Factors and Proteases in Medium and Large Vessel Vasculitis. Clin. Immunol. 2019, 206, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory Checkpoint Deficiency in Medium and Large Vessel Vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; La Barbera, L.; Miceli, G.; Tuttolomondo, A.; Guggino, G. The Innate Face of Giant Cell Arteritis: Insight into Cellular and Molecular Innate Immunity Pathways to Unravel New Possible Biomarkers of Disease. Front. Mol. Med. 2022, 2, 933161. [Google Scholar] [CrossRef]
- Ma-Krupa, W.; Jeon, M.-S.; Spoerl, S.; Tedder, T.F.; Goronzy, J.J.; Weyand, C.M. Activation of Arterial Wall Dendritic Cells and Breakdown of Self-Tolerance in Giant Cell Arteritis. J. Exp. Med. 2004, 199, 173–183. [Google Scholar] [CrossRef]
- Watanabe, R.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Cellular Signaling Pathways in Medium and Large Vessel Vasculitis. Front. Immunol. 2020, 11, 587089. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Getz, T.M.; Padmanabhan, R.; Villa-Forte, A.; Clifford, A.H.; Funchain, P.; Sankunny, M.; Perry, J.D.; Blandford, A.; Kosmorsky, G.; et al. The Microbiome of Temporal Arteries. Pathog. Immun. 2019, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Piggott, K.; Biousse, V.; Newman, N.J.; Goronzy, J.J.; Weyand, C.M. Vascular Damage in Giant Cell Arteritis. Autoimmunity 2009, 42, 596–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, J.; Ma-Krupa, W.; Gewirtz, A.T.; Younge, B.R.; Goronzy, J.J.; Weyand, C.M. Toll-Like Receptors 4 and 5 Induce Distinct Types of Vasculitis. Circ. Res. 2009, 104, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Grayson, P.C.; Merkel, P.A.; Tomasson, G. Infections and the Risk of Incident Giant Cell Arteritis: A Population-Based, Case-Control Study. Ann. Rheum. Dis. 2017, 76, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Macaluso, F.; Fasano, S.; Grasso, G.; Ciccia, F.; Guggino, G. Microbiome Changes in Connective Tissue Diseases and Vasculitis: Focus on Metabolism and Inflammation. Int. J. Mol. Sci. 2022, 23, 6532. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Ma-Krupa, W.; Pryshchep, O.; Gröschel, S.; Bernardino, R.; Goronzy, J.J. Vascular Dendritic Cells in Giant Cell Arteritis. Ann. N. Y. Acad. Sci. 2005, 1062, 195–208. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Micaily, I.; Chernoff, M. An Unknown Reaction to Pembrolizumab: Giant Cell Arteritis. Ann. Oncol. 2017, 28, 2621–2622. [Google Scholar] [CrossRef] [PubMed]
- Narala, R.; Reddy, S.A.; Mruthyunjaya, P. Giant Cell Arteritis Manifesting as Retinal Arterial Occlusion and Paracentral Acute Middle Maculopathy in a Patient on Pembrolizumab for Metastatic Uveal Melanoma. Am. J. Ophthalmol. Case Rep. 2020, 20, 100891. [Google Scholar] [CrossRef]
- Betrains, A.E.; Blockmans, D.E. Immune Checkpoint Inhibitor-Associated Polymyalgia Rheumatica/Giant Cell Arteritis Occurring in a Patient after Treatment with Nivolumab. J. Clin. Rheumatol. 2021, 27, S555–S556. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Zhang, H.; Berry, G.; Goronzy, J.J.; Weyand, C.M. Immune Checkpoint Dysfunction in Large and Medium Vessel Vasculitis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1052–H1059. [Google Scholar] [CrossRef] [PubMed]
- Mirault, T.; Guillet, H.; Messas, E. Immune response in Takayasu arteritis. Presse Médicale 2017, 46, e189–e196. [Google Scholar] [CrossRef] [PubMed]
- Inder, S.J.; Bobryshev, Y.V.; Cherian, S.M.; Albert Lord, R.S.; Masuda, K.; Yutani, C. Accumulation of Lymphocytes, Dendritic Cells, and Granulocytes in the Aortic Wall Affected by Takayasu’s Disease. Angiology 2000, 51, 565–579. [Google Scholar] [CrossRef]
- Kurata, A.; Saito, A.; Hashimoto, H.; Fujita, K.; Ohno, S.; Kamma, H.; Nagao, T.; Kobayashi, S.; Yamashina, A.; Kuroda, M. Difference in Immunohistochemical Characteristics between Takayasu Arteritis and Giant Cell Arteritis: It May Be Better to Distinguish Them in the Same Age. Mod. Rheumatol. 2019, 29, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Muratore, F.; Boiardi, L.; Restuccia, G.; Pipitone, N.; Pazzola, G.; Tagliavini, E.; Ragazzi, M.; Rossi, G.; Salvarani, C. Inflamed Temporal Artery: Histologic Findings in 354 Biopsies, with Clinical Correlations. Am. J. Surg. Pathol. 2014, 38, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Esen, I.; Jiemy, W.F.; Van Sleen, Y.; Van Der Geest, K.S.M.; Sandovici, M.; Heeringa, P.; Boots, A.M.H.; Brouwer, E. Functionally Heterogenous Macrophage Subsets in the Pathogenesis of Giant Cell Arteritis: Novel Targets for Disease Monitoring and Treatment. J. Clin. Med. 2021, 10, 4958. [Google Scholar] [CrossRef]
- Van Sleen, Y.; Wang, Q.; Van Der Geest, K.S.M.; Westra, J.; Abdulahad, W.H.; Heeringa, P.; Boots, A.M.H.; Brouwer, E. Involvement of Monocyte Subsets in the Immunopathology of Giant Cell Arteritis. Sci. Rep. 2017, 7, 6553. [Google Scholar] [CrossRef]
- Santos, J.P.; Artigiani Neto, R.; Mangueira, C.L.P.; Filippi, R.Z.; Gutierrez, P.S.; Westra, J.; Brouwer, E.; De Souza, A.W.S. Associations between Clinical Features and Therapy with Macrophage Subpopulations and T Cells in Inflammatory Lesions in the Aorta from Patients with Takayasu Arteritis. Clin. Exp. Immunol. 2020, 202, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Seo, N.; Torii, M.; Ma, N.; Muraoka, D.; Tawara, I.; Masuya, M.; Tanaka, K.; Takei, Y.; Shiku, H.; et al. Interleukin-17 Induces an Atypical M2-Like Macrophage Subpopulation That Regulates Intestinal Inflammation. PLoS ONE 2014, 9, e108494. [Google Scholar] [CrossRef] [PubMed]
- Van Sleen, Y.; Jiemy, W.F.; Pringle, S.; Van Der Geest, K.S.M.; Abdulahad, W.H.; Sandovici, M.; Brouwer, E.; Heeringa, P.; Boots, A.M.H. A Distinct Macrophage Subset Mediating Tissue Destruction and Neovascularization in Giant Cell Arteritis: Implication of the YKL-40/Interleukin-13 Receptor A2 Axis. Arthritis Rheumatol. 2021, 73, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Jiemy, W.F.; Van Sleen, Y.; Van Der Geest, K.S.; Ten Berge, H.A.; Abdulahad, W.H.; Sandovici, M.; Boots, A.M.; Heeringa, P.; Brouwer, E. Distinct Macrophage Phenotypes Skewed by Local Granulocyte Macrophage Colony-stimulating Factor (GM-CSF) and Macrophage Colony-stimulating Factor (M-CSF) Are Associated with Tissue Destruction and Intimal Hyperplasia in Giant Cell Arteritis. Clin. Transl. Immunol. 2020, 9, e1164. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pla, A.; Bosch-Gil, J.A.; Rosselló-Urgell, J.; Huguet-Redecilla, P.; Stone, J.H.; Vilardell-Tarres, M. Metalloproteinase-2 and -9 in Giant Cell Arteritis: Involvement in Vascular Remodeling. Circulation 2005, 112, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Maeda, T.; Zhang, H.; Berry, G.J.; Zeisbrich, M.; Brockett, R.; Greenstein, A.E.; Tian, L.; Goronzy, J.J.; Weyand, C.M. MMP (Matrix Metalloprotease)-9–Producing Monocytes Enable T Cells to Invade the Vessel Wall and Cause Vasculitis. Circ. Res. 2018, 123, 700–715. [Google Scholar] [CrossRef]
- Kaiser, M.; Younge, B.; Björnsson, J.; Goronzy, J.J.; Weyand, C.M. Formation of New Vasa Vasorum in Vasculitis. Am. J. Pathol. 1999, 155, 765–774. [Google Scholar] [CrossRef]
- Watanabe, R.; Hilhorst, M.; Zhang, H.; Zeisbrich, M.; Berry, G.J.; Wallis, B.B.; Harrison, D.G.; Giacomini, J.C.; Goronzy, J.J.; Weyand, C.M. Glucose Metabolism Controls Disease-Specific Signatures of Macrophage Effector Functions. JCI Insight 2018, 3, e123047. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Wang, C.; Watanabe, R.; Zhang, H.; Akiyama, M.; Bois, M.C.; Maleszewski, J.J.; Warrington, K.J.; Berry, G.J.; Goronzy, J.J.; et al. Deficiency of the CD155-CD96 Immune Checkpoint Controls IL-9 Production in Giant Cell Arteritis. Cell Rep. Med. 2023, 4, 101012. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Rizzo, A.; Guggino, G.; Cavazza, A.; Alessandro, R.; Maugeri, R.; Cannizzaro, A.; Boiardi, L.; Iacopino, D.G.; Salvarani, C.; et al. Difference in the Expression of IL-9 and IL-17 Correlates with Different Histological Pattern of Vascular Wall Injury in Giant Cell Arteritis. Rheumatology 2015, 54, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Khoury, P.; Grayson, P.C.; Klion, A.D. Eosinophils in Vasculitis: Characteristics and Roles in Pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 474–483. [Google Scholar] [CrossRef]
- Schnabel, A.; Csernok, E.; Braun, J.; Gross, W.L. Activation of Neutrophils, Eosinophils, and Lymphocytes in the Lower Respiratory Tract in Wegener’s Granulomatosis. Am. J. Respir. Crit. Care Med. 2000, 161, 399–405. [Google Scholar] [CrossRef]
- Terai, M.; Yasukawa, K.; Honda, T.; Jibiki, T.; Hirano, K.; Sato, J.; Ishiwada, N.; Seguchi, M.; Ueda, S.; Kohno, Y. Peripheral Blood Eosinophilia and Eosinophil Accumulation in Coronary Microvessels in Acute Kawasaki Disease. Pediatr. Infect. Dis. J. 2002, 21, 777–780. [Google Scholar] [CrossRef]
- Bahrami, S.; Malone, J.C.; Webb, K.G.; Callen, J.P. Tissue Eosinophilia as an Indicator of Drug-Induced Cutaneous Small-Vessel Vasculitis. Arch. Dermatol. 2006, 142, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Fujiya, M.; Kashima, S.; Sugiyama, Y.; Iwama, T.; Ijiri, M.; Tanaka, K.; Takahashi, K.; Ando, K.; Nomura, Y.; Ueno, N.; et al. Takayasu’s Arteritis Associated with Eosinophilic Gastroenteritis, Possibly via the Overactivation of Th17. Gut Pathog. 2018, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Benavides, R.; Ramírez-Peralta, A.F.; Muñoz-Urbano, M.; Mejía, L.; Cardona-Cardona, A.F.; Muñoz-Vahos, C.H. Temporal Arteritis Caused by Eosinophilic Vasculitis Associated with a Lymphocytic Variant of the Hypereosinophilic Syndrome: A Case Report. Rev. Colomb. Reumatol. 2023; in press. [Google Scholar] [CrossRef]
- Beaven, M.A. Our Perception of the Mast Cell from Paul Ehrlich to Now. Eur. J. Immunol. 2009, 39, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.F. The Role of Mast Cells in Wound Healing. Int. Wound J. 2010, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- De Souza Junior, D.; Mazucato, V.; Santana, A.; Oliver, C.; Jamur, M. Mast Cells Interact with Endothelial Cells to Accelerate In Vitro Angiogenesis. Int. J. Mol. Sci. 2017, 18, 2674. [Google Scholar] [CrossRef] [PubMed]
- Mäyränpää, M.I.; Trosien, J.A.; Fontaine, V.; Folkesson, M.; Kazi, M.; Eriksson, P.; Swedenborg, J.; Hedin, U. Mast Cells Associate with Neovessels in the Media and Adventitia of Abdominal Aortic Aneurysms. J. Vasc. Surg. 2009, 50, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Sibilano, R.; Frossi, B.; Pucillo, C.E. Mast Cell Activation: A Complex Interplay of Positive and Negative Signaling Pathways. Eur. J. Immunol. 2014, 44, 2558–2566. [Google Scholar] [CrossRef]
- Levick, S.P.; Melendez, G.C.; Plante, E.; McLarty, J.L.; Brower, G.L.; Janicki, J.S. Cardiac Mast Cells: The Centrepiece in Adverse Myocardial Remodelling. Cardiovasc. Res. 2011, 89, 12–19. [Google Scholar] [CrossRef]
- Bot, I.; Biessen, E. Mast Cells in Atherosclerosis. Thromb. Haemost. 2011, 106, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Alessandro, R.; Rizzo, A.; Raimondo, S.; Giardina, A.; Raiata, F.; Boiardi, L.; Cavazza, A.; Guggino, G.; De Leo, G.; et al. IL-33 Is Overexpressed in the Inflamed Arteries of Patients with Giant Cell Arteritis. Ann. Rheum. Dis. 2013, 72, 258–264. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast Cells Enhance T Cell Activation: Importance of Mast Cell Costimulatory Molecules and Secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Mäyränpää, M.I.; Trosien, J.A.; Nikkari, S.T.; Kovanen, P.T. Mast Cells Associate with T-Cells and Neointimal Microvessels in Giant Cell Arteritis. Clin. Exp. Rheumatol. 2008, 26, S63–S66. [Google Scholar] [PubMed]
- Misra, D.P.; Singh, K.; Sharma, A.; Agarwal, V. Arterial Wall Fibrosis in Takayasu Arteritis and Its Potential for Therapeutic Modulation. Front. Immunol. 2023, 14, 1174249. [Google Scholar] [CrossRef]
- Le Joncour, A.; Desbois, A.-C.; Leroyer, A.S.; Tellier, E.; Régnier, P.; Maciejewski-Duval, A.; Comarmond, C.; Barete, S.; Arock, M.; Bruneval, P.; et al. Mast Cells Drive Pathologic Vascular Lesions in Takayasu Arteritis. J. Allergy Clin. Immunol. 2022, 149, 292–301.e3. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-Y.; Smrž, D.; Desai, A.; Bandara, G.; Ito, T.; Iwaki, S.; Kang, J.-H.; Andrade, M.V.; Hilderbrand, S.C.; Brown, J.M.; et al. IL-33 Induces a Hyporesponsive Phenotype in Human and Mouse Mast Cells. J. Immunol. 2013, 190, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast Cells Are Essential Intermediaries in Regulatory T-Cell Tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.; Mustelin, T.; Lood, C. Role of Neutrophils in Systemic Vasculitides. Front. Immunol. 2020, 11, 619705. [Google Scholar] [CrossRef]
- Aymonnier, K.; Amsler, J.; Lamprecht, P.; Salama, A.; Witko-Sarsat, V. The Neutrophil: A Key Resourceful Agent in Immune-mediated Vasculitis. Immunol. Rev. 2023, 314, 326–356. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and Therapeutic Interventions for ANCA-Associated Vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef]
- Michailidou, D.; Duvvuri, B.; Kuley, R.; Cuthbertson, D.; Grayson, P.C.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; Moreland, L.W.; et al. Neutrophil Activation in Patients with Anti-Neutrophil Cytoplasmic Autoantibody-Associated Vasculitis and Large-Vessel Vasculitis. Arthritis Res. Ther. 2022, 24, 160. [Google Scholar] [CrossRef]
- Foell, D.; Hernández-Rodríguez, J.; Sánchez, M.; Vogl, T.; Cid, M.C.; Roth, J. Early Recruitment of Phagocytes Contributes to the Vascular Inflammation of Giant Cell Arteritis. J. Pathol. 2004, 204, 311–316. [Google Scholar] [CrossRef]
- Springer, J.M.; Monach, P.; Cuthbertson, D.; Carette, S.; Khalidi, N.A.; McAlear, C.A.; Pagnoux, C.; Seo, P.; Warrington, K.J.; Ytterberg, S.R.; et al. Serum S100 Proteins as a Marker of Disease Activity in Large Vessel Vasculitis. J. Clin. Rheumatol. 2018, 24, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.-H.; Stirnemann, J.; Liozon, E.; Michel, M.; Fain, O.; Fauchais, A.-L. Interleukin-1 Blockade in Refractory Giant Cell Arteritis. Jt. Bone Spine 2014, 81, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Lindén, A. Interleukin-17 Family Members and Inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, L.; Haroche, J.; Mathian, A.; Gorochov, G.; Amoura, Z. Pathogenesis of Takayasu’s Arteritis: A 2011 Update. Autoimmun. Rev. 2011, 11, 61–67. [Google Scholar] [CrossRef]
- Michailidou, D.; Kuley, R.; Wang, T.; Hermanson, P.; Grayson, P.C.; Cuthbertson, D.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; et al. Neutrophil Extracellular Trap Formation in Anti-Neutrophil Cytoplasmic Antibody-Associated and Large-Vessel Vasculitis. Clin. Immunol. 2023, 249, 109274. [Google Scholar] [CrossRef] [PubMed]
- Palamidas, D.A.; Argyropoulou, O.D.; Georgantzoglou, N.; Karatza, E.; Xingi, E.; Kapsogeorgou, E.K.; Anagnostopoulos, C.D.; Lazaris, A.C.; Ritis, K.; Goules, A.V.; et al. Neutrophil Extracellular Traps in Giant Cell Arteritis Biopsies: Presentation, Localization and Co-Expression with Inflammatory Cytokines. Rheumatology 2022, 61, 1639–1644. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yasuoka, H.; Yoshimoto, K.; Suzuki, K.; Takeuchi, T. Platelet CXCL4 Mediates Neutrophil Extracellular Traps Formation in ANCA-Associated Vasculitis. Sci. Rep. 2021, 11, 222. [Google Scholar] [CrossRef]
- Nakazawa, D.; Tomaru, U.; Yamamoto, C.; Jodo, S.; Ishizu, A. Abundant Neutrophil Extracellular Traps in Thrombus of Patient with Microscopic Polyangiitis. Front. Immun. 2012, 3, 333. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Kamp, V.M.; Van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A Subset of Neutrophils in Human Systemic Inflammation Inhibits T Cell Responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Dalli, J.; Hollywood, J.; Mason, J.C.; Dasgupta, B.; Perretti, M. Investigational Analysis Reveals a Potential Role for Neutrophils in Giant-Cell Arteritis Disease Progression. Circ. Res. 2014, 114, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ai, Z.; Khoyratty, T.; Zec, K.; Eames, H.L.; Van Grinsven, E.; Hudak, A.; Morris, S.; Ahern, D.; Monaco, C.; et al. ROS-Producing Immature Neutrophils in Giant Cell Arteritis Are Linked to Vascular Pathologies. JCI Insight 2020, 5, e139163. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Wannemacher, J.; Christ, S.; Koopmans, T.; Kadri, S.; Zhao, J.; Gouda, M.; Ye, H.; Mück-Häusl, M.; Krenn, P.W.; et al. Neutrophils Direct Preexisting Matrix to Initiate Repair in Damaged Tissues. Nat. Immunol. 2022, 23, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Curaj, A.; Schumacher, D.; Rusu, M.; Staudt, M.; Li, X.; Simsekyilmaz, S.; Jankowski, V.; Jankowski, J.; Dumitraşcu, A.R.; Hausenloy, D.J.; et al. Neutrophils Modulate Fibroblast Function and Promote Healing and Scar Formation after Murine Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 3685. [Google Scholar] [CrossRef] [PubMed]
- Pulli, R.; Dorigo, W.; Pratesi, G.; Fargion, A.; Pratesi, C. Single-Center Experience on Endovascular Repair of Noninfected Extracranial Internal Carotid Artery Pseudoaneurysms. Ann. Vasc. Surg. 2013, 27, e13–e672. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, P.; Rawat, A.; Sharma, M.; Singh, S. Complement in Autoimmune Diseases. Clin. Chim. Acta 2017, 465, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Trivioli, G.; Vaglio, A. The Rise of Complement in ANCA-Associated Vasculitis: From Marginal Player to Target of Modern Therapy. Clin. Exp. Immunol. 2020, 202, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Hou, R.; Xu, K.; Han, Y.; Hu, J.; Zhang, Y.; Su, Y.; Gao, J.; Zhang, G.; Zhang, L. Pentraxin 3 Is More Accurate than C-Reactive Protein for Takayasu Arteritis Activity Assessment: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0245612. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, V.S.; Brossart, P.; Warrington, K.J.; Kurts, C.; Sendtner, G.W.; Aden, C.A. The Role of Autoimmunity and Autoinflammation in Giant Cell Arteritis: A Systematic Literature Review. Autoimmun. Rev. 2023, 22, 103328. [Google Scholar] [CrossRef] [PubMed]
- Jayakanthan, K.; Gupta, A.N.; Mathew, J.; Ravindran, R.; Mahasampth, G.; Danda, D. Clinical Utility of Anti-C1q Antibody in Primary and Secondary Vasculitic Conditions. Int. J. Health Sci. 2017, 11, 3–6. [Google Scholar]
- Potlukova, E.; Kralikova, P. Complement Component C1q and Anti-C1q Antibodies in Theory and in Clinical Practice. Scand. J. Immunol. 2008, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rongyi, C.; Xiaojuan, D.; Jinghua, W.; Lingying, M.; Xiaomin, D.; Lili, M.; Huiyong, C.; Lindi, J.; Ying, S. High Level of Serum Complement 3 Is a Risk Factor for Vascular Stenosis Progression in TA Patients Receiving Tocilizumab: A Prospective Observational Study. Arthritis Res. Ther. 2023, 25, 137. [Google Scholar] [CrossRef]
- Chen, R.; Ma, L.; Lv, P.; Lin, J.; Li, C.; Yan, Y.; Jin, X.; Dai, X.; Ji, Z.; Chen, H.; et al. Serum Complement 3 Is a Potential Biomarker for Assessing Disease Activity in Takayasu Arteritis. Arthritis Res. Ther. 2021, 23, 63. [Google Scholar] [CrossRef]
- Ma, J.; Luo, X.; Wu, Q.; Chen, Z.; Kou, L.; Wang, H. Circulation Levels of Acute Phase Proteins in Patients with Takayasu Arteritis. J. Vasc. Surg. 2010, 51, 700–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Takahashi, M. NLRP3 Inflammasome as a Key Driver of Vascular Disease. Cardiovasc. Res. 2022, 118, 372–385. [Google Scholar] [CrossRef]
- Wortmann, M.; Peters, A.S.; Erhart, P.; Körfer, D.; Böckler, D.; Dihlmann, S. Inflammasomes in the Pathophysiology of Aortic Disease. Cells 2021, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.-D. Caspase-1: The Inflammasome and Beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Kimura, T.; Jain, A.; Choi, S.W.; Mandell, M.A.; Schroder, K.; Johansen, T.; Deretic, V. TRIM-Mediated Precision Autophagy Targets Cytoplasmic Regulators of Innate Immunity. J. Cell Biol. 2015, 210, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Maejima, Y.; Matsumura, T.; Vega, R.B.; Amiya, E.; Ito, Y.; Shiheido-Watanabe, Y.; Ashikaga, T.; Komuro, I.; Kelly, D.P.; et al. Single-Nucleotide Polymorphism of the MLX Gene Is Associated With Takayasu Arteritis. Circ. Genom. Precis. Med. 2018, 11, e002296. [Google Scholar] [CrossRef] [PubMed]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 Forms an IL-1β-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Carmona, F.D.; Castañeda, S.; Solans, R.; Hernández-Rodríguez, J.; Cid, M.C.; Prieto-González, S.; Miranda-Filloy, J.A.; Rodríguez-Rodríguez, L.; Morado, I.C.; et al. Evidence of Association of the NLRP1 Gene with Giant Cell Arteritis. Ann. Rheum. Dis. 2013, 72, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, Y.; Isobe, M.; Takei, S.; Tanaka, Y.; Ishii, T.; Yokota, S.; Nomura, A.; Yoshida, S.; Nishimoto, N. Efficacy and Safety of Tocilizumab in Patients with Refractory Takayasu Arteritis: Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial in Japan (the TAKT Study). Ann. Rheum. Dis. 2018, 77, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Cid, M.C.; Unizony, S.H.; Blockmans, D.; Brouwer, E.; Dagna, L.; Dasgupta, B.; Hellmich, B.; Molloy, E.; Salvarani, C.; Trapnell, B.C.; et al. Efficacy and Safety of Mavrilimumab in Giant Cell Arteritis: A Phase 2, Randomised, Double-Blind, Placebo-Controlled Trial. Ann. Rheum. Dis. 2022, 81, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and Regulation of Endothelial VEGF Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Sano, M. Complexity of Inflammation in the Trajectory of Vascular Disease: Interleukin 6 and Beyond. Ann. Vasc. Dis. 2023, 16, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.A. Colchicine Update: 2008. Semin. Arthritis Rheum. 2009, 38, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I Interferon Inhibits Interleukin-1 Production and Inflammasome Activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Marvisi, C.; Castrignanò, P.; Pipitone, N.; Salvarani, C. Comparing Treatment Options for Large Vessel Vasculitis. Expert. Rev. Clin. Immunol. 2022, 18, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; Furuya, M.; Gunji, N.; Fujiwara, T.; Asano, T.; Onizawa, M.; et al. A Case of Takayasu Arteritis Complicated by Refractory Ulcerative Colitis Successfully Treated with Tofacitinib. Rheumatology 2020, 59, 1773–1775. [Google Scholar] [CrossRef]
- Kuwabara, S.; Tanimura, S.; Matsumoto, S.; Nakamura, H.; Horita, T. Successful Remission with Tofacitinib in a Patient with Refractory Takayasu Arteritis Complicated by Ulcerative Colitis. Ann. Rheum. Dis. 2020, 79, 1125–1126. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M. Perspectives of JAK Inhibitors for Large Vessel Vasculitis. Front. Immunol. 2022, 13, 881705. [Google Scholar] [CrossRef]
| GCA | TAK | |
|---|---|---|
| Mast cells | ||
 VEGF-ST2 receptor VEGF-ST2 receptorPresence in the neointima |  PDGF and TGF-β PDGF and TGF-βWall fibrosis | |
| Neutrophils | ||
Vasa vasorum and small vessels in the temporal arteries S100A12, IL-1β, IL-17, IL-9, IL-8, IL-6 S100A12, IL-1β, IL-17, IL-9, IL-8, IL-6 | Aorta walls; IL-17, IL-8, IFN-γ, TNF-α IL-17, IL-8, IFN-γ, TNF-α  Anti-histone antibodies Anti-histone antibodiesWall fibrosis | |
| NET activation Partnership with platelet activation | ||
| Macrophages | ||
| M1 in adventitia and media M2 in the media-intima border CD206+/YKL-40+/MMP-9+ in media FRβ+/CD206- in adventitia and intima  PD-L1 expression PD-L1 expression CD155-CD96 checkpoint CD155-CD96 checkpoint | All layers of the vessel M1 is more frequent in the aorta | |
| Dendritic cells (vasDCs) | ||
 Expression of CCR7, CCL19 and CCL21 Expression of CCR7, CCL19 and CCL21TLR4 and TLR5 ligands promote vascular damage  IL-6, IL-18, IL-23, IL-32, and IL-33 IL-6, IL-18, IL-23, IL-32, and IL-33 PD-L1 expression PD-L1 expression | T cells co-localize with DC in the adventitia of the aortic wall The involvement of DC remains marginal | |
| Complement | ||
 C3, C4b, and C4BP correlate with disease activity and vascular stenosis progression C3, C4b, and C4BP correlate with disease activity and vascular stenosis progression | ||
| Inflammasome | ||
| Genetic association of NLRP1 with GCA was found genotypinge a single-nucleotide polymorphism (rs8182352) | MLX-Q139R mutation promotes NLRP3 inflammasome formation, leading to increased IL-1β production | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Barbera, L.; Rizzo, C.; Camarda, F.; Miceli, G.; Tuttolomondo, A.; Guggino, G. The Contribution of Innate Immunity in Large-Vessel Vasculitis: Detangling New Pathomechanisms beyond the Onset of Vascular Inflammation. Cells 2024, 13, 271. https://doi.org/10.3390/cells13030271
La Barbera L, Rizzo C, Camarda F, Miceli G, Tuttolomondo A, Guggino G. The Contribution of Innate Immunity in Large-Vessel Vasculitis: Detangling New Pathomechanisms beyond the Onset of Vascular Inflammation. Cells. 2024; 13(3):271. https://doi.org/10.3390/cells13030271
Chicago/Turabian StyleLa Barbera, Lidia, Chiara Rizzo, Federica Camarda, Giuseppe Miceli, Antonino Tuttolomondo, and Giuliana Guggino. 2024. "The Contribution of Innate Immunity in Large-Vessel Vasculitis: Detangling New Pathomechanisms beyond the Onset of Vascular Inflammation" Cells 13, no. 3: 271. https://doi.org/10.3390/cells13030271
APA StyleLa Barbera, L., Rizzo, C., Camarda, F., Miceli, G., Tuttolomondo, A., & Guggino, G. (2024). The Contribution of Innate Immunity in Large-Vessel Vasculitis: Detangling New Pathomechanisms beyond the Onset of Vascular Inflammation. Cells, 13(3), 271. https://doi.org/10.3390/cells13030271










