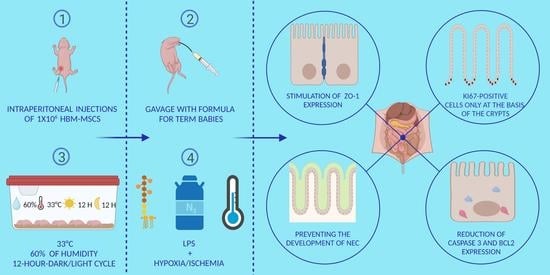
Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and Expansion of Human BM-MSCs
2.3. Human BM-MSC Characterization
2.4. RNA Extraction and RT-PCR
2.5. Mouse Intestine Immunohistochemistry (IHC)
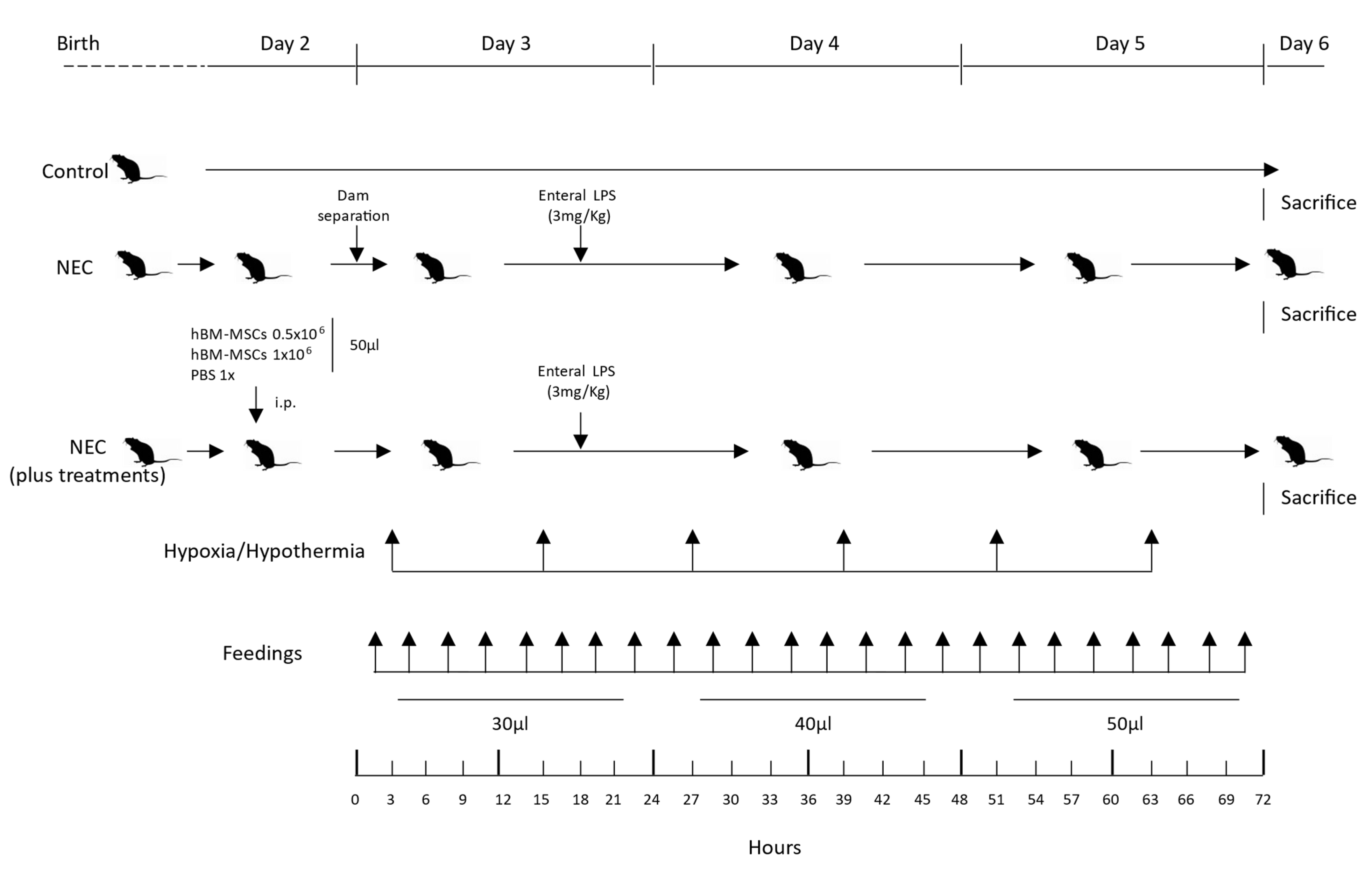
2.6. Experimental NEC Neonatal Mouse Model
2.7. Bodyweight and Survival Rate
2.8. Histological Injury Score of NEC in Neonatal Mice
2.9. Statistical Analysis
3. Results
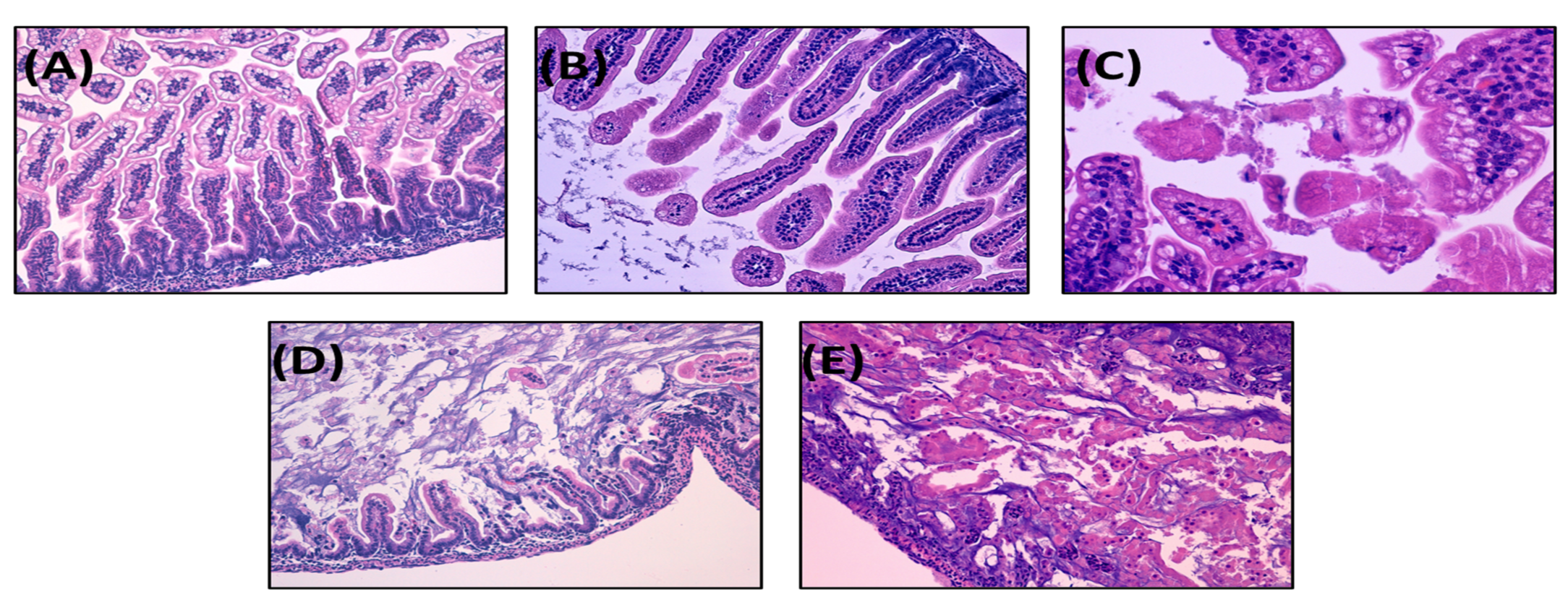
3.1. The Present NEC Model Based on Term Infant Formula Feedings Can Induce the Pathological Hallmarks of the Disease
3.1.1. Survival Rate
3.1.2. Body Weight
3.1.3. NEC Incidence and Histological Score
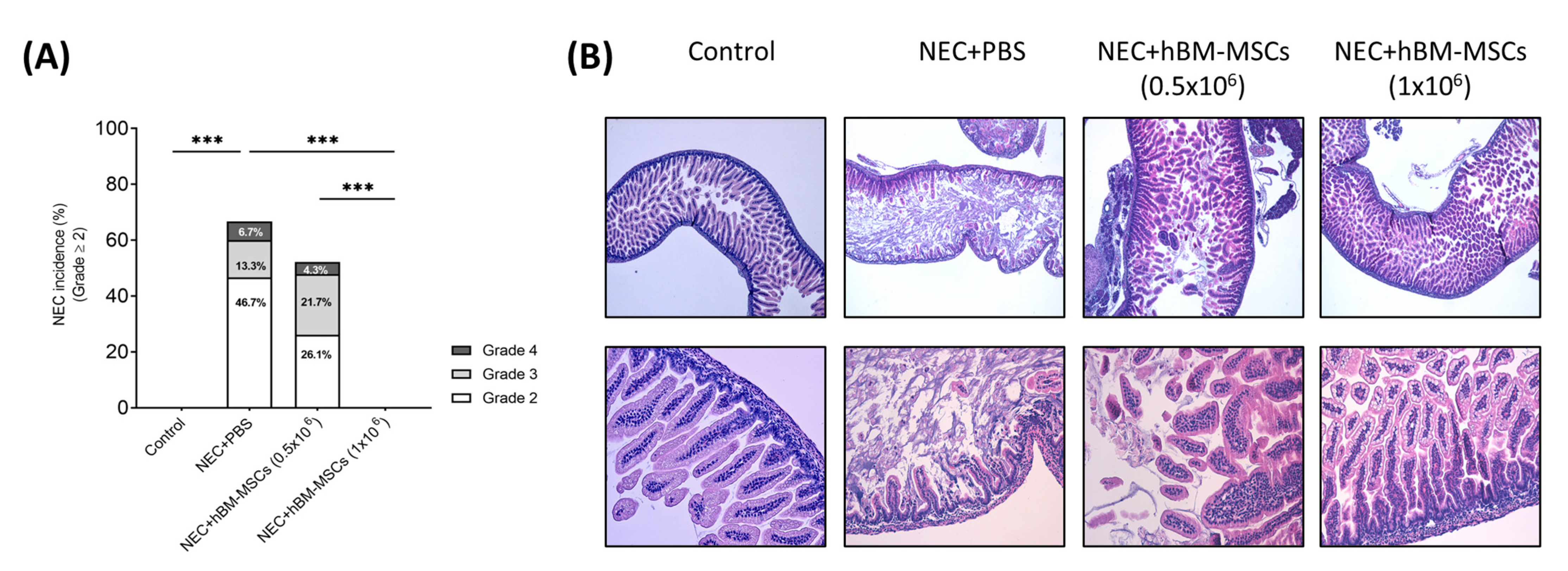
3.2. Treatment with hBM-MSCs Decreases the Incidence and Severity of Experimental NEC in a Concentration-Dependent Fashion in Neonatal Mice
3.2.1. Survival Rates
3.2.2. Body Weight
3.2.3. NEC Incidence and Histological Score
3.3. hBM-MSCs Reduced Apoptosis and Stimulated Tissue Regeneration in a Neonatal Mouse NEC Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Duchon, J.; Barbian, M.E.; Denning, P.W. Necrotizing Enterocolitis. Clin. Perinatol. 2021, 48, 229–250. [Google Scholar] [CrossRef]
- Dominguez, K.M.; Moss, R.L. Necrotizing enterocolitis. Clin. Perinatol. 2012, 39, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Alganabi, M.; Lee, C.; Bindi, E.; Li, B.; Pierro, A. Recent advances in understanding necrotizing enterocolitis. F1000Res 2019, 8, 107. [Google Scholar] [CrossRef]
- Kim, J.H.; Sampath, V.; Canvasser, J. Challenges in diagnosing necrotizing enterocolitis. Pediatr. Res. 2020, 88, 16–20. [Google Scholar] [CrossRef]
- Thompson, A.M.; Bizzarro, M.J. Necrotizing enterocolitis in newborns: Pathogenesis, prevention and management. Drugs 2008, 68, 1227–1238. [Google Scholar] [CrossRef]
- Chatterton, D.E.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Adams-Chapman, I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin. Perinatol. 2018, 45, 453–466. [Google Scholar] [CrossRef]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1075; discussion 1075–1076. [Google Scholar] [CrossRef]
- Kim, J.H. Necrotizing enterocolitis: The road to zero. Semin. Fetal Neonatal Med. 2014, 19, 39–44. [Google Scholar] [CrossRef]
- Villamor-Martinez, E.; Hundscheid, T.; Kramer, B.W.; Hooijmans, C.R.; Villamor, E. Stem Cells as Therapy for Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis of Preclinical Studies. Front Pediatr. 2020, 8, 578984. [Google Scholar] [CrossRef]
- Lopez, C.M.; Sampah, M.E.S.; Duess, J.W.; Ishiyama, A.; Ahmad, R.; Sodhi, C.P.; Hackam, D.J. Models of necrotizing enterocolitis. Semin. Perinatol. 2022, 47, 151695. [Google Scholar] [CrossRef]
- Xing, T.; Camacho Salazar, R.; Chen, Y.H. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers 2017, 5, e1356901. [Google Scholar] [CrossRef]
- Topalian, S.L.; Ziegler, M.M. Necrotizing enterocolitis: A review of animal models. J. Surg. Res. 1984, 37, 320–336. [Google Scholar] [CrossRef]
- Sulistyo, A.; Rahman, A.; Biouss, G.; Antounians, L.; Zani, A. Animal models of necrotizing enterocolitis: Review of the literature and state of the art. Innov. Surg. Sci. 2018, 3, 87–92. [Google Scholar] [CrossRef]
- Sodhi, C.; Richardson, W.; Gribar, S.; Hackam, D.J. The development of animal models for the study of necrotizing enterocolitis. Dis. Model. Mech. 2008, 1, 94–98. [Google Scholar] [CrossRef]
- Lu, P.; Sodhi, C.P.; Jia, H.; Shaffiey, S.; Good, M.; Branca, M.F.; Hackam, D.J. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: Pathophysiology, translational relevance, and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G917–G928. [Google Scholar] [CrossRef]
- Crissinger, K.D. Animal models of necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 17–22. [Google Scholar] [CrossRef]
- Ares, G.J.; McElroy, S.J.; Hunter, C.J. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 29–33. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Cavallaro, G.; Auteri, M.; Caldara, G.; Amodeo, I.; Mastropaolo, M.; Nuzzo, D.; Di Carlo, M.; Fumagalli, M.; Mosca, F.; et al. Postnatal development of the dopaminergic signaling involved in the modulation of intestinal motility in mice. Pediatr. Res. 2016, 80, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Cole, T.J. Breast milk and neonatal necrotising enterocolitis. Lancet 1990, 336, 1519–1523. [Google Scholar] [CrossRef]
- Rose, A.T.; Patel, R.M. A critical analysis of risk factors for necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 374–379. [Google Scholar] [CrossRef]
- Cruz, D.; Bazacliu, C. Enteral feeding composition and necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018, 23, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Ambalavanan, N. Feeding practices and necrotizing enterocolitis. Clin. Perinatol. 2013, 40, 1–10. [Google Scholar] [CrossRef]
- Pearson, F.; Johnson, M.J.; Leaf, A.A. Milk osmolality: Does it matter? Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F166–F169. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Fornabaio, D.M.; McNeill, H.; Mullane, K.M.; Caravella, S.J.; Miller, M.J. Contribution of oxygen-derived free radicals to experimental necrotizing enterocolitis. Am. J. Pathol. 1988, 130, 537–542. [Google Scholar]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Guthrie, S.O.; Gordon, P.V.; Thomas, V.; Thorp, J.A.; Peabody, J.; Clark, R.H. Necrotizing enterocolitis among neonates in the United States. J. Perinatol. 2003, 23, 278–285. [Google Scholar] [CrossRef]
- Young, C.M.; Kingma, S.D.; Neu, J. Ischemia-reperfusion and neonatal intestinal injury. J. Pediatr. 2011, 158, e25–e28. [Google Scholar] [CrossRef]
- Caplan, M.S.; Hedlund, E.; Adler, L.; Hsueh, W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr. Pathol. 1994, 14, 1017–1028. [Google Scholar] [CrossRef]
- Feng, J.; El-Assal, O.N.; Besner, G.E. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 167–174. [Google Scholar] [CrossRef]
- Miyake, H.; Chen, Y.; Koike, Y.; Hock, A.; Li, B.; Lee, C.; Zani, A.; Pierro, A. Osmolality of enteral formula and severity of experimental necrotizing enterocolitis. Pediatr. Surg. Int. 2016, 32, 1153–1156. [Google Scholar] [CrossRef]
- Kelly, E.J.; Newell, S.J.; Brownlee, K.G.; Primrose, J.N.; Dear, P.R. Gastric acid secretion in preterm infants. Early Hum. Dev. 1993, 35, 215–220. [Google Scholar] [CrossRef]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef]
- Emami, C.N.; Petrosyan, M.; Giuliani, S.; Williams, M.; Hunter, C.; Prasadarao, N.V.; Ford, H.R. Role of the host defense system and intestinal microbial flora in the pathogenesis of necrotizing enterocolitis. Surg. Infect. 2009, 10, 407–417. [Google Scholar] [CrossRef]
- Clark, D.A.; Mitchell, A.L. Development of gastrointestinal function: Risk factors for necrotizing enterocolitis. J. Pediatr. Pharmacol. Ther. 2004, 9, 96–103. [Google Scholar] [CrossRef]
- Neu, J.; Rushing, J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef]
- Hodzic, Z.; Bolock, A.M.; Good, M. The Role of Mucosal Immunity in the Pathogenesis of Necrotizing Enterocolitis. Front Pediatr. 2017, 5, 40. [Google Scholar] [CrossRef]
- Santaolalla, R.; Fukata, M.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2011, 27, 125–131. [Google Scholar] [CrossRef]
- Meng, D.; Zhu, W.; Shi, H.N.; Lu, L.; Wijendran, V.; Xu, W.; Walker, W.A. Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: A possible role in necrotizing enterocolitis. Pediatr. Res. 2015, 77, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Gribar, S.C.; Anand, R.J.; Sodhi, C.P.; Hackam, D.J. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J. Leukoc. Biol. 2008, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Jilling, T.; Simon, D.; Lu, J.; Meng, F.J.; Li, D.; Schy, R.; Thomson, R.B.; Soliman, A.; Arditi, M.; Caplan, M.S. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 2006, 177, 3273–3282. [Google Scholar] [CrossRef]
- Obladen, M. Necrotizing enterocolitis--150 years of fruitless search for the cause. Neonatology 2009, 96, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R.M.; Walker, W.A.; Yolken, R.H. Necrotizing enterocolitis: Research agenda for a disease of unknown etiology and pathogenesis. Pediatr. Res. 1993, 34, 701–708. [Google Scholar] [CrossRef]
- Nitkin, C.R.; Rajasingh, J.; Pisano, C.; Besner, G.E.; Thébaud, B.; Sampath, V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr. Res. 2020, 87, 265–276. [Google Scholar] [CrossRef]
- Liau, L.L.; Al-Masawa, M.E.; Koh, B.; Looi, Q.H.; Foo, J.B.; Lee, S.H.; Cheah, F.C.; Law, J.X. The Potential of Mesenchymal Stromal Cell as Therapy in Neonatal Diseases. Front Pediatr. 2020, 8, 591693. [Google Scholar] [CrossRef]
- Secunda, R.; Vennila, R.; Mohanashankar, A.M.; Rajasundari, M.; Jeswanth, S.; Surendran, R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: A comparative study. Cytotechnology 2015, 67, 793–807. [Google Scholar] [CrossRef]
- Talwadekar, M.D.; Kale, V.P.; Limaye, L.S. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci. Rep. 2015, 5, 15784. [Google Scholar] [CrossRef]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Rashedi, I.; Gómez-Aristizábal, A.; Wang, X.H.; Viswanathan, S.; Keating, A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells 2017, 35, 265–275. [Google Scholar] [CrossRef]
- Najar, M.; Krayem, M.; Meuleman, N.; Bron, D.; Lagneaux, L. Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review. Immune Netw. 2017, 17, 89–102. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, B.S.; Ryu, D.B.; Kim, T.W.; Park, G.; Min, C.K. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-γ and poly(I:C) priming through promoting the expression of indoleamine 2,3-dioxygenase. Stem Cell Res. Ther. 2021, 12, 37. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Iwasaki, M.; Koyanagi, M.; Urbich, C.; Rosenthal, N.; Zeiher, A.M.; Dimmeler, S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ. Res. 2008, 103, 203–211. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Klimczak, A.; Kozlowska, U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016, 2016, 4285215. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Sémont, A.; Demarquay, C.; Bessout, R.; Durand, C.; Benderitter, M.; Mathieu, N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS ONE 2013, 8, e70170. [Google Scholar] [CrossRef] [PubMed]
- Sémont, A.; Mouiseddine, M.; François, A.; Demarquay, C.; Mathieu, N.; Chapel, A.; Saché, A.; Thierry, D.; Laloi, P.; Gourmelon, P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010, 17, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Bahmanzadegan Jahromi, F.; Kavousi, S.; Monabati, A.; Kazemi, A. Mesenchymal stem cell transplantation after acute myocardial infarction: A meta-analysis of clinical trials. Stem Cell Res. Ther. 2021, 12, 600. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Ingo, D.M.; Redaelli, D.; Rossella, V.; Perini, O.; Santoleri, L.; Ciceri, F.; Aiuti, A.; Bernardo, M.E. Bone marrow-derived CD34(-) fraction: A rich source of mesenchymal stromal cells for clinical application. Cytotherapy 2016, 18, 1560–1563. [Google Scholar] [CrossRef]
- Tian, R.; Liu, S.X.; Williams, C.; Soltau, T.D.; Dimmitt, R.; Zheng, X.; De Plaen, I.G. Characterization of a necrotizing enterocolitis model in newborn mice. Int. J. Clin. Exp. Med. 2010, 3, 293–302. [Google Scholar]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Moser, V.C.; Walls, I.; Zoetis, T. Direct dosing of preweaning rodents in toxicity testing and research: Deliberations of an ILSI RSI Expert Working Group. Int. J. Toxicol. 2005, 24, 87–94. [Google Scholar] [CrossRef]
- Ramig, R.F. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb. Pathog. 1988, 4, 189–202. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L. Developing early formulations: Practice and perspective. Int. J. Pharm. 2007, 341, 1–19. [Google Scholar] [CrossRef]
- Olson, J.K.; Navarro, J.B.; Allen, J.M.; McCulloh, C.J.; Mashburn-Warren, L.; Wang, Y.; Varaljay, V.A.; Bailey, M.T.; Goodman, S.D.; Besner, G.E. An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest Liver Physiol. 2018, 315, G408–G419. [Google Scholar] [CrossRef]
- Crippa, S.; Conti, A.; Vavassori, V.; Ferrari, S.; Beretta, S.; Rivis, S.; Bosotti, R.; Scala, S.; Pirroni, S.; Jofra-Hernandez, R. Mesenchymal stromal cells improve the transplantation outcome of CRISPR-Cas9 gene-edited human HSPCs. Mol. Ther. 2022, 31, 230–248. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Wu, H.; Guo, K.; Zhuo, Z.; Zeng, R.; Luo, Y.; Yang, Q.; Li, J.; Jiang, R.; Huang, Z.; Sha, W.; et al. Current therapy option for necrotizing enterocolitis: Practicalities and challenge. Front. Pediatr. 2022, 10, 954735. [Google Scholar] [CrossRef]
- Kuca-Warnawin, E.; Olesińska, M.; Szczȩsny, P.; Kontny, E. Impact and possible mechanism (s) of adipose tissue-derived mesenchymal stem cells on T-cell proliferation in patients with rheumatic disease. Front. Physiol. 2022, 12, 2464. [Google Scholar] [CrossRef]
- Shandil, R.K.; Dhup, S.; Narayanan, S. Evaluation of the therapeutic potential of mesenchymal stem cells (MSCs) in preclinical models of autoimmune diseases. Stem Cells Int. 2022, 2022, 6379161. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T. Molecular mechanisms of VEGF-A action during tissue repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef]
- Song, H.; Song, B.W.; Cha, M.J.; Choi, I.G.; Hwang, K.C. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin. Biol. Ther. 2010, 10, 309–319. [Google Scholar] [CrossRef]
- Barnard, W.; Bower, J.; Brown, M.A.; Murphy, M.; Austin, L. Leukemia inhibitory factor (LIF) infusion stimulates skeletal muscle regeneration after injury: Injured muscle expresses lif mRNA. J. Neurol. Sci. 1994, 123, 108–113. [Google Scholar] [CrossRef]
- Bodnar, R.J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care 2013, 2, 24–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Tuazon, J.P.; Lee, J.Y.; Corey, S.; Kvederis, L.; Kingsbury, C.; Kaneko, Y.; Borlongan, C.V. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen. Res. 2019, 14, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yim, H.W.; Park, H.J.; Cho, Y.; Hong, H.; Kim, N.J.; Oh, I.H. Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis. Int. J. Stem Cells 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chern, Y.; Shen, C.K.; Wen, H.L.; Chang, Y.C.; Li, H.; Cheng, T.H.; Hsieh-Li, H.M. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS ONE 2011, 6, e22924. [Google Scholar] [CrossRef]
- Botto, S.; Streblow, D.N.; DeFilippis, V.; White, L.; Kreklywich, C.N.; Smith, P.P.; Caposio, P. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood 2011, 117, 352–361. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Jiang, Y.; Gui, C.; Sun, Y.; Li, J.; Wang, J.A. The antiapoptotic effect of mesenchymal stem cell transplantation on ischemic myocardium is enhanced by anoxic preconditioning. Can. J. Cardiol. 2009, 25, 353–358. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Nusrat, A.; Parkos, C.A. The epithelium in inflammatory bowel disease: Potential role of endocytosis of junctional proteins in barrier disruption. Novartis. Found Symp. 2004, 263, 115–124; discussion 124–132, 211–118. [Google Scholar]
- Chen, J.; Zhang, R.; Wang, J.; Yu, P.; Liu, Q.; Zeng, D.; Song, H.; Kuang, Z. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can. J. Physiol. Pharmacol. 2015, 93, 233–237. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20, quiz 21–22. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Fanning, A.S.; Mitic, L.L.; Anderson, J.M. Transmembrane proteins in the tight junction barrier. J. Am. Soc. Nephrol. 1999, 10, 1337–1345. [Google Scholar] [CrossRef]
- Nusrat, A.; Turner, J.R.; Madara, J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines, and immune cells. Am. J. Physiol. Gastrointest Liver Physiol. 2000, 279, G851–G857. [Google Scholar] [CrossRef]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; MacDonald, T.T.; Collins, J.E. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef]
- Hackam, D.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin. Pediatr. Surg. 2005, 14, 49–57. [Google Scholar] [CrossRef]
- Shiou, S.R.; Yu, Y.; Chen, S.; Ciancio, M.J.; Petrof, E.O.; Sun, J.; Claud, E.C. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J. Biol. Chem. 2011, 286, 12123–12132. [Google Scholar] [CrossRef]
- Hosfield, B.D.; Hunter, C.E.; Li, H.; Drucker, N.A.; Pecoraro, A.R.; Manohar, K.; Shelley, W.C.; Markel, T.A. A hydrogen-sulfide derivative of mesalamine reduces the severity of intestinal and lung injury in necrotizing enterocolitis through endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R422–R431. [Google Scholar] [CrossRef]
- Gonçalves, F.L.; Gallindo, R.M.; Soares, L.M.; Figueira, R.L.; Volpe, F.A.; Pereira-da-Silva, M.A.; Sbragia, L. Validation of protocol of experimental necrotizing enterocolitis in rats and the pitfalls during the procedure. Acta Cir. Bras. 2013, 28 (Suppl. 1), 19–25. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Flahive, C.; Schlegel, A.; Mezoff, E.A. Necrotizing enterocolitis: Updates on morbidity and mortality outcomes. J. Pediatr. 2020, 220, 7–9. [Google Scholar] [CrossRef]


| Target | Primer Forward | Primer Reverse |
|---|---|---|
| Bcl-2 | GAACTGGGGGAGGATTGTGG | GCATGCTGGGGCCATATAGT |
| IL1b | TGCCACCTTTTGACAGTGATG | TGATGTGCTGCTGCGAGATT |
| ZO-1 | TCTTGCAAAGTATCCCTTCTGT | GAAATCGTGCTGATGTGCCA |
| ACTB | CGGAGTCCATCACAATGCCT | GCCATGTACGTAGCCATCCA |
| PND | Control (n = 16) | NEC (n = 23) | NEC + PBS (n = 15) | NEC + hBM-MSCs 0.5 × 106 (n = 23) | NEC + hBM-MSCs 1 × 106 (n = 15) |
|---|---|---|---|---|---|
| 2 | 1.55 (0.04) | 1.54 (0.04) | 1.66 (0.05) | 1.66 (0.02) | 1.66 (0.06) |
| 3 | 1.82 (0.06) | 1.81 (0.04) | 2.07 (0.06) * | 2.03 (0.04) * | 1.92 (0.09) |
| 4 | 2.13 (0.09) | 1.79 (0.05) *** | 1.92 (0.05) | 1.93 (0.05) | 1.95 (0.09) |
| 5 | 2.65 (0.09) | 1.77 (0.05) *** | 1.79 (0.05) *** | 1.81 (0.05) *** | 1.86 (0.08) *** |
| 6 | 3.16 (0.09) | 1.69 (0.05) *** | 1.75 (0.06) *** | 1.85 (0.08) *** | 1.74 (0.06) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provitera, L.; Tomaselli, A.; Raffaeli, G.; Crippa, S.; Arribas, C.; Amodeo, I.; Gulden, S.; Amelio, G.S.; Cortesi, V.; Manzoni, F.; et al. Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner. Cells 2023, 12, 760. https://doi.org/10.3390/cells12050760
Provitera L, Tomaselli A, Raffaeli G, Crippa S, Arribas C, Amodeo I, Gulden S, Amelio GS, Cortesi V, Manzoni F, et al. Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner. Cells. 2023; 12(5):760. https://doi.org/10.3390/cells12050760
Chicago/Turabian StyleProvitera, Livia, Andrea Tomaselli, Genny Raffaeli, Stefania Crippa, Cristina Arribas, Ilaria Amodeo, Silvia Gulden, Giacomo Simeone Amelio, Valeria Cortesi, Francesca Manzoni, and et al. 2023. "Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner" Cells 12, no. 5: 760. https://doi.org/10.3390/cells12050760
APA StyleProvitera, L., Tomaselli, A., Raffaeli, G., Crippa, S., Arribas, C., Amodeo, I., Gulden, S., Amelio, G. S., Cortesi, V., Manzoni, F., Cervellini, G., Cerasani, J., Menis, C., Pesenti, N., Tripodi, M., Santi, L., Maggioni, M., Lonati, C., Oldoni, S., ... Cavallaro, G. (2023). Human Bone Marrow-Derived Mesenchymal Stromal Cells Reduce the Severity of Experimental Necrotizing Enterocolitis in a Concentration-Dependent Manner. Cells, 12(5), 760. https://doi.org/10.3390/cells12050760