Chorioallantoic Membrane Assay at the Cross-Roads of Adipose-Tissue-Derived Stem Cell Research
Abstract
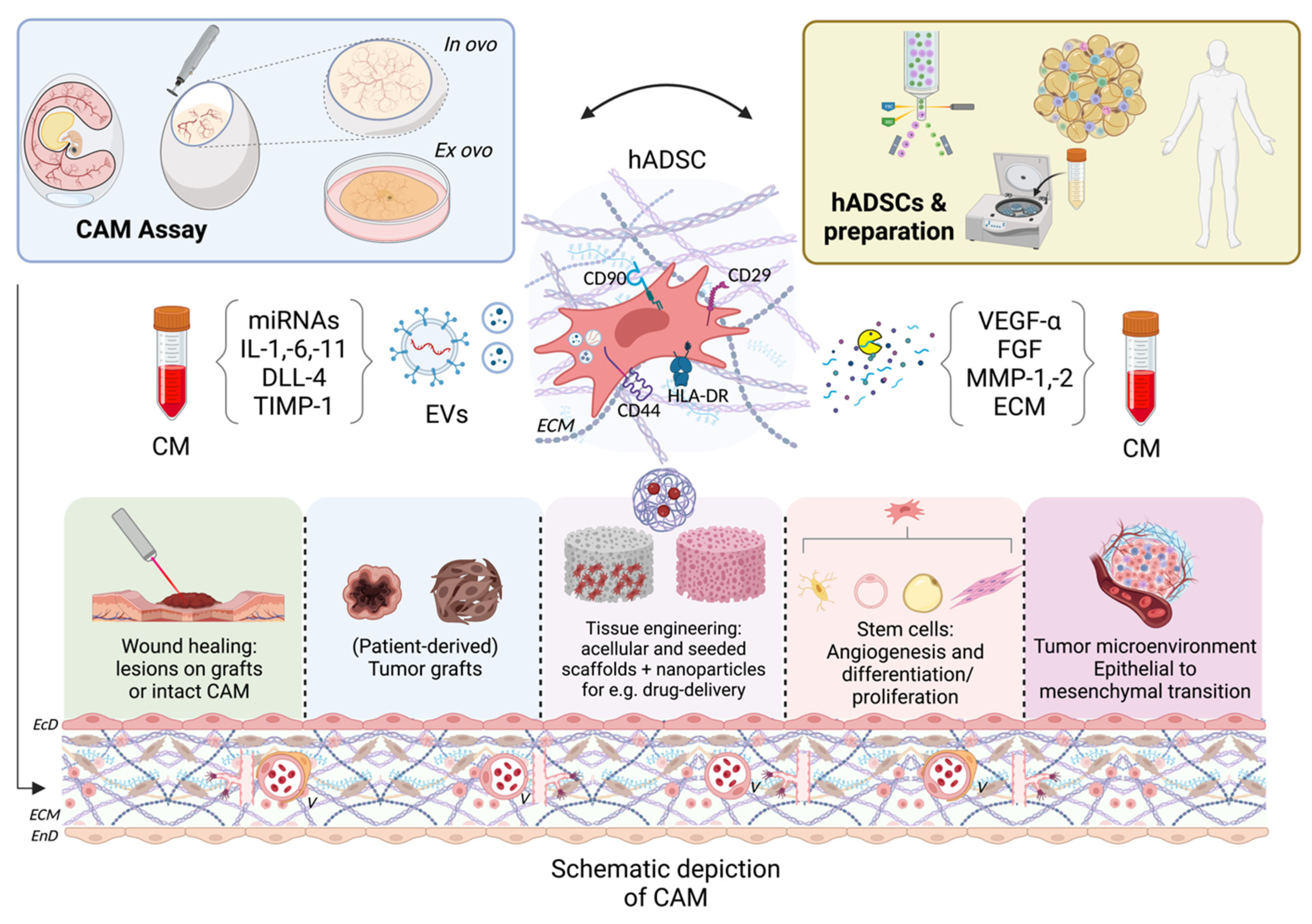
1. Introduction
2. Available CAM Models and Embryology
3. Wound Healing
4. Primary Cell Tissues, Cultures, and Sarcoma Research
5. Tissue Engineering
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Differentiation of Vascular Endothelium in the Chick Chorioallantois: A Structural and Autoradiographic Study. Dev. Biol. 1974, 38, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken Embryo Development: Metabolic and Morphological Basis for in Ovo Feeding Technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- Merckx, G.; Tay, H.; Lo Monaco, M.; van Zandvoort, M.; De Spiegelaere, W.; Lambrichts, I.; Bronckaers, A. Chorioallantoic Membrane Assay as Model for Angiogenesis in Tissue Engineering: Focus on Stem Cells. Tissue Eng. Part B Rev. 2020, 26, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Rous, P.; Murphy James, B. Tumor Implantations In The Developing Embryo. J. Am. Med. Assoc. 1911, 56, 741–742. [Google Scholar] [CrossRef]
- Borges, J.; Tegtmeier, F.T.; Padron, N.T.; Mueller, M.C.; Lang, E.M.; Stark, G.B. Chorioallantoic Membrane Angiogenesis Model for Tissue Engineering: A New Twist on a Classic Model. Tissue Eng. 2003, 9, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Seto, F. Early Development of the Avian Immune System. Poult. Sci. 1981, 60, 1981–1995. [Google Scholar] [CrossRef]
- Ribatti, D. Chorioallantoic Membrane in the Study of Tumor Angiogenesis. In The Chick Embryo Chorioallantoic Membrane in the Study of Angiogenesis and Metastasis; Springer: Dordrecht, The Netherlands, 2010; pp. 41–57. ISBN 978-90-481-3843-2. [Google Scholar]
- The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [CrossRef]
- Miebach, L.; Berner, J.; Bekeschus, S. In Ovo Model in Cancer Research and Tumor Immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Wilson, S.M.; Chambers, A.F. Experimental Metastasis Assays in the Chick Embryo. Curr. Protoc. Cell Biol. 2003, 21, 1906. [Google Scholar] [CrossRef]
- Chambers, A.F.; Schmidt, E.E.; MacDonald, I.C.; Morris, V.L.; Groom, A.C. Early Steps in Hematogenous Metastasis of B16F1 Melanoma Cells in Chick Embryos Studied by High-Resolution Intravital Videomicroscopy. JNCI J. Natl. Cancer Inst. 1992, 84, 797–803. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Goodpasture, E.W.; Woodruff, A.M.; Buddingh, G.J. The Cultivation of Vaccine and Other Viruses in the Chorio-Allantoic Membrane of Chick Embryos. Science 1931, 74, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Ribatti, D.; Raica, M. The Chick Embryo Chorioallantoic Membrane as a Model to Study Tumor Metastasis. Angiogenesis 2008, 11, 311–319. [Google Scholar] [CrossRef]
- Pion, E.; Haerteis, S.; Aung, T. Application of Laser Speckle Contrast Imaging (LSCI) for the Angiogenesis Measurement of Tumors in the Chorioallantoic Membrane (CAM) Model. In Tumor Angiogenesis Assays; Methods in Molecular Biology; Ribatti, D., Ed.; Springer: New York, NY, USA, 2023; Volume 2572, pp. 141–153. ISBN 978-1-07-162702-0. [Google Scholar]
- Pion, E.; Asam, C.; Feder, A.-L.; Felthaus, O.; Heidekrueger, P.I.; Prantl, L.; Haerteis, S.; Aung, T. Laser Speckle Contrast Analysis (LASCA) Technology for the Semiquantitative Measurement of Angiogenesis in in-Ovo-Tumor-Model. Microvasc. Res. 2021, 133, 104072. [Google Scholar] [CrossRef] [PubMed]
- Kuri, P.M.; Pion, E.; Mahl, L.; Kainz, P.; Schwarz, S.; Brochhausen, C.; Aung, T.; Haerteis, S. Deep Learning-Based Image Analysis for the Quantification of Tumor-Induced Angiogenesis in the 3D In Vivo Tumor Model—Establishment and Addition to Laser Speckle Contrast Imaging (LSCI). Cells 2022, 11, 2321. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Schmidt, K.M.; Jordan, K.; Federlin, M.; Milenkovic, V.M.; Liebisch, G.; Artati, A.; Schmidl, C.; Madej, G.; Tokarz, J.; et al. Cancer-Associated Cells Release Citrate to Support Tumour Metastatic Progression. Life Sci. Alliance 2021, 4, e202000903. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. Npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Halvorsen, Y.C.; Wilkison, W.O.; Gimble, J.M. Adipose-Derived Stromal Cells--Their Utility and Potential in Bone Formation. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24 (Suppl. S4), S41–S44. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Svintsitskaya, V.A.; Smirnov, V.N. Searching for Alternative Sources of Postnatal Human Mesenchymal Stem Cells: Candidate MSC-Like Cells from Umbilical Cord. Stem Cells 2003, 21, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosome and Mesenchymal Stem Cell Cross-Talk in the Tumor Microenvironment. Semin. Immunol. 2018, 35, 69–79. [Google Scholar] [CrossRef]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal Stem Cells: Key Players in Cancer Progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat Tissue: An Underappreciated Source of Stem Cells for Biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose Tissue-Derived Stromal Vascular Fraction in Regenerative Medicine: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Reinhard, R.; Siegmund, A.; Heumann, K.; Felthaus, O. Cell-Enriched Lipotransfer (CELT) Improves Tissue Regeneration and Rejuvenation without Substantial Manipulation of the Adipose Tissue Graft. Cells 2022, 11, 3159. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Felthaus, O. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef]
- Mizuno, H.; Zuk, P.A.; Zhu, M.; Lorenz, H.P.; Benhaim, P.; Hedrick, M.H. Myogenic Differentiation by Human Processed Lipoaspirate Cells. Plast. Reconstr. Surg. 2002, 109, 199–209; discussion 210–211. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human Adipose Tissue-Derived Stem Cells Differentiate into Endothelial Cells in Vitro and Improve Postnatal Neovascularization in Vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; Ugarte, D.A.D.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W. Adipose-Derived Stem Cells: Implications in Tissue Regeneration. World J. Stem Cells 2014, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Haubner, F.; Muschter, D.; Schuster, N.; Pohl, F.; Ahrens, N.; Prantl, L.; Gassner, H.G. Platelet-Rich Plasma Stimulates Dermal Microvascular Endothelial Cells and Adipose Derived Stem Cells after External Radiation. Clin. Hemorheol. Microcirc. 2015, 61, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Eyal-Giladi, H.; Kochav, S. From Cleavage to Primitive Streak Formation: A Complementary Normal Table and a New Look at the First Stages of the Development of the Chick. I. General Morphology. Dev. Biol. 1976, 49, 321–337. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Sheng, G. Day-1 Chick Development: Day-1 Chick Development. Dev. Dyn. 2014, 243, 357–367. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The Chicken Chorioallantoic Membrane Model in Biology, Medicine and Bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Bellairs, R.; Osmond, M. Extra-Embryonic Membranes. In Atlas of Chick Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 127–129. ISBN 978-0-12-384951-9. [Google Scholar]
- Nagai, H.; Tanoue, Y.; Nakamura, T.; Chan, C.J.J.; Yamada, S.; Saitou, M.; Fukuda, T.; Sheng, G. Mesothelial Fusion Mediates Chorioallantoic Membrane Formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210263. [Google Scholar] [CrossRef]
- Kurz, H.; Ambrosy, S.; Wilting, J.; Marmé, D.; Christ, B. Proliferation Pattern of Capillary Endothelial Cells in Chorioallantoic Membrane Development Indicates Local Growth Control, Which Is Counteracted by Vascular Endothelial Growth Factor Application. Dev. Dyn. 1995, 203, 174–186. [Google Scholar] [CrossRef]
- Flamme, I.; Schulze-Osthoff, K.; Jacob, H.J. Mitogenic Activity of Chicken Chorioallantoic Fluid Is Temporally Correlated to Vascular Growth in the Chorioallantoic Membrane and Related to Fibroblast Growth Factors. Development 1991, 111, 683–690. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, I.; Zoer, B.; Altimiras, J.; Villamor, E. Reactivity of Chicken Chorioallantoic Arteries, Avian Homologue of Human Fetoplacental Arteries. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2010, 61, 619–628. [Google Scholar]
- Reizis, A.; Hammel, I.; Ar, A. Regional and Developmental Variations of Blood Vessel Morphometry in the Chick Embryo Chorioallantoic Membrane. J. Exp. Biol. 2005, 208, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Cordeiro, C.M.M.; Elebute, O.; Hincke, M.T. Proteomic Analysis of Chicken Chorioallantoic Membrane (CAM) during Embryonic Development Provides Functional Insight. BioMed Res. Int. 2022, 2022, 7813921. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.E.; Romanoff, A.L. The Avian Embryo: Structural and Functional Development. Avian Dis. 1960, 4, 541. [Google Scholar] [CrossRef]
- Ribatti, D. Chicken Chorioallantoic Membrane Angiogenesis Model. In Cardiovascular Development; Methods in Molecular Biology; Peng, X., Antonyak, M., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 843, pp. 47–57. ISBN 978-1-61779-522-0. [Google Scholar]
- Kauffmann, P.; Troeltzsch, M.; Cordesmeyer, R.; Heidekrueger, P.I.; Schliephake, H.; Canis, M.; Wolff, H.A.; Rave-Fraenk, M.; Stroebel, P.; Kehrer, A.; et al. Presentation of a Variation of the Chorioallantoic Membrane Set up as a Potential Model for Individual Therapy for Squamous Cell Carcinoma of the Oropharynx. Clin. Hemorheol. Microcirc. 2017, 67, 453–457. [Google Scholar] [CrossRef]
- Kauffmann, P.; Troeltzsch, M.; Brockmeyer, P.; Bohnenberger, H.; Heidekrüger, P.I.; Manzke, M.; Canis, M.; Gaayathiri, S.; Schliephake, H.; Prantl, L.; et al. First Experience of Chick Chorioallantoic Membrane (CAM) Assay in the Clinical Work Flow with Oral Squamous Cell Carcinoma Patients. Clin. Hemorheol. Microcirc. 2019, 70, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.V.; Berlow, N.E.; Price, L.H.; Mansoor, A.; Cairo, S.; Rugonyi, S.; Keller, C. Preclinical therapeutics ex ovo quail eggs as a biomimetic automation-ready xenograft platform. Sci. Rep. 2021, 11, 23302. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of Chronic Wounds in the General Population: Systematic Review and Meta-Analysis of Observational Studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Augustin, M.; Brocatti, L.K.; Rustenbach, S.J.; Schäfer, I.; Herberger, K. Cost-of-Illness of Leg Ulcers in the Community. Int. Wound J. 2014, 11, 283–292. [Google Scholar] [CrossRef]
- Purwins, S.; Herberger, K.; Debus, E.S.; Rustenbach, S.J.; Pelzer, P.; Rabe, E.; Schäfer, E.; Stadler, R.; Augustin, M. Cost-of-Illness of Chronic Leg Ulcers in Germany. Int. Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration in Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S. The Chemotactic Effect of Mixtures of Antibody and Antigen on Polymorphonuclear Leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical Wound-Healing Assay for Cells in Vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Ranieri, G.; Sorino, S.; Roncali, L. The Chick Embryo Chorioallantoic Membrane as an in Vivo Wound Healing Model. Pathol. Res. Pract. 1996, 192, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Presta, M. Endogenous and Exogenous Fibroblast Growth Factor-2 Modulate Wound Healing in the Chick Embryo Chorioallantoic Membrane. Angiogenesis 1999, 3, 89–95. [Google Scholar] [CrossRef]
- Zaugg, P.; Djonov, V.; Füchtbauer, E.-M.; Draeger, A. Sorting of Murine Vascular Smooth Muscle Cells during Wound Healing in the Chicken Chorioallantoic Membrane. Exp. Cell Res. 1999, 253, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kilarski, W.W.; Jura, N.; Gerwins, P. An Ex Vivo Model for Functional Studies of Myofibroblasts. Lab. Investig. 2005, 85, 643–654. [Google Scholar] [CrossRef]
- Kilarski, W.W.; Samolov, B.; Petersson, L.; Kvanta, A.; Gerwins, P. Biomechanical Regulation of Blood Vessel Growth during Tissue Vascularization. Nat. Med. 2009, 15, 657–664. [Google Scholar] [CrossRef]
- Rezzola, S.; Loda, A.; Corsini, M.; Semeraro, F.; Annese, T.; Presta, M.; Ribatti, D. Angiogenesis-Inflammation Cross Talk in Diabetic Retinopathy: Novel Insights From the Chick Embryo Chorioallantoic Membrane/Human Vitreous Platform. Front. Immunol. 2020, 11, 581288. [Google Scholar] [CrossRef]
- Carre, A.L.; Larson, B.J.; Knowles, J.A.; Kawai, K.; Longaker, M.T.; Lorenz, H.P. Fetal Mouse Skin Heals Scarlessly in a Chick Chorioallantoic Membrane Model System. Ann. Plast. Surg. 2012, 69, 85–90. [Google Scholar] [CrossRef]
- Kunzi-Rapp, K.; Rück, A.; Kaufmann, R. Characterization of the Chick Chorioallantoic Membrane Model as a Short-Term in Vivo System for Human Skin. Arch. Dermatol. Res. 1999, 291, 290–295. [Google Scholar] [CrossRef]
- Sivan, U.; Jayakumar, K.; Krishnan, L.K. Constitution of Fibrin-Based Niche for In Vitro Differentiation of Adipose-Derived Mesenchymal Stem Cells to Keratinocytes. BioResearch Open Access 2014, 3, 339–347. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound Healing Effect of Adipose-Derived Stem Cells: A Critical Role of Secretory Factors on Human Dermal Fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Shingyochi, Y.; Orbay, H.; Mizuno, H. Adipose-Derived Stem Cells for Wound Repair and Regeneration. Expert Opin. Biol. Ther. 2015, 15, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; Kim, S.J.; Lee, J.B.; Bae, Y.C.; Sung, S.M.; Jung, J.S. Human Adipose Tissue-Derived Mesenchymal Stem Cells Improve Postnatal Neovascularization in a Mouse Model of Hindlimb Ischemia. Cell. Physiol. Biochem. 2006, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.I.; Cunha, P.; de Miranda, M.; Faraco, C.C.F.; Barbosa, J.L.; Ferreira, A.; Kunrath Lima, M.; Faria, J.A.Q.A.; Rodrigues, M.Â.; Goes, A.M.; et al. Human Adipose-derived Stromal/Stem Cells Are Distinct from Dermal Fibroblasts as Evaluated by Biological Characterization and RNA Sequencing. Cell Biochem. Funct. 2021, 39, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Jääger, K.; Islam, S.; Zajac, P.; Linnarsson, S.; Neuman, T. RNA-Seq Analysis Reveals Different Dynamics of Differentiation of Human Dermis- and Adipose-Derived Stromal Stem Cells. PLoS ONE 2012, 7, e38833. [Google Scholar] [CrossRef]
- Zych, J.; Spangenberg, L.; Stimamiglio, M.A.; Abud, A.P.R.; Shigunov, P.; Marchini, F.K.; Kuligovski, C.; Cofré, A.R.; Schittini, A.V.; Aguiar, A.M.; et al. Polysome Profiling Shows the Identity of Human Adipose-Derived Stromal/Stem Cells in Detail and Clearly Distinguishes Them from Dermal Fibroblasts. Stem Cells Dev. 2014, 23, 2791–2802. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from Adipose-derived Stem Cells and Application to Skin Wound Healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes Secreted by Human Adipose Mesenchymal Stem Cells Promote Scarless Cutaneous Repair by Regulating Extracellular Matrix Remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose Mesenchymal Stem Cell-derived Exosomes Promote Cell Proliferation, Migration, and Inhibit Cell Apoptosis via Wnt/Β-catenin Signaling in Cutaneous Wound Healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.S.; Wei, Q.; Gurung, A.; Youn, A.; Bright, T.; Poon, R.; Whetstone, H.; Guha, A.; Alman, B.A. Beta-catenin Regulates Wound Size and Mediates the Effect of TGF-beta in Cutaneous Healing. FASEB J. 2006, 20, 692–701. [Google Scholar] [CrossRef]
- Song, Y.H.; Shon, S.H.; Shan, M.; Stroock, A.D.; Fischbach, C. Adipose-Derived Stem Cells Increase Angiogenesis through Matrix Metalloproteinase-Dependent Collagen Remodeling. Integr. Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine Profile of Human Adipose-Derived Stem Cells: Expression of Angiogenic, Hematopoietic, and pro-Inflammatory Factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef]
- Ebrahimian, T.G.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Gourmelon, P.; Benderitter, M.; Casteilla, L.; Tamarat, R. Cell Therapy Based on Adipose Tissue-Derived Stromal Cells Promotes Physiological and Pathological Wound Healing. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar] [CrossRef]
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of MiRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes Secreted by Mesenchymal Stem Cells Promote Endothelial Cell Angiogenesis by Transferring MiR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef]
- Almeida, M.I.; Silva, A.M.; Vasconcelos, D.M.; Almeida, C.R.; Caires, H.; Pinto, M.T.; Calin, G.A.; Santos, S.G.; Barbosa, M.A. MiR-195 in Human Primary Mesenchymal Stromal/Stem Cells Regulates Proliferation, Osteogenesis and Paracrine Effect on Angiogenesis. Oncotarget 2016, 7, 7–22. [Google Scholar] [CrossRef]
- Zhu, H.; Zhuang, Y.; Li, D.; Dong, N.; Ma, H.; Liu, L.; Shi, Q.; Ju, X. Cryo-Temperature Pretreatment Increases the Pro-Angiogenic Capacity of Three-Dimensional Mesenchymal Stem Cells via the PI3K-AKT Pathway. Cell Transplant. 2022, 31, 1–12. [Google Scholar] [CrossRef]
- Lin, C.-H.; Tsai, C.-H.; Yang, I.-C.; Ma, H. Frozen Fat Grafts Maintain Vascular Endothelial Growth Factor Expression and Mediate Angiogenesis During Adipose-Derived Stem Cell Enrichment for Soft Tissue Augmentation. Ann. Plast. Surg. 2022, 88, S4–S12. [Google Scholar] [CrossRef]
- Beugels, J.; Molin, D.G.M.; Ophelders, D.R.M.G.; Rutten, T.; Kessels, L.; Kloosterboer, N.; Grzymala, A.A.P.D.; Kramer, B.W.W.; van der Hulst, R.R.W.J.; Wolfs, T.G.A.M. Electrical Stimulation Promotes the Angiogenic Potential of Adipose-Derived Stem Cells. Sci. Rep. 2019, 9, 12076. [Google Scholar] [CrossRef]
- Teo, J.Y.; Seo, Y.; Ko, E.; Leong, J.; Hong, Y.-T.; Yang, Y.Y.; Kong, H. Surface Tethering of Stem Cells with H2O2-Responsive Anti-Oxidizing Colloidal Particles for Protection against Oxidation-Induced Death. Biomaterials 2019, 201, 1–15. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in Vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Gao, W.; Qiao, X.; Ma, S.; Cui, L. Adipose-Derived Stem Cells Accelerate Neovascularization in Ischaemic Diabetic Skin Flap via Expression of Hypoxia-Inducible Factor-1α. J. Cell. Mol. Med. 2011, 15, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwata, T.; Morikawa, S.; Yamato, M.; Okano, T.; Uchigata, Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes 2015, 64, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Kuroda, H.; Morikawa, S.; Hamada, M.; Ikura, K.; Kaibuchi, N.; Yamato, M.; et al. Creation and Transplantation of an Adipose-Derived Stem Cell (ASC) Sheet in a Diabetic Wound-Healing Model. J. Vis. Exp. 2017, 126, e54539. [Google Scholar] [CrossRef]
- Hamada, M.; Iwata, T.; Kato, Y.; Washio, K.; Morikawa, S.; Sakurai, H.; Yamato, M.; Okano, T.; Uchigata, Y. Xenogeneic Transplantation of Human Adipose-Derived Stem Cell Sheets Accelerate Angiogenesis and the Healing of Skin Wounds in a Zucker Diabetic Fatty Rat Model of Obese Diabetes. Regen. Ther. 2017, 6, 65–73. [Google Scholar] [CrossRef]
- Yu, J.; Hsu, Y.-C.; Lee, J.-K.; Cheng, N.-C. Enhanced Angiogenic Potential of Adipose-Derived Stem Cell Sheets by Integration with Cell Spheroids of the Same Source. Stem Cell Res. Ther. 2022, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; TorÍo-Padrón, N.; Momeni, A.; Mueller, M.C.; Tegtmeier, F.T.; Stark, B.G. Adipose Precursor Cells (Preadipocytes) Induce Formation of New Vessels in Fibrin Glue on the Newly Developed Cylinder Chorioallantoic Membrane Model (CAM). Minim. Invasive Ther. Allied Technol. 2006, 15, 246–252. [Google Scholar] [CrossRef]
- Buschmann, J.; Härter, L.; Gao, S.; Hemmi, S.; Welti, M.; Hild, N.; Schneider, O.D.; Stark, W.J.; Lindenblatt, N.; Werner, C.M.L.; et al. Tissue Engineered Bone Grafts Based on Biomimetic Nanocomposite PLGA/Amorphous Calcium Phosphate Scaffold and Human Adipose-Derived Stem Cells. Injury 2012, 43, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Vagaska, B.; Bulstrode, N.W.; Seifalian, A.M.; Ferretti, P. Chondrogenic Differentiation of Adipose Tissue-Derived Stem Cells within Nanocaged POSS-PCU Scaffolds: A New Tool for Nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 279–289. [Google Scholar] [CrossRef]
- Handel, M.; Hammer, T.R.; Nooeaid, P.; Boccaccini, A.R.; Hoefer, D. 45S5-Bioglass®-Based 3D-Scaffolds Seeded with Human Adipose Tissue-Derived Stem Cells Induce In Vivo Vascularization in the CAM Angiogenesis Assay. Tissue Eng. Part A 2013, 19, 2703–2712. [Google Scholar] [CrossRef]
- Strassburg, S.; Nienhueser, H.; Björn Stark, G.; Finkenzeller, G.; Torio-Padron, N. Co-Culture of Adipose-Derived Stem Cells and Endothelial Cells in Fibrin Induces Angiogenesis and Vasculogenesis in a Chorioallantoic Membrane Model: Neovascularization by ASCs and Endothelial Cells. J. Tissue Eng. Regen. Med. 2016, 10, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Wahl, E.A.; Fierro, F.A.; Peavy, T.R.; Hopfner, U.; Dye, J.F.; Machens, H.-G.; Egaña, J.T.; Schenck, T.L. In Vitro Evaluation of Scaffolds for the Delivery of Mesenchymal Stem Cells to Wounds. BioMed Res. Int. 2015, 2015, 108571. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.P.; Ibrahim, A.; Guasti, L.; Zucchelli, E.; Birchall, M.; Bulstrode, N.W.; Seifalian, A.M.; Ferretti, P. Towards Reconstruction of Epithelialized Cartilages from Autologous Adipose Tissue-Derived Stem Cells: Epithelialized Cartilage from Paediatric Adipose Tissue-Derived Stem Cells. J. Tissue Eng. Regen. Med. 2017, 11, 3078–3089. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained Release of Adipose-Derived Stem Cells by Thermosensitive Chitosan/Gelatin Hydrogel for Therapeutic Angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef]
- Shafaat, S.; Mangir, N.; Regureos, S.R.; Chapple, C.R.; MacNeil, S. Demonstration of Improved Tissue Integration and Angiogenesis with an Elastic, Estradiol Releasing Polyurethane Material Designed for Use in Pelvic Floor Repair. Neurourol. Urodyn. 2018, 37, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, M.; Schaafsma, W.; Grillo, E.; Vliora, M.; Dakou, E.; Corsini, M.; Ravelli, C.; Ronca, R.; Sakellariou, P.; Vanparijs, J.; et al. Natural Histogel-Based Bio-Scaffolds for Sustaining Angiogenesis in Beige Adipose Tissue. Cells 2019, 8, 1457. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.R.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. One-Step Rapid Fabrication of Cell-Only Living Fibers. Adv. Mater. 2020, 32, 1906305. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cheam, N.M.J.; Cao, H.; Lee, M.K.H.; Sze, S.K.; Tan, N.S.; Tay, C.Y. Materials Stiffness-Dependent Redox Metabolic Reprogramming of Mesenchymal Stem Cells for Secretome-Based Therapeutic Angiogenesis. Adv. Healthc. Mater. 2019, 8, 1900929. [Google Scholar] [CrossRef]
- Ratushnyy, A.; Ezdakova, M.; Buravkova, L. Secretome of Senescent Adipose-Derived Mesenchymal Stem Cells Negatively Regulates Angiogenesis. Int. J. Mol. Sci. 2020, 21, 1802. [Google Scholar] [CrossRef]
- Otto, L.; Wolint, P.; Bopp, A.; Woloszyk, A.; Becker, A.S.; Boss, A.; Böni, R.; Calcagni, M.; Giovanoli, P.; Hoerstrup, S.P.; et al. 3D-Microtissue Derived Secretome as a Cell-Free Approach for Enhanced Mineralization of Scaffolds in the Chorioallantoic Membrane Model. Sci. Rep. 2021, 11, 5418. [Google Scholar] [CrossRef]
- Watchararot, T.; Prasongchean, W.; Thongnuek, P. Angiogenic Property of Silk Fibroin Scaffolds with Adipose-Derived Stem Cells on Chick Chorioallantoic Membrane. R. Soc. Open Sci. 2021, 8, 201618. [Google Scholar] [CrossRef] [PubMed]
- Ezdakova, M.I.; Matveeva, D.K.; Andreeva, E.R. Short-Term Interaction with Endothelial Cells Enhances Angiogenic Activity of Growth-Arrested Mesenchymal Stromal Cells In Vitro and In Ovo. Bull. Exp. Biol. Med. 2022, 174, 125–130. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth in Vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef]
- Comşa, Ş.; Ceaușu, A.-R.; Popescu, R.; Sârb, S.; Cîmpean, A.-M.; Raica, M. The MSC-MCF-7 Duet Playing Tumor Vasculogenesis and Angiogenesis onto the Chick Embryo Chorioallantoic Membrane. In Vivo 2020, 34, 3315–3325. [Google Scholar] [CrossRef]
- Comşa, Ş.; Ceaușu, R.A.; Popescu, R.; Cîmpean, A.M.; Raica, M. The Human Mesenchymal Stem Cells and the Chick Embryo Chorioallantoic Membrane: The Key and the Lock in Revealing Vasculogenesis. In Vivo 2017, 31, 1139–1144. [Google Scholar] [CrossRef]
- Hsiao, S.T.-F.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-derived Stem Cells for Wound Healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Pinxteren, J.; Roobrouck, V.D.; Luyckx, A.; Van’t Hof, W.; Deans, R.; Verfaillie, C.M.; Waer, M.; Billiau, A.D.; Van Gool, S.W. Human Multipotent Adult Progenitor Cells Are Nonimmunogenic and Exert Potent Immunomodulatory Effects on Alloreactive T-Cell Responses. Cell Transplant. 2013, 22, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Jennewein, M.; Bubel, M.; Guthörl, S.; Pohlemann, T.; Oberringer, M. Interacting Adipose-Derived Stem Cells and Microvascular Endothelial Cells Provide a Beneficial Milieu for Soft Tissue Healing. Mol. Biol. Rep. 2020, 47, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Est-Witte, S.E.; Farris, A.L.; Tzeng, S.Y.; Hutton, D.L.; Gong, D.H.; Calabresi, K.G.; Grayson, W.L.; Green, J.J. Non-Viral Gene Delivery of HIF-1α Promotes Angiogenesis in Human Adipose-Derived Stem Cells. Acta Biomater. 2020, 113, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Zhao, Y.; Qin, Y.; Zhang, Y.; Pang, H.; Zhou, Y.; Liu, X.; Xiao, Z. Extracellular Vesicles from HIF-1α-Overexpressing Adipose-Derived Stem Cells Restore Diabetic Wounds Through Accelerated Fibroblast Proliferation and Migration. Int. J. Nanomed. 2021, 16, 7943–7957. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef]
- Khatri, M.; Sharma, J.M. Response of Embryonic Chicken Lymphoid Cells to Infectious Bursal Disease Virus. Vet. Immunol. Immunopathol. 2009, 127, 316–324. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J.; Segarra, M.; Vilardell, C.; Sánchez, M.; García-Martínez, A.; Esteban, M.-J.; Grau, J.M.; Urbano-Márquez, A.; Colomer, D.; Kleinman, H.K.; et al. Elevated Production of Interleukin-6 Is Associated With a Lower Incidence of Disease-Related Ischemic Events in Patients With Giant-Cell Arteritis: Angiogenic Activity of Interleukin-6 as a Potential Protective Mechanism. Circulation 2003, 107, 2428–2434. [Google Scholar] [CrossRef]
- Naldini, A.; Leali, D.; Pucci, A.; Morena, E.; Carraro, F.; Nico, B.; Ribatti, D.; Presta, M. Cutting Edge: IL-1β Mediates the Proangiogenic Activity of Osteopontin-Activated Human Monocytes. J. Immunol. 2006, 177, 4267–4270. [Google Scholar] [CrossRef]
- FDA Wound Healing Clinical Focus Groupa Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment. Wound Repair Regen. 2001, 9, 258–268. [CrossRef] [PubMed]
- Feng, C.-J.; Lin, C.-H.; Tsai, C.-H.; Yang, I.-C.; Ma, H. Adipose-Derived Stem Cells-Induced Burn Wound Healing and Regeneration of Skin Appendages in a Novel Skin Island Rat Model. J. Chin. Med. Assoc. 2019, 82, 635–642. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing Potential of Injectable Aloe Vera Hydrogel Loaded by Adipose-Derived Stem Cell in Skin Tissue-Engineering in a Rat Burn Wound Model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019, 28, 1463–1472. [Google Scholar] [CrossRef]
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, C.J.; Mittal, S.; et al. Adipose-Derived Stromal Cells Seeded in Pullulan-Collagen Hydrogels Improve Healing in Murine Burns. Tissue Eng. Part A 2021, 27, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.W.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the Use of Cell Lines in Biomedical Research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef]
- Hurst, E.W.; Cooke, B.; McLennan, G.C. A Note on the Survival and Growth of Human and Rabbit Tissues (Normal and Neoplastic) in the Chorio-Allantois of the Chick and Duck Embryo. Aust. J. Exp. Biol. Med. 1939, 17, 215–224. [Google Scholar] [CrossRef]
- Karnofsky, D.A.; Ridgway, L.P.; Patterson, P.A. Tumor Transplantation to the Chick Embryo. Ann. N. Y. Acad. Sci. 1952, 55, 313–329. [Google Scholar] [CrossRef]
- Dagg, C.P.; Karnofskayn, D. Growth of Transplantable Human Tumors in the Chick Embryo and Hatched Chick. Cancer Res. 1956, 16, 589–594. [Google Scholar] [PubMed]
- Komatsu, A.; Matsumoto, K.; Saito, T.; Muto, M.; Tamanoi, F. Patient Derived Chicken Egg Tumor Model (PDcE Model): Current Status and Critical Issues. Cells 2019, 8, 440. [Google Scholar] [CrossRef]
- Shoin, K.; Yamashita, J.; Enkaku, F.; Sasaki, T.; Tanaka, M.; Endo, Y. Chick Embryo Assay as Chemosensitivity Test for Malignant Glioma. Jpn. J. Cancer Res. Gann 1991, 82, 1165–1170. [Google Scholar] [CrossRef]
- Lucarelli, E.; Sangiorgi, L.; Benassi, S.; Donati, D.; Gobbi, G.A.; Picci, P.; Vacca, A.; Ribatti, D. Angiogenesis in Lipoma: An Experimental Study in the Chick Embryo Chorioallantoic Membrane. Int. J. Mol. Med. 1999, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Gil-Benso, R.; Lopez-Gines, C.; López-Guerrero, J.A.; Carda, C.; Callaghan, R.C.; Navarro, S.; Ferrer, J.; Pellín, A.; Llombart-Bosch, A. Establishment and Characterization of a Continuous Human Chondrosarcoma Cell Line, Ch-2879: Comparative Histologic and Genetic Studies with Its Tumor of Origin. Lab. Investig. 2003, 83, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Sys, G.; Van Bockstal, M.; Forsyth, R.; Balke, M.; Poffyn, B.; Uyttendaele, D.; Bracke, M.; De Wever, O. Tumor Grafts Derived from Sarcoma Patients Retain Tumor Morphology, Viability, and Invasion Potential and Indicate Disease Outcomes in the Chick Chorioallantoic Membrane Model. Cancer Lett. 2012, 326, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Sys, G.M.L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The In Ovo CAM-Assay as a Xenograft Model for Sarcoma. J. Vis. Exp. 2013, 77, e50522. [Google Scholar] [CrossRef]
- Guder, W.K.; Hartmann, W.; Trautmann, M.; Hardes, J.; Wardelmann, E.; Balke, M.; Streitbürger, A. Analysis of Drug Sensitivity of Human High-Grade Osteosarcoma in a Chick Chorioallantoic Membrane (CAM) Model: A Proof of Principle Study. BMC Res. Notes 2020, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.-L.; Pion, E.; Troebs, J.; Lenze, U.; Prantl, L.; Htwe, M.M.; Phyo, A.; Haerteis, S.; Aung, T. Extended Analysis of Intratumoral Heterogeneity of Primary Osteosarcoma Tissue Using 3D-in-Vivo-Tumor-Model. Clin. Hemorheol. Microcirc. 2020, 76, 133–141. [Google Scholar] [CrossRef]
- Rubio, R.; García-Castro, J.; Gutiérrez-Aranda, I.; Paramio, J.; Santos, M.; Catalina, P.; Leone, P.E.; Menendez, P.; Rodríguez, R. Deficiency in P53 but Not Retinoblastoma Induces the Transformation of Mesenchymal Stem Cells In Vitro and Initiates Leiomyosarcoma In Vivo. Cancer Res. 2010, 70, 4185–4194. [Google Scholar] [CrossRef]
- Rubio, R.; Gutierrez-Aranda, I.; Sáez-Castillo, A.I.; Labarga, A.; Rosu-Myles, M.; Gonzalez-Garcia, S.; Toribio, M.L.; Menendez, P.; Rodriguez, R. The Differentiation Stage of P53-Rb-Deficient Bone Marrow Mesenchymal Stem Cells Imposes the Phenotype of in Vivo Sarcoma Development. Oncogene 2013, 32, 4970–4980. [Google Scholar] [CrossRef]
- Velletri, T.; Xie, N.; Wang, Y.; Huang, Y.; Yang, Q.; Chen, X.; Chen, Q.; Shou, P.; Gan, Y.; Cao, G.; et al. P53 Functional Abnormality in Mesenchymal Stem Cells Promotes Osteosarcoma Development. Cell Death Dis. 2016, 7, e2015. [Google Scholar] [CrossRef] [PubMed]
- Mohseny, A.B.; Szuhai, K.; Romeo, S.; Buddingh, E.P.; Briaire-de Bruijn, I.; de Jong, D.; van Pel, M.; Cleton-Jansen, A.-M.; Hogendoorn, P.C. Osteosarcoma Originates from Mesenchymal Stem Cells in Consequence of Aneuploidization and Genomic Loss of Cdkn2: Mesenchymal Stem Cells-Based Model to Study Osteosarcoma. J. Pathol. 2009, 219, 294–305. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting Tumours in Context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Marini, F.C.; Solley, T.N.; Elizondo, P.B.; Zhang, Y.; Sharp, H.J.; Broaddus, R.; Kolonin, M.; Mok, S.C.; Thompson, M.S.; et al. Human Omental-Derived Adipose Stem Cells Increase Ovarian Cancer Proliferation, Migration, and Chemoresistance. PLoS ONE 2013, 8, e81859. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Avnet, S.; Grisendi, G.; Salerno, M.; Granchi, D.; Dominici, M.; Kusuzaki, K.; Baldini, N. Role of Mesenchymal Stem Cells in Osteosarcoma and Metabolic Reprogramming of Tumor Cells. Oncotarget 2014, 5, 7575–7588. [Google Scholar] [CrossRef]
- Tu, B.; Du, L.; Fan, Q.-M.; Tang, Z.; Tang, T.-T. STAT3 Activation by IL-6 from Mesenchymal Stem Cells Promotes the Proliferation and Metastasis of Osteosarcoma. Cancer Lett. 2012, 325, 80–88. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, Y.; Yue, B.; Ma, X.; Zhang, G.; Xiang, H.; Liu, Y.; Wang, T.; Wu, X.; Chen, B. Adipose-Derived Mesenchymal Stem Cells Promote Osteosarcoma Proliferation and Metastasis by Activating the STAT3 Pathway. Oncotarget 2017, 8, 23803–23816. [Google Scholar] [CrossRef]
- Yu, H.; Jove, R. The STATs of Cancer—New Molecular Targets Come of Age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Plava, J.; Burikova, M.; Cihova, M.; Trnkova, L.; Smolkova, B.; Babal, P.; Krivosikova, L.; Janega, P.; Rojikova, L.; Drahosova, S.; et al. Chemotherapy-Triggered Changes in Stromal Compartment Drive Tumor Invasiveness and Progression of Breast Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 302. [Google Scholar] [CrossRef]
- Vallabhaneni, K.C.; Penfornis, P.; Dhule, S.; Guillonneau, F.; Adams, K.V.; Mo, Y.Y.; Xu, R.; Liu, Y.; Watabe, K.; Vemuri, M.C.; et al. Extracellular Vesicles from Bone Marrow Mesenchymal Stem/Stromal Cells Transport Tumor Regulatory MicroRNA, Proteins, and Metabolites. Oncotarget 2015, 6, 4953–4967. [Google Scholar] [CrossRef] [PubMed]
- Cortini, M.; Avnet, S.; Baldini, N. Mesenchymal Stroma: Role in Osteosarcoma Progression. Cancer Lett. 2017, 405, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mannerström, B.; Kornilov, R.; Abu-Shahba, A.G.; Chowdhury, I.M.; Sinha, S.; Seppänen-Kaijansinkko, R.; Kaur, S. Epigenetic Alterations in Mesenchymal Stem Cells by Osteosarcoma-Derived Extracellular Vesicles. Epigenetics 2019, 14, 352–364. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, Y.; Li, K.; Zhang, G.; Guo, Z.; Wu, X.; Qiu, C.; Li, Y.; Wan, X.; Sui, J.; et al. Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front. Cell Dev. Biol. 2020, 8, 353. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of Exosomes on Pre-Metastatic Niche Formation in Tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef]
- Chang, X.; Ma, Z.; Zhu, G.; Lu, Y.; Yang, J. New Perspective into Mesenchymal Stem Cells: Molecular Mechanisms Regulating Osteosarcoma. J. Bone Oncol. 2021, 29, 100372. [Google Scholar] [CrossRef]
- Avril, P.; Duteille, F.; Ridel, P.; Heymann, M.-F.; De Pinieux, G.; Rédini, F.; Blanchard, F.; Heymann, D.; Trichet, V.; Perrot, P. Opposite Effects of Soluble Factors Secreted by Adipose Tissue on Proliferating and Quiescent Osteosarcoma Cells. Plast. Reconstr. Surg. 2016, 137, 865–875. [Google Scholar] [CrossRef]
- Lee, S.-W.; Jeon, T.J.; Biswal, S. Effect of Local Treatment with Adipose Tissue-Derived Mesenchymal Stem Cells in the Early Tumorigenesis of Osteosarcoma. Oncol. Rep. 2015, 33, 1381–1387. [Google Scholar] [CrossRef]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.-C.; Choi, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl. Med. 2019, 8, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Guiho, R.; Biteau, K.; Grisendi, G.; Taurelle, J.; Chatelais, M.; Gantier, M.; Heymann, D.; Dominici, M.; Redini, F. TRAIL Delivered by Mesenchymal Stromal/Stem Cells Counteracts Tumor Development in Orthotopic Ewing Sarcoma Models: MSC-TRAIL Counteract-Ewing Sarcoma Tumor Development. Int. J. Cancer 2016, 139, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Litowczenko, J.; Woźniak-Budych, M.J.; Staszak, K.; Wieszczycka, K.; Jurga, S.; Tylkowski, B. Milestones and Current Achievements in Development of Multifunctional Bioscaffolds for Medical Application. Bioact. Mater. 2021, 6, 2412–2438. [Google Scholar] [CrossRef]
- Muzzio, N.; Moya, S.; Romero, G. Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics 2021, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Two New Applications in the Study of Angiogenesis the CAM Assay: Acellular Scaffolds and Organoids. Microvasc. Res. 2022, 140, 104304. [Google Scholar] [CrossRef]
- Morgan, J.P.; Delnero, P.F.; Zheng, Y.; Verbridge, S.S.; Chen, J.; Craven, M.; Choi, N.W.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; et al. Formation of Microvascular Networks in Vitro. Nat. Protoc. 2013, 8, 1820–1836. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of Decellularized Scaffolds for Stem Cell-Driven Tissue Engineering: Decellularized Scaffolds for Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- D’Arcy, P.F.; Howard, E.M. A New Anti-Inflammatory Test, Utilizing the Chorio-Allantoic Membrane of the Chick Embryo. Br. J. Pharmacol. Chemother. 1967, 29, 378–387. [Google Scholar] [CrossRef]
- Zwadlo-Klarwasser, G.; Görlitz, K.; Hafemann, B.; Klee, D.; Klosterhalfen, B. The Chorioallantoic Membrane of the Chick Embryo as a Simple Model for the Study of the Angiogenic and Inflammatory Response to Biomaterials. J. Mater. Sci. Mater. Med. 2001, 12, 195–199. [Google Scholar] [CrossRef]
- Coombes, A.G.A.; Heckman, J.D. Gel Casting of Resorbable Polymers. Biomaterials 1992, 13, 297–307. [Google Scholar] [CrossRef]
- Chiu, L.L.Y.; Radisic, M.; Vunjak-Novakovic, G. Bioactive Scaffolds for Engineering Vascularized Cardiac Tissues: Bioactive Scaffolds for Engineering Vascularized Cardiac Tissues. Macromol. Biosci. 2010, 10, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Oates, M.; Chen, R.; Duncan, M.; Hunt, J.A. The Angiogenic Potential of Three-Dimensional Open Porous Synthetic Matrix Materials. Biomaterials 2007, 28, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The Effect of Porous Structure on the Cell Proliferation, Tissue Ingrowth and Angiogenic Properties of Poly(Glycerol Sebacate Urethane) Scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.-C.; Brey, E.M.; Cinar, A. An Agent-Based Model for the Investigation of Neovascularization Within Porous Scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef]
- Dreesmann, L.; Ahlers, M.; Schlosshauer, B. The Pro-Angiogenic Characteristics of a Cross-Linked Gelatin Matrix. Biomaterials 2007, 28, 5536–5543. [Google Scholar] [CrossRef]
- Shahzadi, L.; Yar, M.; Jamal, A.; Siddiqi, S.A.; Chaudhry, A.A.; Zahid, S.; Tariq, M.; Rehman, I.U.; MacNeil, S. Triethyl Orthoformate Covalently Cross-Linked Chitosan-(Poly Vinyl) Alcohol Based Biodegradable Scaffolds with Heparin-Binding Ability for Promoting Neovascularisation. J. Biomater. Appl. 2016, 31, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Macchiarini, P.; Ribatti, D. Chorioallantoic Membrane for in Vivo Investigation of Tissue-Engineered Construct Biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1425–1434. [Google Scholar] [CrossRef]
- Grieb, G.; Groger, A.; Piatkowski, A.; Markowicz, M.; Steffens, G.C.M.; Pallua, N. Tissue Substitutes with Improved Angiogenic Capabilities: An in Vitro Investigation with Endothelial Cells and Endothelial Progenitor Cells. Cells Tissues Organs 2010, 191, 96–104. [Google Scholar] [CrossRef]
- Chau, D.Y.S.; Brown, S.V.; Mather, M.L.; Hutter, V.; Tint, N.L.; Dua, H.S.; Rose, F.R.A.J.; Ghaemmaghami, A.M. Tissue Transglutaminase (TG-2) Modified Amniotic Membrane: A Novel Scaffold for Biomedical Applications. Biomed. Mater. 2012, 7, 045011. [Google Scholar] [CrossRef]
- Timmer, M.D.; Shin, H.; Horch, R.A.; Ambrose, C.G.; Mikos, A.G. In Vitro Cytotoxicity of Injectable and Biodegradable Poly(Propylene Fumarate)-Based Networks: Unreacted Macromers, Cross-Linked Networks, and Degradation Products. Biomacromolecules 2003, 4, 1026–1033. [Google Scholar] [CrossRef]
- Lu, H.; Wang, F.; Mei, H.; Wang, S.; Cheng, L. Human Adipose Mesenchymal Stem Cells Show More Efficient Angiogenesis Promotion on Endothelial Colony-Forming Cells than Umbilical Cord and Endometrium. Stem Cells Int. 2018, 2018, 7537589. [Google Scholar] [CrossRef]
- Biagini, G.; Senegaglia, A.C.; Pereira, T.; Berti, L.F.; Marcon, B.H.; Stimamiglio, M.A. 3D Poly(Lactic Acid) Scaffolds Promote Different Behaviors on Endothelial Progenitors and Adipose-Derived Stromal Cells in Comparison With Standard 2D Cultures. Front. Bioeng. Biotechnol. 2021, 9, 700862. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Villalobos, M.A.; Choron, R.L.; Chang, S.; Brown, S.A.; Carpenter, J.P.; Tulenko, T.N.; Zhang, P. Fibroblast Growth Factor and Vascular Endothelial Growth Factor Play a Critical Role in Endotheliogenesis from Human Adipose-Derived Stem Cells. J. Vasc. Surg. 2017, 65, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kakudo, N.; Morimoto, N.; Lai, F.; Taketani, S.; Kusumoto, K. Fibroblast Growth Factor-2 Stimulates Proliferation of Human Adipose-Derived Stem Cells via Src Activation. Stem Cell Res. Ther. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Brugge, J.S. Cellular Functions Regulated by SRC Family Kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Martin, E.; Hayes, D.; Gimble, J.; Devireddy, R. MicroRNA Sequencing of CD34+ Sorted Adipose Stem Cells Undergoing Endotheliogenesis. Stem Cells Dev. 2021, 30, 265–288. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Li, J.; You, C.; Lu, P.; Feng, H.; Kong, Y.; Zhang, H.; Liu, Y.; Jiao, R.; et al. MicroRNA-181a Promotes Angiogenesis in Colorectal Cancer by Targeting SRCIN1 to Promote the SRC/VEGF Signaling Pathway. Cell Death Dis. 2018, 9, 438. [Google Scholar] [CrossRef]
- Winter, R.; Dungel, P.; Reischies, F.M.J.; Rohringer, S.; Slezak, P.; Smolle, C.; Spendel, S.; Kamolz, L.-P.; Ghaffari-Tabrizi-Wizsy, N.; Schicho, K. Photobiomodulation (PBM) Promotes Angiogenesis in-Vitro and in Chick Embryo Chorioallantoic Membrane Model. Sci. Rep. 2018, 8, 17080. [Google Scholar] [CrossRef]
- Dungel, P.; Mittermayr, R.; Haindl, S.; Osipov, A.; Wagner, C.; Redl, H.; Kozlov, A.V. Illumination with Blue Light Reactivates Respiratory Activity of Mitochondria Inhibited by Nitric Oxide, but Not by Glycerol Trinitrate. Arch. Biochem. Biophys. 2008, 471, 109–115. [Google Scholar] [CrossRef]
- Paschke, F.; Rabong, C.; Schuster, C. Red Light as a 12-Oxo-Leukotriene B4 Antagonist: An Explanation for the Efficacy of Intensive Red Light in the Therapy of Peripheral Inflammatory Diseases. Biomed. Eng. Biomed. Tech. 2014, 59, 487–493. [Google Scholar] [CrossRef]
- Conconi, M.T.; Nico, B.; Rebuffat, P.; Crivellato, E.; Parnigotto, P.P.; Nussdorfer, G.G.; Ribatti, D. Angiogenic Response Induced by Acellular Femoral Matrix in Vivo: Angiogenic Response Induced by Acellular Femoral Matrix, M.T. Conconi et Al. J. Anat. 2005, 207, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Bellini, S.; Teoli, D.; de Coppi, P.; Ribatti, D.; Nico, B.; Simonato, E.; Gamba, P.G.; Nussdorfer, G.G.; Morpurgo, M.; et al. In Vitro and in Vivo Evaluation of Acellular Diaphragmatic Matrices Seeded with Muscle Precursors Cells and Coated with VEGF Silica Gels to Repair Muscle Defect of the Diaphragm. J. Biomed. Mater. Res. A 2009, 89, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Gonfiotti, A.; Jaus, M.; Comin, C.E.; Paglierani, M.; Del Gaudio, C.; Bianco, A.; Ribatti, D.; Macchiarini, P. Development of Bioengineered Human Larynx. Biomaterials 2011, 32, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Del Gaudio, C.; Jaus, M.O.; Polizzi, L.; Gonfiotti, A.; Comin, C.E.; Bianco, A.; Ribatti, D.; Taylor, D.A.; Macchiarini, P. Long-Term Changes to in Vitro Preserved Bioengineered Human Trachea and Their Implications for Decellularized Tissues. Biomaterials 2012, 33, 3662–3672. [Google Scholar] [CrossRef]
- Haag, J.; Baiguera, S.; Jungebluth, P.; Barale, D.; Del Gaudio, C.; Castiglione, F.; Bianco, A.; Comin, C.E.; Ribatti, D.; Macchiarini, P. Biomechanical and Angiogenic Properties of Tissue-Engineered Rat Trachea Using Genipin Cross-Linked Decellularized Tissue. Biomaterials 2012, 33, 780–789. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Gálvez-Montón, C.; Prat-Vidal, C.; Jorba, I.; Segú-Vergés, C.; Roura, S.; Soler-Botija, C.; Iborra-Egea, O.; Revuelta-López, E.; Fernández, M.A.; et al. Head-to-Head Comparison of Two Engineered Cardiac Grafts for Myocardial Repair: From Scaffold Characterization to Pre-Clinical Testing. Sci. Rep. 2018, 8, 6708. [Google Scholar] [CrossRef]
- Yokoyama, R.; Ii, M.; Tabata, Y.; Hoshiga, M.; Ishizaka, N.; Asahi, M. Cardiac Regeneration by Statin-Polymer Nanoparticle-Loaded Adipose-Derived Stem Cell Therapy in Myocardial Infarction. Stem Cells Transl. Med. 2019, 8, 1055–1067. [Google Scholar] [CrossRef]
- Li, Z.-H.; Ji, S.-C.; Wang, Y.-Z.; Shen, X.-C.; Liang, H. Silk Fibroin-Based Scaffolds for Tissue Engineering. Front. Mater. Sci. 2013, 7, 237–247. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Silva-Correia, J.; Nascimento, A.I.; da Silva Morais, A.; Marques, A.P.; Ribeiro, A.S.; Silva, C.J.; Bonifácio, G.; Sousa, R.A.; Oliveira, J.M.; et al. Silk-Based Anisotropical 3D Biotextiles for Bone Regeneration. Biomaterials 2017, 123, 92–106. [Google Scholar] [CrossRef]
- Pelto, J.; Björninen, M.; Pälli, A.; Talvitie, E.; Hyttinen, J.; Mannerström, B.; Suuronen Seppanen, R.; Kellomäki, M.; Miettinen, S.; Haimi, S. Novel Polypyrrole-Coated Polylactide Scaffolds Enhance Adipose Stem Cell Proliferation and Early Osteogenic Differentiation. Tissue Eng. Part A 2013, 19, 882–892. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Khademhosseini, A.; Marques, A.P.; Gomes, M.E. The Osteogenic Differentiation of SSEA-4 Sub-Population of Human Adipose Derived Stem Cells Using Silicate Nanoplatelets. Biomaterials 2014, 35, 9087–9099. [Google Scholar] [CrossRef] [PubMed]
- Milat, F.; Ng, K.W. Is Wnt Signalling the Final Common Pathway Leading to Bone Formation? Mol. Cell. Endocrinol. 2009, 310, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, A.S.; Sabliov, C.; Gimble, J.M.; Hayes, D.J. Human Adipose-Derived Stem Cells and Three-Dimensional Scaffold Constructs: A Review of the Biomaterials and Models Currently Used for Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 187–199. [Google Scholar] [CrossRef]
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A Comparative Study of Proliferation and Osteogenic Differentiation of Adipose-Derived Stem Cells on Akermanite and β-TCP Ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef]
- Cordeiro, I.R.; Lopes, D.V.; Abreu, J.G.; Carneiro, K.; Rossi, M.I.D.; Brito, J.M. Chick embryo xenograft model reveals a novel perineural niche for human adipose-derived stromal cells. Biol. Open 2015, 4, 1180–1193. [Google Scholar] [CrossRef]
- Cirligeriu, L.; Cimpean, A.; Calniceanu, H.; Vladau, M.; Sarb, S.; Raica, M.; Nica, L. Hyaluronic Acid/Bone Substitute Complex Implanted on Chick Embryo Chorioallantoic Membrane Induces Osteoblastic Differentiation and Angiogenesis, but Not Inflammation. Int. J. Mol. Sci. 2018, 19, 4119. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Huang, J.-P.; Chen, Y.-Y.; Aplin, J.D.; Wu, Y.-H.; Chen, C.-Y.; Chen, P.-C.; Chen, C.-P. Angiogenesis in Differentiated Placental Multipotent Mesenchymal Stromal Cells Is Dependent on Integrin A5β1. PLoS ONE 2009, 4, e6913. [Google Scholar] [CrossRef]
- Kaushik, K.; Das, A. Cycloxygenase-2 Inhibition Potentiates Trans-Differentiation of Wharton’s Jelly–Mesenchymal Stromal Cells into Endothelial Cells: Transplantation Enhances Neovascularization-Mediated Wound Repair. Cytotherapy 2019, 21, 260–273. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.A.; Janeczek, A.A.; Kontouli, N.; Kanczler, J.M.; Evans, N.D.; Oreffo, R.O. The Chorioallantoic Membrane (CAM) Assay for the Study of Human Bone Regeneration: A Refinement Animal Model for Tissue Engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef]
- Faihs, L.; Firouz, B.; Slezak, P.; Slezak, C.; Weißensteiner, M.; Ebner, T.; Ghaffari Tabrizi-Wizsy, N.; Schicho, K.; Dungel, P. A Novel Artificial Intelligence-Based Approach for Quantitative Assessment of Angiogenesis in the Ex Ovo CAM Model. Cancers 2022, 14, 4273. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Belinha, J.; Mangir, N.; MacNeil, S.; Natal Jorge, R. Simulation of the Process of Angiogenesis: Quantification and Assessment of Vascular Patterning in the Chicken Chorioallantoic Membrane. Comput. Biol. Med. 2021, 136, 104647. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus Guidelines for the Use and Interpretation of Angiogenesis Assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed]
- International Chicken Genome Sequencing Consortium. Sequence and Comparative Analysis of the Chicken Genome Provide Unique Perspectives on Vertebrate Evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | CAM Assay | ADSC Origin | Context of Utilization | Main Findings | Ref. |
|---|---|---|---|---|---|
| Borges et al. (2006) | In ovo | Human subcutaneous tissue derived from surgery | Vascularization and angiogenic effects of ADSCs in fibrin matrix | Significantly increased angiogenesis in the intervention group | [97] |
| Buschmann et al. (2012) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic potential of ADSC-seeded PLGA/a-CaP electrospun scaffolds | Homogeneous vessel distribution within the tubes | [98] |
| Guasti et al. (2013) | In ovo | Lipoaspirated, pretreated paediatric human cells | Vascular response to human ADSCs-seeded POSS-PCU scaffolds and cell survival | Successful vascularization and presence of ADSCs within the scaffold | [99] |
| Handel et al. (2013) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of ADSC-seeded 45S5-Bioglass-Based 3D scaffolds | Significantly increased angiogenesis in the intervention group compared to human-fibroblast-seeded scaffolds | [100] |
| Strassburg et al. (2013) | In ovo | Human subcutaneous tissue derived from surgery | Angiogenic effects of ADSCs in co-culture with endothelial cell and HUVEC spheroids in fibrin matrix | Significantly increased angiogenesis in the intervention group with HUVECs | [101] |
| Wahl et al. (2015) | Ex ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of CM from ADSC-seeded chitosan, fibrin, bovine collagen, and decellularized porcine dermis scaffolds | Significantly increased angiogenesis for CM from seeded COL/GAG matrices | [102] |
| New et al. (2016) | Both | Lipoaspirated, pretreated paediatric human cells | Angiogenesis and compatibility of ADSC-seeded nanoscaffold composites | Proof-of-concept for the intervention group in terms of in vivo biocompatibility, angiogenesis, and vascularization | [103] |
| Cheng et al. (2017) | In ovo | Human subcutaneous tissue derived from abdominoplasty | Angiogenic effects of ADSC-blended collagen/chitosan hydrogels | Significantly increased angiogenesis in the intervention group | [104] |
| Shafaat et al. (2017) | Ex ovo | Human subcutaneous-fat-tissue-derived | Angiogenic effects of ADSC-seeded estradiol-releasing PU scaffolds | Significantly increased angiogenesis in the intervention group | [105] |
| Increased density of ECM in the intervention group | |||||
| Beugels et al. (2019) | In ovo | Lipoaspirated, pretreated, Single-donor human cells | Angiogenic effects of ADSC- derived secretome post-electrostimulation | Significantly increased angiogenesis in the intervention group | [88] |
| Di Somma et al. (2019) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of ADSC-derived beige cells | Significantly increased angiogenesis in the intervention group | [106] |
| Sousa et al. (2019) | In ovo | Human ADSCs (ATCC) | Angiogenic effects of ADSC- derived cell-fibers | Significantly increased angiogenesis in the intervention group with HUVECs | [107] |
| Teo et al. (2019) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of ADSCs equipped with antioxidizing particles exposed to H2O2 | Significantly increased angiogenesis for cells tethered with particles loading EGCG and MnO2 nanocatalysts | [89] |
| Yang et al. (2019) | Ex ovo | hTERT immortalized ADMSCs | Angiogenic effect of CM from ADSCs exposed to low-stiffness hydrogel | Significantly increased EC proliferation, migration, and angiogenesis in the intervention group | [108] |
| Ratushnyy et al. (2020) | In ovo | Non-senescent and senescent (long-term cultivated), lipoaspirated human cells | Comparison of angiogenic effects of CM of both groups | Significantly decreased angiogenesis in the senescent group | [109] |
| Otto et al. (2021) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of combinations of sc-ADSCs, 3D-MT ADSCs, and its secretome in a collagen scaffold | Significantly increased angiogenesis for sc-ADSCs | [110] |
| Significantly increased COL formation for sc-ADSCs | |||||
| Significantly increased mineralization for 3D-MT ADSC secretome | |||||
| Watchararot et al. (2021) | Ex ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of ADSC-seeded vs. acellular SF scaffolds | Significantly increased angiogenesis in the intervention group at day E11 | [111] |
| Ezdakova et al. (2022) | In ovo | hTERT immortalized cells (ASC52telo) | Angiogenic effect of CM from ADSCs and ECs co-culture | Significantly increased angiogenesis in the intervention group | [112] |
| Lin et al. (2022) | In ovo | Lipoaspirated, cryopreserved human cells | Angiogenic effects of cryopreservation | Significantly increased angiogenesis in the intervention group | [87] |
| Yu et al. (2022) | In ovo | Human subcutaneous-fat-tissue-derived | Angiogenic effects of ADSC-spheroid-integrated cell sheets | Significantly increased angiogenesis in the intervention group | [96] |
| Zhu et al. (2022) | In ovo | Lipoaspirated, pretreated human cells | Angiogenic effects of hypothermic pre-treatment | Significantly increased angiogenesis in the intervention group | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliinyk, D.; Eigenberger, A.; Felthaus, O.; Haerteis, S.; Prantl, L. Chorioallantoic Membrane Assay at the Cross-Roads of Adipose-Tissue-Derived Stem Cell Research. Cells 2023, 12, 592. https://doi.org/10.3390/cells12040592
Oliinyk D, Eigenberger A, Felthaus O, Haerteis S, Prantl L. Chorioallantoic Membrane Assay at the Cross-Roads of Adipose-Tissue-Derived Stem Cell Research. Cells. 2023; 12(4):592. https://doi.org/10.3390/cells12040592
Chicago/Turabian StyleOliinyk, Dmytro, Andreas Eigenberger, Oliver Felthaus, Silke Haerteis, and Lukas Prantl. 2023. "Chorioallantoic Membrane Assay at the Cross-Roads of Adipose-Tissue-Derived Stem Cell Research" Cells 12, no. 4: 592. https://doi.org/10.3390/cells12040592
APA StyleOliinyk, D., Eigenberger, A., Felthaus, O., Haerteis, S., & Prantl, L. (2023). Chorioallantoic Membrane Assay at the Cross-Roads of Adipose-Tissue-Derived Stem Cell Research. Cells, 12(4), 592. https://doi.org/10.3390/cells12040592







