Mustard Gas Exposure Actuates SMAD2/3 Signaling to Promote Myofibroblast Generation in the Cornea
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vivo Rabbit SM Studies
2.3. Animal Tissue Processing
2.4. In Vitro NM Studies
2.5. Real-Time PCR
2.6. Western Blot
2.7. Immunocytochemistry
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. SM Exposure Induces Myofibroblast Formation in Rabbit Cornea In Vivo
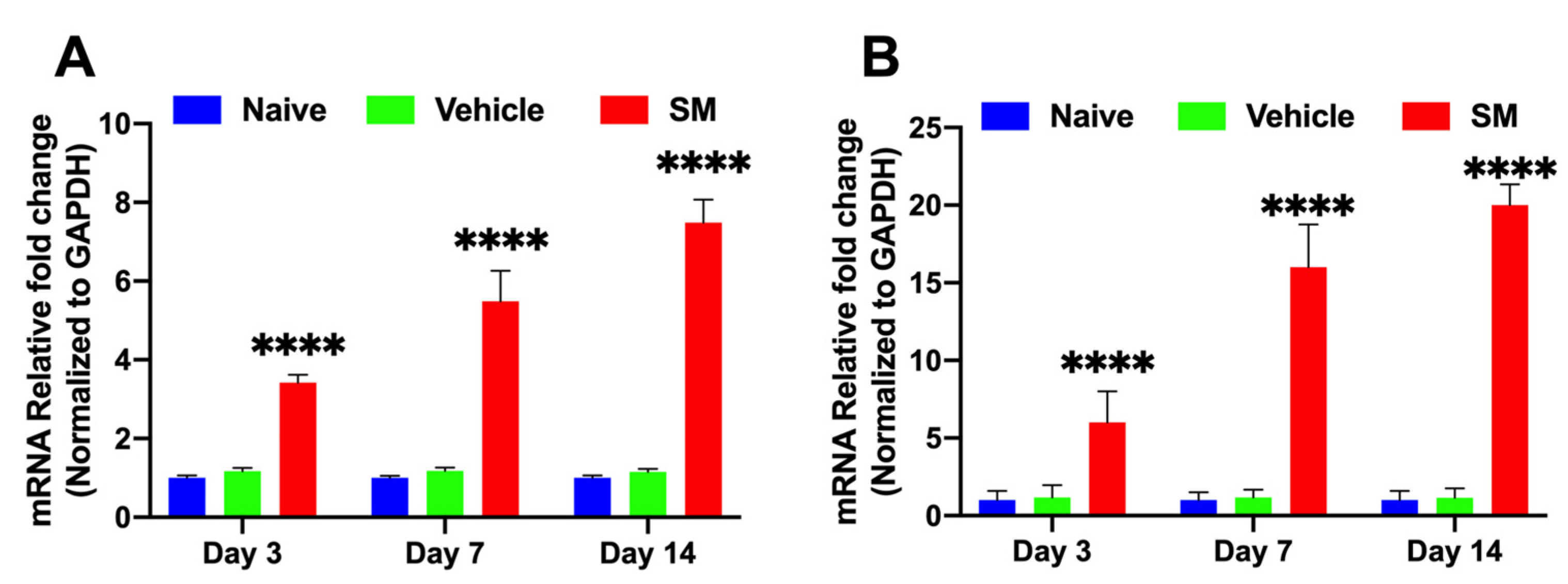
3.1.1. Time-Dependent Expression of Profibrotic Genes in Rabbit CORNEAS Exposed to SM In Vivo
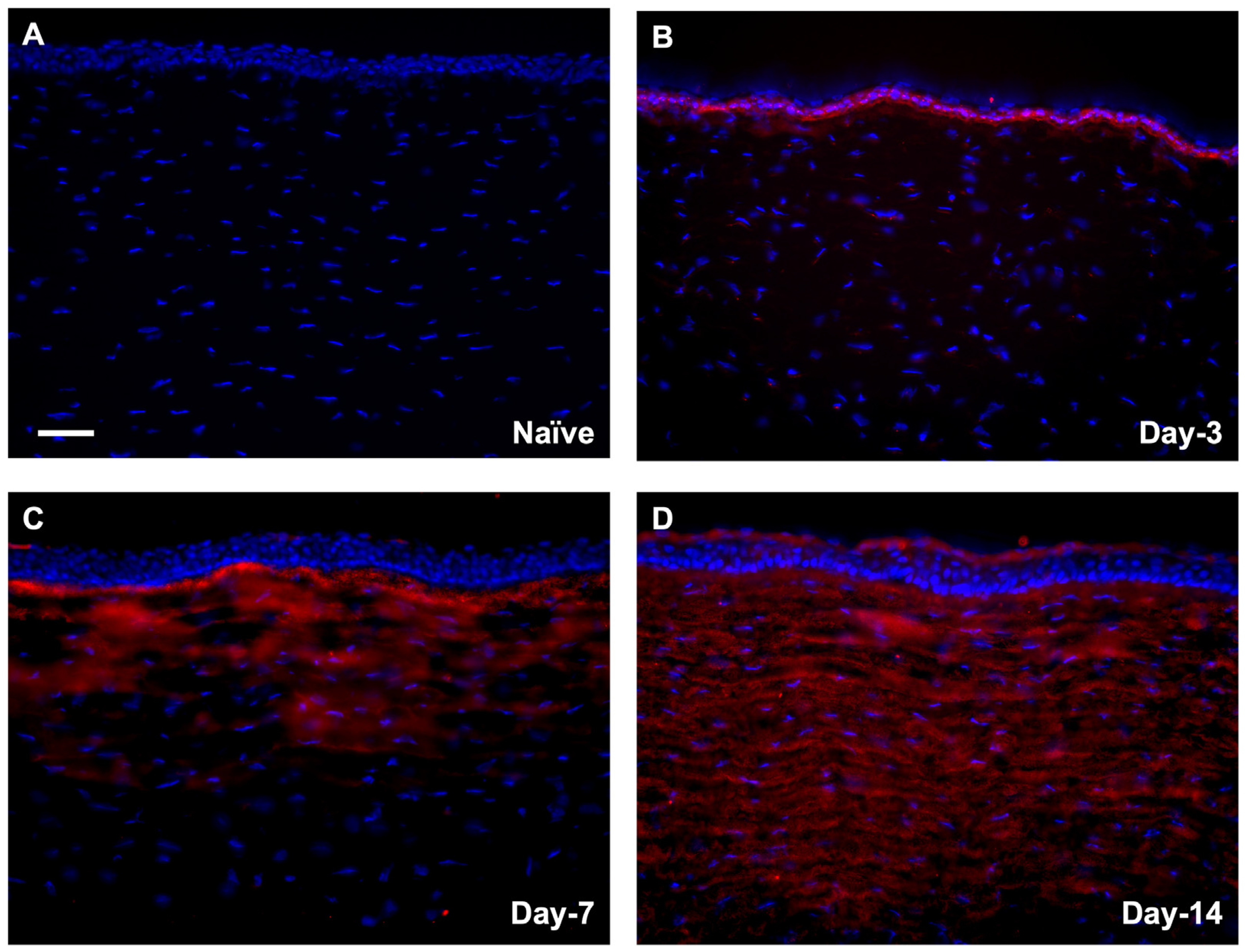
3.1.2. Localization of αSMA in Rabbit Corneas Exposed to SM In Vivo
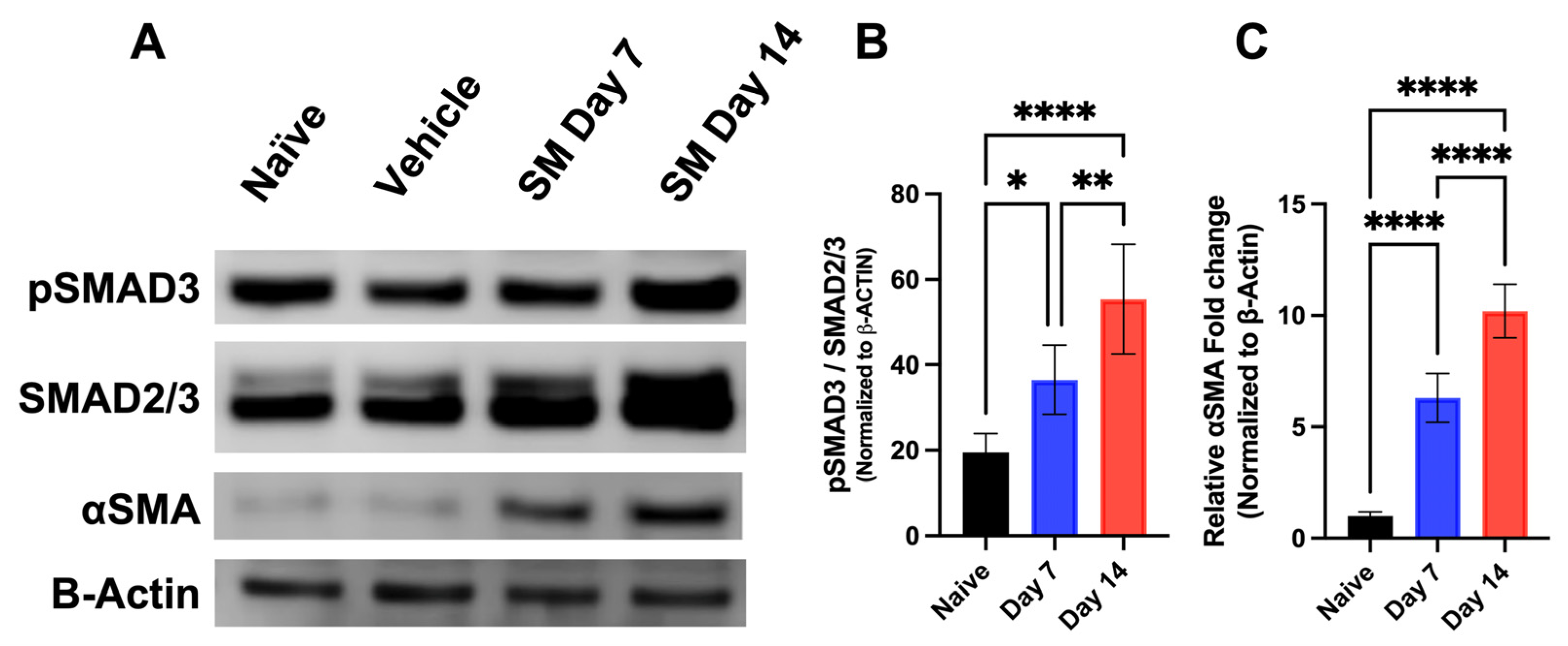
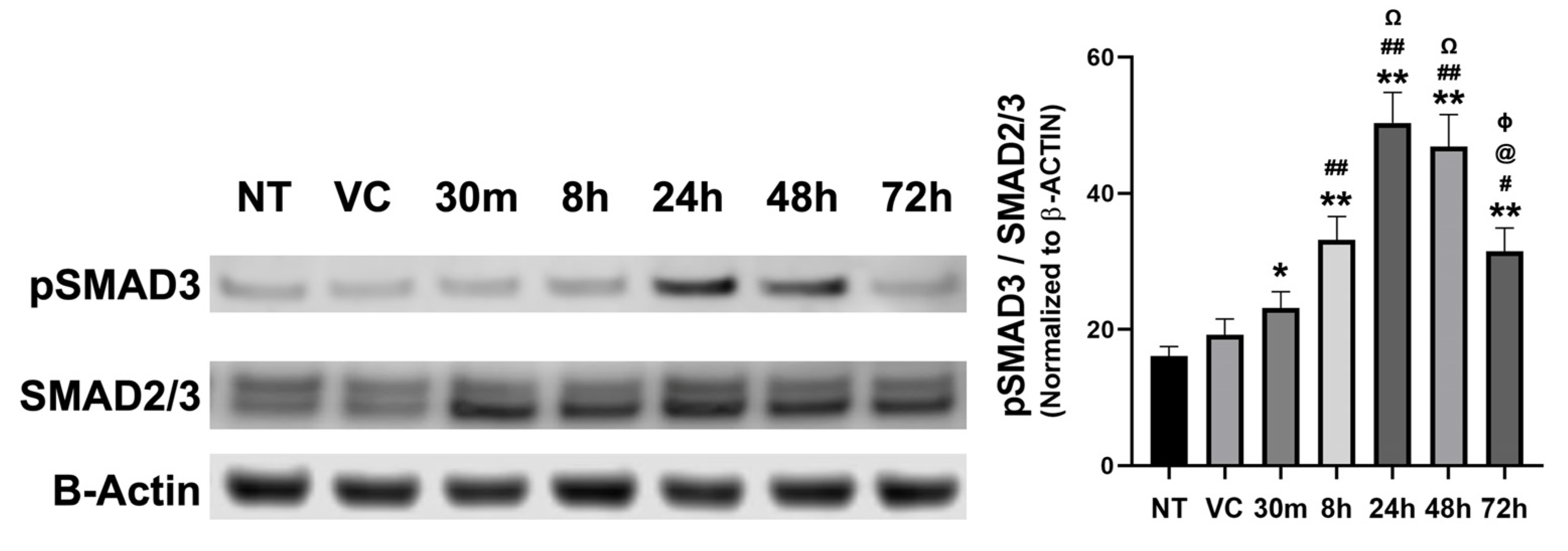
3.1.3. Time-Dependent Expression of SMAD Signaling Proteins in Rabbit Corneas Exposed to SM In Vivo
3.2. NM Exposure Induces Myofibroblast Formation in a Time-Dependent Expression In Vitro
3.2.1. Time-Dependent Analysis of αSMA in hCSFs Treated with NM In Vitro
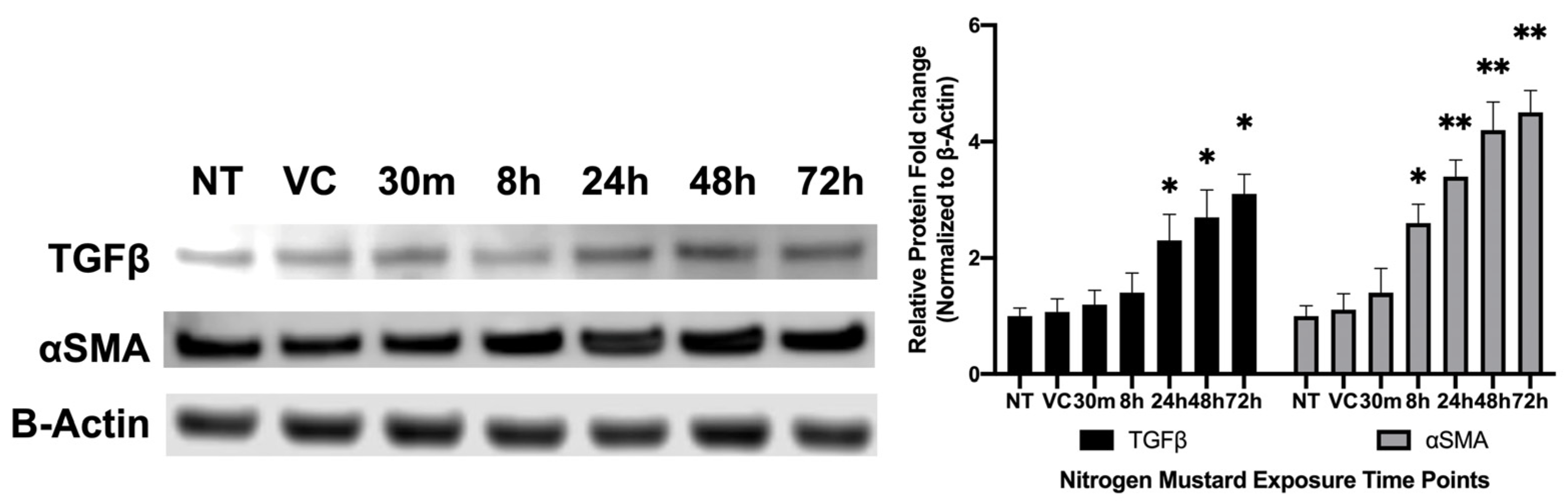
3.2.2. Profibrotic Protein Analysis of hCSFs Treated −/+ NM In Vitro
3.3. NM-Induced Myofibroblast Formation Involves Activation of SMAD Signaling Pathway
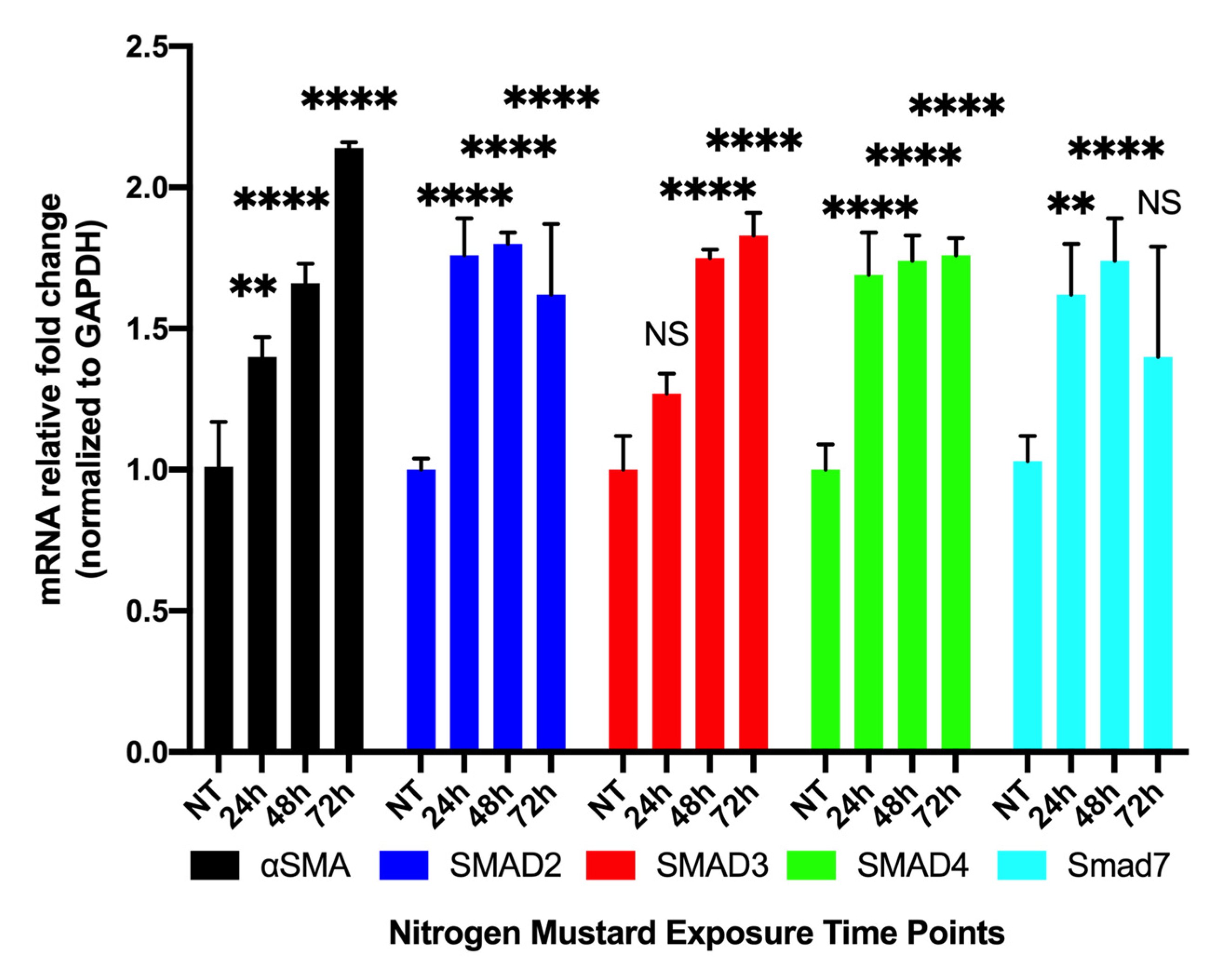
3.3.1. Time-Dependent Expression of Profibrotic Genes in hCSFs Treated with NM In Vitro
3.3.2. Time-Dependent Expression of Profibrotic Proteins in hCSFs Treated with NM In Vitro
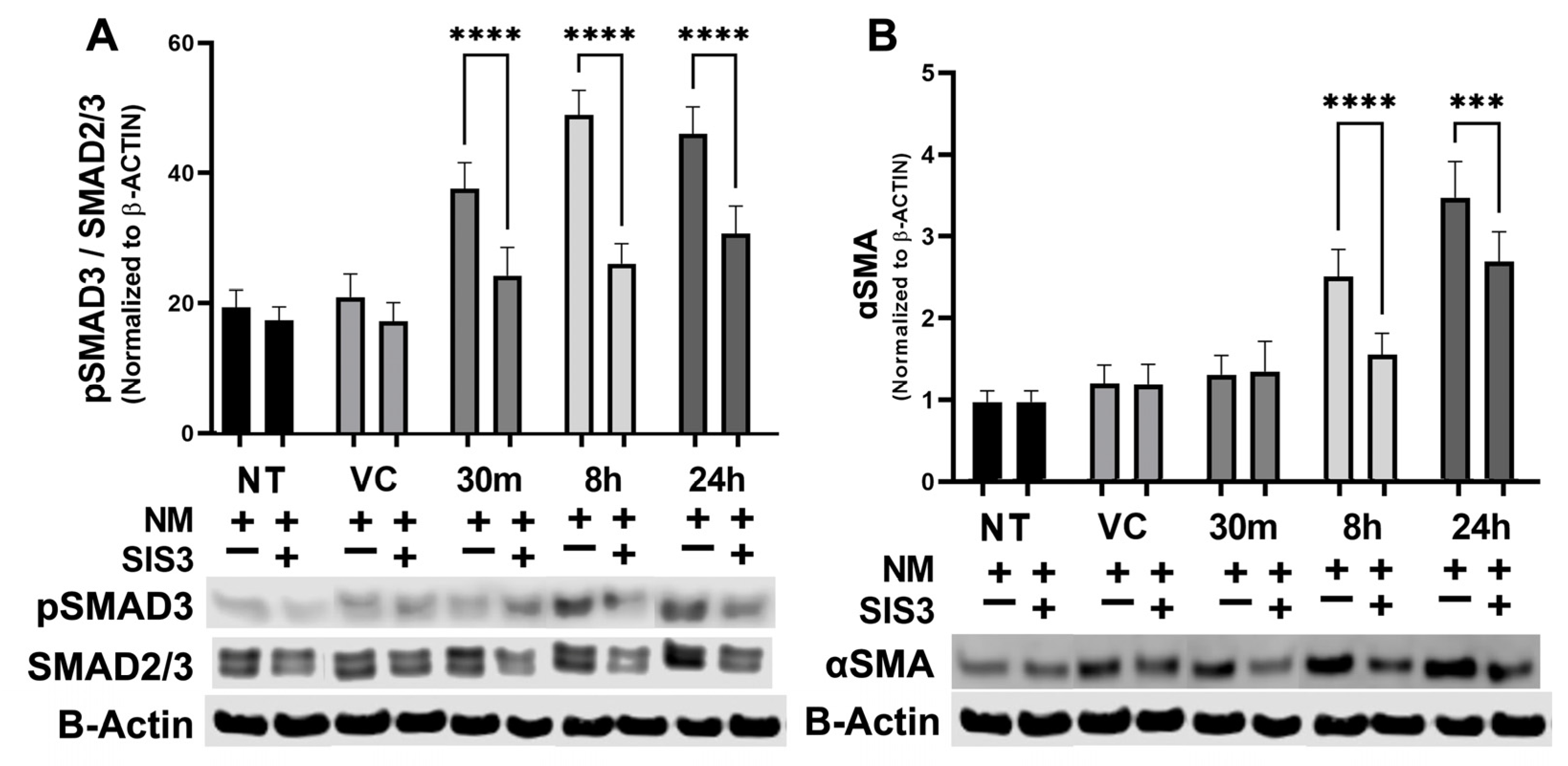
3.4. Modulation of NM-Induced SMAD Expression by Specific Inhibitor In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wattana, M.; Bey, T. Mustard Gas or Sulfur Mustard: An Old Chemical Agent as a New Terrorist Threat. Prehospital Disaster Med. 2009, 24, 19–29. [Google Scholar] [CrossRef] [PubMed]
- OPCW Technical Secretariat Report of the OPCW Fact-Finding Mission in Syria Regarding the Incident of 2 August 2016 as Reported in the Note Verbale of the Syrian Arab Republic Number 69 Dated 16 August 2016. 2016/12/21 2016, S14442016, 30. Available online: https://www.opcw.org/sites/default/files/documents/2018/11/s-1444-2016%28e%29.pdf (accessed on 24 August 2020).
- Smith, S.L. Toxic Legacy: Mustard Gas in the Sea Around Us. J. Law Med. Ethics 2011, 39, 34–40. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Moosavi, S.A.; Montazeri, B. Ocular Injuries Caused by Mustard Gas: Diagnosis, Treatment, and Medical Defense. Mil. Med. 2001, 166, 67–70. [Google Scholar] [CrossRef] [PubMed]
- McNutt, P.; Lyman, M.; Swartz, A.; Tuznik, K.; Kniffin, D.; Whitten, K.; Milhorn, D.; Hamilton, T. Architectural and Biochemical Expressions of Mustard Gas Keratopathy: Preclinical Indicators and Pathogenic Mechanisms. PLoS ONE 2012, 7, e42837. [Google Scholar] [CrossRef]
- Kilic, E.; Ortatatli, M.; Sezigen, S.; Eyison, R.K.; Kenar, L. Acute Intensive Care Unit Management of Mustard Gas Victims: The Turkish Experience*. Cutan. Ocul. Toxicol. 2018, 37, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Ghazanfari, T.; Ghassemi-Broumand, M.; Javadi, M.A.; Babaei, M.; Soroush, M.R.; Yaraee, R.; Faghihzadeh, S.; Poorfarzam, S.; Owlia, P.; et al. Long-Term Ocular Consequences of Sulfur Mustard in Seriously Eye-Injured War Veterans. Cutan. Ocul. Toxicol. 2009, 28, 71–77. [Google Scholar] [CrossRef]
- Javadi, M.A.; Jafarinasab, M.R.; Feizi, S.; Karimian, F.; Negahban, K. Management of Mustard Gas-Induced Limbal Stem Cell Deficiency and Keratitis. Ophthalmology 2011, 118, 1272–1281. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Tseng, S.C.G. Sulfur Mustard-Induced Ocular Surface Disorders. Ocul. Surf. 2011, 9, 163–178. [Google Scholar] [CrossRef]
- Safi, S.; Javadi, M.A.; Jafarinasab, M.R.; Feizi, S.; Moghadam, M.S.; Jadidi, K.; Babaei, M.; Shirvani, A.; Baradaran-Rafii, A.; Mohammad-Rabei, H.; et al. Clinical Practice Guidelines for Prevention, Diagnosis and Management of Early and Delayed-Onset Ocular Injuries Due to Mustard Gas Exposure. J. Ophthalmic Vis. Res. 2017, 12, 65–80. [Google Scholar] [CrossRef]
- Solberg, Y.; Alcalay, M.; Belkin, M. Ocular Injury by Mustard Gas. Surv. Ophthalmol. 1997, 41, 461–466. [Google Scholar] [CrossRef]
- McNutt, P.; Hamilton, T.; Nelson, M.; Adkins, A.; Swartz, A.; Lawrence, R.; Milhorn, D. Pathogenesis of Acute and Delayed Corneal Lesions after Ocular Exposure to Sulfur Mustard Vapor. Cornea 2012, 31, 280–290. [Google Scholar] [CrossRef]
- Milhorn, D.M.; Hamilton, T.A.; Nelson, M.R.; McNutt, P.M. Progression of Ocular Sulfur Mustard Injury: Development of a Model System. Ann. N. Y. Acad. Sci. 2010, 1194, 72–80. [Google Scholar] [CrossRef]
- Rowell, M.; Kehe, K.; Balszuweit, F.; Thiermann, H. The Chronic Effects of Sulfur Mustard Exposure. Toxicology 2009, 263, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Jafarinasab, M.R.; Zarei-Ghanavati, S.; Kanavi, M.R.; Karimian, F.; Soroush, M.R.; Javadi, M.A. Confocal Microscopy in Chronic and Delayed Mustard Gas Keratopathy. Cornea 2010, 29, 889–894. [Google Scholar] [CrossRef] [PubMed]
- McNutt, P.; Tuznik, K.; Nelson, M.; Adkins, A.; Lyman, M.; Glotfelty, E.; Hughes, J.; Hamilton, T. Structural, Morphological, and Functional Correlates of Corneal Endothelial Toxicity Following Corneal Exposure to Sulfur Mustard Vapor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6735–6744. [Google Scholar] [CrossRef]
- Kadar, T.; Horwitz, V.; Sahar, R.; Cohen, M.; Cohen, L.; Gez, R.; Tveria, L.; Gutman, H.; Buch, H.; Fishbine, E.; et al. Delayed Loss of Corneal Epithelial Stem Cells in a Chemical Injury Model Associated with Limbal Stem Cell Deficiency in Rabbits. Curr. Eye Res. 2011, 36, 1098–1107. [Google Scholar] [CrossRef]
- Murray, V.S.G.; Volans, G.N. Management of Injuries Due to Chemical Weapons. Br. Med. J. 1991, 302, 129–130. [Google Scholar] [CrossRef] [PubMed]
- McNutt, P.M.; Mohan, R.R. The Need for Improved Therapeutic Approaches to Protect the Cornea against Chemotoxic Injuries. Transl. Vis. Sci. Technol. 2020, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M. Corneal Collagen-Its Role in Maintaining Corneal Shape and Transparency. Biophys. Rev. 2009, 1, 83–93. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and Proteoglycans of the Corneal Extracellular Matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The Molecular Basis of Corneal Transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Balne, P.K.; Gupta, S.; Zhang, J.; Bristow, D.; Faubion, M.; Heil, S.D.; Sinha, P.R.; Green, S.L.; Iozzo, R.V.; Mohan, R.R. The Functional Role of Decorin in Corneal Neovascularization in Vivo. Exp. Eye Res. 2021, 207, 108610. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Martin, L.M.; Sinha, N.R.; Smith, K.E.; Sinha, P.R.; Emilee, M.; Hesemann, N.P.; Mohan, R.R. Role of Inhibitor of Differentiation 3 Gene in Cellular Differentiation of Human Corneal Stromal Fibroblasts. Mol. Vis. 2020, 26, 742–756. [Google Scholar] [PubMed]
- Kamil, S.; Mohan, R.R. Corneal Stromal Wound Healing: Major Regulators and Therapeutic Targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Chandrasekher, G.; Ma, X.; Lallier, T.E.; Bazan, H.E.P. Delay of Corneal Epithelial Wound Healing and Induction of Keratocyte Apoptosis by Platelet-Activating Factor. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1422–1428. [Google Scholar]
- Bonanno, J.A. Molecular Mechanisms Underlying the Corneal Endothelial Pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The Cellular Basis of Corneal Transparency: Evidence for “Corneal Crystallins”. J. Cell Sci. 1999, 112 Pt 5, 613–622. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in Corneal Wound Healing HHS Public Access. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the Corneal Endothelium in Health and Disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef]
- Sinha, N.R.; Balne, P.K.; Bunyak, F.; Hofmann, A.C.; Lim, R.R.; Rajiv, R. Collagen Matrix Perturbations in Corneal Stroma of Ossabaw Mini Pigs with Type 2 Diabetes. Mol. Vis. 2021, 27, 666–678. [Google Scholar]
- Tandon, A.; Tovey, J.C.K.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of Transforming Growth Factor Beta in Corneal Function, Biology and Pathology. Curr. Mol. Med. 2012, 10, 565–578. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-Epithelial Interactions in the Cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambro, R.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; Wearda, T.; et al. Wound Healing: An Update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef]
- Chen, L.; Mongan, M.; Meng, Q.; Wang, Q.; Kao, W.; Xia, Y. Corneal Wound Healing Requires IKB Kinase β Signaling in Keratocytes. PLoS ONE 2016, 11, e0151869. [Google Scholar] [CrossRef]
- Bazan, H.E.P. Cellular and Molecular Events in Corneal Wound Healing: Significance of Lipid Signalling. Exp. Eye Res. 2005, 80, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Reinach, P.S.; Pokorny, K.S. The Corneal Epithelium: Clinical Relevance of Cytokine-Mediated Responses to Maintenance of Corneal Health. Arq. Bras. Oftalmol. 2008, 71, 80–86. [Google Scholar] [CrossRef]
- Gonzalez-Andrades, M.; Alonso-Pastor, L.; Mauris, J.; Cruzat, A.; Dohlman, C.H.; Argüeso, P. Establishment of a Novel in Vitro Model of Stratified Epithelial Wound Healing with Barrier Function. Sci. Rep. 2016, 6, 19395. [Google Scholar] [CrossRef]
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Gupta, P.C.; Wilson, S.E. Effect of Prophylactic and Therapeutic. J. Refract. Surg. 2006, 562–575. [Google Scholar] [CrossRef]
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Dupps, W.; Wilson, S.E. Stromal Haze, Myofibroblasts, and Surface Irregularity after PRK. Exp. Eye Res. 2006, 82, 788–797. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.K.; Sharma, A.; Tandon, A. Gene Therapy in the Cornea: 2005-Present. Prog. Retin. Eye Res. 2012, 31, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal Wound Healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Balne, P.K.; Sinha, N.R.; Martin, L.M.; Kamil, S.; Landreneau, J.R.; Gupta, S.; Rodier, J.T.; Sinha, P.R.; Hesemann, N.P.; et al. A Novel Topical Ophthalmic Formulation to Mitigate Acute Mustard Gas Keratopathy in Vivo: A Pilot Study. Transl. Vis. Sci. Technol. 2020, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Giuliano, E.A.; Sinha, N.R.; Mohan, R.R. Ocular Toxicity of Mustard Gas: A Concise Review. Toxicol. Lett. 2021, 343, 21–27. [Google Scholar] [CrossRef]
- Mohan, R.R.; Martin, L.M.; Sinha, N.R. Novel Insights into Gene Therapy in the Cornea. Exp. Eye Res. 2020, 202, 108361. [Google Scholar] [CrossRef]
- Mohan, R.R.; Sharma, A.; Cebulko, T.C.; Tandon, A. Vector Delivery Technique Affects Gene Transfer in the Cornea in Vivo. Mol. Vis. 2010, 16, 2494–2501. [Google Scholar]
- Wang, T.; Zhou, X.; Yu, Y.; Zhu, J.; Dai, J.; Qu, X.; Le, Q.; Chu, R. Inhibition of Corneal Fibrosis by Smad7 in Rats after Photorefractive Keratectomy. Chin. Med. J. 2013, 126, 1445–1450. [Google Scholar]
- Wang, T.; Zhou, X.; Yu, Y.; Dai, J.; Qu, X.; LE, Q.; Chu, R. Expression of Smad7 Inhibits Fibrogenic Responses of Keratocytes to Transforming Growth Factor Β2. Chin. Med. J. 2011, 124, 1988–1993. [Google Scholar]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Miyamoto, T.; Ohnishi, Y.; Sato, M.; Muragaki, Y.; Ooshima, A.; Nakajima, Y.; Kao, W.W.-Y.; et al. Expression of Smad7 in Mouse Eyes Accelerates Healing of Corneal Tissue after Exposure to Alkali. Am. J. Pathol. 2005, 166, 1405–1418. [Google Scholar] [CrossRef]
- Gupta, S.; Rodier, J.T.; Sharma, A.; Giuliano, E.A.; Sinha, P.R.; Hesemann, N.P.; Ghosh, A.; Mohan, R.R. Targeted AAV5-Smad7 Gene Therapy Inhibits Corneal Scarring in Vivo. PLoS ONE 2017, 12, e0172928. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, N.R.; Siddiqui, S.; Mohan, R.R. Role of 5′TG3′-Interacting Factors (TGIFs) in Vorinostat (HDAC Inhibitor)-Mediated Corneal Fibrosis Inhibition. Mol. Vis. 2015, 21, 974–984. [Google Scholar] [PubMed]
- Marlo, T.L.; Giuliano, E.A.; Tripathi, R.; Sharma, A.; Mohan, R.R. Altering Equine Corneal Fibroblast Differentiation through Smad Gene Transfer. Vet. Ophthalmol. 2018, 21, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Poojya Anantharam, P.D. Sulfur Mustard Mitigation by Turbo Eye Drops (Chronic Efficacy and Safety Study). 2021. Available online: https://www.mriglobal.org/fda-approved-mustard-gas-burn-product/ (accessed on 21 December 2020).
- Balne, P.K.; Sinha, N.R.; Hofmann, A.C.; Martin, L.M.; Mohan, R.R. Characterization of Hydrogen Sulfide Toxicity to Human Corneal Stromal Fibroblasts. Ann. N. Y. Acad. Sci. 2020, 1480, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Morgan, B.R.; Anumanthan, G.; Sharma, A.; Chaurasia, S.S.; Rieger, F.G. Characterization of Inhibitor of Differentiation (Id) Proteins in Human Cornea. Exp. Eye Res. 2016, 146, 145–153. [Google Scholar] [CrossRef]
- Lim, R.R.; Tan, A.; Liu, Y.-C.C.; Barathi, V.A.; Mohan, R.R.; Mehta, J.S.; Chaurasia, S.S. ITF2357 Transactivates Id3 and Regulate TGFβ/BMP7 Signaling Pathways to Attenuate Corneal Fibrosis. Sci. Rep. 2016, 6, 20841. [Google Scholar] [CrossRef]
- Sinha, N.R.; Tripathi, R.; Balne, P.K.; Green, S.L.; Sinha, P.R.; Bunyak, F.; Giuliano, E.A.; Chaurasia, S.S.; Mohan, R.R. Time-Dependent in Situ Structural and Cellular Aberrations in Rabbit Cornea in Vivo after Mustard Gas Exposure. Exp. Eye Res. 2022, 224, 109247. [Google Scholar] [CrossRef]
- Gupta, S.; Fink, M.K.; Martin, L.M.; Sinha, P.R.; Rodier, J.T.; Sinha, N.R.; Hesemann, N.P.; Chaurasia, S.S.; Mohan, R.R. A Rabbit Model for Evaluating Ocular Damage from Acrolein Toxicity in Vivo. Ann. N. Y. Acad. Sci. 2020, 1480, 233–245. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of Alpha-Smooth Muscle (Alpha-SM) Actin during Corneal Stromal Wound Healing. Investig. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar]

| Target Gene | Species | Accession # | Forward Sequence | Reverse Sequence |
|---|---|---|---|---|
| GAPDH | rabbit, human | NM_002046.3 | GCCTCAAGATCATCAGCAATGCCT | TGTGGTCATGAGTCCTTCCACGAT |
| αSMA | rabbit, human | NM_001613 | AAGATCCTGACTGAGCGT | CAAAGTCCAGAGCGACATAG |
| Smad 2 | human | XM_002713521.3 | CGAAACGCCACAGTAGAA | GCACTATCACTTAGGCACTC |
| Smad 3 | human | NM_001145102.2 | CCCAGAGCAATATTCCAGAG | GTCCATGCTGTGGTTCAT |
| Smad 4 | human | XM_002713541.3 | TGGATGTTCAGGTAGGAGAG | CTGTGGACATTGGAGAGTTG |
| Smad 7 | human | XM_017344169.1 | ATCACCTTAGCCGACTCT | GCACAGCATCTGGACAAT |
| TGFβ1 | rabbit, human | NM_003242 | CGACTACTACGCCAAGGA | GAGAGCAACACGGGTTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, N.R.; Tripathi, R.; Balne, P.K.; Suleiman, L.; Simkins, K.; Chaurasia, S.S.; Mohan, R.R. Mustard Gas Exposure Actuates SMAD2/3 Signaling to Promote Myofibroblast Generation in the Cornea. Cells 2023, 12, 1533. https://doi.org/10.3390/cells12111533
Sinha NR, Tripathi R, Balne PK, Suleiman L, Simkins K, Chaurasia SS, Mohan RR. Mustard Gas Exposure Actuates SMAD2/3 Signaling to Promote Myofibroblast Generation in the Cornea. Cells. 2023; 12(11):1533. https://doi.org/10.3390/cells12111533
Chicago/Turabian StyleSinha, Nishant R., Ratnakar Tripathi, Praveen K. Balne, Laila Suleiman, Katherine Simkins, Shyam S. Chaurasia, and Rajiv R. Mohan. 2023. "Mustard Gas Exposure Actuates SMAD2/3 Signaling to Promote Myofibroblast Generation in the Cornea" Cells 12, no. 11: 1533. https://doi.org/10.3390/cells12111533
APA StyleSinha, N. R., Tripathi, R., Balne, P. K., Suleiman, L., Simkins, K., Chaurasia, S. S., & Mohan, R. R. (2023). Mustard Gas Exposure Actuates SMAD2/3 Signaling to Promote Myofibroblast Generation in the Cornea. Cells, 12(11), 1533. https://doi.org/10.3390/cells12111533







