Abstract
The aim of the present study was to investigate the levels of YKL-40 during and after coronary artery bypass grafting surgery (CABG) and to establish possible connections between YKL-40 and markers of oxidative stress, inflammation, and myocardial injury. Patients undergoing elective CABG utilizing cardiopulmonary bypass (CPB) were recruited into the study. Blood samples were collected at the onset of anesthesia, during surgery and post-operatively. Levels of YKL-40, 8-isoprostane, interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1) and troponin T (TnT) were measured by immunoassay. YKL-40 levels increased significantly 24 h after CPB. Positive correlation was seen between post-operative TnT and YKL-40 levels (r = 0.457, p = 0.016) and, interestingly, baseline YKL-40 predicted post-operative TnT increase (r = 0.374, p = 0.050). There was also a clear association between YKL-40 and the chemotactic factors MCP-1 (r = 0.440, p = 0.028) and IL-8 (r = 0.484, p = 0.011) linking YKL-40 to cardiac inflammation and fibrosis following CABG. The present results show, for the first time, that YKL-40 is associated with myocardial injury and leukocyte-activating factors following coronary artery bypass surgery. YKL-40 may be a factor and/or biomarker of myocardial inflammation and injury and subsequent fibrosis following heart surgery.
1. Introduction
Coronary artery disease (CAD) often requires invasive therapy, i.e., percutaneous coronary invasion (PCI) or coronary artery bypass grafting surgery (CABG). CABG utilizing cardiopulmonary bypass (CPB) is an effective treatment but it is complicated with systemic inflammatory response syndrome (SIRS), ischemia-reperfusion syndrome and morbidity [1]. For instance, preoperative LDL-levels and postoperative rehabilitation have been shown to improve prognosis and recovery from heart surgery [2,3]. Biomarker that could recognize patients with myocardial injury and poor prognosis could be utilized to focus therapeutic interventions to high-risk patients.
YKL-40 is a chitin-binding glycoprotein, known also as chitinase-3-like protein 1 (CHI3L1) describing the lack of chitinase activity that characterizes true chitinases. YKL-40 is expressed in many cell types, including neutrophils, macrophages, and vascular smooth muscle cells [4,5] as well as chondrocytes and cancer cells [5,6]. YKL-40 serves as growth, adhesion and migration factor having a role in proliferation and fibrosis of connective tissue [5,7,8]. Accordingly, associations of YKL-40 with inflammation and tissue remodeling in cancer, liver and lung fibrosis, arthritis and strenuous joint loading have been reported [9,10,11,12,13,14]. The function of YKL-40 is still, however, largely unknown. In the framework of the present study, it is interesting that YKL-40 has been shown to increase in acute myocardial infarction [15,16] and in chronic heart failure [5,17]. In addition, increased levels of YKL-40 expression have been found in arteriosclerotic plaques suggesting that YKL-40 plays a role in plaque formation [5,18]. Poorer prognosis and increased rate of adverse cardiovascular events have been linked to high YKL-40 serum levels in patients with CAD or congestive heart disease (CHD) [16,19,20]. YKL-40 levels also increase with CAD severity [21,22]. YKL-40 has also been identified as an independent risk factor for all-cause mortality in patients acutely admitted to the hospital [23]. Interestingly, the expression of YKL-40 was increased in rat hearts recovering from ischemia-like insult of the myocardium with tissue remodeling when compared to continuously volume-overloaded hearts [24].
In the present study, YKL-40 and markers of myocardial injury (troponin T [TnT]), leukocyte activation (monocyte chemotactic protein-1 [MCP-1] and interleukin 8 [IL-8]) and oxidative stress (8-isoprostane) were analyzed in blood samples collected from patients undergoing elective CABG surgery. We hypothesized that YKL-40 may participate in the inflammatory cascade following open-heart surgery utilizing CPB and might function as a biomarker of inflammation and myocardial injury.
2. Materials and Methods
The present study included thirty-two patients (8 females, BMI 28.5 ± 0.8 kg/m2, age 68 ± 2 years; mean ± SEM) admitted to the Tampere University Hospital Heart Center Co to have an elective on-pump CABG with CPB. Canadian Cardiovascular Society (CCS) angina scale was used to assess the severity of coronary artery disease (CAD) and at least grade II symptoms were set as inclusion criteria. The average CCS value in these patients was grade III. Pre-existent pulmonary disease, pulmonary hypertension (PAP > 40 mmHg), smoking, impaired left ventricular function (LVEF < 50%), myocardial infarction, cardiac failure period or acute coronary syndrome during the previous 3 months, previous malignancy, infection and/or treatment with COX-2 selective nonsteroidal anti-inflammatory drugs or glucocorticoids during the previous month were used as exclusion criteria. Three patients had very high TnT levels (TnT > 1000 ng/L, and one of them had a documented myocardial infarction during the post-operative period) and one patient an aberrantly high MCP-1 levels post-operatively. These four patients were excluded from the final statistical analysis to minimize the confounding effects of post-operative complications. Anesthesia, perfusion, surgical approach, and intensive care unit protocol were performed according to standard procedure as previously described [25]. Patient characteristics are summarized in Table 1.

Table 1.
Demographic Data.
Blood samples were drawn from the radial artery. At the onset of anesthesia, the first sample was taken at the baseline (BL) and the second sample was collected at the onset of CPB (CPB). The third sample was acquired one minute after the removal of aortic cross-clamp (ACC + 1 min) and the fourth sample was drawn 15 min after the removal of aortic cross-clamp upon reinflating lungs (ACC + 15 min). The last two samples were taken 4 and 24 h after the onset of CPB (CPB + 4 h and CPB + 24 h).
Obtained aliquots of plasma were kept at −80 °C until analysis. Concentrations of YKL-40, MCP-1, IL-8 and 8-isoprostane in the plasma samples were determined by immunoassays using following reagents: YKL-40 and MCP-1 (DuoSet ELISA, R&D Systems Europe Ltd., Abingdon, UK), IL-8 (BD Biosciences, Erembodegem, Belgium), and 8-isoprostane (Cayman, Ann Arbor, MI, USA). HUSLAB (Helsinki University Hospital, Helsinki, Finland) measured troponin T (TnT) concentrations by using immunochemiluminometric method validated for clinical use.
Data are shown as mean ± standard error of mean (SEM) or as indicated otherwise. According to data distribution, Spearman’s and Pearson’s correlations were used. A logarithmic transformation was used to create normal distribution when needed. Correlation was detected when R-values were greater than 0.3 and −0.3 [26]. Area under the curve (AUC) was calculated using trapezoid method with Graphpad Prism 8.0.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance of variations in cytokine levels between time points was tested by repeated measures ANOVA and Bonferroni post hoc test using IBM SPSS statistics 22.0 (IBM, Armonk, NY, USA).
3. Results
3.1. YKL-40 Levels Increased Significantly after CABG Surgery
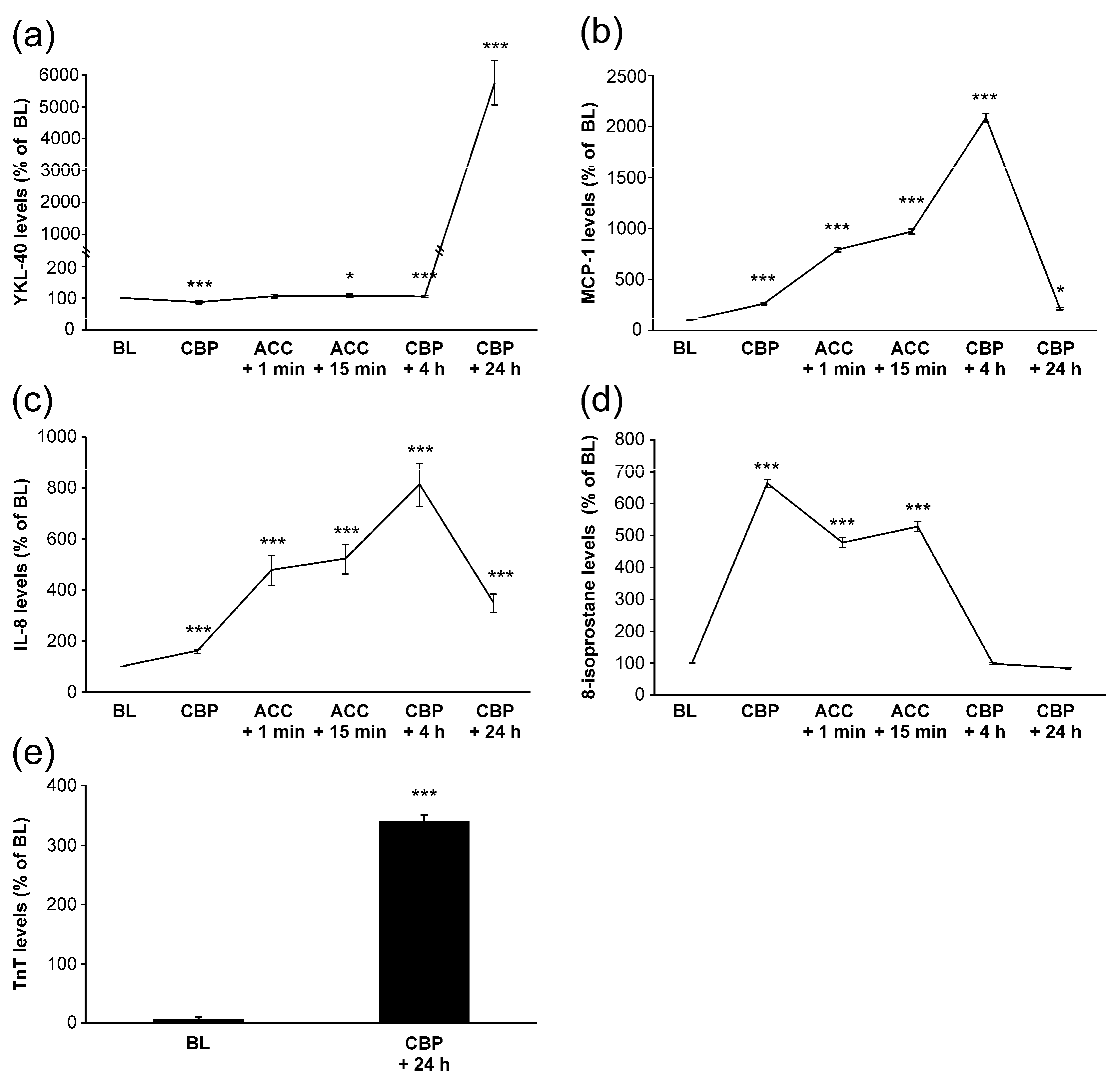
Mean baseline level of YKL-40 was 36 ± 5 ng/mL. During surgery, YKL-40 levels remained relatively constant. Post-operatively, four hours after onset of CPB, YKL-40 concentrations were still close to the baseline levels. Unexpectedly, YKL-40 levels elevated significantly 24 h after the CPB onset to a mean level of 1720 ± 205 ng/mL (Figure 1a).
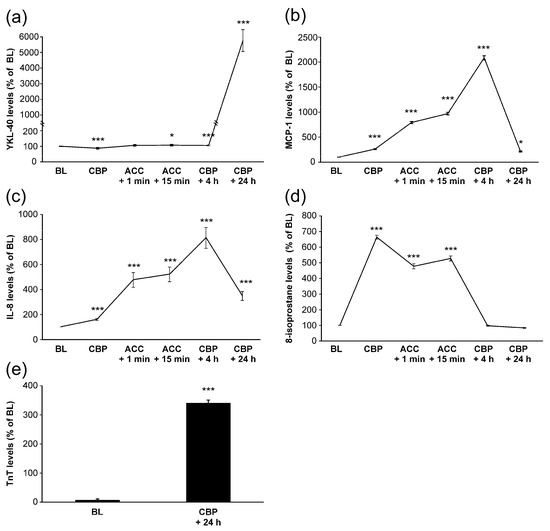
Figure 1.
Levels of YKL-40 (a), monocyte chemotactic protein-1 (MCP-1, (b)), interleukin-8 (IL-8, (c)), 8-isoprostane (d) and troponin T (TnT, (e)) during and after coronary artery bypass grafting operation. Samples were drawn from the radial artery at the following time points: onset of anesthesia (baseline, BL), onset of cardiopulmonary bypass (CPB), 1 min after removal of aortic cross clamp (ACC + 1 min), 15 min after removal of ACC (ACC + 15 min), 4 h after onset of CPB (CPB + 4 h) and 24 h after onset of CPB (CPB + 24 h). Results are presented as mean + SEM, n = 28 patients. * = p < 0.05, *** = p < 0.001.
3.2. YKL-40 Correlated with TnT, A Marker of Myocardial Injury
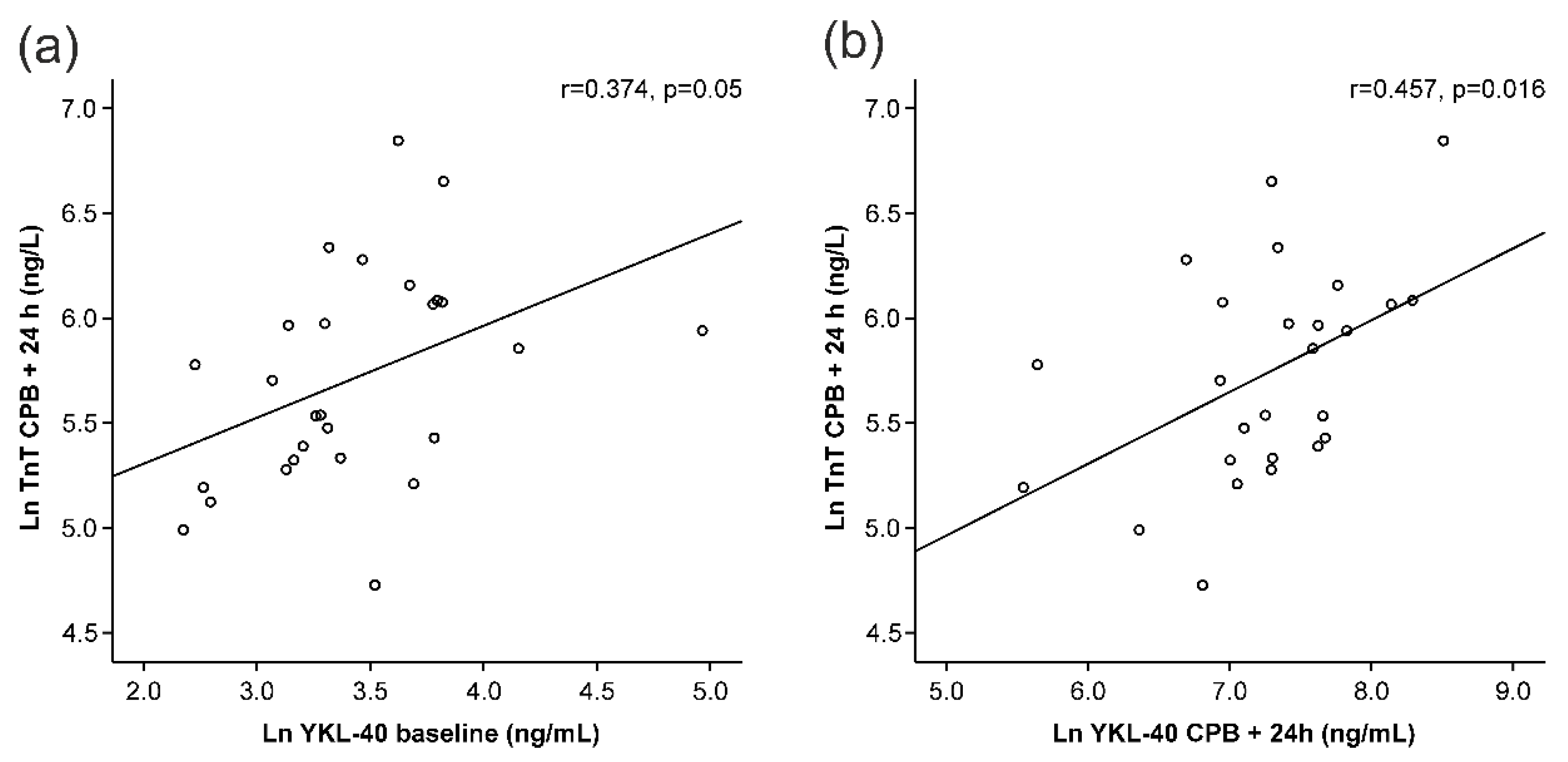
A clear increase in TnT levels was seen during surgery (Figure 1e). Interestingly, baseline YKL-40 levels correlated positively with TnT levels measured 24 h after the onset of surgery (r = 0.374, p = 0.050, Figure 2a, Table 2). YKL-40 measured at the same 24 h timepoint with TnT levels correlated even more strongly (r = 0.457, p = 0.016, Figure 2b, Table 2).
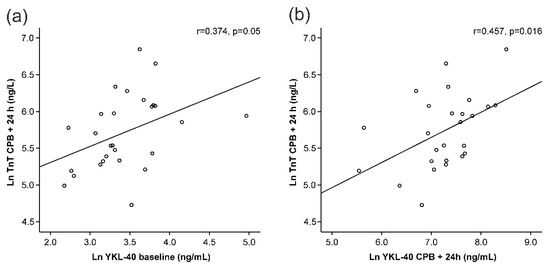
Figure 2.
Correlations of troponin T (TnT) levels 24 h after the onset of cardiopulmonary bypass (CPB) to YKL-40 at baseline (a) and 24 h after the onset of CPB (b). All variables are presented in logarithmic form to obtain normal distributions. Pearson’s correlation was calculated (r). p < 0.05 was considered statistically significant.

Table 2.
Correlations between baseline (Base) and maximum (Max) YKL-40 levels and baseline levels, maximum levels and areas under curve (AUC) of monocyte chemotactic protein-1 (MCP-1), interleukin-8 (IL-8), 8-isoprostane and troponin T (TnT).
3.3. YKL-40 Correlated with Chemotactic Cytokines MCP-1 and IL-8
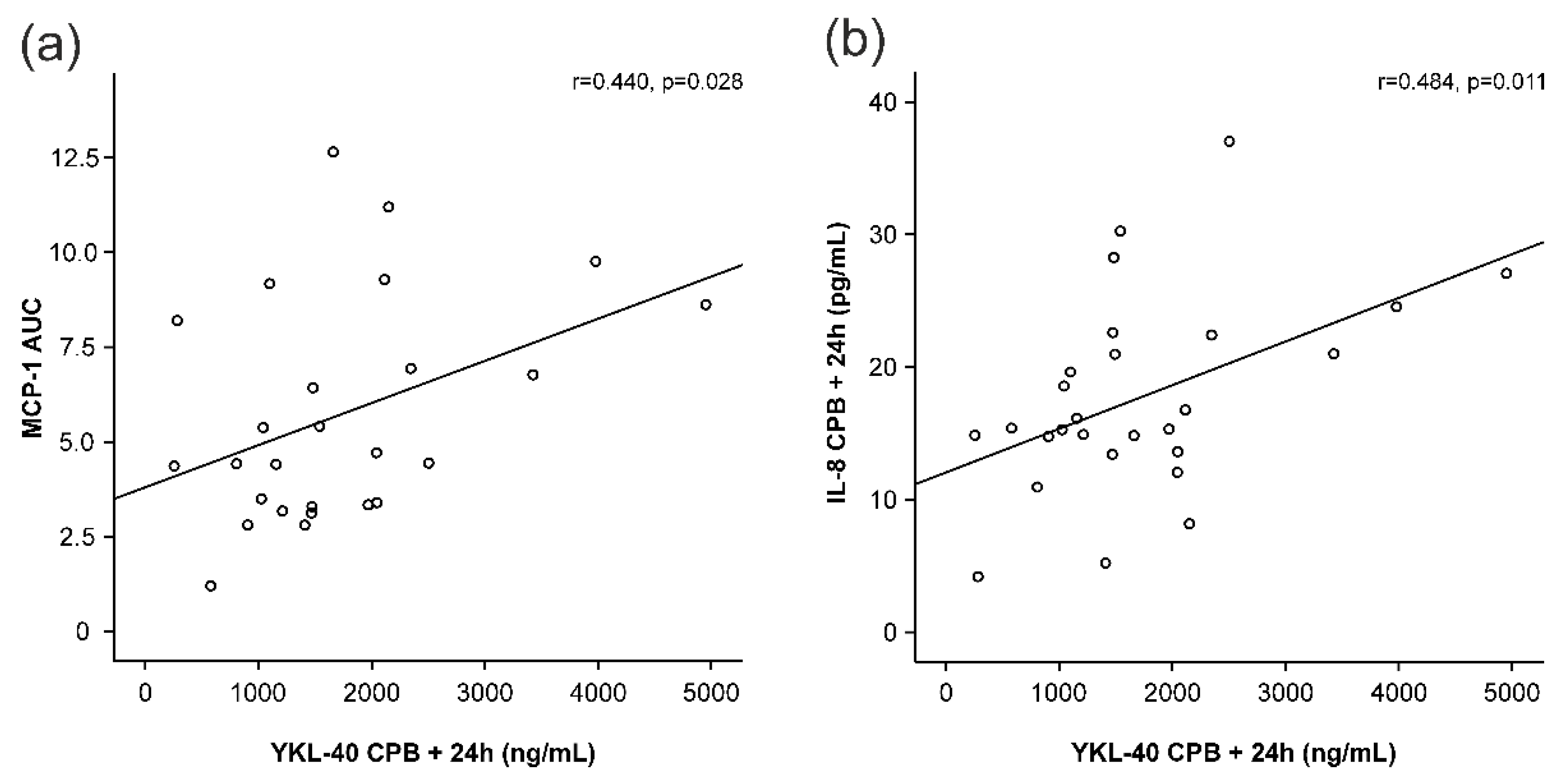
MCP-1 and IL-8 levels started to increase early during surgery and peaked at 4 h after the onset of CPB (Figure 1b,c), i.e., before any increase in YKL-40 was seen. MCP-1 levels had normalized 24 h after the onset of CPB, whereas IL-8 levels were still significantly elevated. Interestingly, AUC of MCP-1 was significantly associated with the peak levels of YKL-40 (r = 0.440, p = 0.028, Figure 3a, Table 2). Furthermore, IL-8 concentrations showed correlation with YKL-40 at baseline (r = 0.500, p = 0.007, Table 2) and at 24 h (r = 0.484, p = 0.011, Figure 3b).
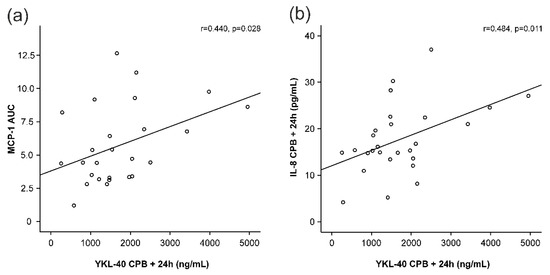
Figure 3.
Correlations of peak YKL-40 levels (YKL-40 levels 24 h after the onset of cardiopulmonary bypass, CPB) to areas under curve (AUC) of monocyte chemotactic protein-1 (MCP-1, (a)) and interleukin-8 (IL-8, (b)) levels 24 h after the onset of cardiopulmonary bypass (CPB). Spearman’s correlation was calculated (r). p < 0.05 was considered statistically significant.
8-isoprostane levels also increased early during surgery as a marker of oxidative stress but values were normalized by 4 h after CPB (Figure 1d). No significant correlations were found between YKL-40 and 8-isoprostane levels. Based on these results, YKL-40 seems to be associated with TnT release and levels of leukocyte-activating factors but not with oxidative stress.
4. Discussion
Our main finding was that YKL-40 levels greatly elevated after CABG surgery, and patients with increased release of the myocardial injury marker TnT also had higher post-operative YKL-40 concentrations. In addition, YKL-40 correlated with the chemotactic cytokines MCP-1 and IL-8 but not with 8-isoprostane, a biomarker of oxidative stress. This is the first study to measure YKL-40 levels during and after CABG surgery, and our findings are in line with the knowledge on biological functions of YKL-40 and with studies on cardiac patients.
Our results are supported by Harutyunyan et al. showing 25% increase in YKL-40 levels the day after percutaneous coronary intervention (PCI) in patients with CAD [27]. In our study, CABG procedure increased YKL-40 concentrations nearly 50-fold and the YKL-40 levels correlated positively with myocardial injury. Use of cardioplegia has made CABG surgery safer, but tissue injury can still occur after myocardial perfusion has been re-established [1,28]. The injury can range from slightly impaired myocardial contractility called myocardial stunning to contraction band necrosis and irreversible damage [29]. Ischemic injury and subsequent reperfusion cause influx of leukocytes into myocardium. This inflammatory response causes additional tissue injury through release of reactive oxygen species (ROS), release of matrix metalloproteinases and inflammatory mediators that strengthen the inflammatory response [29]. Neutrophils peak at 24 h after ischemic injury, and afterwards monocytes become the most prominent leukocyte type found in injured area [30].
Our data show that circulating MCP-1 and IL-8 correlate with plasma YKL-40 levels. MCP-1 is a potent chemotactic cytokine produced by inflammatory cells [31]. MCP-1 is expressed also in myocardium rapidly after myocardial infarction [32]. In the present study, MCP-1 levels rose early during surgery, peaked four hours after the onset of CPB and fell towards baseline levels within 24 h after the onset of CPB, supporting the results reported by Kortekaas et al. [33]. In the present study, the peak levels of MCP-1 were detected before YKL-40 levels were increased and, interestingly, the AUC of MCP-1 concentrations correlated positively with the maximum increase of YKL-40.
IL-8 (also known as chemokine CXCL8) is a potent chemotactic agent produced primarily by monocytes and IL-8 release results in recruitment of neutrophils into injured myocardium [34]. IL-8 levels increased significantly after reperfusion and stayed elevated 24 h after the onset of CPB. After myocardial ischemia, monocytes of splenic origin are recruited into blood stream [35]. Since IL-8 is primarily produced by monocytes, the substantial increase in IL-8 levels found in the present study may be secondary to mobilization of splenic monocytes. YKL-40 may also stimulate IL-8 production as shown by Tang et al. [36]. Association between YKL-40 and IL-8 is supported by the current findings on clear positive correlations between these two factors at baseline and especially post-operatively. It is also known that YKL-40 is produced by macrophages [5], so taking our findings into account, it is possible that a major portion of the significant rise in YKL-40 levels post-operatively is released from leukocytes activated and recruited by chemokines such as MCP-1 and IL-8.
In the present study, 8-isoprostane levels increased significantly at the onset of CPB and returned to baseline levels post-operatively. CPB has been shown to cause oxidative stress due to the inflammatory response it elicits and the high arterial partial oxygen pressures used during CPB [37]. As 8-isoprostane is a product of arachidonic acid oxidation following reactive oxygen species (ROS) release [38], we believe that the increase in 8-isoprostane levels after the onset of CPB is caused by the hyperoxic conditions during CPB and the ROS released secondary to the inflammatory response to CPB.
YKL-40 has been shown to stimulate connective tissue growth and thus may contribute to tissue fibrosis [5,7]. YKL-40 also inhibits cellular responses to interleukin-1 and tumor necrosis factor α resulting in reduced MMP-1, MMP-3 and MMP-13 release [39]. Thus, the marked increase in YKL-40 levels post-operatively may contribute to the development of myocardial fibrosis secondary to myocardial injury. If this is the case, it would be seminal to investigate whether high YKL-40 levels predict development of cardiac fibrosis and impaired left ventricular function post-operatively, as YKL-40 has been linked with poor prognosis among CAD and CHD patients [16,19,20]. The findings reported by Huuskonen et al. support this assumption. In that study, rat hearts recovering from acute volume overload-induced myocardial insult with tissue remodeling showed higher YKL-40 expression when compared to continuously volume overloaded-hearts [24].
The present study was a pilot study looking for changes in YKL-40 and cytokine levels during and after CABG surgery in order to generate hypothesis and research plan for further studies. As a pilot study, we limited the follow-up time to the intra-operative period and up to the first post-operative morning as cytokine levels normally taper off after the first post-operative day following CABG using CPB [40]. In addition, we assumed that possible complications and conditions appearing during the post-surgical period such as myocardial infarctions or infections might confound the data. However, YKL-40 levels were still on the increase at the last 24 h timepoint. In future studies, follow-up time should be extended at least up to the fourth post-operative day to follow YKL-40 levels during the recovery phase. Another limitation is the small patient cohort (n = 32) in which we decided to exclude four patients with aberrantly high TnT or MCP-1 levels from the final analysis to minimize the confounding effect of a post-operative complications and to exclude the risk of type I error. All patients included, the correlation between YKL-40 and TnT would have been even greater (r = 0.518, p = 0.003, n = 32).
In conclusion, we report here, for the first time, that YKL-40 levels significantly increase following open-heart surgery and correlate with markers of inflammation and myocardial injury. Thus, YKL-40 may be an effector and biomarker of myocardial inflammation and injury and subsequent remodeling following heart surgery. These findings warrant further research on the effects of YKL-40 during and after myocardial injury and on the impact of increased YKL-40 levels on cardiac function, morbidity and mortality following coronary artery bypass surgery.
Author Contributions
Conception and design of the study, A.L., K.V., V.T., T.R., T.L., M.H., M.T., J.L. and E.M.; Selecting patients and in acquiring patient samples, V.T., T.R., M.T., J.L.; Acquisition of the laboratory data, A.L., K.V., T.L. and M.H.; Analysis and interpretation of data, A.L., K.V., T.L., M.H., J.L. and E.M.; Drafted the manuscript, A.L., K.V. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from Tampere Tuberculosis Foundation, Tampere, Finland; The Finnish Cultural Foundation, Pirkanmaa Regional Fund, Finland; and Competitive State Research Funding of Tampere University Hospital, Tampere, Finland.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Tampere University Hospital (code R04100).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are included in the manuscript.
Acknowledgments
We wish to thank Meiju Kukkonen, Petra Miikkulainen and Elina Jaakkola for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.K.; Olasińska-Wiśniewska, A.; Michalak, M.; Gąsecka, A.; Rodzki, M.; Perek, B.; Jemielity, M. Cardioprotective effect of low level of LDL cholesterol on perioperative myocardial injury in off-pump coronary artery bypass grafting. Medicina 2021, 57, 875. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Morimoto, Y.; Hanada, M.; Yano, Y.; Sawai, T.; Miura, T.; Eishi, K.; Kozu, R. Determining factors for independent walking in patients undergoing cardiovascular surgery: Differences between coronary artery bypass grafting, heart valve surgery, and aortic surgery. Healthcare 2021, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Shackelton, L.M.; Mann, D.M.; Millis, A.J. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J. Biol. Chem. 1995, 270, 13076–13083. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Hakala, B.E.; White, C.; Recklies, A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [CrossRef]
- Recklies, A.D.; White, C.; Ling, H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem. J. 2002, 365, 119–126. [Google Scholar] [CrossRef]
- Nishikawa, K.C.; Millis, A.J. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp. Cell Res. 2003, 287, 79–87. [Google Scholar] [CrossRef]
- Väänänen, T.; Koskinen, A.; Paukkeri, E.L.; Hämäläinen, M.; Moilanen, T.; Moilanen, E.; Vuolteenaho, K. YKL-40 as a novel factor associated with inflammation and catabolic mechanisms in osteoarthritic joints. Mediat. Inflamm. 2014, 2014, 215140. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Leppänen, T.; Kekkonen, R.; Korpela, R.; Moilanen, E. Running a marathon induces changes in adipokine levels and in markers of cartilage degradation--novel role for resistin. PLoS ONE 2014, 9, e110481. [Google Scholar] [CrossRef]
- Di Rosa, M.; Distefano, G.; Zorena, K.; Malaguarnera, L. Chitinases and immunity: Ancestral molecules with new functions. Immunobiology 2015, 221, 399–411. [Google Scholar] [CrossRef]
- Väänänen, T.; Lehtimäki, L.; Vuolteenaho, K.; Hämäläinen, M.; Oksa, P.; Vierikko, T.; Järvenpää, R.; Uitti, J.; Kankaanranta, H.; Moilanen, E. Glycoprotein YKL-40 levels in plasma are associated with fibrotic changes on HRCT in asbestos-exposed subjects. Mediators Inflamm. 2017, 2017, 1797512. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, T.; Vuolteenaho, K.; Kautiainen, H.; Nieminen, R.; Möttönen, T.; Hannonen, P.; Korpela, M.; Kauppi, M.J.; Laiho, K.; Kaipiainen-Seppänen, O.; et al. Glycoprotein YKL-40: A potential biomarker of disease activity in rheumatoid arthritis during intensive treatment with csDMARDs and infliximab. Evidence from the randomised controlled NEO-RACo trial. PLoS ONE 2017, 12, e0183294. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, T.; Kallio, J.; Vuolteenaho, K.; Ojala, A.; Luukkaala, T.; Hämäläinen, M.; Tammela, T.; Kellokumpu-Lehtinen, P.L.; Moilanen, E. High YKL-40 is associated with poor survival in patients with renal cell carcinoma: A novel independent prognostic marker. Scand. J. Urol. 2017, 51, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Nojgaard, C.; Host, N.B.; Christensen, I.J.; Poulsen, S.H.; Egstrup, K.; Price, P.A.; Johansen, J.S. Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coron. Artery Dis. 2008, 19, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Jakobsen, J.C.; Winkel, P.; Hilden, J.; Jensen, G.B.; Sajadieh, A.; Larsson, A.; Ärnlöv, J.; Harutyunyan, M.; Johansen, J.S.; et al. Prognosis and reclassification by ykl-40 in stable coronary artery disease. J. Am. Heart Assoc. 2020, 9, e014634. [Google Scholar] [CrossRef] [PubMed]
- Rathcke, C.N.; Kistorp, C.; Raymond, I.; Hildebrandt, P.; Gustafsson, F.; Lip, G.Y.; Faber, J.; Vestergaard, H. Plasma YKL-40 levels are elevated in patients with chronic heart failure. Scand. Cardiovasc. J. 2010, 44, 92–99. [Google Scholar] [CrossRef]
- Boot, R.G.; van Achterberg, T.A.; van Aken, B.E.; Renkema, G.H.; Jacobs, M.J.; Aerts, J.M.; de Vries, C.J. Strong induction of members of the chitinase family of proteins in atherosclerosis: Chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 687–694. [Google Scholar] [CrossRef]
- Bilim, O.; Takeishi, Y.; Kitahara, T.; Ishino, M.; Sasaki, T.; Suzuki, S.; Shishido, T.; Kubota, I. Serum YKL-40 predicts adverse clinical outcomes in patients with chronic heart failure. J. Card. Fail. 2010, 16, 873–879. [Google Scholar] [CrossRef]
- Yang, L.; Dong, H.; Lu, H.; Liao, Y.; Zhang, H.; Xu, L.; Tan, Y.; Cao, S.; Tan, J.; Fu, S. Serum YKL-40 predicts long-term outcome in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Medicine 2019, 98, e14920. [Google Scholar] [CrossRef]
- Kucur, M.; Isman, F.K.; Karadag, B.; Vural, V.A.; Tavsanoglu, S. Serum YKL-40 levels in patients with coronary artery disease. Coron. Artery Dis. 2007, 18, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Li, B.; Diao, H.Y.; Wang, J.H.; Shi, Y.; Lu, Y.; Wang, G.; Guo, Z.Y.; Li, Y.X.; Liu, J.G.; et al. Diagnostic value of serum YKL-40 level for coronary artery disease: A meta-analysis. J. Clin. Lab. Anal. 2016, 30, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Mygind, N.D.; Iversen, K.; Kober, L.; Goetze, J.P.; Nielsen, H.; Boesgaard, S.; Bay, M.; Johansen, J.S.; Nielsen, O.W.; Kirk, V.; et al. The inflammatory biomarker YKL-40 at admission is a strong predictor of overall mortality. J. Intern. Med. 2013, 273, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, C.; Hämäläinen, M.; Kivikangas, N.; Paavonen, T.; Moilanen, E.; Mennander, A.A. Early reversibility of histological changes after experimental acute cardiac volume-overload. Am. J. Cardiovasc. Dis. 2022, 12, 205–211. [Google Scholar] [PubMed]
- Laurikka, A.; Vuolteenaho, K.; Toikkanen, V.; Rinne, T.; Leppänen, T.; Tarkka, M.; Laurikka, J.; Moilanen, E. Adipocytokine resistin correlates with oxidative stress and myocardial injury in patients undergoing cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2014, 46, 729–736. [Google Scholar] [CrossRef]
- Cohen, J.W. Statistical Power Analysis for the Behavioral Sciences; Hillsdale, N.J., Ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Harutyunyan, M.; Johansen, J.S.; Mygind, N.D.; Reuter, S.B.; Kastrup, J. Serum YKL-40 for monitoring myocardial ischemia after revascularization in patients with stable coronary artery disease. Biomark. Med. 2014, 8, 977–987. [Google Scholar] [CrossRef]
- Haider, A.; Khwaja, I.A.; Khan, A.H.; Yousaf, M.S.; Zaneb, H.; Qureshi, A.B.; Rehman, H. Efficacy of whole-blood Del Nido cardioplegia compared with diluted Del Nido cardioplegia in coronary artery bypass grafting: A retrospective monocentric analysis of Pakistan. Medicina 2021, 57, 918. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/reperfusion injury following acute myocardial infarction: A critical issue for clinicians and forensic pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Turillazzi, E.; Di Paolo, M.; Neri, M.; Riezzo, I.; Fineschi, V. A theoretical timeline for myocardial infarction: Immunohistochemical evaluation and western blot quantification for interleukin-15 and monocyte chemotactic protein-1 as very early markers. J. Transl. Med. 2014, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.A.; Lindeman, J.H.; Versteegh, M.I.; van Beelen, E.; Kleemann, R.; Klautz, R.J. Heart failure determines the myocardial inflammatory response to injury. Eur. J. Heart Fail. 2013, 15, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Frangogiannis, N.G. Chemokines in myocardial infarction. J. Cardiovasc. Transl. Res. 2021, 14, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Sun, Y.; Shi, Z.; Huang, H.; Fang, Z.; Chen, J.; Xiu, Q.; Li, B. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-B pathways, causing bronchial smooth muscle proliferation and migration. J. Immunol. 2013, 190, 438–446. [Google Scholar] [CrossRef]
- McDonald, C.I.; Fraser, J.F.; Coombes, J.S.; Fung, Y.L. Oxidative stress during extracorporeal circulation. Eur. J. Cardiothorac. Surg. 2014, 46, 937–943. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Ling, H.; Recklies, A.D. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem. J. 2004, 380, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Lante, W.; Fackeldey, V.; Becker, H.P.; Thode, C.; Kuhlmann, W.D.; Markewitz, A. Proinflammatory and antiinflammatory cytokines after cardiac operation: Different cellular sources at different times. Ann. Thorac. Surg. 2002, 74, 363–370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).