Commander Complex—A Multifaceted Operator in Intracellular Signaling and Cargo
Abstract
1. Introduction
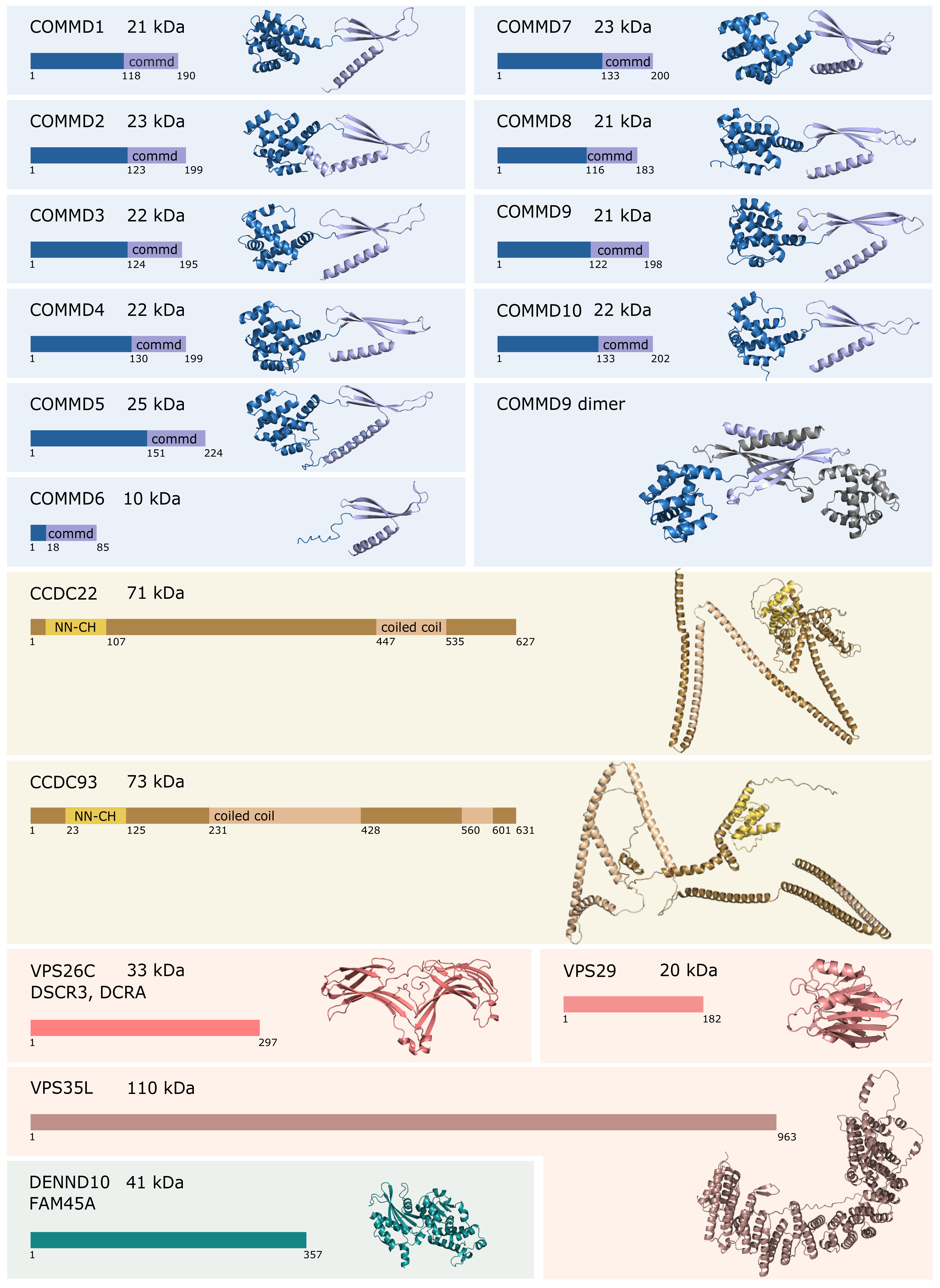
2. Composition and Assembly of the Commander Complex
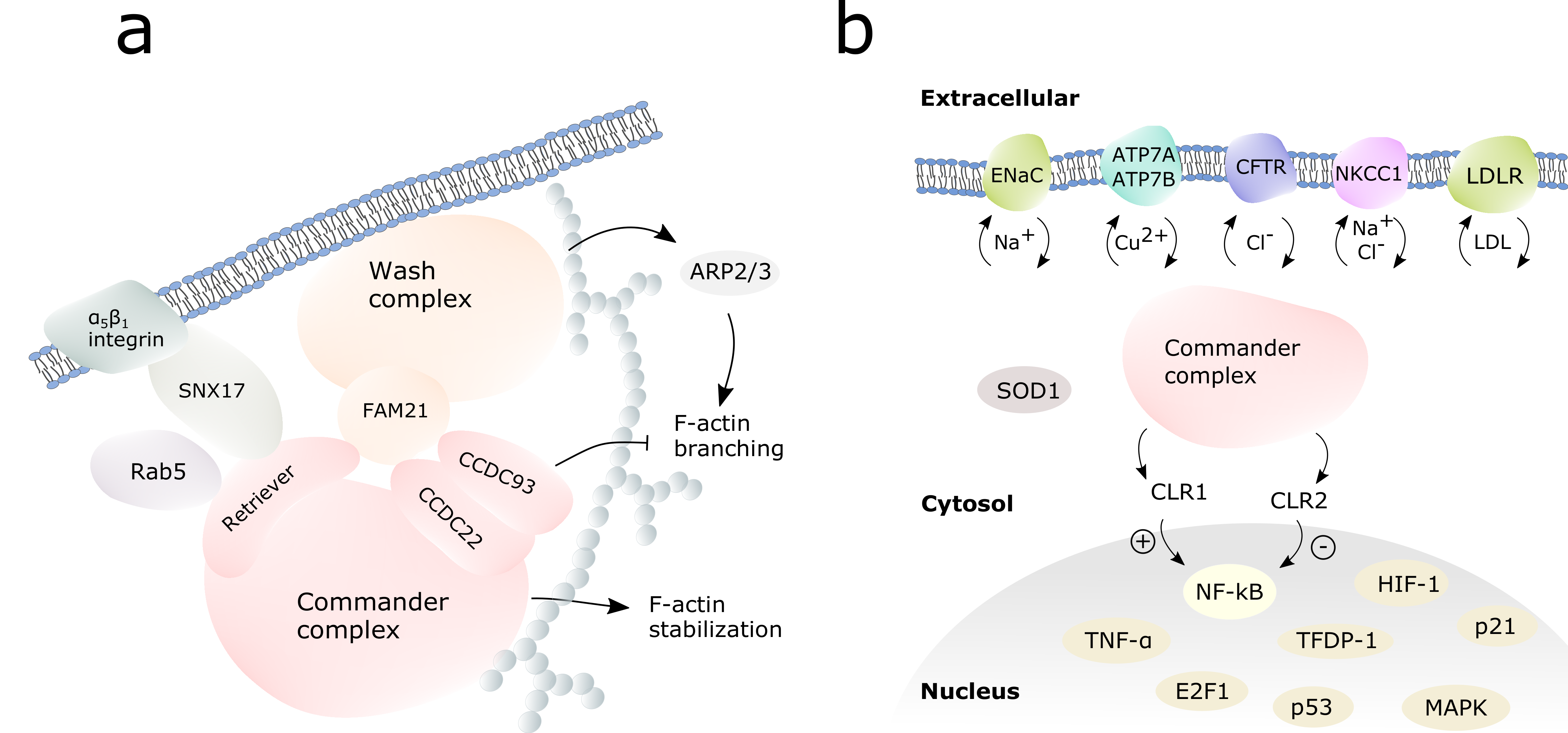
3. Endosomal Trafficking
3.1. Ion Channels and Transporters
3.2. Cholesterol Intake
3.3. Viral-Host Interactions
4. Immune Response Regulation
5. Cell Cycle and Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Sirkis, D.W.; Schekman, R. Protein Sorting at the trans-Golgi Network. Annu. Rev. Cell Dev. Biol. 2014, 30, 169–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fedoseienko, A.; Chen, B.; Burstein, E.; Jia, D.; Billadeau, D.D. Endosomal Receptor Trafficking: Retromer and Beyond. Traffic 2018, 19, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.E.; Healy, M.D.; Collins, B.M. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Borgeson, B.; Phanse, S.; Tu, F.; Drew, K.; Clark, G.; Xiong, X.; Kagan, O.; Kwan, J.; Bezginov, A.; et al. Panorama of ancient metazoan macromolecular complexes. Nature 2015, 525, 339–344. [Google Scholar] [CrossRef]
- Mallam, A.L.; Marcotte, E.M. Systems-wide studies uncover Commander, a multiprotein complex essential to human development. Cell Syst. 2017, 4, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Jaimovich, A.; Collins, S.R.; Seki, A.; Meyer, T. Systematic Discovery of Human Gene Function and Principles of Modular Organization through Phylogenetic Profiling. Cell Rep. 2015, 10, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.Y.; Hubner, N.C.; Poser, I.; Cox, J.; Nagaraj, N.; Toyoda, Y.; Gak, I.A.; Weisswange, I.; Mansfeld, J.; Buchholz, F.; et al. A Human Interactome in Three Quantitative Dimensions Organized by Stoichiometries and Abundances. Cell 2015, 163, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Calvo, S.E.; Gutman, R.; Liu, J.S.; Mootha, V.K. Expansion of biological pathways based on evolutionary inference. Cell 2014, 158, 213–225. [Google Scholar] [CrossRef]
- McNally, K.E.; Faulkner, R.; Steinberg, F.; Gallon, M.; Ghai, R.; Pim, D.; Langton, P.; Pearson, N.; Danson, C.M.; Nägele, H.; et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017, 19, 1214–1225. [Google Scholar] [CrossRef]
- Singla, A.; Fedoseienko, A.; Giridharan, S.S.P.; Overlee, B.L.; Lopez, A.; Jia, D.; Song, J.; Huff-Hardy, K.; Weisman, L.; Burstein, E.; et al. Endosomal PI(3)P regulation by the COMMD/CCDC22/CCDC93 (CCC) complex controls membrane protein recycling. Nat. Commun. 2019, 10, 4271. [Google Scholar] [CrossRef]
- van de Sluis, B.; Rothuizen, J.; Pearson, P.L.; van Oost, B.A.; Wijmenga, C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum. Mol. Genet. 2002, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Biasio, W.; Chang, T.; McIntosh, C.J.; McDonald, F.J. Identification of Murr1 as a Regulator of the Human Epithelial Sodium Channel. J. Biol. Chem. 2004, 279, 5429–5434. [Google Scholar] [CrossRef]
- Drevillon, L.; Tanguy, G.; Hinzpeter, A.; Arous, N.; de Becdelievre, A.; Aissat, A.; Tarze, A.; Goossens, M.; Fanen, P. COMMD1-Mediated Ubiquitination Regulates CFTR Trafficking. PLoS ONE 2011, 6, e18334. [Google Scholar] [CrossRef]
- Smith, L.; Litman, P.; Liedtke, C.M. COMMD1 interacts with the COOH terminus of NKCC1 in Calu-3 airway epithelial cells to modulate NKCC1 ubiquitination. Am. J. Physiol. Cell Physiol. 2013, 305, C133–C146. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Morgan, C.T.; Shinde, U.; Haddock, G.; Lutsenko, S. COMMD1 Forms Oligomeric Complexes Targeted to the Endocytic Membranes via Specific Interactions with Phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 2009, 284, 696–707. [Google Scholar] [CrossRef]
- Fedoseienko, A.; Wijers, M.; Wolters, J.C.; Dekker, D.; Smit, M.; Huijkman, N.; Kloosterhuis, N.; Klug, H.; Schepers, A.; van Dijk, K.W.; et al. The COMMD Family Regulates Plasma LDL Levels and Attenuates Atherosclerosis through Stabilizing the CCC Complex in Endosomal LDLR Trafficking. Circ. Res. 2018, 122, 1648–1660. [Google Scholar] [CrossRef]
- Bartuzi, P.; Billadeau, D.D.; Favier, R.; Rong, S.; Dekker, D.; Fedoseienko, A.; Fieten, H.; Wijers, M.; Levels, J.H.; Huijkman, N.; et al. CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL. Nat. Commun. 2017, 7, 10961. [Google Scholar] [CrossRef]
- Maine, G.N.; Mao, X.; Komarck, C.M.; Burstein, E. COMMD1 promotes the ubiquitination of NF-jB subunits through a cullin-containing ubiquitin ligase. EMBO J. 2007, 26, 436–447. [Google Scholar] [CrossRef]
- Mao, X.; Gluck, N.; Chen, B.; Starokadomskyy, P.; Li, H.; Maine, G.N.; Burstein, E. COMMD1 (Copper Metabolism MURR1 Domain-containing Protein 1) Regulates Cullin RING Ligases by Preventing CAND1 (Cullin-associated Nedd8-dissociated Protein 1) Binding. J. Biol. Chem. 2011, 286, 32355–32365. [Google Scholar] [CrossRef]
- Starokadomskyy, P.; Gluck, N.; Li, H.; Chen, B.; Wallis, M.; Maine, G.N.; Mao, X.; Zaidi, I.W.; Hein, M.Y.; McDonald, F.J.; et al. CCDC22 deficiency in humans blunts activation of proinflammatory NF-κB signaling. J. Clin. Investig. 2013, 123, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Mouhadeb, O.; Shlomo, S.B.; Cohen, K.; Farkash, I.; Gruber, S.; Maharshak, N.; Halpern, Z.; Burstein, E.; Gluck, N.; Varol, C. Impaired COMMD10-Mediated Regulation of Ly6C(hi) Monocyte-Driven Inflammation Disrupts Gut Barrier Function. Front. Immunol. 2018, 9, 2623. [Google Scholar] [CrossRef]
- Nakai, A.; Fujimoto, J.; Miyata, H.; Stumm, R.; Narazaki, M.; Schulz, S.; Baba, Y.; Kumanogoh, A.; Suzuki, K. The COMMD3/8 complex determines GRK6 specificity for chemoattractant receptors. J. Exp. Med. 2019, 216, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.M.; Solban, N.; Tremblay, S.; Gutkowska, J.; Schurch, W.; Orlov, S.N.; Lewanczuk, R.; Hamet, P.; Tremblay, J. HCaRG is a novel regulator of renal epithelial cell growth and differentiation causing G2M arrest. Am. J. Physiol. Renal Physiol. 2003, 284, F753–F762. [Google Scholar] [CrossRef] [PubMed]
- Van de Sluis, B.; Mao, X.; Zhai, Y.; Groot, A.J.; Vermeulen, J.F.; van der Wall, E.; van Diest, P.J.; Hofker, M.H.; Wijmenga, C.; Klomp, L.W.; et al. COMMD1 disrupts HIF-1 dimerization and inhibits human tumor cell invasion. J. Clin. Investig. 2010, 120, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Koo, Y.; Mao, X.; Sifuentes-Dominguez, L.; Morris, L.L.; Jia, D.; Miyata, N.; Faulkner, R.A.; van Deursen, J.M.; Vooijs, M.; et al. Endosomal sorting of Notch receptors through COMMD9- dependent pathways modulates Notch signaling. J. Cell Biol. 2015, 211, 605–617. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, W.; Han, T.; Xie, C.; Zhang, T.; Gan, M.; Wang, J.B. COMMD9 promotes TFDP1/E2F1 transcriptional activity via interaction with TFDP1 in non-small cell lung cancer. Cell Signal 2017, 30, 59–66. [Google Scholar] [CrossRef]
- Bandmann, O.; Heinz Weiss, K.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Vonk, W.I.M.; Kakkar, V.; Bartuzi, P.; Jaarsma, D.; Berger, R.; Hofker, M.H.; Klomp, L.W.; Wijmenga, C.; Kampinga, H.H.; van de Sluis, B. The Copper Metabolism MURR1 Domain Protein 1 (COMMD1) Modulates the Aggregation of Misfolded Protein Species in a Client-Specific Manner. PLoS ONE 2014, 9, e92408. [Google Scholar]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, S.X.; Zheng, X.; Huang, S.; Chen, H.; Chen, H.; Luo, W.; Guo, Z.; He, X.; Zhao, Q. Transcriptional analysis of the expression, prognostic value and immune infiltration activities of the COMMD protein family in hepatocellular carcinoma. BMC Cancer 2021, 21, 1001. [Google Scholar] [CrossRef]
- Matsuda, H.; Campion, C.G.; Fujiwara, K.; Ikeda, J.; Cossette, S.; Verissimo, T.; Ogasawara, M.; Gaboury, L.; Saito, K.; Yamaguchi, K.; et al. HCaRG/COMMD5 inhibits ErbB receptor-driven renal cell carcinoma. Oncotarget 2017, 8, 69559–69576. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Huang, W.; Sun, Y.; Liang, H.; Chen, M.; Wu, X.; Wang, X.; Zhang, L.; Cheng, X.; Fan, Y.; et al. Prognosis and modulation mechanisms of COMMD6 in human tumours based on expression profiling and comprehensive bioinformatics analysis. Br. J. Cancer 2019, 121, 699–709. [Google Scholar] [CrossRef]
- Riera-Romo, M. COMMD1: A Multifunctional Regulatory Protein. J. Cell Biochem. 2018, 119, 34–51. [Google Scholar] [CrossRef]
- Burstein, E.; Hoberg, J.E.; Wilkinson, A.S.; Rumble, J.M.; Csomos, R.A.; Komarck, C.M.; Maine, G.N.; Wilkinson, J.C.; Mayo, M.W.; Duckett, C.S. COMMD Proteins, a Novel Family of Structural and Functional Homologs of MURR1. J. Biol. Chem. 2005, 280, 22222–22232. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.D.; Hospenthal, M.K.; Hall, R.J.; Chandra, M.; Chilton, M.; Tillu, V.; Chen, K.E.; Celligoi, J.; McDonald, F.J.; Cullen, P.J.; et al. Structural insights into the architecture and membrane interactions of the conserved COMMD proteins. eLife 2018, 7, e35898. [Google Scholar] [CrossRef] [PubMed]
- Narindrasorasak, S.; Kulkarni, P.; Deschamps, P.; She, Y.M.; Sarkar, B. Characterization and Copper Binding Properties of Human COMMD1 (MURR1). Biochemistry 2007, 46, 3116–3128. [Google Scholar] [CrossRef]
- AlphaFold Protein Structure Database. Available online: Alphafold.ebi.ac.uk (accessed on 1 October 2021).
- Phillips-Krawczak, C.A.; Singla, A.; Starokadomskyy, P.; Deng, Z.; Osborne, D.G.; Li, H.; Dick, C.J.; Gomez, T.S.; Koenecke, M.; Zhang, J.S.; et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol. Biol. Cell 2015, 26, 91–103. [Google Scholar] [CrossRef]
- Schou, K.B.; Andersen, J.S.; Pedersen, L.B. A divergent calponin homology (NN–CH) domain defines a novel family: Implications for evolution of ciliary IFT complex B proteins. Bioinformatics 2014, 30, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.J.; Ahmad, Y.; Larance, M.; Lamond, A.I. Characterization of native protein complexes and protein isoform variation using size-fractionation-based quantitative proteomics. Mol. Cell Proteom. 2013, 12, 3851–3873. [Google Scholar] [CrossRef]
- Kendall, A.K.; Xie, B.; Xu, P.; Wang, J.; Burcham, R.; Frazier, M.N.; Binshtein, E.; Wei, H.; Graham, T.R.; Nakagawa, T.; et al. Mammalian Retromer Is an Adaptable Scaffold for Cargo Sorting from Endosomes. Structure 2020, 28, 393–405. [Google Scholar] [CrossRef]
- Harbour, M.E.; Breusegem, S.Y.; Seaman, M.N.J. Recruitment of the endosomal WASH complex is mediated by the extended ‘tail’ of Fam21 binding to the retromer protein Vps35. Biochem. J. 2012, 442, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC.: New York, NY, USA, 2015. [Google Scholar]
- Zhang, T.J.; Zhang, K.; Qi, L.; Hu, Q.; Shen, Z.; Liu, B.; Deng, J.; Zhang, C.; Zhang, Y. DENN domain-containing protein FAM45A regulates the homeostasis of late/multivesicular endosomes. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 916–929. [Google Scholar] [CrossRef] [PubMed]
- GnomAD Genome Aggregation Database v2.1.1. Available online: Gnomad.broadinstitute.org (accessed on 1 October 2021).
- The Human Protein Atlas. Available online: www.proteinatlas.org (accessed on 1 October 2021).
- Linardopoulou, E.V.; Parghi, S.S.; Friedman, C.; Osborn, G.E.; Parkhurst, S.M.; Trask, B.J. Human Subtelomeric WASH Genes Encode a New Subclass of the WASP Family. PLoS Gen. 2007, 3, e237. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; van Vlijmen, T.; Mardones, G.A.; Prabhu, Y.; Rojas, A.L.; Mohammed, S.; Heck, A.J.R.; Raposo, G.; van der Sluijs, P.; Bonifacino, J.S. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 2008, 183, 513–526. [Google Scholar] [CrossRef]
- Campion, C.G.; Zaoui, K.; Verissimo, T.; Cossette, S.; Matsuda, H.; Solban, N.; Hamet, P.; Tremblay, J. COMMD5/HCaRG Hooks Endosomes on Cytoskeleton and Coordinates EGFR Trafficking. Cell Rep. 2018, 24, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Kalaidzidis, I.V.; Kalaidzidis, Y.; Lambright, D.; Datta, S. Molecular Insights into Rab7-Mediated Endosomal Recruitment of Core Retromer: Deciphering the Role of Vps26 and Vps35. Traffic 2015, 16, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Duleh, S.N.; Welch, M.D. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton 2010, 67, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.S.; Billadeau, D.D. A FAM21-Containing WASH Complex Regulates Retromer-Dependent Sorting. Dev. Cell 2009, 17, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hodgkinson, V.; Zhu, S.; Weisman, G.A.; Petris, M.J. Advances in the Understanding of Mammalian Copper Transporters. Adv. Nutr. 2011, 2, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Materia, S.; Cater, M.A.; Klomp, L.W.J.; Mercer, J.F.B.; La Fontaine, S. Clusterin and COMMD1 Independently Regulate Degradation of the Mammalian Copper ATPases ATP7A and ATP7B. J. Biol. Chem. 2012, 287, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Voineagu, I.; Huang, L.; Winden, K.; Lazaro, M.; Haan, E.; Nelson, J.; McGaughran, J.; Nguyen, L.S.; Friend, K.; Hackett, A.; et al. CCDC22: A novel candidate gene for syndromic X-linked intellectual disability. Mol. Psychiatry 2012, 17, 4–7. [Google Scholar] [CrossRef]
- Tao, T.Y.; Liu, F.; Klomp, L.; Wijmenga, C.; Gitlin, J.D. The Copper Toxicosis Gene Product Murr1 Directly Interacts with the Wilson Disease Protein. J. Biol. Chem. 2003, 278, 41593–41596. [Google Scholar] [CrossRef] [PubMed]
- De Bie, P.; van de Sluis, B.; Burstein, E.; van de Berghe, P.V.E.; Muller, P.; Berger, R.; Gitlin, J.D.; Wijmenga, C.; Klomp, L.W.J. Distinct Wilson-disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B. Gastroenterology 2007, 133, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Ke, Y.; Ly, K.; McDonald, F.J. COMMD1 regulates the delta epithelial sodium channel (dENaC) through trafficking and ubiquitination. Biochem. Biophys. Res. Commun. 2011, 411, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Swart, M.; Ke, Y.; Ly, K.; McDonald, F.J. Functional interaction of COMMD3 and COMMD9 with the epithelial sodium channel. Am. J. Physiol. Renal Physiol. 2013, 305, F80–F89. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.W.; Cheung, T.T.; Rasulov, S.; Burstein, E.; McDonald, F.J. Epithelial Na+ Channel: Reciprocal Control by COMMD10 and Nedd4-2. Front. Physiol. 2018, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Patel, S.V.; Snyder, P.M. Nedd4-2 Catalyzes Ubiquitination and Degradation of Cell Surface ENaC. J. Biol. Chem. 2007, 282, 20207–20212. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Butt, A.G.; Swart, M.; Liu, Y.F.; McDonald, F.J. COMMD1 downregulates the epithelial sodium channel through Nedd4–2. Am. J. Physiol. Renal Physiol. 2010, 298, F1445–F1456. [Google Scholar] [CrossRef] [PubMed]
- Solban, N.; Jia, H.P.; Richard, S.; Tremblay, S.; Devlin, A.M.; Peng, J.; Gossard, F.; Guo, D.F.; Morel, G.; Hamet, P.; et al. HCaRG, a novel calcium-regulated gene coding for a nuclear protein, is potentially involved in the regulation of cell proliferation. J. Biol. Chem. 2000, 275, 32234–32243. [Google Scholar] [CrossRef] [PubMed]
- Groppelli, E.; Len, A.C.; Granger, L.A.; Jolly, C. Retromer regulates HIV-1 envelope glycoprotein trafficking and incorporation into virions. PLoS Pathog. 2014, 10, e1004518. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.; Zhang, W.; Harrison, M.S.; Goodner, K.; Kazakov, T.; Goodwin, E.C.; Lipovsky, A.; Burd, C.G.; DiMaio, D. Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection. PLoS Pathog. 2015, 11, e1004699. [Google Scholar] [CrossRef]
- Mirrashidi, K.M.; Elwell, C.A.; Verschueren, E.; Johnson, J.R.; Frando, A.; von Dollen, J.; Rosenberg, O.; Gulbahce, N.; Jang, G.; Johnson, T.; et al. Global mapping of the Inc-human Interactome reveals that Retromer restricts chlamydia infection. Cell Host Microbe. 2015, 18, 109–121. [Google Scholar] [CrossRef]
- Yao, J.; Yang, F.; Sun, X.; Wang, S.; Gan, N.; Liu, Q.; Liu, D.; Zhang, X.; Niu, D.; Wei, Y.; et al. Mechanism of inhibition of retromer transport by the bacterial effector RidL. Proc. Natl. Acad. Sci. USA 2018, 115, E1446–E1454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; You, N.; Huang, X.; Gu, H.; Wu, K.; Mi, N.; Li, J. COMMD7 Regulates NF-kB Signaling Pathway in Hepatocellular Carcinoma Stem-like Cells. Mol. Ther. Oncolytics 2019, 12, 112–123. [Google Scholar] [CrossRef]
- Esposito, E.; Napolitano, G.; Pescatore, A.; Calculli, G.; Incoronato, M.R.; Leonardi, A.; Ursini, M.V. COMMD7 as a novel NEMO interacting protein involved in the termination of NF-κB signaling. J. Cell Physiol. 2016, 231, 152–161. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Li, J.; Huang, X.; Wu, K.; Tang, Y.; Wang, L.; Li, H.; Mi, N.; Zheng, L. COMMD7 promotes hepatocellular carcinoma through regulating CXCL10. Biomed. Pharmacother. 2017, 88, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Sperger, J.M.; Chen, X.; Draper, J.S.; Antosiewicz, J.E.; Chon, C.H.; Jones, S.B.; Brooks, J.D.; Andrews, P.W.; Brown, P.O.; Thomson, J.A. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 13350–13355. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, L.; Sun, Y.; Yang, M.; Wang, X.; Wu, X.; Huang, W.; Chen, L.; Pan, S.; Guan, J. Expression profile and bioinformatics analysis of COMMD10 in BALB/C mice and human. Cancer Gene Ther. 2020, 27, 216–225. [Google Scholar] [CrossRef]
- Yang, S.S.; Li, X.M.; Yang, M.; Ren, X.L.; Hu, J.L.; Zhu, X.H.; Wang, F.F.; Zeng, Z.C.; Li, J.Y.; Cheng, Z.Q.; et al. FMNL2 destabilises COMMD10 to activate NF-kB pathway in invasion and metastasis of colorectal cancer. Br. J. Cancer 2017, 117, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Li, J.; Gong, Z.; Huang, X.; Wang, W.; Wang, L.; Wu, K.; Zheng, L. COMMD7 Functions as Molecular Target in Pancreatic Ductal Adenocarcinoma. Mol. Carcinog. 2017, 56, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Van de Sluis, B.; Muller, P.; Duran, K.; Chen, A.; Groot, A.J.; Klomp, L.W.; Liu, P.P.; Wijmenga, C. Increased Activity of Hypoxia-Inducible Factor 1 Is Associated with Early Embryonic Lethality in Commd1 Null Mice. Mol. Cell Biol. 2007, 27, 4142–4156. [Google Scholar] [CrossRef]
- Mu, P.; Akashi, T.; Lu, F.; Kishida, S.; Kadomatsu, K. A novel nuclear complex of DRR1, F-actin and COMMD1 involved in NF-κB degradation and cell growth suppression in neuroblastoma. Oncogene 2017, 36, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- Vonk, W.I.M.; Wijmenga, C.; Berger, R.; van de Sluis, B.; Klomp, L.W.J. Cu,Zn Superoxide Dismutase Maturation and Activity Are Regulated by COMMD1. J. Biol. Chem. 2010, 285, 28991–29000. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, B.; Ufer, C.; Stehling, S.; Heydeck, D.; Kuhn, H.; Sofi, S. Identification of the COMM-domain containing protein 1 as specific binding partner for the guanine-rich RNA sequence binding factor 1. Biochem. Biophys. Acta Gen. Subj. 2020, 1864, 129678. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Li, J.; Huang, X.; Wu, K.; Tang, Y.; Wang, L.; Li, H.; Mi, N.; Zheng, L. COMMD7 activates CXCL10 production by regulating NF-κB and the production of reactive oxygen species. Mol. Med. Rep. 2018, 17, 6784–6788. [Google Scholar] [CrossRef]
- Matsuda, H.; Hamet, P.; Tremblay, J. Hypertension-related, calcium-regulated gene (HCaRG/COMMD5) and kidney diseases: HCaRG accelerates tubular repair. J. Nephrol. 2014, 27, 351–360. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laulumaa, S.; Varjosalo, M. Commander Complex—A Multifaceted Operator in Intracellular Signaling and Cargo. Cells 2021, 10, 3447. https://doi.org/10.3390/cells10123447
Laulumaa S, Varjosalo M. Commander Complex—A Multifaceted Operator in Intracellular Signaling and Cargo. Cells. 2021; 10(12):3447. https://doi.org/10.3390/cells10123447
Chicago/Turabian StyleLaulumaa, Saara, and Markku Varjosalo. 2021. "Commander Complex—A Multifaceted Operator in Intracellular Signaling and Cargo" Cells 10, no. 12: 3447. https://doi.org/10.3390/cells10123447
APA StyleLaulumaa, S., & Varjosalo, M. (2021). Commander Complex—A Multifaceted Operator in Intracellular Signaling and Cargo. Cells, 10(12), 3447. https://doi.org/10.3390/cells10123447





