MicroRNA Bta-miR-24-3p Suppressed Galectin-9 Expression through TLR4/NF-ĸB Signaling Pathway in LPS-Stimulated Bovine Endometrial Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Chemical
2.2. CellIsolation, Culture, and Preservation
2.3. Transfection of Bovine Endometrial Epithelial Cell with miRNA and si-LGALS9
2.4. Cell Counting Kit-8 (CCK-8) Assay
2.5. RNA Extraction, cDNA Synthesis, and Reverse Transcription Quantitative Polymerase Chain Reaction
2.6. Extraction of Protein and Western Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Immunofluorescence Techniques
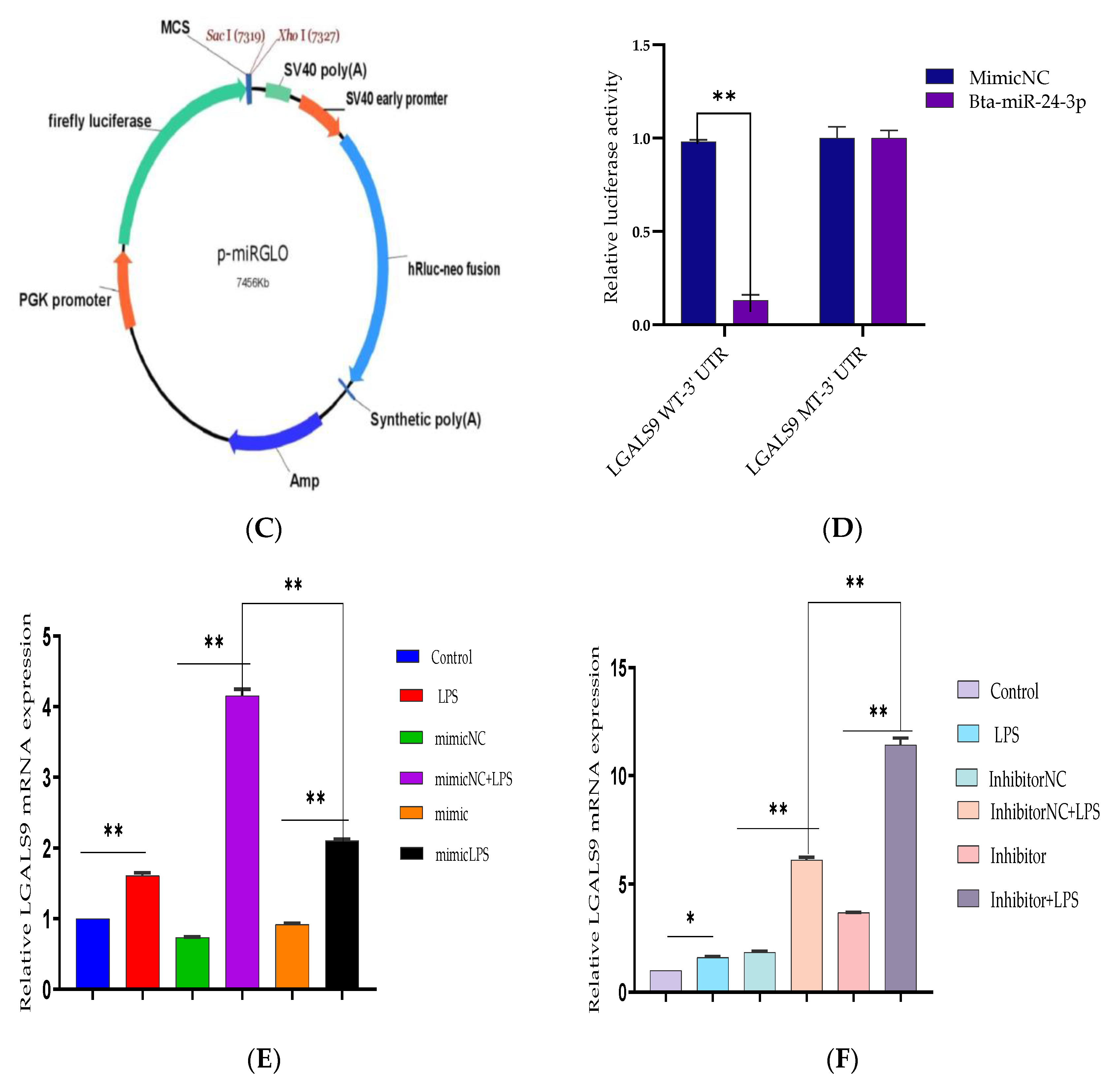
2.9. Target Prediction of Bta-miR-24-3p and Luciferase Reporter Assay
2.10. Recombinant Plasmid Construction and Transfection
2.11. Data Statistics
3. Results
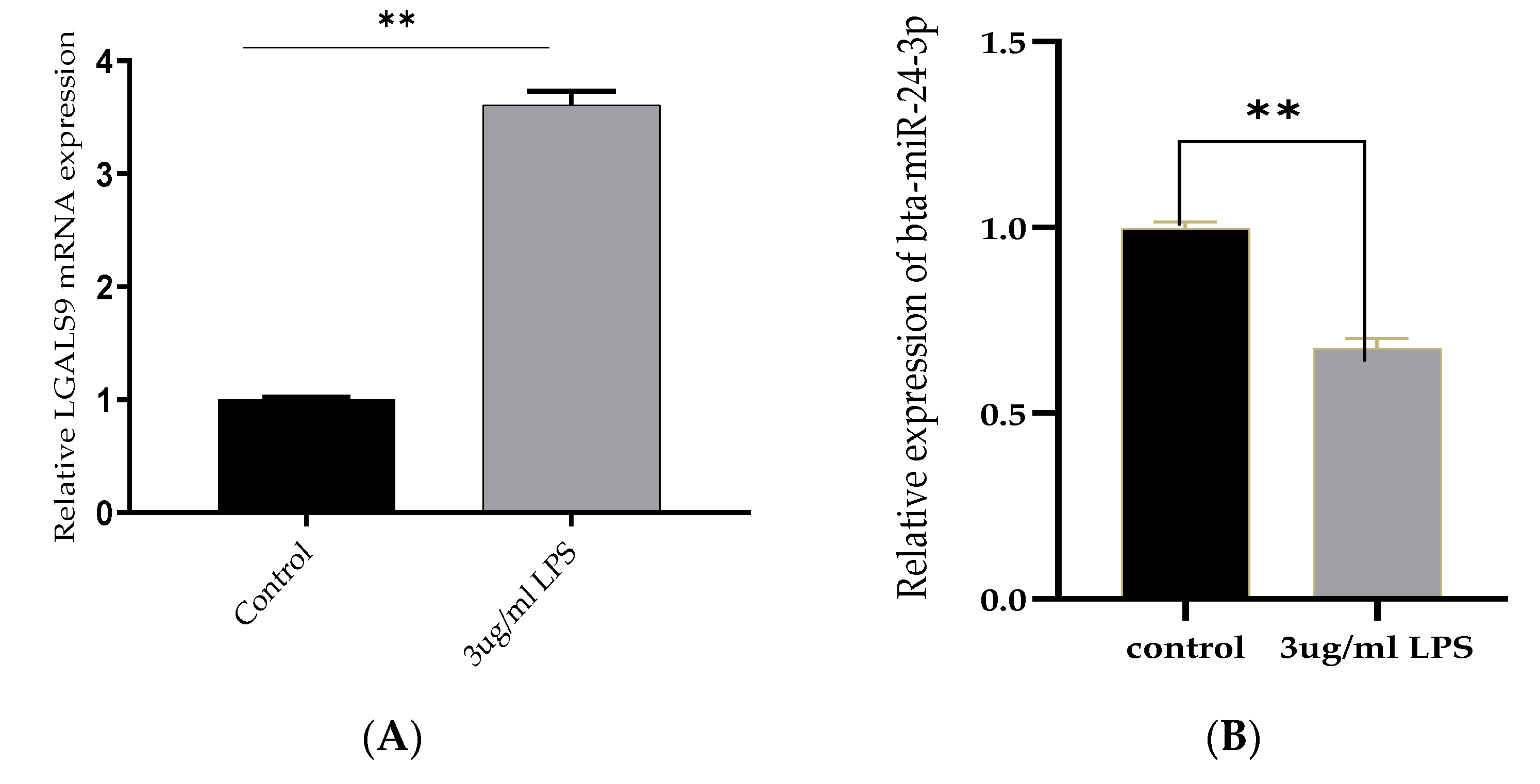
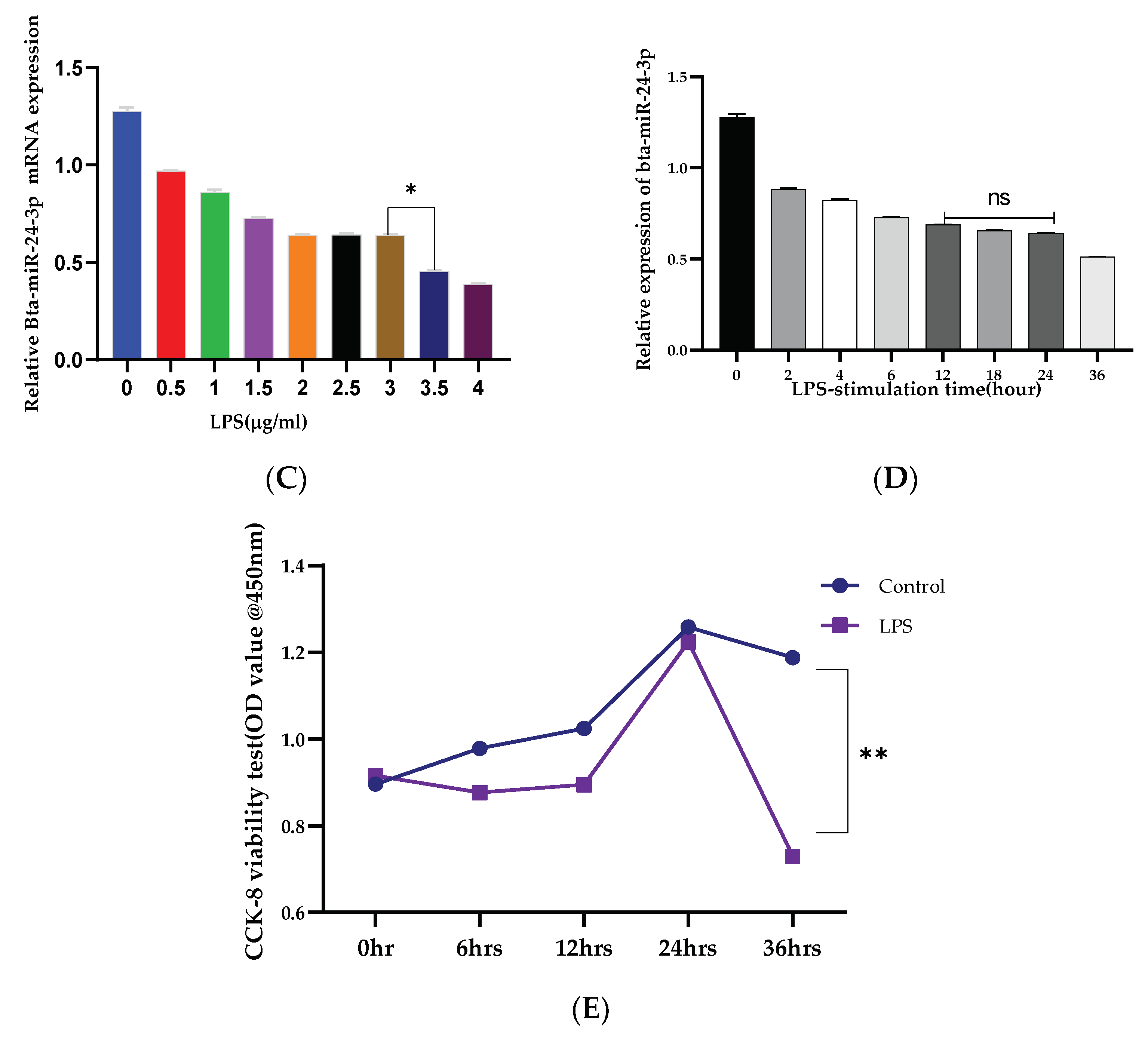
3.1. Reduced Expression of Bta-miR-24-3p and Elevation of LGALS9 following LPS-Stimulation In Vitro
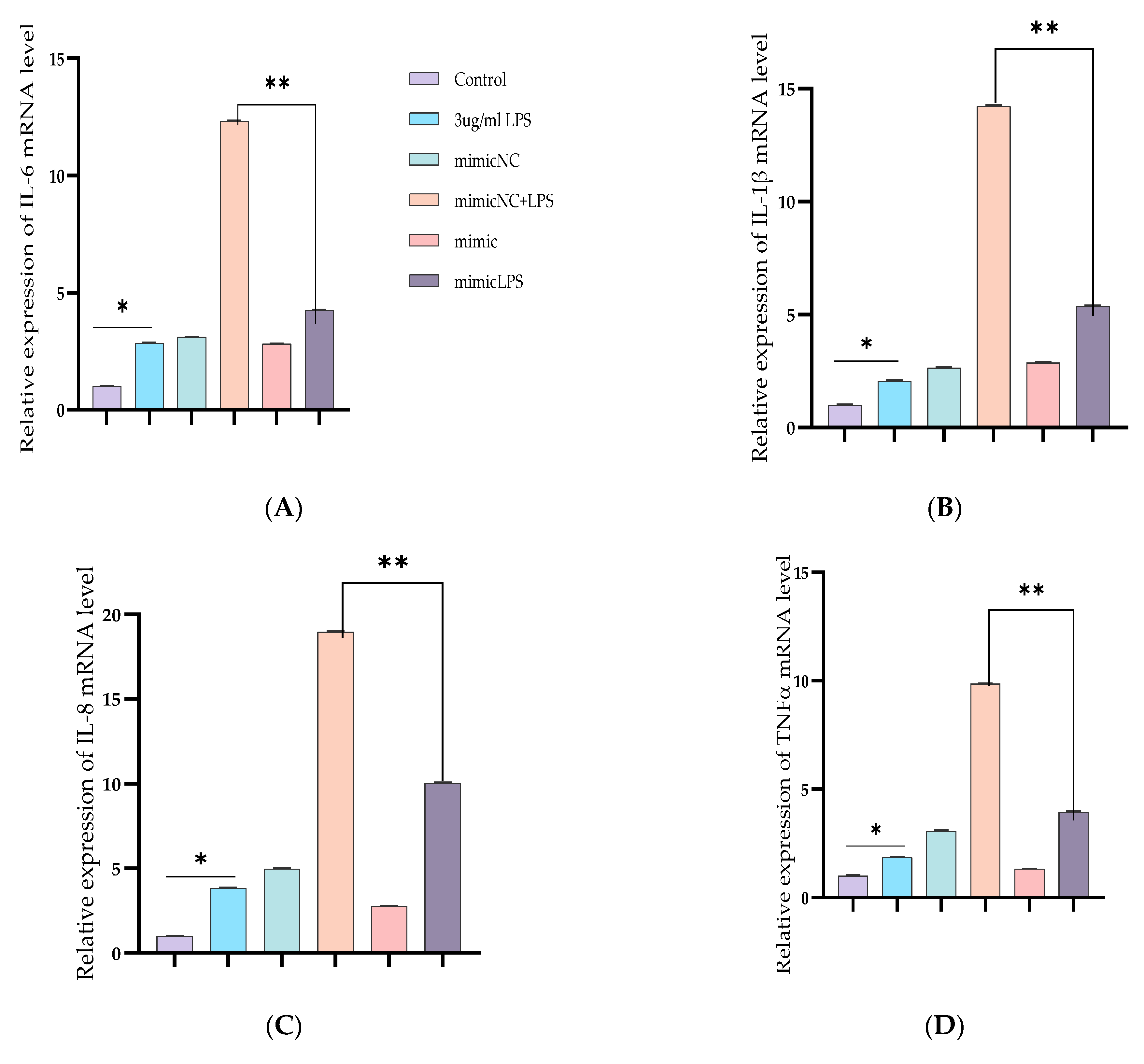
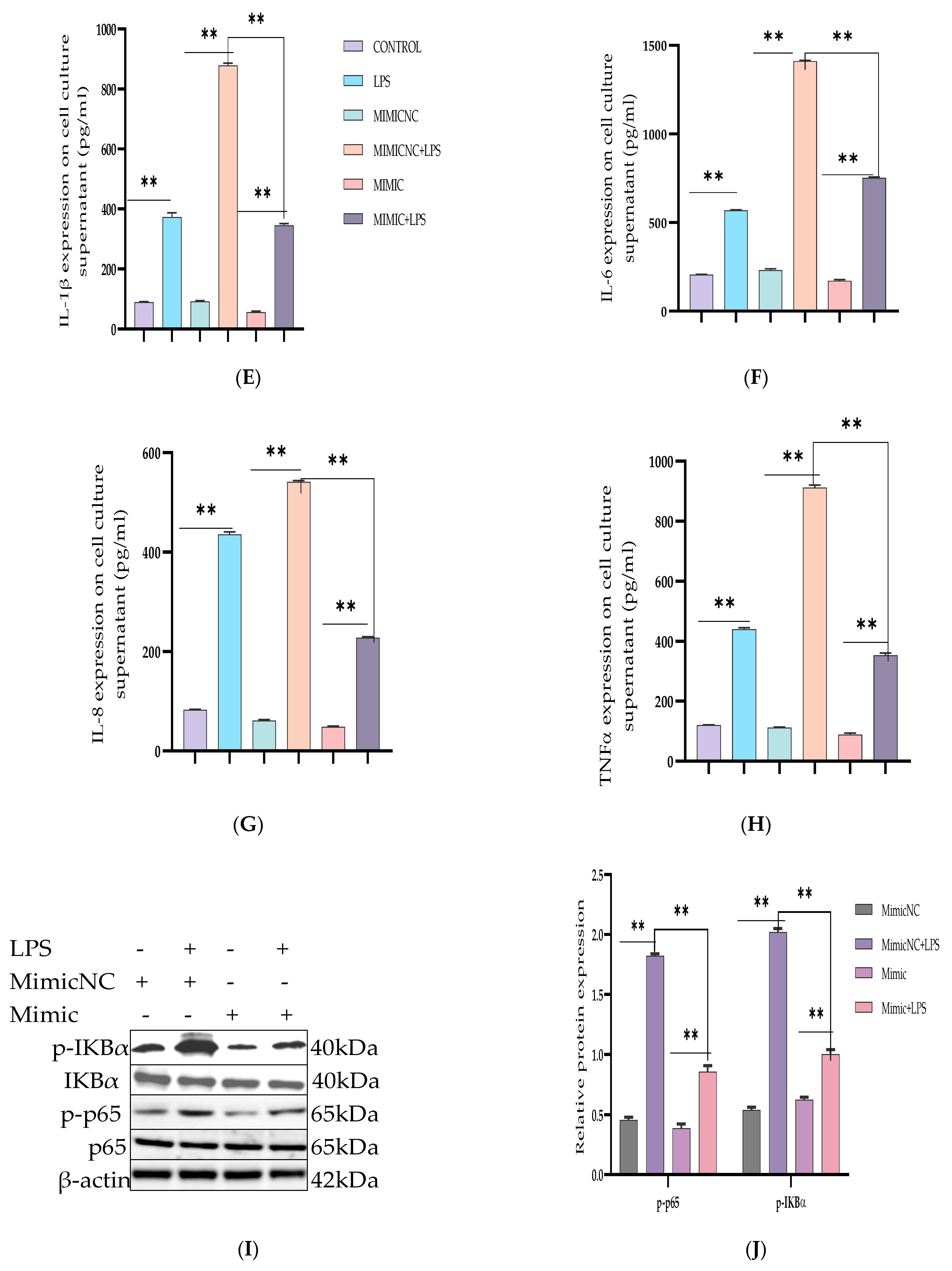
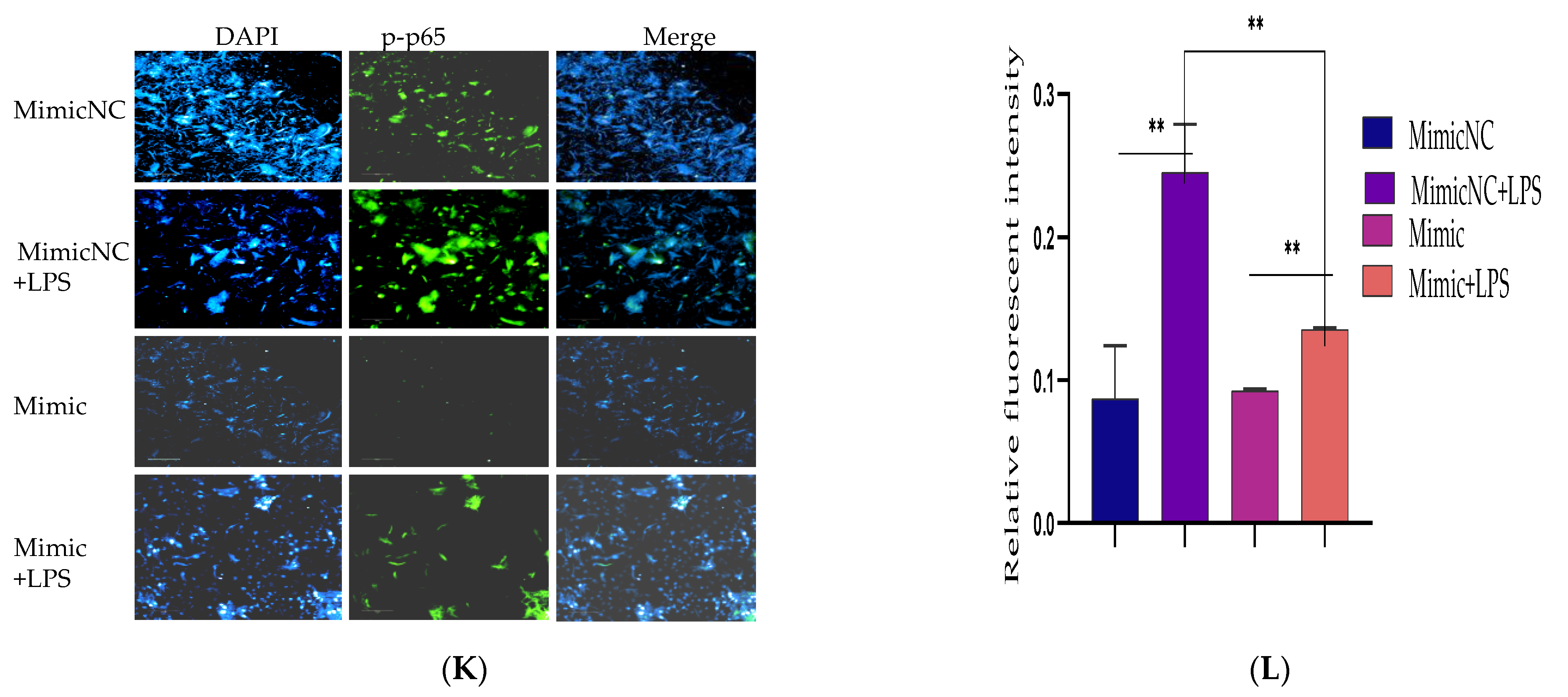
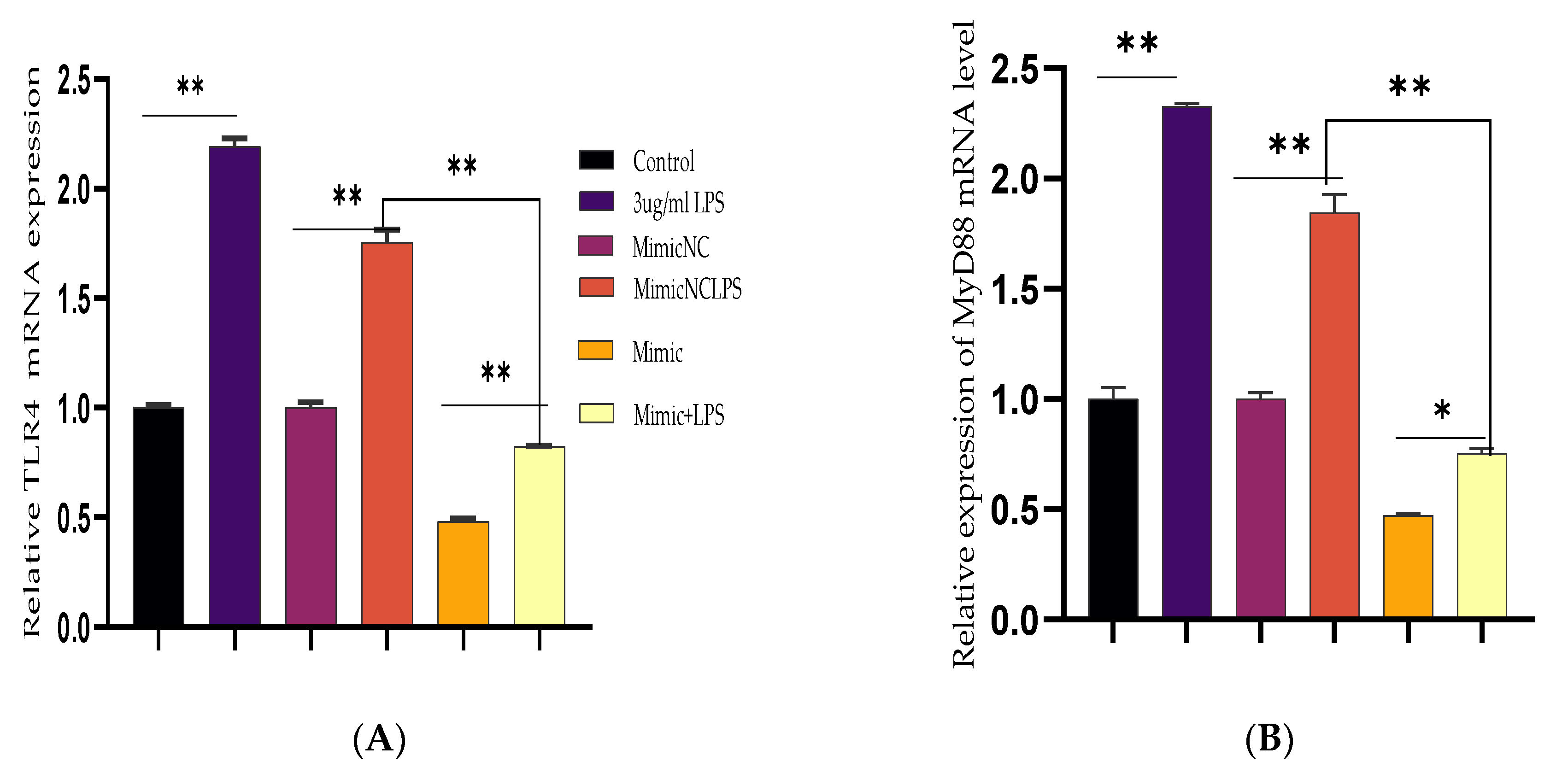
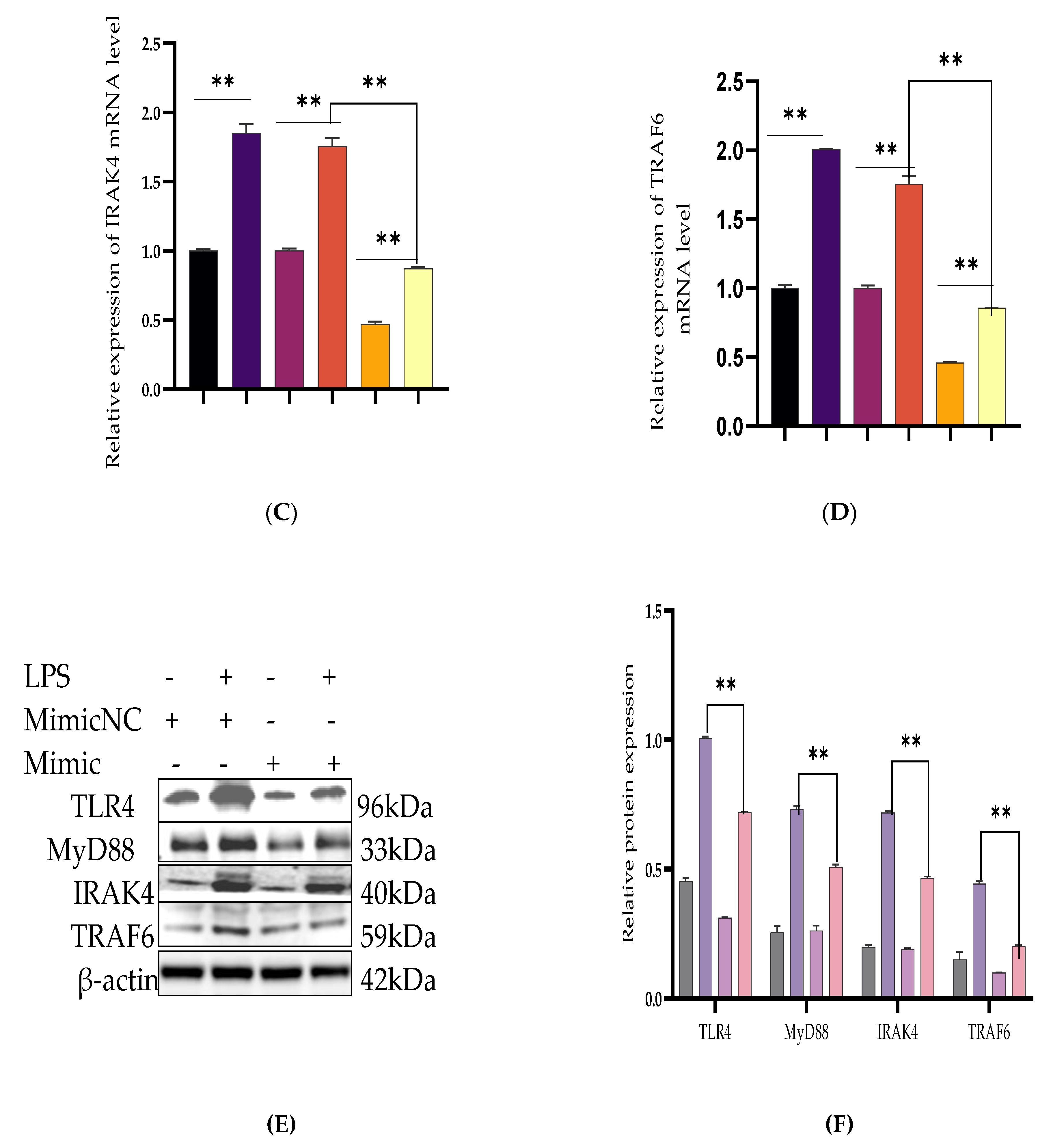
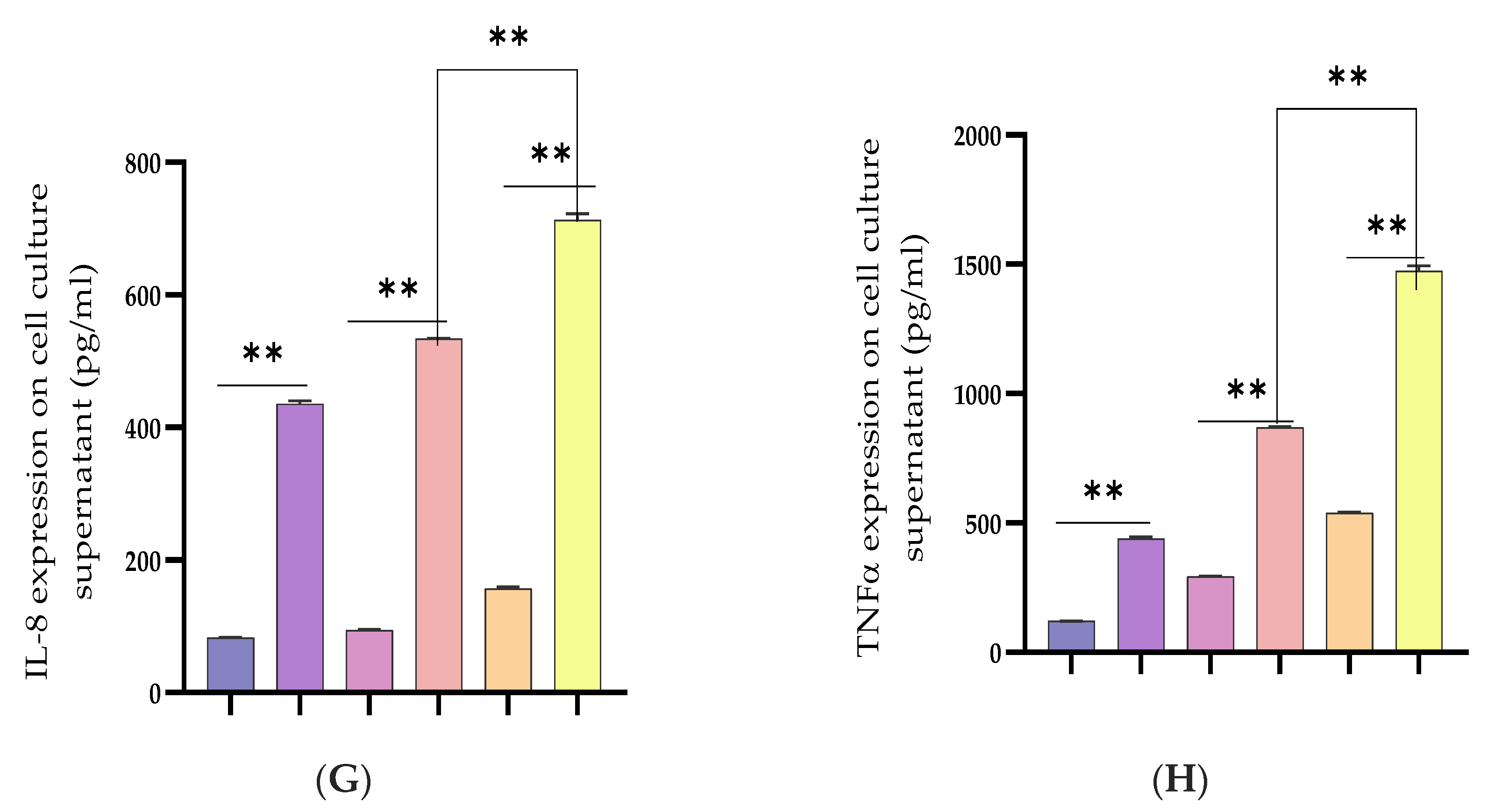
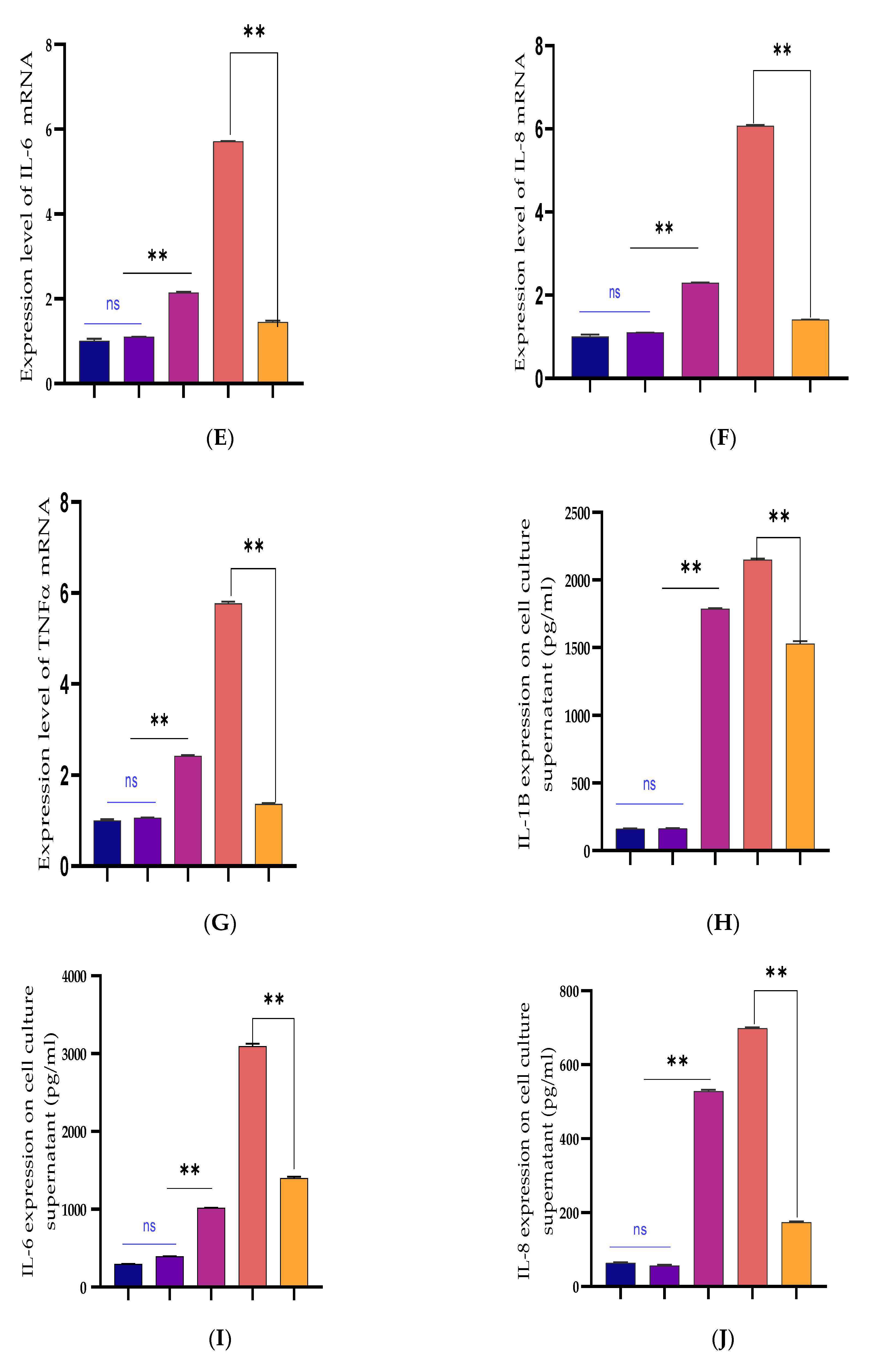
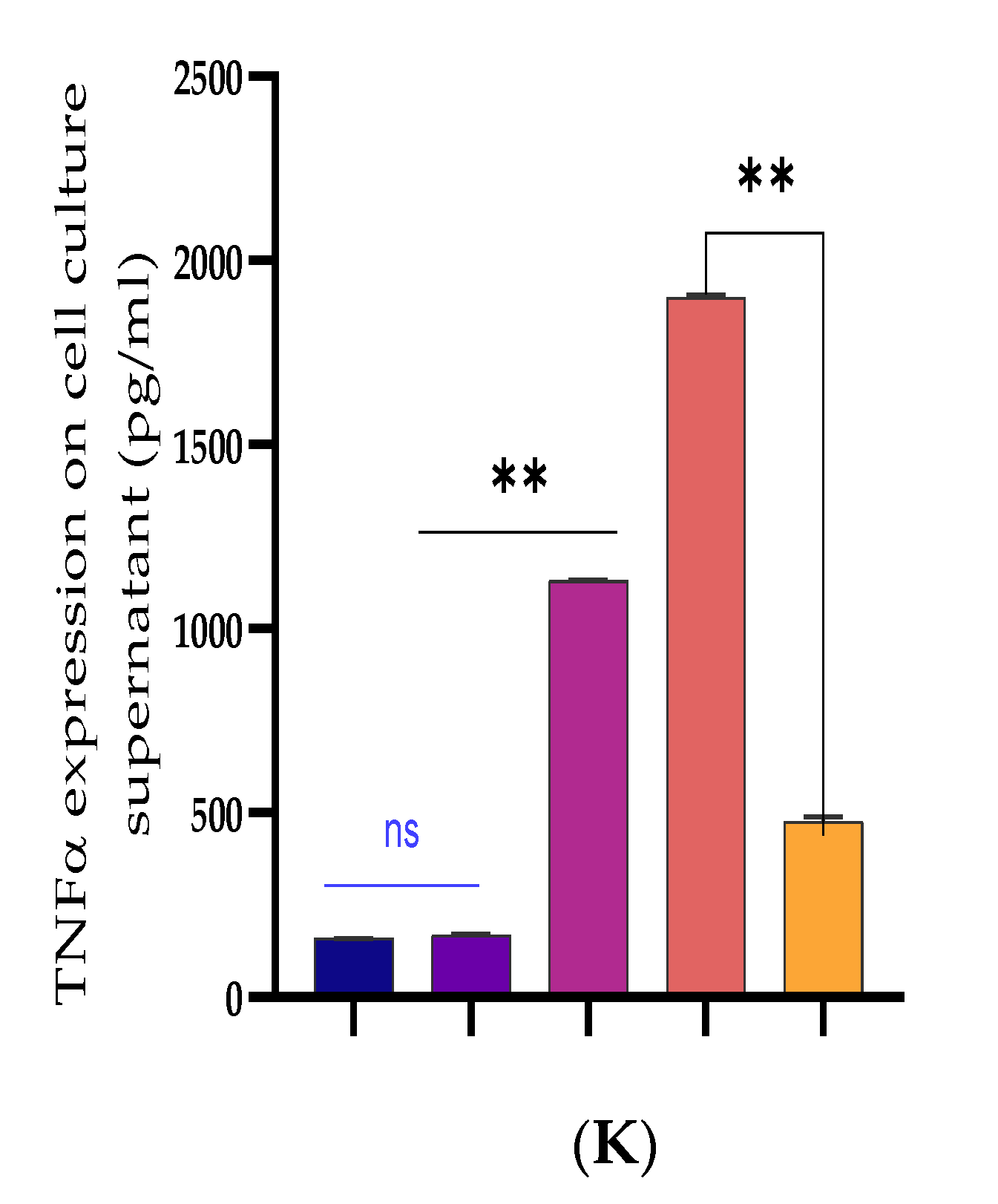
3.2. Inhibiting the Expression of Pro-Inflammatory Cytokines and Restrains Activation of the TLR4/NF-κB Pathway by Bta-miR-24-3p in BEECs
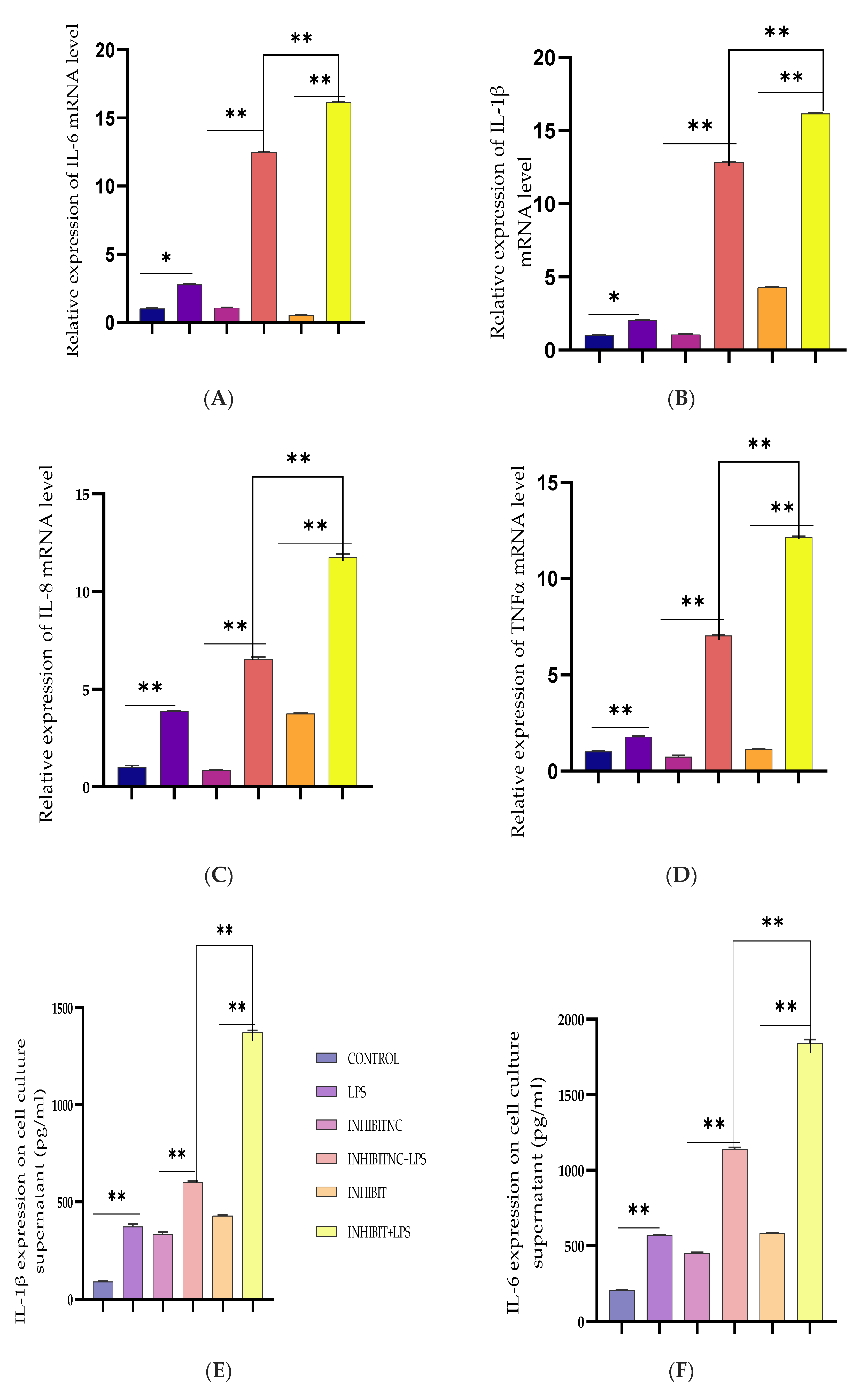
3.3. Upregulation of Pro-Inflammatory Cytokines by Bta-miR-24-3p Inhibitor in BEECs
3.4. LGALS9 as a Direct Molecular Target of Bta-miR-24-3p
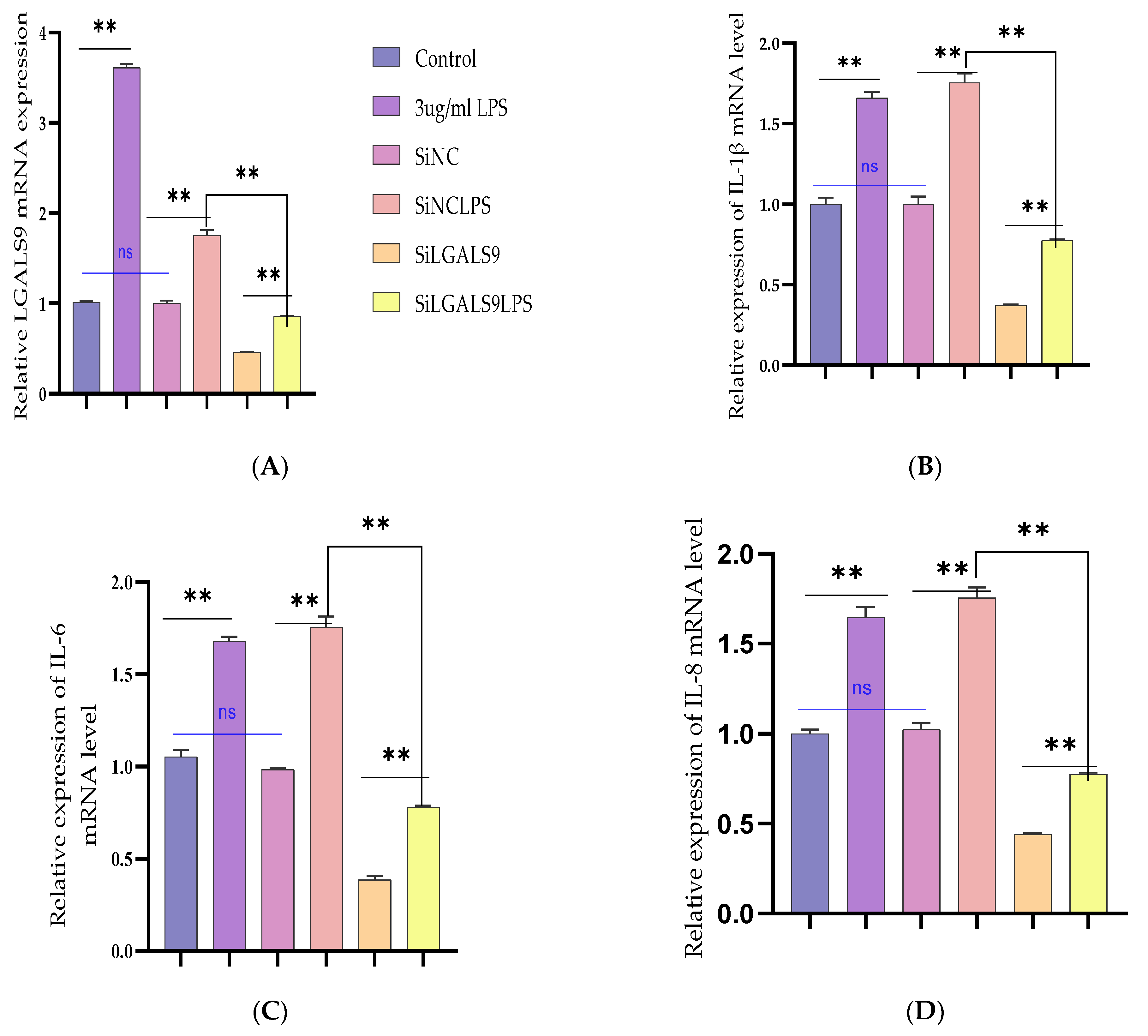
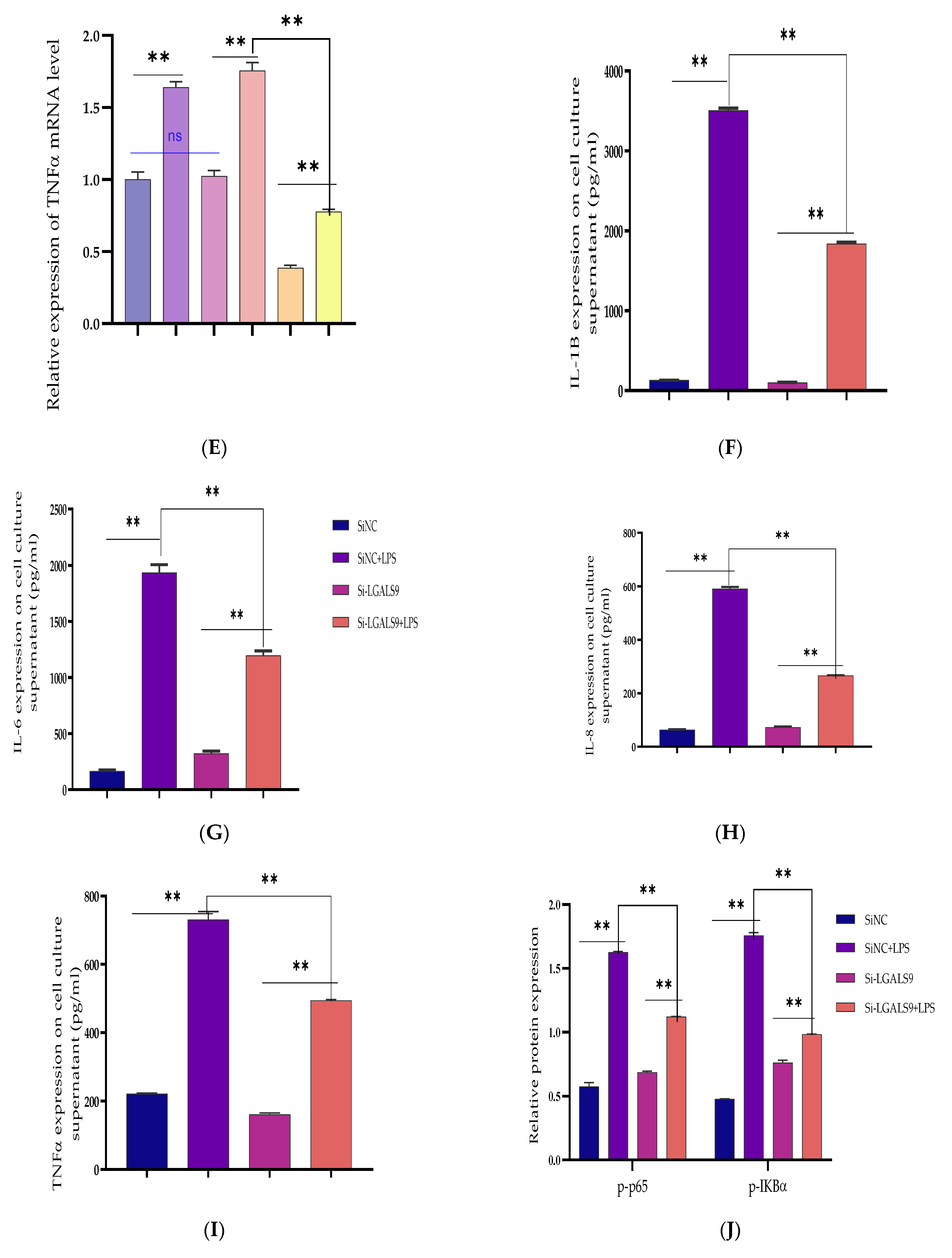
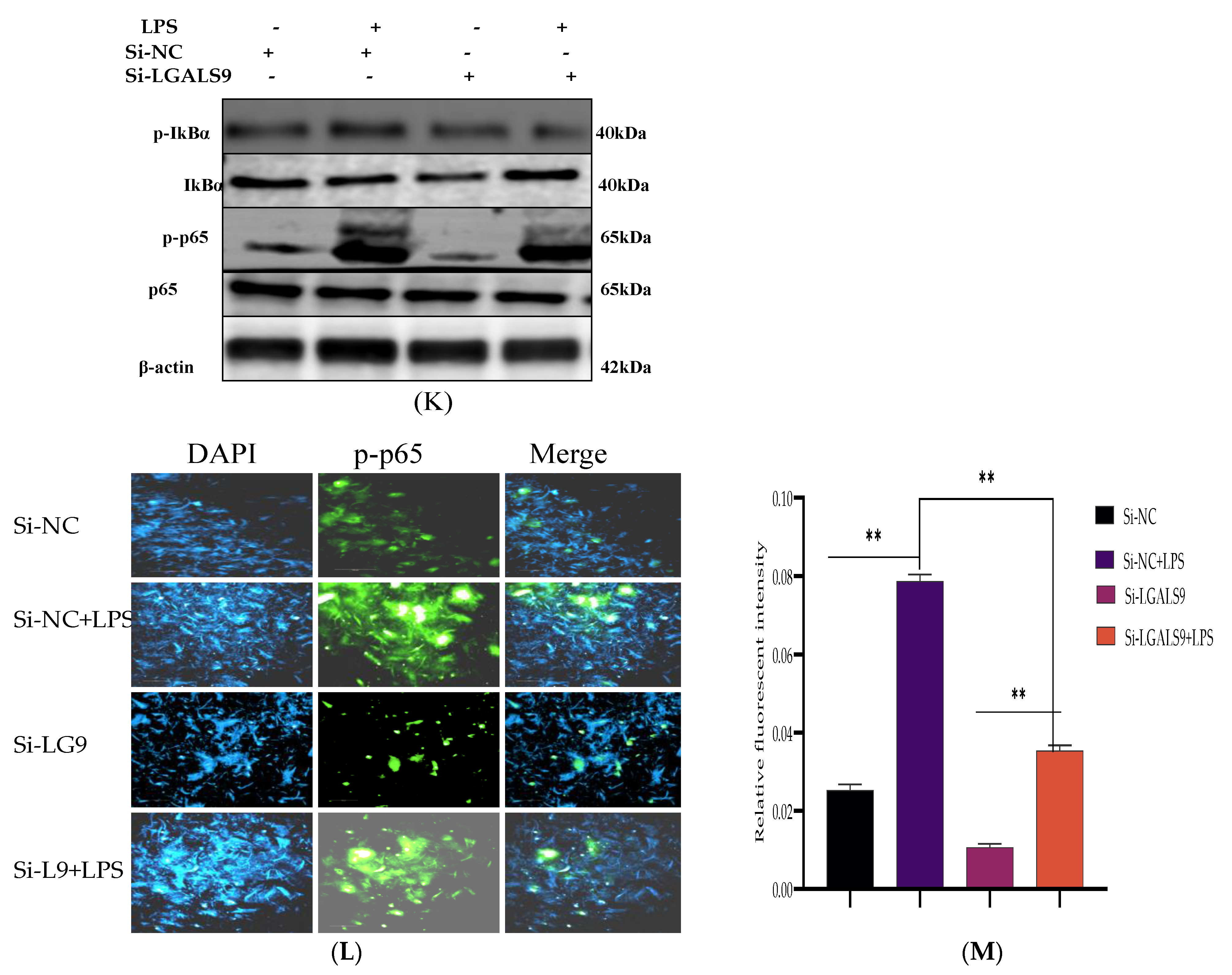
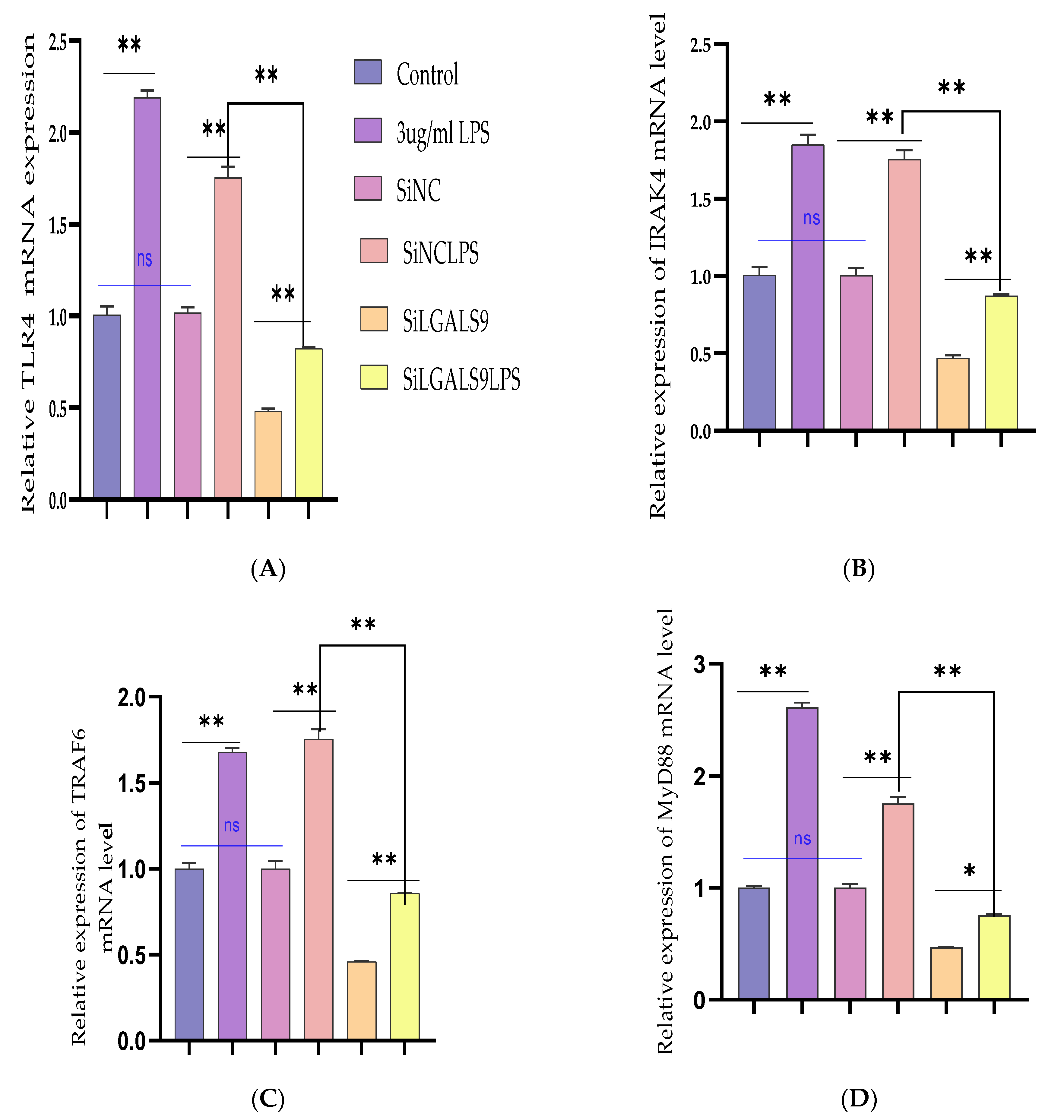
3.5. Silencing LGALS9 Inhibits LPS-Mediated Inflammation in BEECs
3.6. Bta-miR-24-3p Regulates LPS-Induced Inflammatory Response by Targeting LGALS9
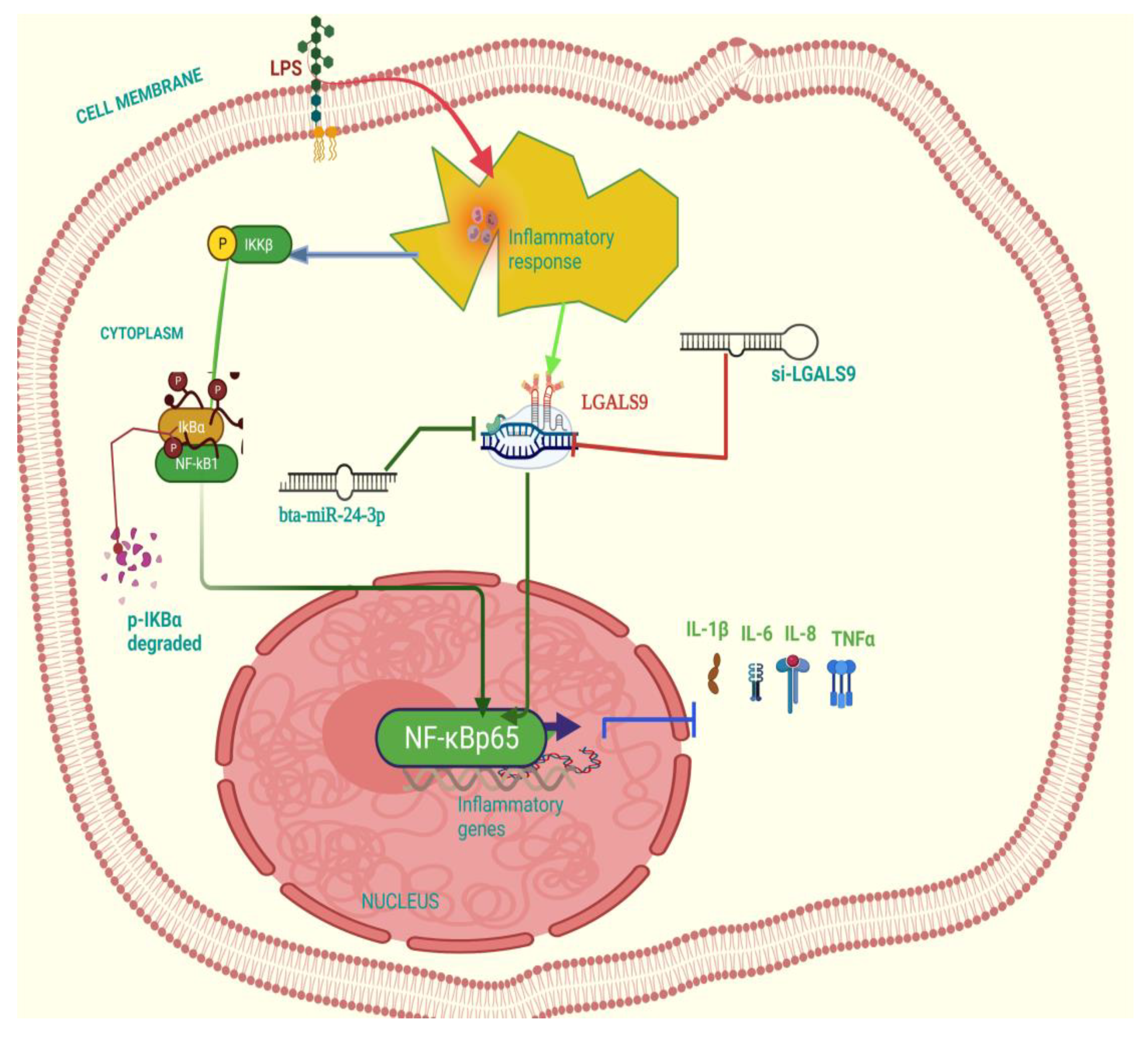
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, I.M.; Cronin, J.; Borges, A. The Postpartum Period and Modern Dairy Cow Fertility. Part 1: Uterine Function. Livestock 2011, 16, 14–18. [Google Scholar] [CrossRef]
- Gilbert, R.O. The Effects of Endometritis on the Establishment of Pregnancy in Cattle. Reprod. Fertil. Dev. 2012, 24, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S.; Duffield, T.; Leslie, K.; Bateman, K.; Keefe, G.; Walton, J.; Johnson, W. Defining and Diagnosing Postpartum Clinical Endometritis and its Impact on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of Endometritis and its Effects on Reproductive Performance of Dairy Cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Borsberry, S.; Dobson, H. Periparturient Diseases and their Effect on Reproductive Performance in Five Dairy Herds. Veter. Rec. 1989, 124, 217–219. [Google Scholar] [CrossRef]
- Minten, M.A.; Bilby, T.R.; Bruno, R.G.S.; Allen, C.C.; Madsen, C.A.; Wang, Z.; Sawyer, J.; Tibary, A.; Neibergs, H.L.; Geary, T.W.; et al. Effects of Fertility on Gene Expression and Function of the Bovine Endometrium. PLoS ONE 2013, 8, e69444. [Google Scholar] [CrossRef]
- Bonneville-Hébert, A.; Bouchard, E.; Du Tremblay, D.; Lefebvre, R. Effect of Reproductive Disorders and Parity on Repeat Breeder Status and Culling of Dairy Cows in Quebec. Can. J. Veter. Res. Rev. Can. Rech. Veter. 2011, 75, 147–151. [Google Scholar]
- Yusuf, M.; Nakao, T.; Ranasinghe, R.B.K.; Gautam, G.; Long, S.T.; Yoshida, C.; Koike, K.; Hayashi, A. Reproductive Performance of Repeat Breeders in Dairy Herds. Theriogenology 2010, 73, 1220–1229. [Google Scholar] [CrossRef]
- Salasel, B.; Mokhtari, A.; Taktaz, T. Prevalence, Risk Factors for and Impact of Subclinical Endometritis in Repeat Breeder Dairy Cows. Theriogenology 2010, 74, 1271–1278. [Google Scholar] [CrossRef]
- Janowski, T.; Barański, W.; Łukasik, K.; Skarzynski, D.J.; Rudowska, M.; Zduńczyk, S. Prevalence of Subclinical Endometritis in Repeat Breeding Cows and mRNA Expression of Tumor Necrosis Factor α and Inducible Nitric Oxide Synthase in the Endometrium of Repeat Breeding Cows with and Without Subclinical Endometritis. Pol. J. Veter. Sci. 2013, 16, 693–699. [Google Scholar] [CrossRef]
- Mesquita, F.S.; Ramos, R.S.; Pugliesi, G.; Andrade, S.; Van Hoeck, V.; Langbeen, A.; Oliveira, M.L.; Gonella-Diaza, A.M.; Gasparin, G.; Fukumasu, H.; et al. The Receptive Endometrial Transcriptomic Signature Indicates an Earlier Shift from Proliferation to Metabolism at Early Diestrus in the Cow1. Biol. Reprod. 2015, 93, 52. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; LaRusso, N.F.; Burgart, L.J.; Gores, G.J. Inflammatory Cytokines Induce DNA Damage and Inhibit DNA Repair in Cholangiocarcinoma Cells by a Nitric Oxide-Dependent Mechanism. Cancer Res. 2000, 60, 184–190. [Google Scholar] [PubMed]
- Oguejiofor, C.; Cheng, Z.; Abudureyimu, A.; Fouladi-Nashta, A.A.; Wathes, D.C. Global Transcriptomic Profiling of Bovine Endometrial Immune Response In Vitro. I. Effect of Lipopolysaccharide on Innate Immunity1. Biol. Reprod. 2015, 93, 100. [Google Scholar] [CrossRef][Green Version]
- Granot, I.; Gnainsky, Y.; Dekel, N.; Jiménez-Trejo, F.; Tapia-Rodríguez, M.; Cerbón, M.; Kuhn, D.M.; Manjarrez-Gutiérrez, G.; Mendoza-Rodríguez, C.A.; Picazo, O. Endometrial Inflammation and Effect on Implantation Improvement and Pregnancy Outcome. Reproduction 2012, 144, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Yongzhi, G.; Tom, V.S.; Jhamat, N.; Adnan Niaz, C.M.; Gilles Charpigny, V.J.F.; Erik, B.; Patrice, H. Differential Gene Expression in Bovine Endometrial Epithelial Cells after Challenge with LPS; Specific Implications for Genes Involved in Embryo Maternal Interactions. PLoS ONE 2019, 14, e0222081. [Google Scholar]
- Fortier, M.A.; Guilbault, L.A.; Grasso, F. Specific Properties of Epithelial and Stromal Cells from the Endometrium of Cows. Reproduction 1988, 83, 239–248. [Google Scholar] [CrossRef]
- Gómez-Chávez, F.; Castro-Leyva, V.; Espejel-Núñez, A.; Zamora-Mendoza, R.G.; Rosas-Vargas, H.; Cancino-Díaz, J.C.; Cancino-Díaz, M.E.; Estrada-Gutierrez, G.; Rodríguez-Martínez, S. Galectin-1 Reduced the Effect of LPS on the IL-6 Production in Decidual Cells by Inhibiting LPS on the Stimulation of IκBζ. J. Reprod. Immunol. 2015, 112, 46–52. [Google Scholar] [CrossRef]
- Heusschen, R.; Griffioen, A.W.; Thijssen, V. Galectin-9 in Tumor Biology: A Jack of Multiple Trades. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1836, 177–185. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kabir-Salmani, M.; Azadbakht, M.; Sugihara, K.; Sakai, K.; Iwashita, M. Expression and Localization of Galectin-9 in the Human Uterodome. Endocr. J. 2008, 55, 879–887. [Google Scholar] [CrossRef]
- Heusschen, R.; Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Moschansky, P.; Muñoz-Fernández, R.; Leno-Duran, E.; Klapp, B.F.; Thijssen, V.L.; Blois, S.M. Profiling Lgals9 Splice Variant Expression at the Fetal-Maternal Interface: Implications in Normal and Pathological Human Pregnancy1. Biol. Reprod. 2013, 88, 22. [Google Scholar] [CrossRef]
- Popovici, R.M.; Krause, M.S.; Germeyer, A.; Strowitzki, T.; von Wolff, M. Galectin-9: A New Endometrial Epithelial Marker for the Mid- and Late-Secretory and Decidual Phases in Humans. J. Clin. Endocrinol. Metab. 2005, 90, 6170–6176. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Hulsmans, S.; Griffioen, A.W. The Galectin Profile of the Endothelium: Altered Expression and Localization Inactivated and Tumor Endothelial Cells. Am. J. Pathol. 2008, 172, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, R.; Hambruch, N.; Haeger, J.-D.; Dilly, M.; Kaltner, H.; Gabius, H.-J.; Pfarrer, C. Galectin Fingerprinting Detects Differences in Expression Profiles between Bovine Endometrium and Placentomes as Well as Early and Late Gestational Stages. Placenta 2012, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mitko, K.G. Dynamic Transcriptome Profiling of Bovine Endometrium during the Oestrous Cycle. Master’s Thesis, LMU Munich, München, Germany, 2008. [Google Scholar]
- Norling, L.V.; Perretti, M.; Cooper, D. Endogenous Galectins and the Control of the Host Inflammatory Response. J. Endocrinol. 2009, 201, 169–184. [Google Scholar] [CrossRef]
- Piersanti, R.L.; Block, J.; Ma, Z.; Jeong, K.C.; Santos, J.E.P.; Yu, F.; Sheldon, I.M.; Bromfield, J.J. Uterine Infusion of Bacteria Alters the Transcriptome of Bovine Oocytes. FASEB BioAdv. 2020, 2, 506–520. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. Review Article the Role of Galectins as Modulators of Metabolism and Inflammation. Mediat. Inflamm. 2008, 2018, 9186940. [Google Scholar]
- Jiang, K.; Yang, J.; Yang, C.; Zhang, T.; Shaukat, A.; Yang, X.; Dai, A.; Wu, H.; Deng, G. miR-148a Suppresses Inflammation in Lipopolysaccharide-Induced Endometritis. J. Cell. Mol. Med. 2020, 24, 405–417. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, R.; Defrere, S.; Devoto, L. Nuclear Factor-kappaB: A Main Regulator of Inflammation and Cell Survival in Endometriosis Pathophysiology. Fertil. Steril. 2012, 98, 520–528. [Google Scholar] [CrossRef]
- Kagan, J.C.; Medzhitov, R. Phosphoinositide-Mediated Adaptor Recruitment controls Toll-Like Receptor Signaling. Cell 2006, 125, 943–955. [Google Scholar] [CrossRef]
- Torchinsky, A.; Markert, U.R.; Toder, V. TNF-&agr;-Mediated Stress-Induced Early Pregnancy Loss: A Possible Role of Leukemia Inhibitory Factor. Anaphylaxis 2005, 89, 62–71. [Google Scholar] [CrossRef]
- Piras, C.; Guo, Y.; Soggiu, A.; Chanrot, M.; Greco, V.; Urbani, A.; Charpigny, G.; Bonizzi, L.; Roncada, P.; Humblot, P. Changes in Protein Expression Profiles in Bovine Endometrial Epithelial Cells Exposed to E. Coli LPS Challenge. Mol. BioSyst. 2017, 13, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.; Lee, J.-W.; Ibeagha, A.E.; Bannerman, D.D.; Paape, M.J.; Zhao, X. Bacterial Lipopolysaccharide induces Increased Expression of Toll-Like Receptor (TLR) 4 and Downstream TLR Signaling Molecules in Bovine Mammary Epithelial Cells. Veter. Res. 2008, 39, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-Like Receptor 4 and MYD88-Dependent Signaling Mechanisms of the Innate Immune System are Essential for the Response to Lipopolysaccharide by Epithelial and Stromal Cells of the Bovine Endometrium. Biol. Reprod. 2012, 86, 51. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Roberts, M.H. Toll-Like Receptor 4 Mediates the Response of Epithelial and Stromal Cells to Lipopolysaccharide in the Endometrium. PLoS ONE 2010, 5, e12906. [Google Scholar] [CrossRef]
- Meijer, H.A.; Kong, Y.W.; Lu, W.T.; Wilczynska, A.; Spriggs, R.V.; Robinson, S.W.; Godfrey, J.D.; Willis, A.E.; Bushell, M. Translational Repression and Eif4a2 Activity are Critical for Microrna-Mediated Gene Regulation. Science 2013, 340, 82–85. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Chekulaeva, M.; Filipowicz, W. Mechanisms of miRNA-Mediated Post-Transcriptional Regulation in Animal Cells. Curr. Opin. Cell Biol. 2009, 21, 452–460. [Google Scholar] [CrossRef]
- Ambros, V. The Functions of Animal MicroRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Yin, N.; Yang, Y.; Wang, X.; Yang, C.; Ma, X.; Shaukat, A.; Zhao, G.; Deng, G. MiR-19a Mediates the Negative Regulation of the NF-Κb Pathway in Lipopolysaccharide-Induced Endometritis by Targeting TBK1. Inflamm. Res. 2019, 68, 231–240. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Santos, N.R.; Gilbert, O.R.; Goetze, L.; Bryant, C.E.; White, O.J.; Cronin, J.; Sheldon, I.M. Expression of Genes Associated with Immunity in the Endometrium of Cattle with Disparate Postpartum Uterine Disease and Fertility. Reprod. Biol. Endocrinol. 2009, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Nam, J.W. Genomics of microRNA. Trends Genet. 2006, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Sponchiado, M.; Gomes, N.S.; Fontes, P.; Martins, T.; del Collado, M.; Pastore, A.D.A.; Pugliesi, G.; Nogueira, M.F.G.; Binelli, M. Pre-Hatching Embryo-Dependent and -Independent Programming of Endometrial Function in Cattle. PLoS ONE 2017, 12, e0175954. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Kim, I.-H. Pregnancy Loss in Dairy Cows: The Contributing Factors, the Effects on Reproductive Performance and the Economic Impact. J. Vet. Sci. 2007, 8, 283–288. [Google Scholar] [CrossRef]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.R.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial Lipopolysaccharide Induces an Endocrine Switch from Prostaglandin F2alpha to Prostaglandin E2 in Bovine Endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef]
- Mateus, L.; Lopes-Da-Costa, L.; Carvalho, H.; Serra, P.; Silva, J.R. Blood and Intrauterine Leukocyte Profile and Function in Dairy Cows that Spontaneously Recovered from Postpartum Endometritis. Reprod. Domest. Anim. 2002, 37, 176–180. [Google Scholar] [CrossRef]
- Sheldon, I.; Dobson, H. Postpartum Uterine Health in Cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef]
- Turner, M.; Healey, G.; Sheldon, I.M. Immunity and Inflammation in the Uterus. Reprod. Domest. Anim. 2012, 47, 402–409. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-Like Receptor Signaling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, Questions, and Possibilities in Toll-Like Receptor Signaling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, D.; Ibrahim, S.; Hoelker, M.; Drillich, M.; Heuwieser, W.; Looft, C.; Cinar, M.U.; Tholen, E.; Schellander, K.; Tesfaye, D. MicroRNA-Regulated Molecular Mechanism Underlying Bovine Subclinical Endometritis. Reprod. Fertil. Dev. 2014, 26, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xing, Y.; Ren, L.; Wang, Y.; Li, Q.; Fu, X.; Yang, Q.; Xu, L.; Willems, L.; Li, J.; et al. Bta-miR-24-3p Controls the Myogenic Differentiation and Proliferation of Fetal, Bovine, Skeletal Muscle-Derived Progenitor Cells by Targeting ACVR1B. Animals 2019, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Qi, J.; Fan, B.-Y.; Zhang, J.; Su, F.-F.; Wang, H.-T. MicroRNA-24-3p Attenuates Myocardial Ischemia/Reperfusion Injury by Suppressing RIPK1 Expression in Mice. Cell. Physiol. Biochem. 2018, 51, 46–62. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Liu, G.; Qi, X.; Cao, X. MicroRNA-24 Inhibits the Proliferation and Migration of Endothelial Cells in Patients with Atherosclerosis by Targeting Importin-A3 and Regulating Inflammatory Responses. Exp. Ther. Med. 2018, 15, 338–344. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y. MiR-24 Inhibits Inflammatory Responses in LPS-Induced Acute Lung Injury of Neonatal Rats through Targeting NLRP3. Pathol. Res. Pract. 2019, 215, 683–688. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Yang, G.; Chen, X.; Zhang, Y.; Cao, G.; Wang, J.; Sun, Y.; Zhang, P.; Fan, M.; et al. Transforming Growth Factor-Beta-Regulated Mir-24 Promotes Skeletal Muscle Differentiation. Nucleic Acids Res. 2008, 36, 2690–2699. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusama, K.; Ideta, A.; Kimura, K.; Hori, M.; Imakawa, K. Effects of miR-98 in Intrauterine Extracellular Vesicles on Maternal Immune Regulation during the Peri-Implantation Period in Cattle. Sci. Rep. 2019, 9, 20330. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, S.; Yang, J.; Liu, J.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Matrine Alleviates Staphylococcus Aureus Lipoteichoic Acid-Induced Endometritis via Suppression of TLR2-Mediated NF-κB Activation. Int. Immunopharmacol. 2019, 70, 201–207. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladejo, A.O.; Li, Y.; Shen, W.; Imam, B.H.; Wu, X.; Yang, J.; Ma, X.; Lv, Y.; Jiang, W.; Ding, X.; et al. MicroRNA Bta-miR-24-3p Suppressed Galectin-9 Expression through TLR4/NF-ĸB Signaling Pathway in LPS-Stimulated Bovine Endometrial Epithelial Cells. Cells 2021, 10, 3299. https://doi.org/10.3390/cells10123299
Oladejo AO, Li Y, Shen W, Imam BH, Wu X, Yang J, Ma X, Lv Y, Jiang W, Ding X, et al. MicroRNA Bta-miR-24-3p Suppressed Galectin-9 Expression through TLR4/NF-ĸB Signaling Pathway in LPS-Stimulated Bovine Endometrial Epithelial Cells. Cells. 2021; 10(12):3299. https://doi.org/10.3390/cells10123299
Chicago/Turabian StyleOladejo, Ayodele Olaolu, Yajuan Li, Wenxiang Shen, Bereket Habte Imam, Xiaohu Wu, Jie Yang, Xiaoyu Ma, Yanan Lv, Wei Jiang, Xuezhi Ding, and et al. 2021. "MicroRNA Bta-miR-24-3p Suppressed Galectin-9 Expression through TLR4/NF-ĸB Signaling Pathway in LPS-Stimulated Bovine Endometrial Epithelial Cells" Cells 10, no. 12: 3299. https://doi.org/10.3390/cells10123299
APA StyleOladejo, A. O., Li, Y., Shen, W., Imam, B. H., Wu, X., Yang, J., Ma, X., Lv, Y., Jiang, W., Ding, X., Wang, S., & Yan, Z. (2021). MicroRNA Bta-miR-24-3p Suppressed Galectin-9 Expression through TLR4/NF-ĸB Signaling Pathway in LPS-Stimulated Bovine Endometrial Epithelial Cells. Cells, 10(12), 3299. https://doi.org/10.3390/cells10123299





