Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism
Abstract
1. Introduction
2. Caveats and Challenges Relevant to the Study of the GH-Mediated Regulation of (Hepatocyte) Metabolism
2.1. Metabolic Actions of GH/GHR Can Be Mediated by Multiple Intracellular Signaling Pathways
2.2. Limitations of In Vitro Models and Choice of Ligand Used
2.3. Challenges Related to In Vivo Approaches (Species, Exogenous/Endogenous Pattern of GH Delivery, Sex, and Metabolic Context)
2.4. Power and Limitations of Genetically Engineered Mouse Models
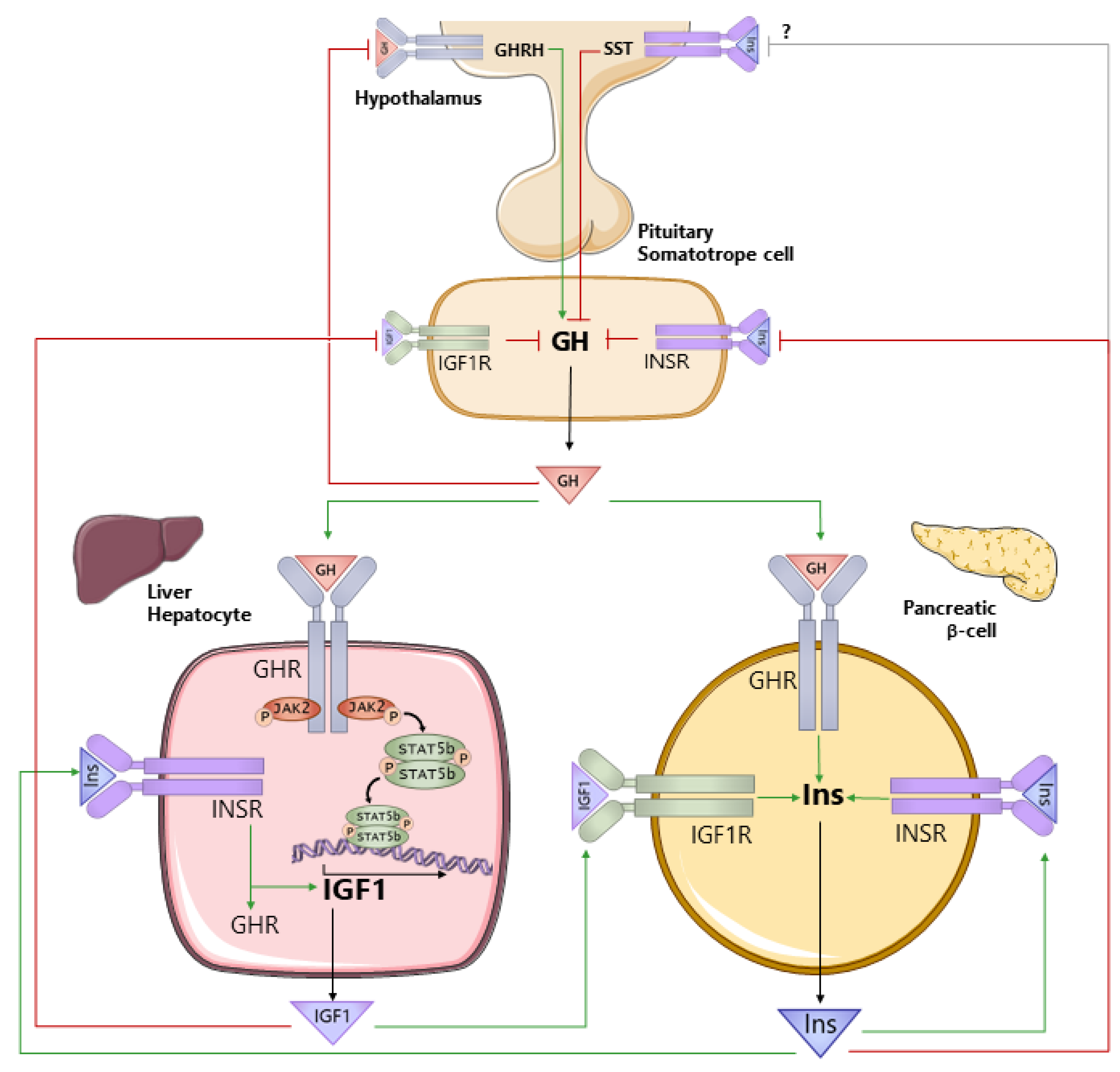
3. Understanding the Interconnection between GH, IGF1, and Insulin Is Critical to Understand How GH Impacts Hepatocyte Metabolism
3.1. Tissue-Specific Metabolic Effects of Insulin and IGF1—Common and Divergent
3.2. IGF1 and Insulin Regulate GH Production and Vice Versa
3.3. GH Antagonizes the Actions of Insulin in a Tissue- and Context-Specific Manner
4. Circulating GH Levels Are Dependent on Age, Time of Day, and Nutritional Status
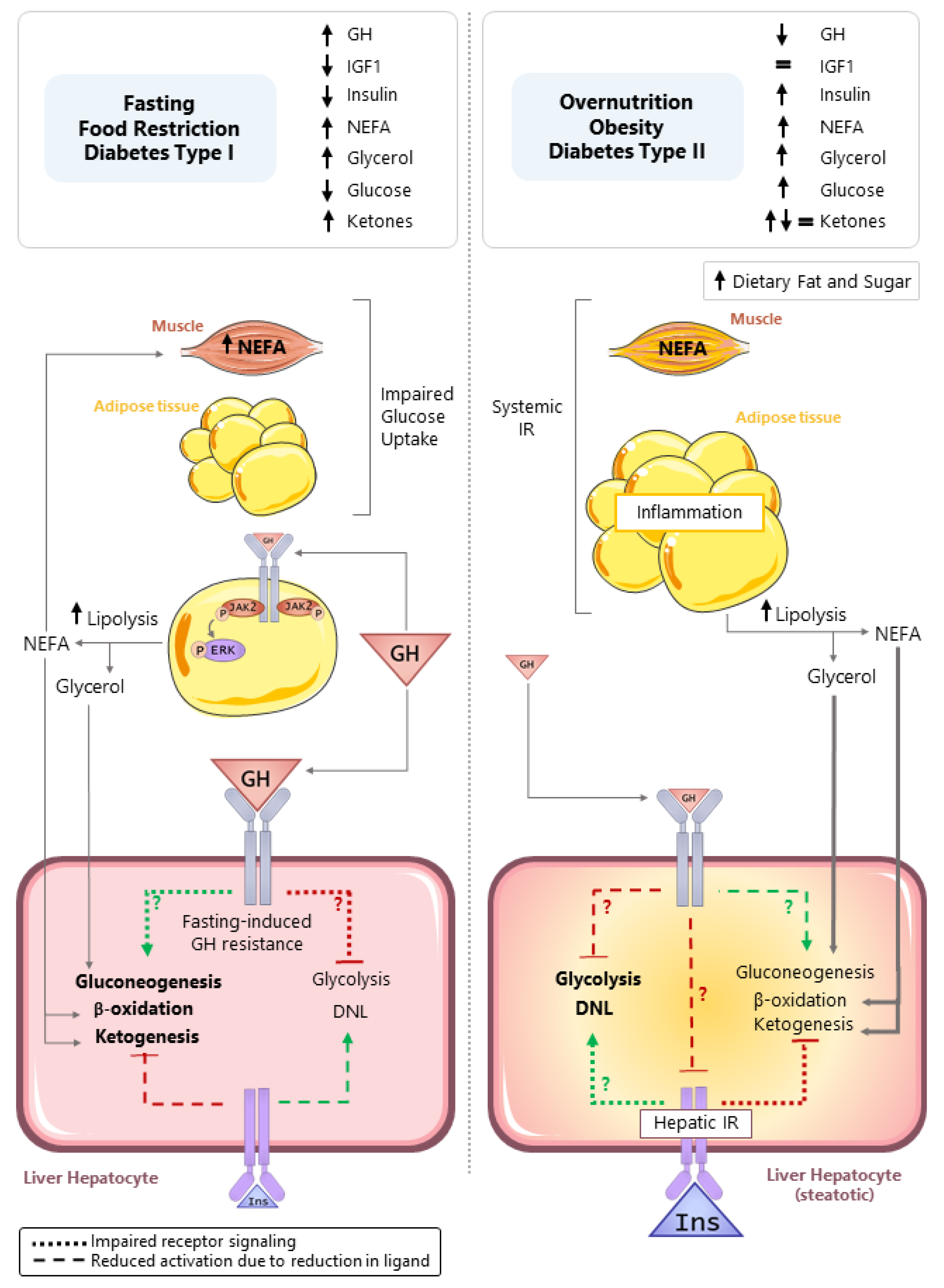
5. Role of GH in Regulating Hepatocyte Metabolism in Catabolic States
5.1. Fasting-Induced Hepatic GH Resistance
5.2. Does GH Directly Regulate Hepatocyte Glycogenolysis, Gluconeogenesis, Ketogenesis, or Fatty Acid Oxidation?
5.2.1. HGP (Glycogenolysis and Gluconeogenesis)
5.2.2. Ketogenesis
5.2.3. Fatty Acid Oxidation
6. Role of GH in Regulating Hepatocyte Metabolism in States of Nutrient Excess
6.1. Associations between Alterations in GH Production and Signaling and Metabolic Disease
6.2. Indirect vs. Direct Effects of GH on Steatosis
6.3. Potential Mechanisms by Which GH Directly Regulates Hepatocyte Lipid Accumulation
7. Past and Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef]
- Frank, S.J. Classical and novel GH receptor signaling pathways. Mol. Cell Endocrinol. 2020, 518, 110999. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Lee, C.M.M.; Muller, A.F.; Brooks, A.J. GHR signalling: Receptor activation and degradation mechanisms. Mol. Cell Endocrinol. 2021, 520, 111075. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.M.; Kopchick, J.J. Nuclear localization of growth hormone receptor: Another age of discovery for cytokine action? Sci. STKE 2007, 2007, pe69. [Google Scholar] [CrossRef] [PubMed]
- Guillouzo, A. Liver cell models in in vitro toxicology. Environ. Health Perspect. 1998, 106 (Suppl. S2), 511–532. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, G.; Su, X.; Jin, C.; Yu, B.; Yu, X.; Lv, Z.; Ma, H.; Zhang, M.; Wei, W.; et al. Maintenance of Primary Hepatocyte Functions In Vitro by Inhibiting Mechanical Tension-Induced YAP Activation. Cell Rep. 2019, 29, 3212–3222. [Google Scholar] [CrossRef]
- Crabb, D.W.; Roepke, J. Loss of growth hormone-dependent characteristics of rat hepatocytes in culture in vitro. Cell Dev. Biol. 1987, 23, 303–307. [Google Scholar] [CrossRef]
- Harrison, S.P.; Baumgarten, S.F.; Verma, R.; Lunov, O.; Dejneka, A.; Sullivan, G.J. Liver Organoids: Recent Developments, Limitations and Potential. Front. Med. 2021, 8, 574047. [Google Scholar] [CrossRef]
- Baumann, G. Growth without a pituitary?—Lessons from the guinea pig. Endocrinology 1997, 138, 3575–3576. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, L.; Curto, L.M.; Miquet, J.G.; Bartke, A.; Turyn, D.; Sotelo, A.I. Differential regulation of membrane associated-growth hormone binding protein (MA-GHBP) and growth hormone receptor (GHR) expression by growth hormone (GH) in mouse liver. Growth Horm. IGF Res. 2007, 17, 104–112. [Google Scholar] [CrossRef]
- Bartke, A.; Kopchick, J.J. The forgotten lactogenic activity of growth hormone: Important implications for rodent studies. Endocrinology 2015, 156, 1620–1622. [Google Scholar] [CrossRef]
- Lopez-Vicchi, F.; De Winne, C.; Brie, B.; Sorianello, E.; Ladyman, S.R.; Becu-Villalobos, D. Metabolic functions of prolactin: Physiological and pathological aspects. J. Neuroendocrinol. 2020, 32, e12888. [Google Scholar] [CrossRef]
- Nagano, M.; Kelly, P.A. Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J. Biol. Chem. 1994, 269, 13337–13345. [Google Scholar] [CrossRef]
- Jahn, G.A.; Daniel, N.; Jolivet, G.; Belair, L.; Bole-Feysot, C.; Kelly, P.A.; Djiane, J. In vivo study of prolactin (PRL) intracellular signalling during lactogenesis in the rat: JAK/STAT pathway is activated by PRL in the mammary gland but not in the liver. Biol. Reprod. 1997, 57, 894–900. [Google Scholar] [CrossRef]
- Le Stunff, C.; Gronowski, A.M.; Rotwein, P. Contrasting acute in vivo nuclear actions of growth hormone and prolactin. Mol. Cell Endocrinol. 1996, 121, 109–117. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hogild, M.L.; Bak, A.M.; Pedersen, S.B.; Rungby, J.; Frystyk, J.; Moller, N.; Jessen, N.; Jorgensen, J.O.L. Growth hormone signaling and action in obese versus lean human subjects. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E333–E344. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Otero-Corchon, V.; Hammond, G.L.; Veldhuis, J.D.; Qi, N.; Low, M.J. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology 2015, 156, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Steyn, F.J.; Huang, L.; Ngo, S.T.; Leong, J.W.; Tan, H.Y.; Xie, T.Y.; Parlow, A.F.; Veldhuis, J.D.; Waters, M.J.; Chen, C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 2011, 152, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Steyn, F.J.; Leong, J.W.; Huang, L.; Tan, H.Y.; Xie, T.Y.; Nelson, C.; Waters, M.J.; Veldhuis, J.D.; Epelbaum, J.; Chen, C. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology 2012, 153, 273–282. [Google Scholar] [CrossRef]
- Steyn, F.J.; Xie, T.Y.; Huang, L.; Ngo, S.T.; Veldhuis, J.D.; Waters, M.J.; Chen, C. Increased adiposity and insulin correlates with the progressive suppression of pulsatile GH secretion during weight gain. J. Endocrinol. 2013, 218, 233–244. [Google Scholar] [CrossRef]
- Giustina, A.; Veldhuis, J.D. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, G.S.; Choi, H.K.; Gurd, W.; Waxman, D.J. Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology 2001, 142, 4599–4606. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Bekaert, A.J.; Dupont, J.; Rouve, S.; Annesi-Maesano, I.; De Magalhaes Filho, C.D.; Kappeler, L.; Holzenberger, M. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J. Endocrinol. 2011, 208, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; Veldhuis, J.D. Growth Hormone Dynamics in Healthy Adults Are Related to Age and Sex and Strongly Dependent on Body Mass Index. Neuroendocrinology 2016, 103, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; O’Connor, C. Growth hormone regulation of sex-dependent liver gene expression. Mol. Endocrinol. 2006, 20, 2613–2629. [Google Scholar] [CrossRef] [PubMed]
- Connerney, J.; Lau-Corona, D.; Rampersaud, A.; Waxman, D.J. Activation of Male Liver Chromatin Accessibility and STAT5-Dependent Gene Transcription by Plasma Growth Hormone Pulses. Endocrinology 2017, 158, 1386–1405. [Google Scholar] [CrossRef]
- Zhang, Y.; Laz, E.V.; Waxman, D.J. Dynamic, sex-differential STAT5 and BCL6 binding to sex-biased, growth hormone-regulated genes in adult mouse liver. Mol. Cell Biol. 2012, 32, 880–896. [Google Scholar] [CrossRef]
- Meinhardt, U.J.; Ho, K.K. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. 2006, 65, 413–422. [Google Scholar] [CrossRef]
- Sidhom, S.; Schneider, A.; Fang, Y.; McFadden, S.; Darcy, J.; Sathiaseelan, R.; Palmer, A.K.; Steyn, F.J.; Grillari, J.; Kopchick, J.J.; et al. 17alpha-Estradiol Modulates IGF1 and Hepatic Gene Expression in a Sex-Specific Manner. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, M.; Pincelli, A.I.; Cavagnini, F. Growth hormone in obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Kineman, R.D. Impact of obesity on the growth hormone axis: Evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 2006, 147, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Hjelholt, A.; Hogild, M.; Bak, A.M.; Arlien-Soborg, M.C.; Baek, A.; Jessen, N.; Richelsen, B.; Pedersen, S.B.; Moller, N.; Lunde Jorgensen, J.O. Growth Hormone and Obesity. Endocrinol. Metab. Clin. N. Am. 2020, 49, 239–250. [Google Scholar] [CrossRef]
- Ho, K.Y.; Veldhuis, J.D.; Johnson, M.L.; Furlanetto, R.; Evans, W.S.; Alberti, K.G.; Thorner, M.O. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Investig. 1988, 81, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Park, S.; Kineman, R.D. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: Potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology 2007, 148, 300–309. [Google Scholar] [CrossRef]
- Gahete, M.D.; Cordoba-Chacon, J.; Luque, R.M.; Kineman, R.D. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology 2013, 154, 263–269. [Google Scholar] [CrossRef][Green Version]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef]
- Asplin, C.M.; Faria, A.C.; Carlsen, E.C.; Vaccaro, V.A.; Barr, R.E.; Iranmanesh, A.; Lee, M.M.; Veldhuis, J.D.; Evans, W.S. Alterations in the pulsatile mode of growth hormone release in men and women with insulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1989, 69, 239–245. [Google Scholar] [CrossRef]
- Flyvbjerg, A.; Bennett, W.F.; Rasch, R.; Kopchick, J.J.; Scarlett, J.A. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes 1999, 48, 377–382. [Google Scholar] [CrossRef]
- Muller, E.E.; Miedico, D.; Giustina, G.; Cocchi, D. Ineffectiveness of hypoglycemia, cold exposure and fasting in stimulating GH secretion in the mouse. Endocrinology 1971, 88, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Steyn, F.J.; Ngo, S.T. Endocrine rhythms of growth hormone release: Insights from animal studies. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, G.S.; Rorstad, O.; Brazeau, P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology 1979, 104, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.L.; Kiersgaard, M.K.; Sorensen, D.B.; Mikkelsen, L.F. Fasting of mice: A review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Lo Martire, V.; Valli, A.; Bingaman, M.J.; Zoccoli, G.; Silvani, A.; Swoap, S.J. Changes in blood glucose as a function of body temperature in laboratory mice: Implications for daily torpor. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E662–E670. [Google Scholar] [CrossRef]
- Ravussin, Y.; LeDuc, C.A.; Watanabe, K.; Leibel, R.L. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R438–R448. [Google Scholar] [CrossRef]
- Bastias-Perez, M.; Zagmutt, S.; Soler-Vazquez, M.C.; Serra, D.; Mera, P.; Herrero, L. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells 2020, 9, 316. [Google Scholar] [CrossRef]
- Bartke, A.; Westbrook, R. Metabolic characteristics of long-lived mice. Front. Genet. 2012, 3, 288. [Google Scholar] [CrossRef]
- Braxton, T.M.; Sarpong, D.E.; Dovey, J.L.; Guillou, A.; Evans, B.; Castellano, J.M.; Keenan, B.E.; Baraghithy, S.; Evans, S.L.; Tena-Sempere, M.; et al. Thermoneutrality improves skeletal impairment in adult Prader-Willi syndrome mice. J. Endocrinol. 2019, 243, 175–186. [Google Scholar] [CrossRef]
- Richard, A.J.; Hang, H.; Allerton, T.D.; Zhao, P.; Ghosh, S.; Elks, C.M.; Stephens, J.M. Loss of STAT5 in adipocytes increases subcutaneous fat mass via sex-dependent and depot-specific pathways. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Bellush, L.L.; Coschigano, K.T. Transgenic models of growth hormone action. Annu. Rev. Nutr. 1999, 19, 437–461. [Google Scholar] [CrossRef]
- Young, J.; Bell, S.; Qian, Y.; Hyman, C.; Berryman, D.E. Mouse models of growth hormone insensitivity. Rev. Endocr. Metab. Disord. 2021, 22, 17–29. [Google Scholar] [CrossRef]
- List, E.O.; Duran-Ortiz, S.; Kopchick, J.J. Effects of tissue-specific GH receptor knockouts in mice. Mol. Cell Endocrinol. 2020, 515, 110919. [Google Scholar] [CrossRef]
- Kaltenecker, D.; Themanns, M.; Mueller, K.M.; Spirk, K.; Suske, T.; Merkel, O.; Kenner, L.; Luis, A.; Kozlov, A.; Haybaeck, J.; et al. Hepatic growth hormone—JAK2–STAT5 signalling: Metabolic function, non-alcoholic fatty liver disease and hepatocellular carcinoma progression. Cytokine 2019, 124, 154569. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; Duran-Ortiz, S.; Suer, O.; Sustarsic, E.G.; Berryman, D.E.; List, E.O.; Kopchick, J.J. Disruption of the GH Receptor Gene in Adult Mice Increases Maximal Lifespan in Females. Endocrinology 2016, 157, 4502–4513. [Google Scholar] [CrossRef] [PubMed]
- Duran-Ortiz, S.; Bell, S.; Kopchick, J.J. Standardizing protocols dealing with growth hormone receptor gene disruption in mice using the Cre-lox system. Growth Horm. IGF Res. 2018, 42–43, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Duran-Ortiz, S.; Corbin, K.L.; Jahan, I.; Whitticar, N.B.; Morris, S.E.; Bartholomew, A.N.; Slepchenko, K.G.; West, H.L.; Max Harry, I.M.; List, E.O.; et al. Loss of growth hormone signaling in the mouse germline or in adulthood reduces islet mass and alters islet function with notable sex differences. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1158–E1172. [Google Scholar] [CrossRef] [PubMed]
- Kiourtis, C.; Wilczynska, A.; Nixon, C.; Clark, W.; May, S.; Bird, T.G. Genetic manipulation using hepatocyte-targeting adeno-associated viral vectors has minimal off-target effects. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cordoba-Chacon, J.; Majumdar, N.; List, E.O.; Diaz-Ruiz, A.; Frank, S.J.; Manzano, A.; Bartrons, R.; Puchowicz, M.; Kopchick, J.J.; Kineman, R.D. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes 2015, 64, 3093–3103. [Google Scholar] [CrossRef] [PubMed]
- Kineman, R.; Majumdar, N.; Subbaiah, P.V.; Cordoba-Chacon, J. Hepatic PPARγ is not essential for the rapid development of steatosis following loss of hepatic GH signaling, in adult male mice. Endocrinology 2016, 157, 1728–1735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vazquez-Borrego, M.C.; Kineman, R.D. Meeting abstracts from the 2020 International Meeting on GH/IGF: Actions in the shadow of COVID19 (T05). Pituitary 2020, 23, 2–35. [Google Scholar] [CrossRef]
- Sarmento-Cabral, A.; Del Rio-Moreno, M.; Vazquez-Borrego, M.C.; Mahmood, M.; Gutierrez-Casado, E.; Pelke, N.; Guzman, G.; Subbaiah, P.V.; Cordoba-Chacon, J.; Yakar, S.; et al. GH directly inhibits steatosis and liver injury in a sex-dependent and IGF1-independent manner. J. Endocrinol. 2021, 248, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Deyle, D.R.; Russell, D.W. Adeno-associated virus vector integration. Curr. Opin. Mol. Ther. 2009, 11, 442–447. [Google Scholar] [PubMed]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Slaaby, R.; Schaffer, L.; Lautrup-Larsen, I.; Andersen, A.S.; Shaw, A.C.; Mathiasen, I.S.; Brandt, J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 2006, 281, 25869–25874. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef]
- Kineman, R.D.; Del Rio-Moreno, M.; Sarmento-Cabral, A. 40 YEARS of IGF1: Understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Sun, H.; Zhao, H.; Pennisi, P.; Toyoshima, Y.; Setser, J.; Stannard, B.; Scavo, L.; Leroith, D. Metabolic effects of IGF-I deficiency: Lessons from mouse models. Pediatr. Endocrinol. Rev. 2005, 3, 11–19. [Google Scholar]
- Boucher, J.; Softic, S.; El Ouaamari, A.; Krumpoch, M.T.; Kleinridders, A.; Kulkarni, R.N.; O’Neill, B.T.; Kahn, C.R. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 2016, 65, 2201–2213. [Google Scholar] [CrossRef]
- Softic, S.; Boucher, J.; Solheim, M.H.; Fujisaka, S.; Haering, M.F.; Homan, E.P.; Winnay, J.; Perez-Atayde, A.R.; Kahn, C.R. Lipodystrophy Due to Adipose Tissue-Specific Insulin Receptor Knockout Results in Progressive NAFLD. Diabetes 2016, 65, 2187–2200. [Google Scholar] [CrossRef]
- Jonsson, C.; Castor Batista, A.P.; Kjolhede, P.; Stralfors, P. Insulin and beta-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochem. J. 2019, 476, 2883–2908. [Google Scholar] [CrossRef]
- Mavalli, M.D.; DiGirolamo, D.J.; Fan, Y.; Riddle, R.C.; Campbell, K.S.; van Groen, T.; Frank, S.J.; Sperling, M.A.; Esser, K.A.; Bamman, M.M.; et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Investig. 2010, 120, 4007–4020. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Lauritzen, H.P.; Hirshman, M.F.; Smyth, G.; Goodyear, L.J.; Kahn, C.R. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Rep. 2015, 11, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Waraky, A.; Aleem, E.; Larsson, O. Downregulation of IGF-1 receptor occurs after hepatic linage commitment during hepatocyte differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2016, 478, 1575–1581. [Google Scholar] [CrossRef]
- The Human Protein Atlas—GHR. Available online: https://www.proteinatlas.org/ENSG00000112964-GHR/tissue (accessed on 20 September 2021).
- The Human Protein Atlas—IGF1. Available online: https://www.proteinatlas.org/ENSG00000017427-IGF1/tissue (accessed on 20 September 2021).
- Yakar, S.; Liu, J.L.; Stannard, B.; Butler, A.; Accili, D.; Sauer, B.; LeRoith, D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 1999, 96, 7324–7329. [Google Scholar] [CrossRef] [PubMed]
- Boisclair, Y.R.; Rhoads, R.P.; Ueki, I.; Wang, J.; Ooi, G.T. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: An important but forgotten component of the circulating IGF system. J. Endocrinol. 2001, 170, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J. Mol. Endocrinol. 2018, 61, T139–T169. [Google Scholar] [CrossRef]
- Wasinski, F.; Pedroso, J.A.B.; Dos Santos, W.O.; Furigo, I.C.; Garcia-Galiano, D.; Elias, C.F.; List, E.O.; Kopchick, J.J.; Szawka, R.E.; Donato, J., Jr. Tyrosine Hydroxylase Neurons Regulate Growth Hormone Secretion via Short-Loop Negative Feedback. J. Neurosci. 2020, 40, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Harel, Z.; Tannenbaum, G.S. Synergistic interaction between insulin-like growth factors-I and -II in central regulation of pulsatile growth hormone secretion. Endocrinology 1992, 131, 758–764. [Google Scholar] [CrossRef]
- Fletcher, T.P.; Thomas, G.B.; Dunshea, F.R.; Moore, L.G.; Clarke, I.J. IGF feedback effects on growth hormone secretion in ewes: Evidence for action at the pituitary but not the hypothalamic level. J. Endocrinol. 1995, 144, 323–331. [Google Scholar] [CrossRef]
- Wallenius, K.; Sjogren, K.; Peng, X.D.; Park, S.; Wallenius, V.; Liu, J.L.; Umaerus, M.; Wennbo, H.; Isaksson, O.; Frohman, L.; et al. Liver-derived IGF-I regulates GH secretion at the pituitary level in mice. Endocrinology 2001, 142, 4762–4770. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Frohman, L.A. Differential effects of central and peripheral administration of growth hormone (GH) and insulin-like growth factor on hypothalamic GH-releasing hormone and somatostatin gene expression in GH-deficient dwarf rats. Endocrinology 1993, 133, 793–799. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Aloqaily, B.; Negron, A.; Wondisford, F.; Radovick, S. Interrupted IGF-1 Feedback in GHRH Neurons and Somatotrophs Results in Impaired Weight Gain and Increased Energy Expenditure. J. Endocr. Soc. 2021, 5, A52. [Google Scholar] [CrossRef]
- Melmed, S.; Yamashita, S.; Yamasaki, H.; Fagin, J.; Namba, H.; Yamamoto, H.; Weber, M.; Morita, S.; Webster, J.; Prager, D. IGF-I receptor signalling: Lessons from the somatotroph. Recent Prog. Horm. Res. 1996, 51, 189–215. [Google Scholar]
- Gahete, M.D.; Cordoba-Chacon, J.; Lin, Q.; Bruning, J.C.; Kahn, C.R.; Castano, J.P.; Christian, H.; Luque, R.M.; Kineman, R.D. Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology 2013, 154, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.J.; Ng, Y.; Luque, R.M.; Kineman, R.D.; Koch, L.; Bruning, J.C.; Radovick, S. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol. Endocrinol. 2010, 24, 1077–1089. [Google Scholar] [CrossRef]
- Rhodes, C.J. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J. Mol. Endocrinol. 2000, 24, 303–311. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Liu, C.; Sun, H.; Vijayakumar, A.; Giglou, P.R.; Qiao, R.; Oppenheimer, J.; Yakar, S.; LeRoith, D. Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J. Clin. Investig. 2011, 121, 2422–2426. [Google Scholar] [CrossRef]
- Brouwers, B.; de Faudeur, G.; Osipovich, A.B.; Goyvaerts, L.; Lemaire, K.; Boesmans, L.; Cauwelier, E.J.; Granvik, M.; Pruniau, V.P.; Van Lommel, L.; et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 2014, 20, 979–990. [Google Scholar] [CrossRef]
- Cordoba-Chacon, J.; Gahete, M.D.; Pokala, N.K.; Geldermann, D.; Alba, M.; Salvatori, R.; Luque, R.M.; Kineman, R.D. Long- but not short-term adult-onset, isolated GH deficiency in male mice leads to deterioration of beta-cell function, which cannot be accounted for by changes in beta-cell mass. Endocrinology 2014, 155, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Scheinman, E.J.; Damouni, R.; Caspi, A.; Shen-Orr, Z.; Tiosano, D.; LeRoith, D. The beneficial effect of growth hormone treatment on islet mass in streptozotocin-treated mice. Diabetes Metab. Res. Rev. 2015, 31, 492–499. [Google Scholar] [CrossRef]
- Cordoba-Chacon, J.; Majumdar, N.; Pokala, N.K.; Gahete, M.D.; Kineman, R.D. Islet insulin content and release are increased in male mice with elevated endogenous GH and IGF-I, without evidence of systemic insulin resistance or alterations in beta-cell mass. Growth Horm. IGF Res. 2015, 25, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Holzenberger, M.; Shih, D.Q.; Ozcan, U.; Stoffel, M.; Magnuson, M.A.; Kahn, C.R. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 2002, 31, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Kitamura, T.; Nakae, J.; Politi, K.; Kido, Y.; Fisher, P.E.; Morroni, M.; Cinti, S.; White, M.F.; Herrera, P.L.; et al. Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J. Clin. Investig. 2002, 110, 1011–1019. [Google Scholar] [CrossRef]
- Ma, F.; Wei, Z.; Shi, C.; Gan, Y.; Lu, J.; Frank, S.J.; Balducci, J.; Huang, Y. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet beta-cells. Mol. Endocrinol. 2011, 25, 2119–2133. [Google Scholar] [CrossRef]
- Phillips, L.S.; Pao, C.I.; Villafuerte, B.C. Molecular regulation of insulin-like growth factor-I and its principal binding protein, IGFBP-3. Prog. Nucleic Acid Res. Mol. Biol. 1998, 60, 195–265. [Google Scholar] [PubMed]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef]
- Melmed, S. Insulin suppresses growth hormone secretion by rat pituitary cells. J. Clin. Investig. 1984, 73, 1425–1433. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Cho, J.H.; Bae, J.Y.; O’Leary, T.P.; Johnson, J.D.; Bae, Y.C.; Kim, E.K. Insulin synthesized in the paraventricular nucleus of the hypothalamus regulates pituitary growth hormone production. JCI Insight 2020, 5, e135412. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef] [PubMed]
- List, E.O.; Berryman, D.E.; Buchman, M.; Parker, C.; Funk, K.; Bell, S.; Duran-Ortiz, S.; Qian, Y.; Young, J.A.; Wilson, C.; et al. Adipocyte-Specific GH Receptor-Null (AdGHRKO) Mice Have Enhanced Insulin Sensitivity with Reduced Liver Triglycerides. Endocrinology 2019, 160, 68–80. [Google Scholar] [CrossRef]
- Kaltenecker, D.; Mueller, K.M.; Benedikt, P.; Feiler, U.; Themanns, M.; Schlederer, M.; Kenner, L.; Schweiger, M.; Haemmerle, G.; Moriggl, R. Adipocyte STAT5 deficiency promotes adiposity and impairs lipid mobilisation in mice. Diabetologia 2017, 60, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.M.; Vestergaard, E.T.; Jessen, N.; Kolind-Thomsen, P.; Nellemann, B.; Nielsen, T.S.; Vendelbo, M.H.; Moller, N.; Sharma, R.; Lee, K.Y.; et al. Growth hormone acts along the PPARgamma-FSP27 axis to stimulate lipolysis in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E34–E42. [Google Scholar] [CrossRef]
- Sharma, R.; Luong, Q.; Sharma, V.M.; Harberson, M.; Harper, B.; Colborn, A.; Berryman, D.E.; Jessen, N.; Jorgensen, J.O.L.; Kopchick, J.J.; et al. Growth hormone controls lipolysis by regulation of FSP27 expression. J. Endocrinol. 2018, 239, 289–301. [Google Scholar] [CrossRef]
- Hjelholt, A.J.; Lee, K.Y.; Arlien-Soborg, M.C.; Pedersen, S.B.; Kopchick, J.J.; Puri, V.; Jessen, N.; Jorgensen, J.O.L. Temporal patterns of lipolytic regulators in adipose tissue after acute growth hormone exposure in human subjects: A randomized controlled crossover trial. Mol. Metab. 2019, 29, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nellemann, B.; Vendelbo, M.H.; Nielsen, T.S.; Bak, A.M.; Hogild, M.; Pedersen, S.B.; Bienso, R.S.; Pilegaard, H.; Moller, N.; Jessen, N.; et al. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol. 2014, 210, 392–402. [Google Scholar] [CrossRef]
- Hjelholt, A.J.; Charidemou, E.; Griffin, J.L.; Pedersen, S.B.; Gudiksen, A.; Pilegaard, H.; Jessen, N.; Moller, N.; Jorgensen, J.O.L. Insulin resistance induced by growth hormone is linked to lipolysis and associated with suppressed pyruvate dehydrogenase activity in skeletal muscle: A 2 × 2 factorial, randomised, crossover study in human individuals. Diabetologia 2020, 63, 2641–2653. [Google Scholar] [CrossRef]
- Zhang, F.; Icyuz, M.; Liu, Z.; Fitch, M.; Sun, L.Y. Insulin sensitivity in long-lived growth hormone-releasing hormone knockout mice. Aging 2020, 12, 18033–18051. [Google Scholar] [CrossRef]
- Chhabra, Y.; Nelson, C.N.; Plescher, M.; Barclay, J.L.; Smith, A.G.; Andrikopoulos, S.; Mangiafico, S.; Waxman, D.J.; Brooks, A.J.; Waters, M.J. Loss of growth hormone-mediated signal transducer and activator of transcription 5 (STAT5) signaling in mice results in insulin sensitivity with obesity. FASEB J. 2019, 33, 6412–6430. [Google Scholar] [CrossRef] [PubMed]
- Cordoba-Chacon, J.; Gahete, M.D.; McGuinness, O.P.; Kineman, R.D. Differential impact of selective GH deficiency and endogenous GH excess on insulin-mediated actions in muscle and liver of male mice. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E928–E934. [Google Scholar] [CrossRef] [PubMed]
- Haluzik, M.; Yakar, S.; Gavrilova, O.; Setser, J.; Boisclair, Y.; LeRoith, D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: Relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes 2003, 52, 2483–2489. [Google Scholar] [CrossRef]
- Yakar, S.; Setser, J.; Zhao, H.; Stannard, B.; Haluzik, M.; Glatt, V.; Bouxsein, M.L.; Kopchick, J.J.; LeRoith, D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J. Clin. Investig. 2004, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Corbit, K.C.; Camporez, J.P.G.; Edmunds, L.R.; Tran, J.L.; Vera, N.B.; Erion, D.M.; Deo, R.C.; Perry, R.J.; Shulman, G.I.; Jurczak, M.J.; et al. Adipocyte JAK2 Regulates Hepatic Insulin Sensitivity Independently of Body Composition, Liver Lipid Content, and Hepatic Insulin Signaling. Diabetes 2018, 67, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Messina, J.L. Crosstalk between growth hormone and insulin signaling. Vitam. Horm. 2009, 80, 125–153. [Google Scholar] [PubMed]
- Cho, Y.; Ariga, M.; Uchijima, Y.; Kimura, K.; Rho, J.Y.; Furuhata, Y.; Hakuno, F.; Yamanouchi, K.; Nishihara, M.; Takahashi, S. The novel roles of liver for compensation of insulin resistance in human growth hormone transgenic rats. Endocrinology 2006, 147, 5374–5384. [Google Scholar] [CrossRef]
- Jiang, Q.; Bai, J.; He, M.; Yuen, K.W.Y.; Wong, A.O.L. Mechanisms Underlying the Synergistic Action of Insulin and Growth Hormone on IGF-I and -II Expression in Grass Carp Hepatocytes. Front. Endocrinol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, F.; Zhang, Q.; Liu, B.; Guo, Y.; Lv, Z.; Xia, T.; Chen, S.; Li, K.; Du, Y.; et al. PRLR regulates hepatic insulin sensitivity in mice via STAT5. Diabetes 2013, 62, 3103–3113. [Google Scholar] [CrossRef]
- Corpas, E.; Harman, S.M.; Blackman, M.R. Human growth hormone and human aging. Endocr. Rev. 1993, 14, 20–39. [Google Scholar] [CrossRef]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Mens Health 2019, 37, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Bassant, M.H.; Zizzari, P.; Poindessous-Jazat, F.; Tomasetto, C.; Epelbaum, J.; Bluet-Pajot, M.T. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology 2002, 143, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Iwamoto, A.; Otsuka, T.; Hishida, Y.; Akiduki, S.; Aoki, M.; Furuse, M.; Yasuo, S. Effects of time of L-ornithine administration on the diurnal rhythms of plasma growth hormone, melatonin, and corticosterone levels in mice. Chronobiol. Int. 2015, 32, 225–234. [Google Scholar] [CrossRef]
- Heuck, C.; Skjaerbaek, C.; Orskov, H.; Wolthers, O.D. Circadian variation in serum free ultrafiltrable insulin-like growth factor I concentrations in healthy children. Pediatr. Res. 1999, 45, 733–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahmani, J.; Kord Varkaneh, H.; Clark, C.; Zand, H.; Bawadi, H.; Ryan, P.M.; Fatahi, S.; Zhang, Y. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 53, 100910. [Google Scholar] [CrossRef] [PubMed]
- Peris-Sampedro, F.; Stoltenborg, I.; Le May, M.V.; Zigman, J.M.; Adan, R.A.H.; Dickson, S.L. Genetic deletion of the ghrelin receptor (GHSR) impairs growth and blunts endocrine response to fasting in Ghsr-IRES-Cre mice. Mol. Metab. 2021, 51, 101223. [Google Scholar] [CrossRef]
- Gupta, D.; Patterson, A.M.; Osborne-Lawrence, S.; Bookout, A.L.; Varshney, S.; Shankar, K.; Singh, O.; Metzger, N.P.; Richard, C.P.; Wyler, S.C.; et al. Disrupting the ghrelin-growth hormone axis limits ghrelin’s orexigenic but not glucoregulatory actions. Mol. Metab. 2021, 53, 101258. [Google Scholar] [CrossRef]
- Cornford, A.S.; Barkan, A.L.; Horowitz, J.F. Rapid suppression of growth hormone concentration by overeating: Potential mediation by hyperinsulinemia. J. Clin. Endocrinol. Metab. 2011, 96, 824–830. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Yuen, K.C.; Bredella, M.A.; Gerweck, A.V.; Russell, B.M.; Riccio, A.D.; Gurel, M.H.; Sluss, P.M.; Biller, B.M.; Miller, K.K. Overweight/Obese adults with pituitary disorders require lower peak growth hormone cutoff values on glucagon stimulation testing to avoid overdiagnosis of growth hormone deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 4712–4719. [Google Scholar] [CrossRef]
- Shalet, S.M.; Toogood, A.; Rahim, A.; Brennan, B.M. The diagnosis of growth hormone deficiency in children and adults. Endocr. Rev. 1998, 19, 203–223. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Misdraji, J.; Bredella, M.A.; Schorr, M.; Osganian, S.A.; Young, B.J.; Sung, J.C.; Miller, K.K. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2017, 8, e217. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Perakakis, N.; Boutari, C.; Kountouras, J.; Ghaly, W.; Anastasilakis, A.D.; Karagiannis, A.; Mantzoros, C.S. Targeted Analysis of Three Hormonal Systems Identifies Molecules Associated with the Presence and Severity of NAFLD. J. Clin. Endocrinol. Metab. 2020, 105, e390–e400. [Google Scholar] [CrossRef]
- Stanley, T.L.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 106, e520–e533. [Google Scholar] [CrossRef]
- Rasmussen, M.H. Obesity, growth hormone and weight loss. Mol. Cell Endocrinol. 2010, 316, 147–153. [Google Scholar] [CrossRef]
- Al-Regaiey, K.; Alshubrami, S.; Al-Beeshi, I.; Alnasser, T.; Alwabel, A.; Al-Beladi, H.; Al-Tujjar, O.; Alnasser, A.; Alfadda, A.A.; Iqbal, M. Effects of gastric sleeve surgery on the serum levels of GH, IGF-1 and IGF-binding protein 2 in healthy obese patients. BMC Gastroenterol. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Gahete, M.D.; Cordoba-Chacon, J.; Childs, G.V.; Kineman, R.D. Does the pituitary somatotrope play a primary role in regulating GH output in metabolic extremes? Ann. N. Y. Acad. Sci. 2011, 1220, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.P.; Goldstein, J.L.; Brown, M.S.; Liang, G. Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice. J. Biol. Chem. 2009, 284, 24644–24652. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Wasinski, F.; Furigo, I.C.; Metzger, M.; Frazao, R. Central Regulation of Metabolism by Growth Hormone. Cells 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.B.M.; Ubah, C.; Debarba, L.K.; Ayyar, I.; Didyuk, O.; Sadagurski, M. Hypothalamic GHR-SIRT1 Axis in Fasting. Cells 2021, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.B.M.; Debarba, L.K.; Rupp, A.C.; Qi, N.; Ubah, C.; Khan, M.; Didyuk, O.; Ayyar, I.; Koch, M.; Sandoval, D.A.; et al. ARC(GHR) Neurons Regulate Muscle Glucose Uptake. Cells 2021, 10, 93. [Google Scholar] [CrossRef]
- Beauloye, V.; Willems, B.; de Coninck, V.; Frank, S.J.; Edery, M.; Thissen, J.P. Impairment of liver GH receptor signaling by fasting. Endocrinology 2002, 143, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gevers, E.F.; Hannah, M.J.; Waters, M.J.; Robinson, I.C. Regulation of rapid signal transducer and activator of transcription-5 phosphorylation in the resting cells of the growth plate and in the liver by growth hormone and feeding. Endocrinology 2009, 150, 3627–3636. [Google Scholar] [CrossRef][Green Version]
- Touvier, T.; Conte-Auriol, F.; Briand, O.; Cudejko, C.; Paumelle, R.; Caron, S.; Bauge, E.; Rouille, Y.; Salles, J.P.; Staels, B.; et al. LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice. J. Clin. Investig. 2009, 119, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Iguchi, G.; Fukuoka, H.; Suda, K.; Bando, H.; Takahashi, M.; Nishizawa, H.; Seino, S.; Takahashi, Y. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proc. Natl. Acad. Sci. USA 2013, 110, 14948–14953. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, L.; Wang, A.; Eleswarapu, S.; Ge, X.; Chen, D.; Jiang, H. Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology 2012, 153, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.M.; Waxman, D.J. Simultaneous, bidirectional inhibitory crosstalk between PPAR and STAT5b. Toxicol. Appl. Pharmacol. 2004, 199, 275–284. [Google Scholar] [CrossRef]
- Ljungberg, A.; Linden, D.; Ameen, C.; Bergstrom, G.; Oscarsson, J. Importance of PPAR alpha for the effects of growth hormone on hepatic lipid and lipoprotein metabolism. Growth Horm. IGF Res. 2007, 17, 154–164. [Google Scholar] [CrossRef]
- Sakharova, A.A.; Horowitz, J.F.; Surya, S.; Goldenberg, N.; Harber, M.P.; Symons, K.; Barkan, A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 2008, 93, 2755–2759. [Google Scholar] [CrossRef]
- Piatti, P.M.; Monti, L.D.; Caumo, A.; Conti, M.; Magni, F.; Galli-Kienle, M.; Fochesato, E.; Pizzini, A.; Baldi, L.; Valsecchi, G.; et al. Mediation of the hepatic effects of growth hormone by its lipolytic activity. J. Clin. Endocrinol. Metab. 1999, 84, 1658–1663. [Google Scholar] [CrossRef]
- Orskov, L.; Schmitz, O.; Jorgensen, J.O.; Arnfred, J.; Abildgaard, N.; Christiansen, J.S.; Alberti, K.G.; Orskov, H. Influence of growth hormone on glucose-induced glucose uptake in normal men as assessed by the hyperglycemic clamp technique. J. Clin. Endocrinol. Metab. 1989, 68, 276–282. [Google Scholar] [CrossRef]
- Ghanaat, F.; Tayek, J.A. Growth hormone administration increases glucose production by preventing the expected decrease in glycogenolysis seen with fasting in healthy volunteers. Metabolism 2005, 54, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hoybye, C.; Chandramouli, V.; Efendic, S.; Hulting, A.L.; Landau, B.R.; Schumann, W.C.; Wajngot, A. Contribution of gluconeogenesis and glycogenolysis to hepatic glucose production in acromegaly before and after pituitary microsurgery. Horm. Metab. Res. 2008, 40, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Karlander, S.; Vranic, M.; Efendic, S. Increased glucose turnover and glucose cycling in acromegalic patients with normal glucose tolerance. Diabetologia 1986, 29, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Jorgensen, J.O.L.; Luger, A.; Stalla, G.K. Insulin Resistance in Patients with Acromegaly. Front. Endocrinol. 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cordoba-Chacon, J.; Kineman, R.D.; Cronstein, B.N.; Muzumdar, R.; Gong, Z.; Werner, H.; Yakar, S. Growth Hormone Control of Hepatic Lipid Metabolism. Diabetes 2016, 65, 3598–3609. [Google Scholar] [CrossRef]
- Liu, J.; Nie, C.; Xue, L.; Yan, Y.; Liu, S.; Sun, J.; Fan, M.; Qian, H.; Ying, H.; Wang, L.; et al. Growth hormone receptor disrupts glucose homeostasis via promoting and stabilizing retinol binding protein 4. Theranostics 2021, 11, 8283–8300. [Google Scholar] [CrossRef]
- Hughey, C.C.; Wasserman, D.H.; Lee-Young, R.S.; Lantier, L. Approach to assessing determinants of glucose homeostasis in the conscious mouse. Mamm. Genome 2014, 25, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Emmison, N.; Agius, L.; Zammit, V.A. Regulation of fatty acid metabolism and gluconeogenesis by growth hormone and insulin in sheep hepatocyte cultures. Effects of lactation and pregnancy. Biochem. J. 1991, 274, 21–26. [Google Scholar] [CrossRef]
- Elias, A.E.; Kun, B.; Sabula, I.M.C.; Golomb-Mello, G.; Cespedes Zablah, A.; Kreiling, J.A. The mir-465 family is upregulated with age and attenuates growth hormone signaling in mouse liver. Aging Cell 2019, 18, e12892. [Google Scholar] [CrossRef]
- Kim, Y.D.; Li, T.; Ahn, S.W.; Kim, D.K.; Lee, J.M.; Hwang, S.L.; Kim, Y.H.; Lee, C.H.; Lee, I.K.; Chiang, J.Y.; et al. Orphan nuclear receptor small heterodimer partner negatively regulates growth hormone-mediated induction of hepatic gluconeogenesis through inhibition of signal transducer and activator of transcription 5 (STAT5) transactivation. J. Biol. Chem. 2012, 287, 37098–37108. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, Y.H.; Tadi, S.; Yu, J.H.; Yim, Y.H.; Jeoung, N.H.; Shong, M.; Hennighausen, L.; Harris, R.A.; Lee, I.K.; et al. Metformin inhibits growth hormone-mediated hepatic PDK4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes 2012, 61, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Shi, X.; Brown, M.S.; Goldstein, J.L.; Liang, G. Growth hormone acts on liver to stimulate autophagy, support glucose production, and preserve blood glucose in chronically starved mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7449–7454. [Google Scholar] [CrossRef]
- Metcalfe, P.; Johnston, D.G.; Nosadini, R.; Orksov, H.; Alberti, K.G. Metabolic effects of acute and prolonged growth hormone excess in normal and insulin-deficient man. Diabetologia 1981, 20, 123–128. [Google Scholar] [CrossRef]
- Keller, U.; Schnell, H.; Girard, J.; Stauffacher, W. Effect of physiological elevation of plasma growth hormone levels on ketone body kinetics and lipolysis in normal and acutely insulin-deficient man. Diabetologia 1984, 26, 103–108. [Google Scholar] [CrossRef]
- Moller, L.; Norrelund, H.; Jessen, N.; Flyvbjerg, A.; Pedersen, S.B.; Gaylinn, B.D.; Liu, J.; Thorner, M.O.; Moller, N.; Lunde Jorgensen, J.O. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J. Clin. Endocrinol. Metab. 2009, 94, 4524–4532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- da Rocha, A.F.; Pereira Junior, P.S.; Calefi, G.S.; Marquezine, G.F.; Morimoto, H.K.; Mazzuco, T.L.; de Faria, E.C.; Urbano, M.R.; Carrilho, A.J.F. Growth hormone directly favors hepatic ketogenesis in persons with prediabetes or type 2 diabetes mellitus treated with empagliflozin. Endocrine 2021, 73, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, P.; Wolf, P.; Pfleger, L.; Krumpolec, P.; Krssak, M.; Klavins, K.; Wolfsberger, S.; Micko, A.; Carey, P.; Gurtl, B.; et al. Increased ATP synthesis might counteract hepatic lipid accumulation in acromegaly. JCI Insight 2020, 5, e134638. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.; Steele, R.; Altszuler, N.; Debodo, R.C. Effect of Growth Hormone on Free Fatty Acid Metabolism. Am. J. Physiol. 1964, 206, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Medes, G.; Weinhouse, S. A study of the effects of growth hormone on fatty acid metabolism in vitro. J. Biol. Chem. 1956, 221, 333–345. [Google Scholar] [CrossRef]
- Leung, K.C.; Ho, K.K. Stimulation of mitochondrial fatty acid oxidation by growth hormone in human fibroblasts. J. Clin. Endocrinol. Metab. 1997, 82, 4208–4213. [Google Scholar] [CrossRef]
- Meyer, R.D.; Laz, E.V.; Su, T.; Waxman, D.J. Male-specific hepatic Bcl6: Growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5. Mol. Endocrinol. 2009, 23, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Sommars, M.A.; Ramachandran, K.; Senagolage, M.D.; Futtner, C.R.; Germain, D.M.; Allred, A.L.; Omura, Y.; Bederman, I.R.; Barish, G.D. Dynamic repression by BCL6 controls the genome-wide liver response to fasting and steatosis. eLife 2019, 8, e43922. [Google Scholar] [CrossRef]
- Cariou, B.; Byrne, C.D.; Loomba, R.; Sanyal, A.J. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab. 2021, 23, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef]
- Ter Horst, K.W.; Vatner, D.F.; Zhang, D.; Cline, G.W.; Ackermans, M.T.; Nederveen, A.J.; Verheij, J.; Demirkiran, A.; van Wagensveld, B.A.; Dallinga-Thie, G.M.; et al. Hepatic Insulin Resistance Is Not Pathway Selective in Humans with Nonalcoholic Fatty Liver Disease. Diabetes Care 2021, 44, 489–498. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Tomlinson, J.W. Non-alcoholic fatty liver disease in common endocrine disorders. Eur. J. Endocrinol. 2013, 169, R27–R37. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Yu, C.; Miao, M.; Zhang, X.; Zhu, Z.; Ding, X.; Li, Y. Association between serum growth hormone levels and nonalcoholic fatty liver disease: A cross-sectional study. PLoS ONE 2012, 7, e44136. [Google Scholar] [CrossRef]
- Arturi, F.; Succurro, E.; Procopio, C.; Pedace, E.; Mannino, G.C.; Lugara, M.; Procopio, T.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J. Clin. Endocrinol. Metab. 2011, 96, E1640–E1644. [Google Scholar] [CrossRef]
- Fusco, A.; Miele, L.; D’Uonnolo, A.; Forgione, A.; Riccardi, L.; Cefalo, C.; Barini, A.; Bianchi, A.; Giampietro, A.; Cimino, V.; et al. Nonalcoholic fatty liver disease is associated with increased GHBP and reduced GH/IGF-I levels. Clin. Endocrinol. 2012, 77, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, S.; Inzaghi, E.; Alisi, A.; Germani, D.; Puglianiello, A.; Nobili, V. Insulin-like growth factor-I and -II levels are associated with the progression of nonalcoholic fatty liver disease in obese children. J. Pediatr. 2014, 165, 92–98. [Google Scholar] [CrossRef]
- Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Espejo, I.; Montero, J.L.; Costan, G.; Marchal, T.; Membrives, A.; Gallardo-Valverde, J.M.; Munoz-Castaneda, J.R.; Arevalo, E.; et al. IL-6 and IGF-1 are independent prognostic factors of liver steatosis and non-alcoholic steatohepatitis in morbidly obese patients. Obes. Surg. 2007, 17, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Hribal, M.L.; Procopio, T.; Petta, S.; Sciacqua, A.; Grimaudo, S.; Pipitone, R.M.; Perticone, F.; Sesti, G. Insulin-like growth factor-I, inflammatory proteins, and fibrosis in subjects with nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2013, 98, E304–E308. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakao, K.; Hamasaki, K.; Furukawa, R.; Tsuruta, S.; Ueda, Y.; Taura, N.; Shibata, H.; Fujimoto, M.; Toriyama, K.; et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol. Int. 2007, 1, 287–294. [Google Scholar] [CrossRef]
- Runchey, S.S.; Boyko, E.J.; Ioannou, G.N.; Utzschneider, K.M. Relationship between serum circulating insulin-like growth factor-1 and liver fat in the United States. J. Gastroenterol. Hepatol. 2014, 29, 589–596. [Google Scholar] [CrossRef]
- Sesti, G.; Hribal, M.L.; Procopio, T.; Fiorentino, T.V.; Sciacqua, A.; Andreozzi, F.; Marini, M.A.; Perticone, F. Low circulating insulin-like growth factor-1 levels are associated with high serum uric acid in nondiabetic adult subjects. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, A.; Gologan, S.; Arbanas, T.; Copaescu, C.; Martin, S.; Albu, A.; Barbu, C.; Pirvulescu, I.; Fica, S. Adiponectin, body mass index and hepatic steatosis are independently associated with IGF-I status in obese non-diabetic women. Growth Horm. IGF Res. 2013, 23, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Yonei, Y.; Tanaka, S.; Mori, K.; Kanemasa, K.; Imai, S.; Taketani, H.; Hara, T.; Seko, Y.; Ishiba, H.; et al. Lower levels of insulin-like growth factor-1 standard deviation score are associated with histological severity of non-alcoholic fatty liver disease. Hepatol. Res. 2014, 45, 771–781. [Google Scholar] [CrossRef]
- Laron, Z.; Ginsberg, S.; Webb, M. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion—Preliminary report. Growth Horm. IGF Res. 2008, 18, 434–438. [Google Scholar] [CrossRef]
- Chen, Q.R.; Braun, R.; Hu, Y.; Yan, C.; Brunt, E.M.; Meerzaman, D.; Sanyal, A.J.; Buetow, K. Multi-SNP analysis of GWAS data identifies pathways associated with nonalcoholic fatty liver disease. PLoS ONE 2013, 8, e65982. [Google Scholar] [CrossRef]
- Bredella, M.A.; Gerweck, A.V.; Lin, E.; Landa, M.G.; Torriani, M.; Schoenfeld, D.A.; Hemphill, L.C.; Miller, K.K. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J. Clin. Endocrinol. Metab. 2013, 98, 3864–3872. [Google Scholar] [CrossRef]
- Stanley, T.L.; Feldpausch, M.N.; Oh, J.; Branch, K.L.; Lee, H.; Torriani, M.; Grinspoon, S.K. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: A randomized clinical trial. JAMA 2014, 312, 380–389. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Mulligan, K.; Lee, J.; Lo, J.C.; Wen, M.; Noor, M.A.; Grunfeld, C.; Schambelan, M. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J. Clin. Endocrinol. Metab. 2002, 87, 942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, Y.; Iida, K.; Takahashi, K.; Yoshioka, S.; Fukuoka, H.; Takeno, R.; Imanaka, M.; Nishizawa, H.; Takahashi, M.; Seo, Y.; et al. Growth hormone reverses nonalcoholic steatohepatitis in a patient with adult growth hormone deficiency. Gastroenterology 2007, 132, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Iguchi, G.; Murawaki, A.; Fukuoka, H.; Hayashi, Y.; Kaji, H.; Yamamoto, M.; Suda, K.; Takahashi, M.; Seo, Y.; et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur. J. Endocrinol. 2012, 167, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Krusenstjerna-Hafstrom, T.; Moller, L.; Christensen, B.; Vendelbo, M.H.; Pedersen, S.B.; Frystyk, J.; Jessen, N.; Hansen, T.K.; Stodkilde-Jorgensen, H.; et al. Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a GH receptor antagonist: A randomized clinical trial. J. Clin. Endocrinol. Metab. 2012, 97, 1227–1235. [Google Scholar] [CrossRef]
- Yang, T.; Householder, L.A.; Lubbers, E.R.; List, E.O.; Troike, K.; Vesel, C.; Duran-Ortiz, S.; Kopchick, J.J.; Berryman, D.E. Growth hormone receptor antagonist transgenic mice are protected from hyperinsulinemia and glucose intolerance despite obesity when placed on a HF diet. Endocrinology 2015, 156, 555–564. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.P. Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats. Lipids Health Dis. 2010, 9, 78. [Google Scholar] [CrossRef]
- List, E.O.; Palmer, A.J.; Berryman, D.E.; Bower, B.; Kelder, B.; Kopchick, J.J. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia 2009, 52, 1647–1655. [Google Scholar] [CrossRef]
- Tateno, C.; Kataoka, M.; Utoh, R.; Tachibana, A.; Itamoto, T.; Asahara, T.; Miya, F.; Tsunoda, T.; Yoshizato, K. Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology 2011, 152, 1479–1491. [Google Scholar] [CrossRef]
- Greenhalgh, C.J.; Rico-Bautista, E.; Lorentzon, M.; Thaus, A.L.; Morgan, P.O.; Willson, T.A.; Zervoudakis, P.; Metcalf, D.; Street, I.; Nicola, N.A.; et al. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J. Clin. Investig. 2005, 115, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hosui, A.; Sun, R.; Shen, K.; Gavrilova, O.; Chen, W.; Cam, M.C.; Gao, B.; Robinson, G.W.; Hennighausen, L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 2007, 46, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Menon, R.K.; Cohen, P.; Hwang, D.; Clemens, T.; DiGirolamo, D.J.; Kopchick, J.J.; Le Roith, D.; Trucco, M.; Sperling, M.A. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 2009, 284, 19937–19944. [Google Scholar] [CrossRef]
- List, E.O.; Berryman, D.E.; Funk, K.; Jara, A.; Kelder, B.; Wang, F.; Stout, M.B.; Zhi, X.; Sun, L.; White, T.A.; et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 2014, 155, 1793–1805. [Google Scholar] [CrossRef]
- Mueller, K.M.; Kornfeld, J.W.; Friedbichler, K.; Blaas, L.; Egger, G.; Esterbauer, H.; Hasselblatt, P.; Schlederer, M.; Haindl, S.; Wagner, K.U.; et al. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology 2011, 54, 1398–1409. [Google Scholar] [CrossRef]
- Sos, B.C.; Harris, C.; Nordstrom, S.M.; Tran, J.L.; Balazs, M.; Caplazi, P.; Febbraio, M.; Applegate, M.A.; Wagner, K.U.; Weiss, E.J. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J. Clin. Investig. 2011, 121, 1412–1423. [Google Scholar] [CrossRef]
- Nordstrom, S.M.; Tran, J.L.; Sos, B.C.; Wagner, K.-U.; Weiss, E.J. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol. Endocrinol. 2013, 27, 1333–1342. [Google Scholar] [CrossRef]
- Cordoba-Chacon, J.; Sarmento-Cabral, A.; Del Rio-Moreno, M.; Diaz-Ruiz, A.; Subbaiah, P.V.; Kineman, R.D. Adult-Onset Hepatocyte GH Resistance Promotes NASH in Male Mice, without Severe Systemic Metabolic Dysfunction. Endocrinology 2018, 159, 3761–3774. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef]
- Ortega-Prieto, P.; Postic, C. Carbohydrate Sensing Through the Transcription Factor ChREBP. Front. Genet. 2019, 10, 472. [Google Scholar] [CrossRef]
- Agius, L.; Chachra, S.S.; Ford, B.E. The Protective Role of the Carbohydrate Response Element Binding Protein in the Liver: The Metabolite Perspective. Front. Endocrinol. 2020, 11, 594041. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, Y.; Zhang, G.; Deng, H.; Chen, C.; Pan, X.; Wu, K.; Fan, J.; Pan, Q.; Wang, K.; et al. Deficiency of gluconeogenic enzyme PCK1 promotes non-alcoholic steatohepatitis progression by activation of PI3K/AKT/PDGF axis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Li, C.H.; Gordon, D.; Knorr, J. The primary structure of sheep pituitary growth hormone. Arch. Biochem. Biophys. 1973, 156, 493–508. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Borrego, M.C.; del Rio-Moreno, M.; Kineman, R.D. Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism. Cells 2021, 10, 2532. https://doi.org/10.3390/cells10102532
Vázquez-Borrego MC, del Rio-Moreno M, Kineman RD. Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism. Cells. 2021; 10(10):2532. https://doi.org/10.3390/cells10102532
Chicago/Turabian StyleVázquez-Borrego, Mari C., Mercedes del Rio-Moreno, and Rhonda D. Kineman. 2021. "Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism" Cells 10, no. 10: 2532. https://doi.org/10.3390/cells10102532
APA StyleVázquez-Borrego, M. C., del Rio-Moreno, M., & Kineman, R. D. (2021). Towards Understanding the Direct and Indirect Actions of Growth Hormone in Controlling Hepatocyte Carbohydrate and Lipid Metabolism. Cells, 10(10), 2532. https://doi.org/10.3390/cells10102532




