Regulation of GH and GH Signaling by Nutrients
Abstract
1. Introduction
2. GH and IGF-I Structure, Regulation, and Signaling
3. GH Secretion during Fasting and Feeding
3.1. Fasting
- -
- -
- Down-regulation of the GHR in the liver: studies in animal models of starvation found low IGF-I levels coupled with decreased hepatic GHR mRNA levels and decreased GH binding [44,45]. Low insulin levels, as those seen in the fasting state, may in part mediate GH resistance by reducing surface expression of GHR in the liver [46];
- -
- Post-receptor mechanisms resulting in the inability of GH to stimulate IGF-I production: for example, fibroblast growth factor-21 (FGF-21) and Sirtuin 1 (SIRT1) have been shown to play a role in GH resistance in states of nutritional deprivation acting via STAT5 inhibition [42].
3.2. Feeding
3.3. Role of the Ghrelin-GH Axis in Fasting and Feeding
4. Regulation of GH and GH Signaling from Macronutrients
4.1. Carbohydrates
Fibers
4.2. Amino Acids and Proteins
4.3. Lipids and Free Fatty Acids
5. Regulation of GH and GH Signaling from Micronutrients
5.1. Vitamins
5.1.1. Vitamin D
5.1.2. Vitamin A
5.1.3. Vitamin E
5.1.4. Vitamins of the B Complex
5.2. Minerals
5.2.1. Sodium, Potassium, and Water
5.2.2. Calcium
5.2.3. Phosphorus
5.2.4. Magnesium
5.2.5. Zinc
5.2.6. Iron
5.2.7. Iodine
5.2.8. Selenium
5.2.9. Manganese
5.2.10. Copper
5.2.11. Chromium
6. Regulation of GH and GH Signaling in Diets and Dietary Habits
6.1. Isocaloric and Hypercaloric Diet Regimens
6.2. Caloric Restriction Regimens
6.3. Mediterranean Diet
7. Healthy Eating Patterns for Patients with GH-Related Clinical Conditions: Are We Ready to Recommend a Personalized Diet?
7.1. GHD
7.2. Acromegaly
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moøller, N.; Joørgensen, J.O.L. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Darcy, J. GH and ageing: Pitfalls and new insights. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Dixit, M.; Neginskaya, M.; Nagaraj, K.; Pavlov, E.; Werner, H.; Yakar, S. Effects of GH/IGF on the Aging Mitochondria. Cells 2020, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.L.; Lysaght, J.; O’Sullivan, J.; Reynolds, J.V. Emerging Concepts Linking Obesity with the Hallmarks of Cancer. Trends Endocrinol. Metab. 2017, 28, 46–62. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Chen, E.Y.; Liao, Y.C.; Smith, D.H.; Barrera-Saldaña, H.A.; Gelinas, R.E.; Seeburg, P.H. The human growth hormone locus: Nucleotide sequence, biology, and evolution. Genomics 1989, 4, 479–497. [Google Scholar] [CrossRef]
- Baumann, G.P. Growth hormone isoforms. Growth Horm. IGF Res. 2009, 19, 333–340. [Google Scholar] [CrossRef]
- Ho, K.Y.; Veldhuis, J.D.; Johnson, M.L.; Furlanetto, R.; Evans, W.S.; Alberti, K.G.M.M.; Thorner, M.O. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Investig. 1988, 81, 968–975. [Google Scholar] [CrossRef]
- Hartman, M.L.; Veldhuis, J.D.; Thorner, M.O. Normal control of growth hormone secretion. Horm. Res. 1993, 40, 37–47. [Google Scholar] [CrossRef]
- Bonert, V.; Melmed, S. The Pituitary, 4th ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Giustina, A.; Veldhuis, J.D. Pathophysiology of the Neuroregulation of Growth Hormone Secretion in Experimental Animals and the Human. Endocr. Rev. 1998, 19, 717–797. [Google Scholar] [CrossRef]
- Eigler, T.; Ben-Shlomo, A. Somatostatin system: Molecular mechanisms regulating anterior pituitary hormones. J. Mol. Endocrinol. 2014, 53. [Google Scholar] [CrossRef]
- Ghigo, E.; Arvat, E.; Bellone, J.; Ramunni, J.; Camanni, F. Neurotransmitter Control of Growth Hormone Secretion in Humans. J. Pediatr. Endocrinol. Metab. 1993, 6, 263–266. [Google Scholar] [CrossRef]
- Vance, M.L.; Hartman, M.L.; Thorner, M.O. Growth hormone and nutrition. Horm. Res. Paediatr. 1992, 38, 85–88. [Google Scholar] [CrossRef]
- Hartman, M.L.; Clayton, P.E.; Johnson, M.L.; Celniker, A.; Perlman, A.J.; Alberti, K.G.M.M.; Thorner, M.O. A low dose euglycemic infusion of recombinant human insulin-like growth factor I rapidly suppresses fasting-enhanced pulsatile growth hormone secretion in humans. J. Clin. Investig. 1993, 91, 2453–2462. [Google Scholar] [CrossRef]
- Vottero, A.; Guzzetti, C.; Loche, S. New aspects of the physiology of the GH-IGF-1 axis. In Hormone Resistance and Hypersensitivity: From Genetics to Clinical Management; S. Karger: Basel, Switzerland, 2013; Volume 24, pp. 96–105. ISBN 9783318022681. [Google Scholar]
- Mertani, H.C.; Delehaye-Zervas, M.C.; Martini, J.F.; Postel-Vinay, M.C.; Morel, G. Localization of growth hormone receptor messenger RNA in human tissues. Endocrine 1995, 3, 135–142. [Google Scholar] [CrossRef]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The growth hormone receptor: Mechanism of receptor activation, cell signaling, and physiological aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef]
- Lanning, N.J.; Carter-Su, C. Recent advances in growth hormone signaling. Rev. Endocr. Metab. Disord. 2006, 7, 225–235. [Google Scholar] [CrossRef]
- Carter-Su, C.; Schwartz, J.; Argetsinger, L.S. Growth hormone signaling pathways. Growth Horm. IGF Res. 2016, 28, 11–15. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Cohen, P. Review: The somatomedin hypothesis 2007: 50 Years later. J. Clin. Endocrinol. Metab. 2007, 92, 4529–4535. [Google Scholar] [CrossRef]
- Siddle, K. Signalling by insulin and IGF receptors: Supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef]
- Messina, J. Insulin as a growth-promoting hormone. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hong, S.; Mannan, A.M.; Inoki, K. Evaluation of the nutrient-sensing mTOR pathway. Methods Mol. Biol. 2012, 821, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.E.; Blyth, A.J.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell. Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Baylink, D.J.; Mohan, S. Insulin-Like Growth Factor-Binding Proteins in Serum and Other Biological Fluids: Regulation and Functions. Endocr. Rev. 1997, 18, 801–831. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.A. What’s new in the IGF-binding proteins? Growth Horm. IGF Res. 2004, 14, 329–336. [Google Scholar] [CrossRef]
- Blum, W.F.; Albertsson-Wikland, K.; Rosberg, S.; Ranke, M.B. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. J. Clin. Endocrinol. Metab. 1993, 76, 1610–1616. [Google Scholar] [CrossRef]
- Maures, T.J.; Duan, C. Structure, developmental expression, and physiological regulation of zebrafish IGF binding protein-1. Endocrinology 2002, 143, 2722–2731. [Google Scholar] [CrossRef]
- Kajimura, S.; Aida, K.; Duan, C. Understanding Hypoxia-Induced Gene Expression in Early Development: In Vitro and In Vivo Analysis of Hypoxia-Inducible Factor 1-Regulated Zebra Fish Insulin-Like Growth Factor Binding Protein 1 Gene Expression. Mol. Cell. Biol. 2006, 26, 1142–1155. [Google Scholar] [CrossRef]
- O’Brien, R.M.; Noisin, E.L.; Suwanichkul, A.; Yamasaki, T.; Lucas, P.C.; Wang, J.C.; Powell, D.R.; Granner, D.K. Hepatic nuclear factor 3- and hormone-regulated expression of the phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein 1 genes. Mol. Cell. Biol. 1995, 15, 1747–1758. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Johnson, M.L. Cluster analysis: A simple, versatile, and robust algorithm for endocrine pulse detection. Am. J. Physiol. Endocrinol. Metab. 1986, 250. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Carlson, M.L.; Johnson, M.L. The pituitary gland secretes in bursts: Appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc. Natl. Acad. Sci. USA 1987, 84, 7686–7690. [Google Scholar] [CrossRef]
- Hartman, M.L.; Veldhuis, J.D.; Johnson, M.L.; Lee, M.M.; Alberti, K.G.M.M.; Samojlik, E.; Thorner, M.O. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J. Clin. Endocrinol. Metab. 1992, 74, 757–765. [Google Scholar] [CrossRef]
- Riedel, M.; Hoeft, B.; Blum, W.F.; von zur Mühlen, A.; Brabant, G. Pulsatile growth hormone secretion in normal-weight and obese men: Differential metabolic regulation during energy restriction. Metabolism 1995, 44, 605–610. [Google Scholar] [CrossRef]
- Sakharova, A.A.; Horowitz, J.F.; Surya, S.; Goldenberg, N.; Harber, M.P.; Symons, K.; Barkan, A. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 2008, 93, 2755–2759. [Google Scholar] [CrossRef]
- Clemmons, D.R.; Klibanski, A.; Underwood, L.E.; McArthur, J.W.; Ridgway, E.C.; Beitins, I.Z.; Van Wyk, J.J. Reduction of plasma immunoreactive somatomedin C during fasting in humans. J. Clin. Endocrinol. Metab. 1981, 53, 1247–1250. [Google Scholar] [CrossRef]
- Merimee, T.J.; Zapf, J.; Froesch, E.R. Insulin-Like Growth Factors in the Fed and Fasted States. J. Clin. Endocrinol. Metab. 1982, 55, 999–1002. [Google Scholar] [CrossRef]
- Underwood, L.E.; Thissen, E.P.; Ketelslegers, J.M. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994, 15, 80–101. [Google Scholar] [CrossRef]
- Counts, D.R.; Gwirtsman, H.; Carlsson, L.M.S.; Lesem, M. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J. Clin. Endocrinol. Metab. 1992, 75, 762–767. [Google Scholar] [CrossRef]
- Argente, J.; Caballo, N.; Barrios, V.; Muñoz, M.T.; Pozo, J.; Chowen, J.A.; Morandé, G.; Hernández, M. Multiple Endocrine Abnormalities of the Growth Hormone and Insulin-Like Growth Factor Axis in Patients with Anorexia Nervosa: Effect of Short- and Long-Term Weight Recuperation 1. J. Clin. Endocrinol. Metab. 1997, 82, 2084–2092. [Google Scholar] [CrossRef][Green Version]
- Fazeli, P.K.; Klibanski, A. Determinants of GH resistance in malnutrition. J. Endocrinol. 2014, 220, R57. [Google Scholar] [CrossRef]
- Clemmons, D.R. Metabolic Actions of Insulin-Like Growth Factor-I in Normal Physiology and Diabetes. Endocrinol. Metab. Clin. N. Am. 2012, 41, 425–443. [Google Scholar] [CrossRef]
- Baxter, R.C.; Bryson, J.M.; Turtle, J.R. The effect of fasting on liver receptors for prolactin and growth hormone. Metabolism 1981, 30, 1086–1090. [Google Scholar] [CrossRef]
- Straus, D.S.; Takemoto, C.D. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol. Endocrinol. 1990, 4, 91–100. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Waters, M.J.; Chen, C. Insulin and Growth Hormone Balance: Implications for Obesity. Trends Endocrinol. Metab. 2020, 31, 642–654. [Google Scholar] [CrossRef]
- Aimaretti, G.; Colao, A.; Corneli, G.; Pivonello, R.; Maccario, M.; Morrison, K.; Pflaum, C.D.; Strasburger, C.J.; Lombardi, G.; Ghigo, E. The study of spontaneous GH secretion after 36-h fasting distinguishes between GH-deficient and normal adults. Clin. Endocrinol. (Oxf.) 1999, 51, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Grottoli, S.; Gasco, V.; Mainolfi, A.; Beccuti, G.; Corneli, G.; Aimaretti, G.; Dieguez, C.; Casanueva, F.; Ghigo, E. Growth hormone/insulin-like growth factor I axis, glucose metabolism, and lypolisis but not leptin show some degree of refractoriness to short-term fasting in acromegaly. J. Endocrinol. Investig. 2008, 31, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Thorner, M.O. Fasting-induced enhancement of pulsatile growth hormone (GH) secretion is rapidly abolished by refeeding. In Proceedings of the 72nd Meeting of the Endocrine Society, Atlanta, GA, USA, 1 March 1990. [Google Scholar]
- Cornford, A.S.; Barkan, A.L.; Horowitz, J.F. Rapid suppression of growth hormone concentration by overeating: Potential mediation by hyperinsulinemia. J. Clin. Endocrinol. Metab. 2011, 96, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Briard, N.; Rico-Gomez, M.; Guillaume, V.; Sauze, N.; Vuaroqueaux, V.; Dadoun, F.; Le Bouc, Y.; Oliver, C.; Dutour, A. Hypothalamic mediated action of free fatty acid on growth hormone secretion in sheep. Endocrinology 1998, 139, 4811–4819. [Google Scholar] [CrossRef][Green Version]
- Casanueva, F.F.; Villanueva, L.; Dieguez, C.; Diaz, Y.; Cabranes, J.A.; Szoke, B.; Scanlon, M.F.; Schally, A.V.; Fernandez-Cruz, A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated gh secretion in man directly at the pituitary. J. Clin. Endocrinol. Metab. 1987, 65, 634–642. [Google Scholar] [CrossRef]
- Bang, P.; Brismar, K.; Rosenfeld, R.G.; Hall, K. Fasting affects serum insulin-like growth factors (IGFs) and IGF-binding proteins differently in patients with noninsulin-dependent diabetes mellitus versus healthy nonobese and obese subjects. J. Clin. Endocrinol. Metab. 1994, 78, 960–967. [Google Scholar] [CrossRef]
- Frystyk, J.; Delhanty, P.J.D.; Skjærbæk, C.; Baxter, R.C. Changes in the circulating IGF system during short-term fasting and refeeding in rats. Am. J. Physiol. Endocrinol. Metab. 1999, 277. [Google Scholar] [CrossRef]
- Maccario, M.; Aimaretti, G.; Grottoli, S.; Gauna, C.; Tassone, F.; Corneli, G.; Rossetto, R.; Wu, Z.; Strasburger, C.J.; Ghigo, E. Effects of 36 hour fasting on GH/IGF-I axis and metabolic parameters in patients with simple obesity. Comparison with normal subjects and hypopituitary patients with severe GH deficiency. Int. J. Obes. 2001, 25, 1233–1239. [Google Scholar] [CrossRef]
- Luque, R.M.; Kineman, R.D. Impact of obesity on the growth hormone axis: Evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 2006, 147, 2754–2763. [Google Scholar] [CrossRef]
- Gahete, M.D.; Córdoba-Chaćon, J.; Lin, Q.; Brüning, J.C.; Kahn, C.R.; Castaño, J.P.; Christian, H.; Luque, R.M.; Kineman, R.D. Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology 2013, 154, 2410–2420. [Google Scholar] [CrossRef]
- Gasco, V.; Pagano, L.; Prodam, F.; Marzullo, P.; Ghigo, E.A.G. Hypothalamic-Pituitary Disease and Obesity; Clemmons, D., Attanasio, A., Eds.; BioScentifica Ltd.: Bristol, UK, 2009; pp. 109–118. [Google Scholar]
- Berryman, D.E.; Glad, C.A.M.; List, E.O.; Johannsson, G. The GH/IGF-1 axis in obesity: Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013, 9, 346–356. [Google Scholar] [CrossRef]
- Grottoli, S.; Gauna, C.; Tassone, F.; Aimaretti, G.; Corneli, G.; Wu, Z.; Strasburger, C.J.; Dieguez, C.; Casanueva, F.F.; Ghigo, E.; et al. Both fasting-induced leptin reduction and GH increase are blunted in Cushing’s syndrome and in simple obesity. Clin. Endocrinol. (Oxf.) 2003, 58, 220–228. [Google Scholar] [CrossRef]
- Bonert, V.S.; Elashoff, J.D.; Barnett, P.; Melmed, S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: Diagnostic caveat for adult GH deficiency. J. Clin. Endocrinol. Metab. 2004, 89, 3397–3401. [Google Scholar] [CrossRef]
- Qu, X.D.; Gaw Gonzalo, I.T.; Al Sayed, M.Y.; Cohan, P.; Christenson, P.D.; Swerdloff, R.S.; Kelly, D.F.; Wang, C. Influence of body mass index and gender on growth hormone (GH) responses to GH-releasing hormone plus arginine and insulin tolerance tests. J. Clin. Endocrinol. Metab. 2005, 90, 1563–1569. [Google Scholar] [CrossRef]
- Williams, T.; Berelowitz, M.; Joffe, S.N.; Thorner, M.O.; Rivier, J.; Vale, W.; Frohman, L.A. Impaired Growth Hormone Responses to Growth Hormone–Releasing Factor in Obesity. N. Engl. J. Med. 1984, 311, 1403–1407. [Google Scholar] [CrossRef]
- Rasmussen, M.H.; Hvidberg, A.; Juul, A.; Main, K.M.; Gotfredsen, A.; Skakkebaek, N.E.; Hilsted, J. Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J. Clin. Endocrinol. Metab. 1995, 80, 1407–1415. [Google Scholar] [CrossRef]
- De Marinis, L.; Bianchi, A.; Mancini, A.; Gentilella, R.; Perrelli, M.; Giampietro, A.; Porcelli, T.; Tilaro, L.; Fusco, A.; Valle, D.; et al. Growth Hormone Secretion and Leptin in Morbid Obesity before and after Biliopancreatic Diversion: Relationships with Insulin and Body Composition. J. Clin. Endocrinol. Metab. 2004, 89, 174–180. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the Acyltransferase that Octanoylates Ghrelin, an Appetite-Stimulating Peptide Hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J. The Complex World of Regulation of Pituitary Growth Hormone Secretion: The Role of Ghrelin, Klotho, and Nesfatins in It. Front. Endocrinol. (Lausanne) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Devesa, J.; Lima, L.; Tresguerres, J.A.F. Neuroendocrine control of growth hormone secretion in humans. Trends Endocrinol. Metab. 1992, 3, 175–183. [Google Scholar] [CrossRef]
- Root, A.W.; Root, M.J. Clinical pharmacology of human growth hormone and its secretagogues. Curr. Drug Targets. Immune. Endocr. Metabol. Disord. 2002, 2, 27–52. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Malik, S.; McGlone, F.; Bedrossian, D.; Dagher, A. Ghrelin Modulates Brain Activity in Areas that Control Appetitive Behavior. Cell Metab. 2008, 7, 400–409. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Ricks, K.M.; Grill, H.J. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 2013, 73, 915–923. [Google Scholar] [CrossRef]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The Homeostatic Force of Ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Barazzoni, R. Ghrelin forms in the modulation of energy balance and metabolism. Eat. Weight Disord. 2019, 24, 997–1013. [Google Scholar] [CrossRef]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; Del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef]
- Duerrschmid, C.; He, Y.; Wang, C.; Li, C.; Bournat, J.C.; Romere, C.; Saha, P.K.; Lee, M.E.; Phillips, K.J.; Jain, M.; et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017, 23, 1444–1453. [Google Scholar] [CrossRef]
- Prodam, F.; Me, E.; Riganti, F.; Gramaglia, E.; Bellone, S.; Baldelli, R.; Rapa, A.; Van Der Lely, A.J.; Bona, G.; Ghigo, E.; et al. The nutritional control of ghrelin secretion in humans: The effects of enteral vs. parenteral nutrition. Eur. J. Nutr. 2006, 45, 399–405. [Google Scholar] [CrossRef]
- Prodam, F.; Monzani, A.; Ricotti, R.; Marolda, A.; Bellone, S.; Aimaretti, G.; Roccio, M.; Bona, G. Systematic review of ghrelin response to food intake in pediatric age, from neonates to adolescents. J. Clin. Endocrinol. Metab. 2014, 99, 1556–1568. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Muller, A.F.; Lamberts, S.W.J.; Janssen, J.A.M.J.L.; Hofland, L.J.; Van Koetsveld, P.; Bidlingmaier, M.; Strasburger, C.J.; Ghigo, E.; Van der Lely, A.J. Ghrelin drives GH secretion during fasting in man. Eur. J. Endocrinol. 2002, 146, 203–207. [Google Scholar] [CrossRef][Green Version]
- Koutkia, P.; Canavan, B.; Breu, J.; Johnson, M.L.; Grinspoon, S.K. Nocturnal ghrelin pulsatility and response to growth hormone secretagogues in healthy men. Am. J. Physiol. Endocrinol. Metab. 2004, 287. [Google Scholar] [CrossRef]
- Nass, R.; Farhy, L.S.; Liu, J.; Prudom, C.E.; Johnson, M.L.; Veldhuis, P.; Pezzoli, S.S.; Oliveri, M.C.; Gaylinn, B.D.; Geysen, H.M.; et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J. Clin. Endocrinol. Metab. 2008, 93, 1988–1994. [Google Scholar] [CrossRef]
- Broglio, F.; Prodam, F.; Riganti, F.; Gottero, C.; Destefanis, S.; Granata, R.; Muccioli, G.; Abribat, T.; van der Lely, A.J.; Ghigo, E. The continuous infusion of acylated ghrelin enhances growth hormone secretion and worsens glucose metabolism in humans. J. Endocrinol. Investig. 2008, 31, 788–794. [Google Scholar] [CrossRef]
- Espelund, U.; Hansen, T.K.; Højlund, K.; Beck-Nielsen, H.; Clausen, J.T.; Hansen, B.S.; Ørskov, H.; Jørgensen, J.O.L.; Frystyk, J. Fasting unmasks a strong inverse association between Ghrelin and Cortisol in serum: Studies in obese and normal-weight subjects. J. Clin. Endocrinol. Metab. 2005, 90, 741–746. [Google Scholar] [CrossRef]
- Nørrelund, H.; Hansen, T.K.; Ørskov, H.; Hosoda, H.; Kojima, M.; Kangawa, K.; Weeke, J.; Møller, N.; Christiansen, J.S.; Jørgensen, J.O.L. Ghrelin immunoreactivity in human plasma is suppressed by somatostatin. Clin. Endocrinol. (Oxf.) 2002, 57, 539–546. [Google Scholar] [CrossRef]
- Natalucci, G.; Riedl, S.; Gleiss, A.; Zidek, T.; Frisch, H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005, 152, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Jaffe, C.A.; Symons, K.V.; Barkan, A.L. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J. Clin. Endocrinol. Metab. 2005, 90, 2982–2987. [Google Scholar] [CrossRef]
- Zhao, T.J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Zhao, T.J.; Li, R.L.; Sherbet, D.P.; Liang, G.; Brown, M.S. Surviving starvation: Essential role of the ghrelin-growth hormone axis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 121–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Goldstein, J.L.; Brown, M.S.; Zhao, T.J. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: Reversal by growth hormone. Proc. Natl. Acad. Sci. USA 2015, 112, 1226–1231. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469. [Google Scholar] [CrossRef]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Louise Thomas, E.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Investig. 2019, 129, 3909–3923. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman: New York, NY, USA, 2017. [Google Scholar]
- SINU—Società Italiana di Nutrizione Umana. Available online: https://sinu.it/ (accessed on 16 April 2021).
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans, 2020–2025 and Online Materials|Dietary Guidelines for Americans. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 16 April 2021).
- Dominici, F.P.; Turyn, D. Growth hormone-induced alterations in the insulin-signaling system. Exp. Biol. Med. 2002, 227, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.P.; Meneguz-Moreno, R.A.; Aguiar-Oliveira, M.H.; Barreto-Filho, J.A.S. Emerging role of the GH/IGF-I on cardiometabolic control. Arq. Bras. Cardiol. 2011, 97, 434–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiarelli, F.; Giannini, C.; Mohn, A. Growth, growth factors and diabetes. Eur. J. Endocrinol. 2004, 151 (Suppl. 3), U109–U117. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, P.; Van Obberghen, E. SOCS proteins causing trouble in insulin action. Acta Physiol. (Oxf.) 2008, 192, 29–36. [Google Scholar] [CrossRef]
- Del Rincon, J.P.; Iida, K.; Gaylinn, B.D.; McCurdy, C.E.; Leitner, J.W.; Barbour, L.A.; Kopchick, J.J.; Friedman, J.E.; Draznin, B.; Thorner, M.O. Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue: Mechanism for growth hormone-mediated insulin resistance. Diabetes 2007, 56, 1638–1646. [Google Scholar] [CrossRef]
- Barbour, L.A.; Rahman, S.M.; Gurevich, I.; Leitner, J.W.; Fischer, S.J.; Roper, M.D.; Knotts, T.A.; Vo, Y.; McCurdy, C.E.; Yakar, S.; et al. Increased P85α is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J. Biol. Chem. 2005, 280, 37489–37494. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Frystyk, J.; White, D.K.; Twickler, T.B.; Koppeschaar, H.P.F.; Harris, P.E.; Fryklund, L.; Murgatroyd, P.R.; Dunger, D.B. Improvement in insulin sensitivity without concomitant changes in body composition and cardiovascular risk markers following fixed administration of a very low growth hormone (GH) dose in adults with severe GH deficiency. Clin. Endocrinol. (Oxf.) 2005, 63, 428–436. [Google Scholar] [CrossRef]
- Hunter, W.M.; Willoughby, J.M.; Strong, J.A. Plasma insulin and growth hormone during 22-hour fasts and after graded glucose loads in six healthy adults. J. Endocrinol. 1968, 40, 297–311. [Google Scholar] [CrossRef]
- Roth, J.; Glick, S.M.; Yalow, R.S.; Berson, S.A. Hypoglycemia: A potent stimulus to secretion of growth hormone. Science 1963, 140, 987–988. [Google Scholar] [CrossRef]
- Roth, J.; Glick, S.; Yalow, R.; Berson, S. Secretion of human growth hormone: Physiologic and experimental modification. Metabolism 1963, 12, 577–579. [Google Scholar]
- Yalow, R.S.; Goldsmith, S.J.; Berson, S.A. Influence of physiologic fluctuations in plasma growth hormone on glucose tolerance. Diabetes 1969, 18, 402–408. [Google Scholar] [CrossRef]
- Penalva, A.; Burguera, B.; Cusahie, X.; Tresguerres, J.A.F.; Dieguez, C.; Casanueva, F.F. Activation of cholinergic neurotransmission by pyridostigmine reverses the inhibitory effect of hyperglycemia on growth hormone (GH) releasing hormone-induced GH secretion in man: Does acute hyperglycemia act through hypothalamic release of somatostatin? Neuroendocrinology 1989, 49, 551–554. [Google Scholar] [CrossRef]
- Masuda, A.; Shibasaki, T.; Nakahara, M.; Imaki, T.; Kiyosawa, Y.; Jibiki, K.; Demura, H.; Shizume, K.; Ling, N. The effect of glucose on growth hormone (gh)-releasing hormone-mediated gh secretion in man. J. Clin. Endocrinol. Metab. 1985, 60, 523–526. [Google Scholar] [CrossRef]
- Gasperi, M.; Cecconi, E.; Grasso, L.; Bartalena, L.; Centoni, R.; Aimaretti, G.; Broglio, F.; Miccoli, P.; Marcocci, C.; Ghigo, E.; et al. GH secretion is impaired in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2002, 87, 1961–1964. [Google Scholar] [CrossRef][Green Version]
- Friend, K.; Iranmanesh, A.; Login, I.S.; Veldhuis, J.D. Pyridostigmine treatment selectively amplifies the mass of GH secreted per burst without altering GH burst frequency, half-life, basal GH secretion or the orderliness of GH release. Eur. J. Endocrinol. 1997, 137, 377–386. [Google Scholar] [CrossRef][Green Version]
- Nakagawa, E.; Nagaya, N.; Okumura, H.; Enomoto, M.; Oya, H.; Ono, F.; Hosoda, H.; Kojima, M.; Kangawa, K. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth-hormone-releasing peptide: Responses to the intravenous and oral administration of glucose. Clin. Sci. 2002, 103, 325–328. [Google Scholar] [CrossRef]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.I.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef]
- Teff, K.L.; Elliott, S.S.; Tschöp, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’Alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef]
- Pena-Bello, L.; Pertega-Diaz, S.; Outeiriño-Blanco, E.; Garcia-Buela, J.; Tovar, S.; Sangiao-Alvarellos, S.; Dieguez, C.; Cordido, F. Effect of oral glucose administration on rebound growth hormone release in normal and obese women: The role of adiposity, insulin sensitivity and ghrelin. PLoS ONE 2015, 10, e0121087. [Google Scholar] [CrossRef]
- Gottero, C.; Bellone, S.; Rapa, A.; van Koetsveld, P.; Vivenza, D.; Prodam, F.; Benso, A.; Destefanis, S.; Gauna, C.; Bellone, J.; et al. Standard light breakfast inhibits circulating ghrelin level to the same extent of oral glucose load in humans, despite different impact on glucose and insulin levels. J. Endocrinol. Investig. 2003, 26, 1203–1207. [Google Scholar] [CrossRef]
- Lucidi, P.; Murdolo, G.; Di Loreto, C.; De Cicco, A.; Parlanti, N.; Fanelli, C.; Santeusanio, F.; Bolli, G.B.; De Feo, P. Ghrelin is not necessary for adequate hormonal counterregulation of insulin-induced hypoglycemia. Diabetes 2002, 51, 2911–2914. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C.; Maykel, J.A.; Bistrian, B.R.; Ling, P.R. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. J. Endocrinol. 2002, 175, R7–R11. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Overduin, J.; Prudom, C.E.; Liu, J.; Callahan, H.S.; Gaylinn, B.D.; Thorner, M.O.; Cummings, D.E. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008, 93, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a Natural GH Secretagogue Produced by the Stomach, Induces Hyperglycemia and Reduces Insulin Secretion in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Aas, A.M.; Astrup, A.; Atkinson, F.S.; Baer-Sinnott, S.; Barclay, A.W.; Brand-Miller, J.C.; Brighenti, F.; Bullo, M.; Buyken, A.E.; et al. Dietary fibre consensus from the international carbohydrate quality consortium (Icqc). Nutrients 2020, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Tungland, B.C.; Meyer, D. Nondigestible oligo-and polysaccharides (dietary fiber): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002, 1, 90–109. [Google Scholar] [CrossRef]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Cani, P.D. Targeting gut microbiota with a complex mix of dietary fibers improves metabolic diseases. Kidney Int. 2019, 95, 14–16. [Google Scholar] [CrossRef]
- Denny-Brown, S.; Stanley, T.L.; Grinspoon, S.K.; Makimura, H. The association of macro- and micronutrient intake with growth hormone secretion. Growth Horm. IGF Res. 2012, 22, 102–107. [Google Scholar] [CrossRef]
- Lopez, M.; Mohiuddin, S. Biochemistry, Essential Amino Acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Griminger, P.; Scanes, C.G. Protein Metabolism. In Avian Physiology; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- USDA. Available online: https://www.usda.gov/ (accessed on 20 April 2021).
- Chromiak, J.A.; Antonio, J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition 2002, 18, 657–661. [Google Scholar] [CrossRef]
- Knopf, R.F.; Conn, J.W.; Fajans, S.S.; Floyd, J.C.; Guntsche, E.M.; Rull, J.A. Plasma Growth Hormone Response to Intravenous Administration of Amino Acids. J. Clin. Endocrinol. Metab. 1965, 25, 1140–1144. [Google Scholar] [CrossRef]
- Alba-Roth, J.; Müller, O.A.; Schopohl, J.; Von Werder, K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J. Clin. Endocrinol. Metab. 1988, 67, 1186–1189. [Google Scholar] [CrossRef]
- Ghigo, E.; Aimaretti, G.; Corneli, G. Diagnosis of adult GH deficiency. Growth Horm. IGF Res. 2008, 18, 1–16. [Google Scholar] [CrossRef]
- Prodam, F.; Pagano, L.; Corneli, G.; Golisano, G.; Belcastro, S.; Busti, A.; Gasco, V.; Beccuti, G.; Grottoli, S.; Di Somma, C.; et al. Update on epidemiology, etiology, and diagnosis of adult growth hormone deficiency. J. Endocrinol. Investig. 2008, 31, 6–11. [Google Scholar]
- Gröschl, M.; Knerr, I.; Topf, H.G.; Schmid, P.; Rascher, W.; Rauh, M. Endocrine responses to the oral ingestion of a physiological dose of essential amino acids in humans. J. Endocrinol. 2003, 179, 237–244. [Google Scholar] [CrossRef]
- Collier, S.R.; Casey, D.P.; Kanaley, J.A. Growth hormone responses to varying doses of oral arginine. Growth Horm. IGF Res. 2005, 15, 136–139. [Google Scholar] [CrossRef]
- Welbourne, T.C. Increased plasma bicarbonate and growth hormone after an oral glutamine load. Am. J. Clin. Nutr. 1995, 61, 1058–1061. [Google Scholar] [CrossRef]
- Isidori, A.; Lo Monaco, A.; Cappa, M. A study of growth hormone release in man after oral administration of amino acids. Curr. Med. Res. Opin. 1981, 7, 475–481. [Google Scholar] [CrossRef]
- Suminski, R.R.; Robertson, R.J.; Goss, F.L.; Arslanian, S.; Kang, J.; DaSilva, S.; Utter, A.C.; Metz, K.F. Acute effect of amino acid ingestion and resistance exercise on plasma growth hormone concentration in young men. Int. J. Sport Nutr. Exerc. Metab. 1997, 7, 48–60. [Google Scholar] [CrossRef]
- Lambert, M.I.; Hefer, J.A.; Millar, R.P.; Macfarlane, P.W. Failure of commercial oral amino acid supplements to increase serum growth hormone concentrations in male body-builders. Int. J. Sport Nutr. 1993, 3, 298–305. [Google Scholar] [CrossRef]
- Hickson, J.F.J.; Bucci, L.; Pivarnik, J.M.; Wolinsky, I.; Mcmahon, J.C.; Turner, S.D. Ornithine ingestion and growth hormone release in bodybuilders. Nutr. Res. 1990, 3, 239. [Google Scholar]
- Merimee, T.J.; Rabinowitz, D.; Fineberg, S.E. Arginine-Initiated Release of Human Growth Hormone. N. Engl. J. Med. 1969, 280, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Inoue, S.; Shiraki, J.; Shishido, T.; Saito, M.; Numata, K.; Takamura, Y. Age-related decrease in plasma growth hormone: Response to growth hormone-releasing hormone, arginine, and l-dopa in obesity. Metabolism 1991, 40, 1257–1262. [Google Scholar] [CrossRef]
- Van Vught, A.J.A.H.; Nieuwenhuizen, A.G.; Brummer, R.J.M.; Westerterp-Plantenga, M.S. Effects of oral ingestion of amino acids and proteins on the somatotropic axis. J. Clin. Endocrinol. Metab. 2008, 93, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Sellini, M.; Fierro, A.; Marchesi, L.; Manzo, G.; Giovannini, C. Behavior of Basal Values and Circadian Rhythm of ACTH, Cortisol, PRL and GH in a High-Protein Diet. Boll. Soc. Ital. Biol. Sper. 1981, 57, 963–969. [Google Scholar] [PubMed]
- Galbo, H.; Christensen, N.J.; Mikines, K.J.; Sonne, B.; Hilsted, J.; Hagen, C.; Fahrenkrug, J. The effect of fasting on the hormonal response to graded exercise. J. Clin. Endocrinol. Metab. 1981, 52, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Quirion, A.; Brisson, G.; De Carufel, D.; Laurencelle, L.; Therminarias, A.; Vogelaere, P. Influence of exercise and dietary modifications on plasma human growth hormone, insulin and FFA. J Sport. Med. Phys. Fit. 1988, 28, 352–353. [Google Scholar]
- Fleddermann, M.; Demmelmair, H.; Grote, V.; Bidlingmaier, M.; Grimminger, P.; Bielohuby, M.; Koletzko, B. Role of selected amino acids on plasma IGF-I concentration in infants. Eur. J. Nutr. 2017, 56, 613–620. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Allen, N.; Appleby, P.; Davey, G.; Kaaks, R.; Rinaldi, S.; Key, T. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1441–1448. [Google Scholar]
- Hoppe, C.; Udam, T.R.; Lauritzen, L.; Mølgaard, C.; Juul, A.; Michaelsen, K.F. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am. J. Clin. Nutr. 2004, 80, 447–452. [Google Scholar] [CrossRef]
- Romo Ventura, E.; Konigorski, S.; Rohrmann, S.; Schneider, H.; Stalla, G.K.; Pischon, T.; Linseisen, J.; Nimptsch, K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr. 2020, 59, 1413–1420. [Google Scholar] [CrossRef]
- Crowe, F.L.; Key, T.J.; Allen, N.E.; Appleby, P.N.; Roddam, A.; Overvad, K.; Grønbæk, H.; Tjønneland, A.; Halkjær, J.; Dossus, L.; et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1333–1340. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, K.; Brismar, K.; Wolk, A. Association of diet with serum insulin-like growth factor I in middle-aged and elderly men. Am. J. Clin. Nutr. 2005, 81, 1163–1167. [Google Scholar] [CrossRef]
- Norat, T.; Dossus, L.; Rinaldi, S.; Overvad, K.; Grønbæk, H.; Tjønneland, A.; Olsen, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Boeing, H.; et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur. J. Clin. Nutr. 2007, 61, 91–98. [Google Scholar] [CrossRef]
- Beasley, J.M.; Gunter, M.J.; Lacroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Vitolins, M.Z.; Strickler, H.D. Associations of serum insulin-like growth factor-I and insulin-like growth factor-binding protein 3 levels with biomarker-calibrated protein, dairy product and milk intake in the Women’s Health Initiative. Br. J. Nutr. 2014, 111, 847–853. [Google Scholar] [CrossRef]
- Takahashi, S.; Kajikawa, M.; Umezawa, T.; Takahashi, S.; Kato, H.; Miura, Y.; Nam, T.; Noguchi, T.; Naito, H. Effect of dietary proteins on the plasma immunoreactive insulin-like growth factor-1/somatomedin C concentration in the rat. Br. J. Nutr. 1990, 63, 521–534. [Google Scholar] [CrossRef]
- Takenaka, A.; Oki, N.; Takahashi, S.I.; Noguchi, T. Dietary restriction of single essential amino acids reduces plasma insulin-like growth factor-I (IGF-I) but does not affect plasma IGF-binding protein-1 in rats. J. Nutr. 2000, 130, 2910–2914. [Google Scholar] [CrossRef]
- Thissen, J.P.; Pucilowska, J.B.; Underwood, L.E. Differential regulation of insulin-like growth factor I (IGF-I) and IGF Binding Protein-1 messenger ribonucleic acids by amino acid availability and growth hormone in rat hepatocyte primary culture. Endocrinology 1994, 134, 1570–1576. [Google Scholar] [CrossRef]
- Philipps, A.F.; Dvořák, B.; Kling, P.J.; Grille, J.G.; Koldovský, O. Absorption of Milk-Borne Insulin-Like Growth Factor-I into Portal Blood of Suckling Rats. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberger, G.; Senn, H.J. Insulin-like growth factors and cancer. Lancet Oncol. 2002, 3, 298–302. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Paszynska, E.; Dmitrzak-Weglarz, M.; Slopien, A.; Tyszkiewicz-Nwafor, M.; Rajewski, A. Salivary and serum insulin-like growth factor (IGF-1) assays in anorexic patients. World J. Biol. Psychiatry 2016, 17, 615–621. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef]
- Tucker, L.A.; Erickson, A.; Lecheminant, J.D.; Bailey, B.W. Dairy Consumption and Insulin Resistance: The Role of Body Fat, Physical Activity, and Energy Intake. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar] [CrossRef]
- Orgeron, M.L.; Stone, K.P.; Wanders, D.; Cortez, C.C.; Van, N.T.; Gettys, T.W. The impact of dietary methionine restriction on biomarkers of metabolic health. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 121, pp. 351–376. ISBN 9780128001011. [Google Scholar]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med. Hypotheses 2009, 72, 125–128. [Google Scholar] [CrossRef]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam. Repub. Iran 2019, 33. [Google Scholar] [CrossRef]
- Le Marchand-Brustel, Y.; Heydrick, S.J.; Jullien, D.; Gautier, N.; Van Obberghen, E. Effect of insulin and insulin-like growth factor-1 on glucose transport and its transporters in soleus muscle of lean and obese mice. Metabolism 1995, 44, 18–23. [Google Scholar] [CrossRef]
- Gjedsted, J.; Gormsen, L.C.; Nielsen, S.; Schmitz, O.; Djurhuus, C.B.; Keiding, S.; Ørskov, H.; Tønnesen, E.; Møller, N. Effects of a 3-day fast on regional lipid and glucose metabolism in human skeletal muscle and adipose tissue. Acta Physiol. 2007, 191, 205–216. [Google Scholar] [CrossRef]
- Wurzburger, M.I.; Prelevic, G.M.; Sonksen, P.H.; Balint-Peric, L.A.; Wheeler, M. The effect of recombinant human growth hormone on regulation of growth hormone secretion and blood glucose in insulin-dependent diabetes. J. Clin. Endocrinol. Metab. 1993, 77, 267–272. [Google Scholar] [CrossRef]
- Li, R.; Ferreira, M.; Cooke, M.; La Bounty, P.; Campbell, B.; Greenwood, M.; Willoughby, D.; Kreider, R. Co-ingestion of carbohydrate with branched-chain amino acids or L-leucine does not preferentially increase serum IGF-1 and expression of myogenic-related genes in response to a single bout of resistance exercise. Amino Acids 2015, 47, 1203–1213. [Google Scholar] [CrossRef]
- Church, D.D.; Schwarz, N.A.; Spillane, M.B.; McKinley-Barnard, S.K.; Andre, T.L.; Ramirez, A.J.; Willoughby, D.S. l-Leucine Increases Skeletal Muscle IGF-1 but Does Not Differentially Increase Akt/mTORC1 Signaling and Serum IGF-1 Compared to Ursolic Acid in Response to Resistance Exercise in Resistance-Trained Men. J. Am. Coll. Nutr. 2016, 35, 627–638. [Google Scholar] [CrossRef]
- Biagetti, B.; Herance, J.R.; Ferrer, R.; Aulinas, A.; Palomino-Schätzlein, M.; Mesa, J.; Castaño, J.P.; Luque, R.M.; Simó, R. Metabolic Fingerprint of Acromegaly and its Potential Usefulness in Clinical Practice. J. Clin. Med. 2019, 8, 1549. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Martos-Moreno, G.; Rupérez, F.J.; Chowen, J.A.; Barbas, C.; Argente, J. Metabolomics changes in patients with PAPP-A2 deficiency in response to rhIGF1 treatment. Growth Horm. IGF Res. 2018, 42–43, 28–31. [Google Scholar] [CrossRef]
- Sawa, R.; Nishida, H.; Yamamoto, Y.; Wake, I.; Kai, N.; Kikkawa, U.; Okimura, Y. Growth hormone and Insulin-like growth factor-I (IGF-I) modulate the expression of L-type amino acid transporters in the muscles of spontaneous dwarf rats and L6 and C2C12 myocytes. Growth Horm. IGF Res. 2018, 42–43, 66–73. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Quabbe, H.J.; Bratzke, H.J.; Siegers, U.; Elban, K. Studies on the relationship between plasma free fatty acids and growth hormone secretion in man. J. Clin. Investig. 1972, 51, 2388–2398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moller, N.; Jorgensen, J.O.L.; Schmitz, O.; Moller, J.; Christiansen, J.S.; Alberti, K.G.M.M.; Orskov, H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am. J. Physiol. Endocrinol. Metab. 1990, 258. [Google Scholar] [CrossRef] [PubMed]
- Neely, R.D.G.; Rooney, D.P.; Bell, P.M.; Bell, N.P.; Sheridan, B.; Atkinson, A.B.; Trimble, E.R. Influence of growth hormone on glucose-glucose 6-phosphate cycle and insulin action in normal humans. Am. J. Physiol. Endocrinol. Metab. 1992, 263. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, A.T.M.G.; Boden, G.; Silva, M.E.R.; Rocha, D.M.; Santos, R.F.; Ursich, M.J.M.; Strassmann, P.G.; Wajchenberg, B.L. Overnight lowering of free fatty acids with acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999, 48, 1836–1841. [Google Scholar] [CrossRef]
- Zachmann, M.; Fernandez, F.; Tassinari, D.; Thakker, R.; Prader, A. Anthropometric measurements in patients with growth hormone deficiency before treatment with human growth hormone. Eur. J. Pediatr. 1980, 133, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Salomon, F.; Cuneo, R.C.; Hesp, R.; Sönksen, P.H. The Effects of Treatment with Recombinant Human Growth Hormone on Body Composition and Metabolism in Adults with Growth Hormone Deficiency. N. Engl. J. Med. 1989, 321, 1797–1803. [Google Scholar] [CrossRef]
- Davidson, M.B. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr. Rev. 1987, 8, 115–131. [Google Scholar] [CrossRef]
- Kreitschmann-Andermahr, I.; Suarez, P.; Jennings, R.; Evers, N.; Brabant, G. GH/IGF-I regulation in obesity—Mechanisms and practical consequences in children and adults. Horm. Res. Paediatr. 2010, 73, 153–160. [Google Scholar] [CrossRef]
- Fuccella, L.M.; Goidaniga, G.; Lovisolo, P.; Maggi, E.; Musatti, L.; Mandelli, V.; Sirtori, C.R. Inhibition of lipolysis by nicotinic acid and by acipimox. Clin. Pharmacol. Ther. 1980, 28, 790–795. [Google Scholar] [CrossRef]
- Cordido, F.; Alvarez-Castro, P.; Isidro, M.L.; Casanueva, F.F.; Dieguez, C. Comparison between insulin tolerance test, growth hormone (GH)-releasing hormone (GHRH), GHRH plus acipimox and GHRH plus GH-releasing peptide-6 for the diagnosis of adult GH deficiency in normal subjects, obese hypopituitary patients. Eur. J. Endocrinol. 2003, 149, 117–122. [Google Scholar] [CrossRef]
- Glass, A.R.; Burman, K.D.; Dahms, W.T.; Boehm, T.M. Endocrine function in human obesity. Metabolism 1981, 30, 89–104. [Google Scholar] [CrossRef]
- Peino, R.; Cordido, F.; Peñalva, A.; Alvarez, C.V.; Dieguez, C.; Casanueva, F.F. Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. J. Clin. Endocrinol. Metab. 1996, 81, 909–913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imaki, T.; Shibasaki, T.; Masuda, A.; Hotta, M.; Yamauchi, N.; Demura, H.; Shizume, K.; Wakabayashi, I.; Ling, N. The effect of glucose and free fatty acids on growth hormone (gh)-releasing factor-mediated gh secretion in rats. Endocrinology 1986, 118, 2390–2394. [Google Scholar] [CrossRef]
- Casanueva, F.F. Physiology of growth hormone secretion and action. Endocrinol. Metab. Clin. N. Am. 1992, 21, 483–517. [Google Scholar] [CrossRef]
- Iranmanesh, A.; Veldhuis, J. Clinical pathophysiology of the somatotropic (GH) axis in adults. Endocrinol. Metab. Clin. North Am. 1992, 21, 783–816. [Google Scholar] [CrossRef]
- Jenkins, R.C.; Ross, R.J.M. Acquired growth hormone resistance in catabolic states. Baillieres. Clin. Endocrinol. Metab. 1996, 10, 411–419. [Google Scholar] [CrossRef]
- Scanlon, M.F.; Issa, B.G.; Dieguez, C. Regulation of growth hormone secretion. Horm. Res. Paediatr. 1996, 46, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Lindblad, A.; Engfeldt, P.; Arner, P. Studies of acute effects of insulin-like growth factors I and II in human fat cells. J. Clin. Endocrinol. Metab. 1987, 65, 732–737. [Google Scholar] [CrossRef]
- Pratipanawatr, T.; Pratipanawatr, W.; Rosen, C.; Berria, R.; Bajaj, M.; Cusi, K.; Mandarino, L.; Kashyap, S.; Belfort, R.; DeFronzo, R.A. Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 282. [Google Scholar] [CrossRef]
- Fang, X.L.; Shu, G.; Zhang, Z.Q.; Wang, S.B.; Zhu, X.T.; Gao, P.; Xi, Q.Y.; Zhang, Y.L.; Jiang, Q.Y. Roles of α-linolenic acid on IGF-I secretion and GH/IGF system gene expression in porcine primary hepatocytes. Mol. Biol. Rep. 2012, 39, 10987–10996. [Google Scholar] [CrossRef]
- Yen, P.M.; Tashjian, A.H. Short chain fatty acids increase prolactin and growth hormone production and alter cell morphology in the GH3 strain of rat pituitary cells. Endocrinology 1981, 109, 17–22. [Google Scholar] [CrossRef]
- Kato, S.I.; Sato, K.; Chida, H.; Roh, S.G.; Ohwada, S.; Sato, S.; Guilloteau, P.; Katoh, K. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J. Endocrinol. 2011, 211, 241–248. [Google Scholar] [CrossRef]
- Ishiwata, H.; Nagano, M.; Sasaki, Y.; Chen, C.; Katoh, K. Short-chain fatty acids inhibit the release and content of growth hormone in anterior pituitary cells of the goat. Gen. Comp. Endocrinol. 2000, 118, 400–406. [Google Scholar] [CrossRef]
- Wang, J.F.; Fu, S.P.; Li, S.N.; Hu, Z.M.; Xue, W.J.; Li, Z.Q.; Huang, B.X.; Lv, Q.K.; Liu, J.X.; Wang, W. Short-chain fatty acids inhibit growth hormone and prolactin gene transcription via cAMP/PKA/CREB signaling pathway in dairy cow anterior pituitary cells. Int. J. Mol. Sci. 2013, 14, 21474–21488. [Google Scholar] [CrossRef]
- Fu, S.P.; Liu, B.R.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Zeng, Y.L.; Huang, B.X.; Li, S.N.; Lv, Q.K.; Wang, W.; et al. β-hydroxybutyric acid inhibits growth hormone-releasing hormone synthesis and secretion through the GPR109A/extracellular signal-regulated 1/2 signalling pathway in the hypothalamus. J. Neuroendocrinol. 2015, 27, 212–222. [Google Scholar] [CrossRef]
- Pérez-Fernandez, R.; Alonso, M.; Segura, C.; Muñoz, I.; García-Caballero, T.; Diéguez, C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. 1996, 60, 35–42. [Google Scholar] [CrossRef]
- Giordano, M.; Godi, M.; Mellone, S.; Petri, A.; Vivenza, D.; Tiradani, L.; Carlomagno, Y.; Ferrante, D.; Arrigo, T.; Corneli, G.; et al. A functional common polymorphism in the vitamin D-responsive element of the GH1 promoter contributes to isolated growth hormone deficiency. J. Clin. Endocrinol. Metab. 2008, 93, 1005–1012. [Google Scholar] [CrossRef][Green Version]
- Seoane, S.; Perez-Fernandez, R. The vitamin D receptor represses transcription of the pituitary transcription factor Pit-1 gene without involvement of the retinoid X receptor. Mol. Endocrinol. 2006, 20, 735–748. [Google Scholar] [CrossRef][Green Version]
- Ameri, P.; Giusti, A.; Boschetti, M.; Murialdo, G.; Minuto, F.; Ferone, D. Interactions between vitamin D and IGF-I: From physiology to clinical practice. Clin. Endocrinol. (Oxf.) 2013, 79, 457–463. [Google Scholar] [CrossRef]
- Keisala, T.; Minasyan, A.; Lou, Y.R.; Zou, J.; Kalueff, A.V.; Pyykkö, I.; Tuohimaa, P. Premature aging in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol. 2009, 115, 91–97. [Google Scholar] [CrossRef]
- Ciresi, A.; Giordano, C. Vitamin D across growth hormone (GH) disorders: From GH deficiency to GH excess. Growth Horm. IGF Res. 2017, 33, 35–42. [Google Scholar] [CrossRef]
- Robson, H.; Siebler, T.; Shalet, S.M.; Williams, G.R. Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr. Res. 2002, 52, 137–147. [Google Scholar] [CrossRef]
- Song, Y.; Kato, S.; Fleet, J.C. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J. Nutr. 2003, 133, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Chen, X.; Wang, S.; Parlow, A.F.; Xu, J. Steroid Receptor Coactivator 3 Maintains Circulating Insulin-Like Growth Factor I (IGF-I) by Controlling IGF-Binding Protein 3 Expression. Mol. Cell. Biol. 2008, 28, 2460–2469. [Google Scholar] [CrossRef]
- Wei, S.; Tanaka, H.; Kubo, T.; Ono, T.; Kanzaki, S.; Seino, Y. Growth hormone increases serum 1,25-dihydroxyvitamin D levels and decreases 24,25-dihydroxyvitamin D levels in children with growth hormone deficiency. Eur. J. Endocrinol. 1997, 136, 45–51. [Google Scholar] [CrossRef][Green Version]
- Halhali, A.; Díaz, L.; Sánchez, I.; Garabédian, M.; Bourges, H.; Larrea, F. Effects of IGF-I on 1,25-dihydroxyvitamin D3 synthesis by human placenta in culture. Mol. Hum. Reprod. 1999, 5, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Guibourdenche, J.; Djakouré, C.; Porquet, D.; Pagésy, P.; Rochette-Egly, C.; Peillon, F.; Li, J.Y.; Evain-Brion, D. Retinoic acid stimulates growth hormone synthesis in human somatotropic adenoma cells: Characterization of its nuclear receptors. J. Cell. Biochem. 1997, 65, 25–31. [Google Scholar] [CrossRef]
- Prodam, F.; Caputo, M.; Mele, C.; Marzullo, P.; Aimaretti, G. Insights into non-classic and emerging causes of hypopituitarism. Nat. Rev. Endocrinol. 2021, 17, 114–129. [Google Scholar] [CrossRef]
- Djakoure, C.; Guibourdenche, J.; Porquet, D.; Pagesy, P.; Peillon, F.; Li, J.Y.; Evain-Brion, D. Vitamin A and retinoic acid stimulate within minutes cAMP release and growth hormone secretion in human pituitary cells. J. Clin. Endocrinol. Metab. 1996, 81, 3123–3126. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Butler, A.M.; Fiala-Beer, E.; Su, G.M. Phospho-STAT5 accumulation in nuclear fractions from vitamin A-deficient rat liver. FEBS Lett. 2005, 579, 3669–3673. [Google Scholar] [CrossRef][Green Version]
- Raifen, R.; Altman, Y.; Zadik, Z. Vitamin A levels and growth hormone axis. Horm. Res. Paediatr. 1996, 46, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Evain-Brion, D.; Fjellestad-Paulsen, A.; Czernichow, P.; Porquet, D.; Thérond, P.; Grenèche, M.O.; François, L. Vitamin A deficiency and nocturnal growth hormone secretion in short children. Lancet 1994, 343, 87–88. [Google Scholar] [CrossRef]
- Mohn, A.; Di Marzio, D.; Giannini, C.; Capanna, R.; Marcovecchio, M.; Chiarelli, F. Alterations in the oxidant-antioxidant status in prepubertal children with growth hormone deficiency: Effect of growth hormone replacement therapy. Clin. Endocrinol. (Oxf.) 2005, 63, 537–542. [Google Scholar] [CrossRef]
- Ren, S.G.; Melmed, S. Pyridoxal phosphate inhibits pituitary cell proliferation and hormone secretion. Endocrinology 2006, 147, 3936–3942. [Google Scholar] [CrossRef]
- Báez-Saldaña, A.; Gutiérrez-Ospina, G.; Chimal-Monroy, J.; Fernandez-Mejia, C.; Saavedra, R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-I. Eur. J. Nutr. 2009, 48, 137–144. [Google Scholar] [CrossRef]
- Waters, M.J.; Shang, C.A.; Behncken, S.N.; Tam, S.P.; Li, H.; Shen, B.; Lobie, P.E. Growth hormone as a cytokine. Clin. Exp. Pharmacol. Physiol. 1999, 26, 760–764. [Google Scholar] [CrossRef]
- Roman-Garcia, P.; Quiros-Gonzalez, I.; Mottram, L.; Lieben, L.; Sharan, K.; Wangwiwatsin, A.; Tubio, J.; Lewis, K.; Wilkinson, D.; Santhanam, B.; et al. Vitamin B12-dependent taurine synthesis regulates growth and bone mass. J. Clin. Investig. 2014, 124, 2988–3002. [Google Scholar] [CrossRef]
- Kamenický, P.; Mazziotti, G.; Lombès, M.; Giustina, A.; Chanson, P. Growth hormone, insulin-like growth factor-1, and the kidney: Pathophysiological and clinical implications. Endocr. Rev. 2014, 35, 234–281. [Google Scholar] [CrossRef]
- Feld, S.; Hirschberg, R. Growth Hormone, the Insulin-Like Growth Factor System, and the Kidney. Endocr. Rev. 1996, 17, 423–480. [Google Scholar] [CrossRef]
- McCormick, S.D.; Bradshaw, D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Rosen, T.; Bosaeus, I.; Tolli, J.; Lindstedt, G.; Bengtsson, B.A. Increased body fat mass and decreased extracellular fluid volume in adults with growth hormone deficiency. Clin. Endocrinol. 1993, 38, 63–71. [Google Scholar] [CrossRef]
- Dimke, H.; Flyvbjerg, A.; Frische, S. Acute and chronic effects of growth hormone on renal regulation of electrolyte and water homeostasis. Growth Horm. IGF Res. 2007, 17, 353–368. [Google Scholar] [CrossRef]
- Ikkos, D.; Luft, R.; Sjogren, B. Body water and sodium in patients with acromegaly. J. Clin. Investig. 1954, 33, 989–994. [Google Scholar] [CrossRef]
- Flyvbjerg, A.; Dørup, I.; Everts, M.E.; Ørskov, H. Evidence that potassium deficiency induces growth retardation through reduced circulating levels of growth hormone and insulin-like growth factor I. Metabolism 1991, 40, 769–775. [Google Scholar] [CrossRef]
- Flyvbjerg, A.; Marshall, S.M.; Frystyk, J.; Rasch, R.; Bornfeldt, K.E.; Arnqvist, H.; Jensen, P.K.; Pallesen, G.; Orskov, H. Insulin-like growth factor I in initial renal hypertrophy in potassium- depleted rats. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1992, 262. [Google Scholar] [CrossRef]
- Zivadinovic, D.; Tomić, M.; Yuan, D.; Stojilkovic, S.S. Cell-type specific messenger functions of extracellular calcium in the anterior pituitary. Endocrinology 2002, 143, 445–455. [Google Scholar] [CrossRef]
- Nussinovitch, I. Tive supervision enhance. Endocrinology 2018, 159, 4043–4055. [Google Scholar] [CrossRef]
- Coiro, V.; Volpi, R.; Capretti, L.; Finardi, L.; Magotti, M.G.; Manfredi, G.; Chiodera, P.; Jotti, G.S. Inhibition of growth hormone secretion in mild primary hyperparathyroidism. Horm. Res. 2004, 62, 88–91. [Google Scholar] [CrossRef]
- Romoli, R.; Lania, A.; Mantovani, G.; Corbetta, S.; Persani, L.; Spada, A. Expression of Calcium-Sensing Receptor and Characterization of Intracellular Signaling in Human Pituitary Adenomas1. J. Clin. Endocrinol. Metab. 1999, 84, 2848–2853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikema, S.; Horikawa, R.; Nakano, M.; Yokouchi, K.; Yamazaki, H.; Tanaka, T.; Tanae, A. Growth and metabolic disturbances in a patient with total parenteral nutrition: A case of hypercalciuric hypercalcemia. Endocr. J. 2000, 47. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Bruns, M.E.; Hock, J.M.; Wood, R.J. Growth hormone and parathyroid hormone stimulate intestinal calcium absorption in aged female rats. Endocrinology 1994, 134, 1755–1760. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Müller, D.; Van Der Kemp, A.W.; Hartog, A.; Suzuki, M.; Ishibashi, K.; Imai, M.; Sweep, F.; Willems, P.H.; Van Os, C.H.; et al. Calcitriol controls the epithelial calcium channel in kidney. J. Am. Soc. Nephrol. 2001, 12, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Kamenický, P.; Blanchard, A.; Gauci, C.; Salenave, S.; Letierce, A.; Lombès, M.; Brailly-Tabard, S.; Azizi, M.; Prié, D.; Souberbielle, J.C.; et al. Pathophysiology of renal calcium handling in acromegaly: What lies behind hypercalciuria? J. Clin. Endocrinol. Metab. 2012, 97, 2124–2133. [Google Scholar] [CrossRef]
- Halloran, B.P.; Spencer, E.M. Dietary phosphorus and 1,25-dihydroxyvitamin d metabolism: Influence of insulin-like growth factor i. Endocrinology 1988, 123, 1225–1229. [Google Scholar] [CrossRef]
- Harbison, M.D.; Gertner, J.M. Permissive action of growth hormone on the renal response to dietary phosphorus deprivation. J. Clin. Endocrinol. Metab. 1990, 70, 1035–1040. [Google Scholar] [CrossRef]
- Saggese, G.; Baroncelli, G.I.; Federico, G.; Bertelloni, S. Effects of growth hormone on phosphocalcium homeostasis and bone metabolism. Horm. Res. Paediatr. 1995, 44, 55–63. [Google Scholar] [CrossRef]
- Efthymiadou, A.; Kritikou, D.; Mantagos, S.; Chrysis, D. The effect of GH treatment on serum FGF23 and Klotho in GH-deficient children. Eur. J. Endocrinol. 2016, 174, 473–479. [Google Scholar] [CrossRef]
- Hirschberg, R.; Brunori, G.; Kopple, J.D.; Guler, H.P. Effects of insulin-like growth factor I on renal function in normal men. Kidney Int. 1993, 43, 387–397. [Google Scholar] [CrossRef]
- Giordano, M.; DeFronzo, R.A. Acute effect of human recombinant insulin-like growth factor I on renal function in humans. Nephron 1995, 71, 10–15. [Google Scholar] [CrossRef]
- Woda, C.B.; Halaihel, N.; Wilson, P.V.; Haramati, A.; Levi, M.; Mulroney, S.E. Regulation of renal NaPi-2 expression and tubular phosphate reabsorption by growth hormone in the juvenile rat. Am. J. Physiol. Ren. Physiol. 2004, 287. [Google Scholar] [CrossRef]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Schmid, C.; Neidert, M.C.; Tschopp, O.; Sze, L.; Bernays, R.L. Growth hormone and Klotho. J. Endocrinol. 2013, 219, R37–R57. [Google Scholar] [CrossRef]
- Narayanan, N.; Lussier, B.; French, M.; Moor, B.; Kraicer, J. Growth hormone-releasing factor-sensitive adenylate cyclase system of purified somatotrophs: Effects of guanine nucleotides, somatostatin, calcium, and magnesium. Endocrinology 1989, 124, 484–495. [Google Scholar] [CrossRef]
- Dørup, I.; Flyvbjerg, A.; Everts, M.E.; Clausen, T. Role of insulin-like growth factor-1 and growth hormone in growth inhibition induced by magnesium and zinc deficiencies. Br. J. Nutr. 1991, 66, 505–521. [Google Scholar] [CrossRef]
- Henneman, P.H.; Forbes, A.P.; Moldawer, M.; Dempsey, E.F.; Carroll, E.L. Effects of human growth hormone in man. J. Clin. Investig. 1960, 39, 1223–1238. [Google Scholar] [CrossRef]
- Mahlbacher, K.; Sicuro, A.; Gerber, H.; Hulter, H.N.; Krapf, R. Growth hormone corrects acidosis-induced renal nitrogen wasting and renal phosphate depletion and attenuates renal magnesium wasting in humans. Metabolism 1999, 48, 763–770. [Google Scholar] [CrossRef]
- Estívariz, C.F.; Ziegler, T.R. Nutrition and the Insulin-Like Growth Factor System. Endocrine 1997, 7, 65–71. [Google Scholar] [CrossRef]
- Millward, D.J. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef]
- Cunningham, B.C.; Mulkerrin, M.G.; Wells, J.A. Dimerization of human growth hormone by zinc. Science 1991, 253, 545–548. [Google Scholar] [CrossRef]
- Li, R.; Hui, J.; Luo, G.; Hong, P.; Li, L.; Yang, Y.; Zheng, X.; Lan, H. Zinc ions increase GH signaling ability through regulation of available plasma membrane-localized GHR. J. Cell. Physiol. 2019, 234, 23388–23397. [Google Scholar] [CrossRef]
- Petkovic, V.; Miletta, M.C.; Eblé, A.; Iliev, D.I.; Binder, G.; Flück, C.E.; Mullis, P.E. Effect of zinc binding residues in growth hormone (GH) and altered intracellular zinc content on regulated GH secretion. Endocrinology 2013, 154, 4215–4225. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.S.; Das, S.; Ghosh, S.; Anoop, A.; Jha, N.N.; Khan, T.; Singru, P.; Kumar, A.; Maji, S.K. Amyloid formation of growth hormone in presence of zinc: Relevance to its storage in secretory granules. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Balaz, M.; Ukropcova, B.; Kurdiova, T.; Gajdosechova, L.; Vlcek, M.; Janakova, Z.; Fedeles, J.; Pura, M.; Gasperikova, D.; Smith, S.R.; et al. Adipokine zinc-α2-glycoprotein regulated by growth hormone and linked to insulin sensitivity. Obesity 2015, 23, 322–328. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.P.; Kirchgessner, M. Influence of alimentary zinc deficiency on the concentration of growth hormone (GH), insulin-like growth factor I (IGF-I) and insulin in the serum of force-fed rats. Horm. Metab. Res. 1994, 26, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ninh, N.X.; Thissen, J.P.; Maiter, D.; Adam, E.; Mulumba, N.; Ketelslegers, J.M. Reduced liver insulin-like growth factor-I gene expression in young zinc-deprived rats is associated with a decrease in liver growth hormone (GH) receptors and serum GH-binding protein. J. Endocrinol. 1995, 144, 449–456. [Google Scholar] [CrossRef]
- Hamza, R.T.; Hamed, A.I.; Sallam, M.T. Effect of zinc supplementation on growth Hormone Insulin growth factor axis in short Egyptian children with zinc deficiency. Ital. J. Pediatr. 2012, 38. [Google Scholar] [CrossRef]
- Ekbote, V.; Khadilkar, A.; Chiplonkar, S.; Mughal, Z.; Khadilkar, V. Enhanced effect of zinc and calcium supplementation on bone status in growth hormone-deficient children treated with growth hormone: A pilot randomized controlled trial. Endocrine 2013, 43, 686–695. [Google Scholar] [CrossRef]
- de Medeiros Rocha, É.D.; de Brito, N.J.N.; Dantas, M.M.G.; de Araújo Silva, A.; das Graças Almeida, M.; Brandão-Neto, J. Effect of Zinc Supplementation on GH, IGF1, IGFBP3, OCN, and ALP in Non-Zinc-Deficient Children. J. Am. Coll. Nutr. 2015, 34, 290–299. [Google Scholar] [CrossRef]
- Prodam, F.; Aimaretti, G. Could zinc supplementation improve bone status in growth hormone (GH) deficient children? Endocrine 2013, 43, 467–468. [Google Scholar] [CrossRef][Green Version]
- Bouglé, D.; Laroche, D.; Bureau, F. Zinc and iron status and growth in healthy infants. Eur. J. Clin. Nutr. 2000, 54, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Stred, S.E.; Messina, J.L. Identification of hemopexin as a GH-regulated gene. Mol. Cell. Endocrinol. 2003, 204, 101–110. [Google Scholar] [CrossRef]
- Weinzimer, S.A.; Gibson, T.B.; Collett-Solberg, P.F.; Khare, A.; Liu, B.; Cohen, P. Transferrin Is an Insulin-Like Growth Factor-Binding Protein-3 Binding Protein1. J. Clin. Endocrinol. Metab. 2001, 86, 1806–1813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyagawa, S.I.; Kobayashi, M.; Konishi, N.; Sato, T.; Ueda, K. Insulin and insulin-like growth factor I support the proliferation of erythroid progenitor cells in bone marrow through the sharing of receptors. Br. J. Haematol. 2000, 109, 555–562. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S. Association of serum insulin-like growth factor-I and erythropoiesis in relation to body iron status. Ann. Clin. Lab. Sci. 2004, 34, 324–328. [Google Scholar]
- De Vita, F.; Maggio, M.; Lauretani, F.; Crucitti, L.; Bandinelli, S.; Mammarella, F.; Landi, F.; Ferrucci, L.; Ceda, G.P. Insulin-like growth factor-1 and anemia in older subjects: The inchianti study. Endocr. Pract. 2015, 21, 1211–1218. [Google Scholar] [CrossRef][Green Version]
- Vihervuori, E.; Cook, J.D.; Siimes, M.A. Iron status of children with short stature during accelerated growth due to growth hormone treatment. Acta Paediatr. Int. J. Paediatr. 1997, 86, 588–593. [Google Scholar] [CrossRef]
- Anwar, U.; ZR, A.; Filteau, S.; Sullivan, K.; Tomkins, A. The impact of maternal supplementation with a single dose of oral iodized poppyseed oil on infant thyroid status in rural Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 499–501. [Google Scholar]
- Zimmermann, M.B.; Jooste, P.L.; Mabapa, N.S.; Mbhenyane, X.; Schoeman, S.; Biebinger, R.; Chaouki, N.; Bozo, M.; Grimci, L.; Bridson, J. Treatment of iodine deficiency in school-age children increases insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations and improves somatic growth. J. Clin. Endocrinol. Metab. 2007, 92, 437–442. [Google Scholar] [CrossRef]
- Crew, M.; Spindler, S. Thyroid hormone regulation of the transfected rat growth hormone promoter. J. Biol. Chem. 1986, 261, 5018–5022. [Google Scholar] [CrossRef]
- Miell, J.P.; Taylor, A.M.; Zini, M.; Maheshwari, H.G.; Ross, R.J.M.; Valcavi, R. Effects of hypothyroidism and hyperthyroidism on insulin-like growth factors (IGFs) and growth hormone- and IGF-binding proteins. J. Clin. Endocrinol. Metab. 1993, 76, 950–955. [Google Scholar] [CrossRef]
- Nanto-Salonen, K.; Muller, H.L.; Hoffman, A.R.; Vu, T.H.; Rosenfeld, R.G. Mechanisms of thyroid hormone action on the insulinlike growth factor system: All thyroid hormone effects are not growth hormone mediated. Endocrinology 1993, 132, 781–788. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, O.; Flyvbjerg, A.; Esmann, J. Evidence that selenium induces growth retardation through reduced growth hormone and somatomedin c production. Endocrinology 1987, 120, 659–663. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Ren, G.; Ali, T.; Chen, W.; Han, D.; Zhang, L.; Gu, X.; Zhang, S.; Ding, L.; Fanning, S.; Han, B. The role of selenium in insulin-like growth factor I receptor (IGF-IR) expression and regulation of apoptosis in mouse osteoblasts. Chemosphere 2016, 144, 2158–2164. [Google Scholar] [CrossRef]
- Wang, J.; Lian, S.; He, X.; Yu, D.; Liang, J.; Sun, D.; Wu, R. Selenium deficiency induces splenic growth retardation by deactivating the IGF-1R/PI3K/Akt/mTOR pathway. Metallomics 2018, 10, 1570–1575. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.D.; Lauretani, F.; Buttò, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Agli, E.; Ceda, G.P. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef]
- Aydin, K.; Bideci, A.; Kendirci, M.; Cinaz, P.; Kurtoglu, S. Insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels of children living in an iodine- and selenium-deficient endemic goiter area. Biol. Trace Elem. Res. 2002, 90, 25–30. [Google Scholar] [CrossRef]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Dall’Aglio, E.; Guralnik, J.M.; Paolisso, G.; Semba, R.D.; Nouvenne, A.; Borghi, L.; et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: The InCHIANTI study. Clin. Nutr. 2010, 29, 674–677. [Google Scholar] [CrossRef]
- Young, N.J.; Metcalfe, C.; Gunnell, D.; Rowlands, M.A.; Lane, J.A.; Gilbert, R.; Avery, K.N.L.; Davis, M.; Neal, D.E.; Hamdy, F.C.; et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes Control 2012, 23, 907–917. [Google Scholar] [CrossRef]
- Janjuha, R.; Bunn, D.; Hayhoe, R.; Hooper, L.; Abdelhamid, A.; Mahmood, S.; Hayden-Case, J.; Appleyard, W.; Morris, S.; Welch, A. Effects of dietary or supplementary micronutrients on sex hormones and IGF-1 in middle and older age: A systematic review and meta-analysis. Nutrients 2020, 12, 1457. [Google Scholar] [CrossRef]
- Werner, L.; Korc, M.; Brannon, P.M. Effects of manganese deficiency and dietary composition on rat pancreatic enzyme content. J. Nutr. 1987, 117, 2079–2085. [Google Scholar] [CrossRef]
- Clegg, M.S.; Donovan, S.M.; Monaco, M.H.; Baly, D.L.; Ensunsa, J.L.; Keen, C.L. The influence of manganese deficiency on serum IGF-1 and IGF binding proteins in the male rat. Proc. Soc. Exp. Biol. Med. 1998, 219, 41–47. [Google Scholar] [CrossRef]
- Bryan, M.R.; Nordham, K.D.; Rose, D.I.R.; O’Brien, M.T.; Joshi, P.; Foshage, A.M.; Gonçalves, F.M.; Nitin, R.; Uhouse, M.A.; Aschner, M.; et al. Manganese Acts upon Insulin/IGF Receptors to Phosphorylate AKT and Increase Glucose Uptake in Huntington’s Disease Cells. Mol. Neurobiol. 2020, 57, 1570–1593. [Google Scholar] [CrossRef]
- Hiney, J.K.; Srivastava, V.K.; Dees, W. Les Manganese induces IGF-1 and cyclooxygenase-2 gene expressions in the basal hypothalamus during prepubertal female development. Toxicol. Sci. 2011, 121, 389–396. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Zhu, X.; Gao, Y.; Liu, Z.; Zhang, L.; Chen, H.; Shi, X.; Yang, L.; Liu, G. High lever dietary copper promote ghrelin gene expression in the fundic gland of growing pigs. Biol. Trace Elem. Res. 2012, 150, 154–157. [Google Scholar] [CrossRef]
- Roughead, Z.K.F.; Lukaski, H.C. Inadequate copper intake reduces serum insulin-like growth factor-I and bone strength in growing rats fed graded amounts of copper and zinc. J. Nutr. 2003, 33, 442–448. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Liu, L.; Zhu, X.; Wang, X.; Liu, Z.; Wang, Z.; Yang, L.; Liu, G. Effect of high dietary copper on somatostatin and growth hormone-releasing hormone levels in the hypothalami of growing pigs. Biol. Trace Elem. Res. 2011, 143, 893–900. [Google Scholar] [CrossRef]
- Wang, M.Q.; Xu, Z.R.; Li, W.F.; Jiang, Z.G. Effect of chromium nanocomposite supplementation on growth hormone pulsatile secretion and mRNA expression in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2009, 93, 520–525. [Google Scholar] [CrossRef]
- Merimee, T.J.; Pulkkinen, A.J.; Burton, C.E. Diet-induced alterations of hgh secretion in man. J. Clin. Endocrinol. Metab. 1976, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Schenk, S.; Barkan, A.L.; Horowitz, J.F. Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J. Clin. Endocrinol. Metab. 2005, 90, 5175–5181. [Google Scholar] [CrossRef] [PubMed]
- McCargar, L.J.; Clandinin, M.T.; Belcastro, A.N.; Walker, K. Dietary carbohydrate-to-fat ratio: Influence on whole-body nitrogen retention, substrate utilization, and hormone response in healthy male subjects. Am. J. Clin. Nutr. 1989, 49, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Darling-Raedeke, M.; Thornton, W.H.; MacDonald, R.S. Growth hormone and igf-i plasma concentrations and macronutrient intake measured in a free-living elderly population during a one-year period. J. Am. Coll. Nutr. 1998, 17, 392–397. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span-from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef]
- Holloszy, J.O.; Fontana, L. Caloric restriction in humans. Exp. Gerontol. 2007, 42, 709–712. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S. Aging, adiposity, and calorie restriction. J. Am. Med. Assoc. 2007, 297, 986–994. [Google Scholar] [CrossRef]
- Mercken, E.M.; Crosby, S.D.; Lamming, D.W.; Jebailey, L.; Krzysik-Walker, S.; Villareal, D.T.; Capri, M.; Franceschi, C.; Zhang, Y.; Becker, K.; et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell 2013, 12, 645–651. [Google Scholar] [CrossRef]
- Milman, S.; Atzmon, G.; Huffman, D.M.; Wan, J.; Crandall, J.P.; Cohen, P.; Barzilai, N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 2014, 13, 769–771. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Bartke, A. Pleiotropic effects of growth hormone signaling in aging. Trends Endocrinol. Metab. 2011, 22, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Al-Regaiey, K.A.; Masternak, M.M.; Bonkowski, M.; Sun, L.; Bartke, A. Long-lived growth hormone receptor knockout mice: Interaction of reduced insulin-like growth factor I/insulin signaling and caloric restriction. Endocrinology 2005, 146, 851–860. [Google Scholar] [CrossRef]
- Bartke, A. Role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 2005, 146, 3718–3723. [Google Scholar] [CrossRef]
- Longo, V.D.; Fontana, L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol. Sci. 2010, 31, 89–98. [Google Scholar] [CrossRef]
- Sabatino, F.; Masoro, E.J.; McMahan, C.A.; Kuhn, R.W. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J. Gerontol. 1991, 46. [Google Scholar] [CrossRef]
- Redman, L.M.; Veldhuis, J.D.; Rood, J.; Smith, S.R.; Williamson, D.; Ravussin, E. The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell 2010, 9, 32–39. [Google Scholar] [CrossRef]
- Cohen, D.E.; Supinski, A.M.; Bonkowski, M.S.; Donmez, G.; Guarente, L.P. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009, 23, 2812–2817. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Xu, X.; Ingram, R.L.; D’Costa, A. Moderate caloric restriction alters the subcellular distribution of somatostatin mRNA and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology 1995, 61, 601–608. [Google Scholar] [CrossRef]
- Caputo, M.; Mele, C.; Ferrero, A.; Leone, I.; Daffara, T.; Marzullo, P.; Prodam, F.; Aimaretti, G. Dynamic tests in pituitary endocrinology: Pitfalls in interpretation during aging. Neuroendocrinology 2021. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A.; Wright, J.C.; Mattison, J.A.; Ingram, D.K.; Miller, R.A.; Roth, G.S. Extending the lifespan of long-lived mice. Nature 2001, 414, 412. [Google Scholar] [CrossRef] [PubMed]
- Dani, S.U.; Dani, M.A.C.; Freire, I.L.; Gouvea, S.P.; Knackfuss, F.B.; Lima, F.P.; Mercadante, M.E.Z.; Monteiro, E.; Paggiaro, S.M.G.; Razook, A.G.; et al. Survival of the thriftiest: Restricted nurture reveals the thrifty nature of a growth gene in Bos indicus. Genet. Mol. Res. 2010, 9, 1032–1044. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Csiszar, A.; De Cabo, R.; Ferrucci, L.; Ungvari, Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: Progress and controversies. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 587–598. [Google Scholar] [CrossRef]
- Suh, Y.; Atzmon, G.; Cho, M.O.; Hwang, D.; Liu, B.; Leahy, D.J.; Barzilai, N.; Cohen, P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. USA 2008, 105, 3438–3442. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef]
- Gavrilova, N.S.; Gavrilov, L.A. Comments on dietary restriction, okinawa diet and longevity. Gerontology 2012, 58, 221–223. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Curb, J.D.; Suzuki, M. Caloric restriction and human longevity: What can we learn from the Okinawans? Biogerontology 2006, 7, 173–177. [Google Scholar] [CrossRef]
- Willcox, D.; Willcox, B.; Yasura, S.; Ashitomi, I.; Suzuki, M. Gender gap in healthspan and life expectancy in Okinawa: Health behaviours. Asian J. Gerontol. Geriatr. 2012, 7, 49. [Google Scholar]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12. [Google Scholar] [CrossRef]
- Varady, K.A.; Roohk, D.J.; Loe, Y.C.; McEvoy-Hein, B.K.; Hellerstein, M.K. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism, and plasma adiponectin levels in mice. J. Lipid Res. 2007, 48, 2212–2219. [Google Scholar] [CrossRef]
- Varady, K.A.; Allister, C.A.; Roohk, D.J.; Hellerstein, M.K. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J. Nutr. Biochem. 2010, 21, 188–195. [Google Scholar] [CrossRef]
- Varady, K.A.; Hudak, C.S.; Hellerstein, M.K. Modified alternate-day fasting and cardioprotection: Relation to adipose tissue dynamics and dietary fat intake. Metabolism. 2009, 58, 803–811. [Google Scholar] [CrossRef]
- Ash, S.; Reeves, M.M.; Yeo, S.; Morrison, G.; Carey, D.; Capra, S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: A randomised trial. Int. J. Obes. 2003, 27, 797–802. [Google Scholar] [CrossRef]
- Klempel, M.C.; Kroeger, C.M.; Bhutani, S.; Trepanowski, J.F.; Varady, K.A. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity 2014, 22, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; He, F.; Tinsley, G.M.; Pannell, B.K.; Ward, E.; Arciero, P.J. Comparison of high-protein, intermittent fasting low-calorie diet and heart healthy diet for vascular health of the obese. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- Harvie, M.N.; Sims, A.H.; Pegington, M.; Spence, K.; Mitchell, A.; Vaughan, A.A.; Allwood, J.W.; Xu, Y.; Rattray, N.J.W.; Goodacre, R.; et al. Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Res. 2016, 18. [Google Scholar] [CrossRef]
- Wegman, M.P.; Guo, M.H.; Bennion, D.M.; Shankar, M.N.; Chrzanowski, S.M.; Goldberg, L.A.; Xu, J.; Williams, T.A.; Lu, X.; Hsu, S.I.; et al. Practicality of Intermittent Fasting in Humans and its Effect on Oxidative Stress and Genes Related to Aging and Metabolism. Rejuvenation Res. 2015, 18, 162–172. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Pluta, R.; Januszewski, S. Ketogenic diet and epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Coopmans, E.C.; Berk, K.A.C.; El-Sayed, N.; Neggers, S.J.C.M.M.; van der Lely, A.J. Eucaloric Very-Low-Carbohydrate Ketogenic Diet in Acromegaly Treatment. N. Engl. J. Med. 2020, 382, 2161–2162. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Peterson, S.J.; Tangney, C.C.; Pimentel-Zablah, E.M.; Hjelmgren, B.; Booth, G.; Berry-Kravis, E. Changes in growth and seizure reduction in children on the ketogenic diet as a treatment for intractable epilepsy. J. Am. Diet. Assoc. 2005, 105, 718–724. [Google Scholar] [CrossRef]
- Spulber, G.; Spulber, S.; Hagenäs, L.; Åmark, P.; Dahlin, M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia 2009, 50, 297–303. [Google Scholar] [CrossRef]
- Vining, E.P.G.; Pyzik, P.; McGrogan, J.; Hladky, H.; Anand, A.; Kriegler, S.; Freeman, J.M. Growth of children on the ketogenic diet. Dev. Med. Child Neurol. 2002, 44, 796–802. [Google Scholar] [CrossRef]
- Wiĺliams, S.; Basualdo-Hammond, C.; Curtis, R.; Schuller, R. Growth retardation in children with epilepsy on the ketogenic diet: A retrospective chart review. J. Am. Diet. Assoc. 2002, 102, 405–407. [Google Scholar] [CrossRef]
- Groleau, V.; Schall, J.I.; Stallings, V.A.; Bergqvist, C.A. Long-term impact of the ketogenic diet on growth and resting energy expenditure in children with intractable epilepsy. Dev. Med. Child Neurol. 2014, 56, 898–904. [Google Scholar] [CrossRef]
- Kim, J.T.; Kang, H.C.; Song, J.E.; Lee, M.J.; Lee, Y.J.; Lee, E.J.; Lee, J.S.; Kim, H.D. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin. Nutr. 2013, 32, 98–103. [Google Scholar] [CrossRef]
- Marchiò, M.; Roli, L.; Lucchi, C.; Costa, A.M.; Borghi, M.; Iughetti, L.; Trenti, T.; Guerra, A.; Biagini, G. Ghrelin Plasma Levels After 1 Year of Ketogenic Diet in Children With Refractory Epilepsy. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef]
- Wibisono, C.; Rowe, N.; Beavis, E.; Kepreotes, H.; Mackie, F.E.; Lawson, J.A.; Cardamone, M. Ten-year single-center experience of the ketogenic diet: Factors influencing efficacy, tolerability, and compliance. J. Pediatr. 2015, 166, 1030–1036.e1. [Google Scholar] [CrossRef]
- Lambrechts, D.A.J.E.; de Kinderen, R.J.A.; Vles, H.S.H.; de Louw, A.J.; Aldenkamp, A.P.; Majoie, M.J.M. The MCT-ketogenic diet as a treatment option in refractory childhood epilepsy: A prospective study with 2-year follow-up. Epilepsy Behav. 2015, 51, 261–266. [Google Scholar] [CrossRef]
- Dressler, A.; Stöcklin, B.; Reithofer, E.; Benninger, F.; Freilinger, M.; Hauser, E.; Reiter-Fink, E.; Seidl, R.; Trimmel-Schwahofer, P.; Feucht, M. Long-term outcome and tolerability of the ketogenic diet in drug-resistant childhood epilepsy-the austrian experience. Seizure 2010, 19, 404–408. [Google Scholar] [CrossRef]
- Ferraris, C.; Guglielmetti, M.; Pasca, L.; De Giorgis, V.; Ferraro, O.E.; Brambilla, I.; Leone, A.; De Amicis, R.; Bertoli, S.; Veggiotti, P.; et al. Impact of the ketogenic diet on linear growth in children: A single-center retrospective analysis of 34 cases. Nutrients 2019, 11, 1442. [Google Scholar] [CrossRef]
- Armeno, M.; Verini, A.; del Pino, M.; Araujo, M.B.; Mestre, G.; Reyes, G.; Caraballo, R.H. A prospective study on changes in nutritional status and growth following two years of ketogenic diet (KD) therapy in children with refractory epilepsy. Nutrients 2019, 11, 1596. [Google Scholar] [CrossRef]
- Nation, J.; Humphrey, M.; Mackay, M.; Boneh, A. Linear growth of children on a ketogenic diet: Does the protein-to-energy ratio matter? J. Child Neurol. 2014, 29, 1496–1501. [Google Scholar] [CrossRef]
- Svedlund, A.; Hallböök, T.; Magnusson, P.; Dahlgren, J.; Swolin-Eide, D. Prospective study of growth and bone mass in Swedish children treated with the modified Atkins diet. Eur. J. Paediatr. Neurol. 2019, 23, 629–638. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Di Somma, C.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. Somatotropic axis and obesity: Is there any role for the Mediterranean diet? Nutrients 2019, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Jørgensen, J.O.L.; Luger, A.; Stalla, G.K. Insulin resistance in patients with acromegaly. Front. Endocrinol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Jørgensen, J.O.L. Treatment guidelines for acromegaly. Report from a Scandinavian workshop. Growth Horm. IGF Res. 2001, 11, 72–74. [Google Scholar]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Whitehead, T.; Robinson, D.; Allaway, S.; Syms, J.; Hale, A. Effect of red wine ingestion on the antioxidant capacity of serum. Clin. Chem. 1995, 41, 32–35. [Google Scholar] [CrossRef]
- Lasheras, C.; Gonzalez, S.; Huerta, J.M.; Lombardia, C.; Ibañez, R.; Patterson, A.M.; Fernandez, S. Food habits are associated with lipid peroxidation in an elderly population. J. Am. Diet. Assoc. 2003, 103, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Antonini, F.M.; Petruzzi, E.; Pinzani, P.; Orlando, C.; Petruzzi, I.; Pazzagli, M.; Masotti, G. Effect of diet and red wine consumption on serum total antioxidant capacity (TAC), dehydroepiandrosterone-sulphate (DHEAS) and insulin-like growth factor-1 (IGF-1) in Italian centenarians. Arch. Gerontol. Geriatr. 2005, 41, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.N.; Chen, J.S.; March, L.M.; Cameron, I.D.; Cumming, R.G.; Lord, S.R.; Zochling, J.; Sitoh, Y.Y.; Lau, T.C.; Schwarz, J.; et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J. Clin. Endocrinol. Metab. 2004, 89, 1572–1576. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Berry, E.M.; Blaak, E.E.; Burlingame, B.; le Coutre, J.; van Eden, W.; El-Sohemy, A.; German, J.B.; Knorr, D.; Lacroix, C.; et al. Goals in Nutrition Science 2020–2025. Front. Nutr. 2021, 7, 606378. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Mitchell, S.J.; de Cabo, R.; Raubenheimer, D.; Le Couteur, D.G.; Simpson, S.J. Macronutrients and caloric intake in health and longevity. J. Endocrinol. 2015, 226, R17–R28. [Google Scholar] [CrossRef]
- Johnson, S.C. Nutrient sensing, signaling and ageing: The role of IGF-1 and mTOR in ageing and age-related disease. In Subcellular Biochemistry; Springer: New York, NY, USA, 2018; Volume 90, pp. 49–97. [Google Scholar]
- Brown-Borg, H.M. The somatotropic axis and longevity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E503–E510. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. Caloric restriction and aging revisited: The need for a geometric analysis of the nutritional bases of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 707–713. [Google Scholar] [CrossRef]
- Simpson, S.; Raubenheimer, D. The Nature of Nutrition. A Unifying Framework Form Animal Adaption to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012. [Google Scholar]
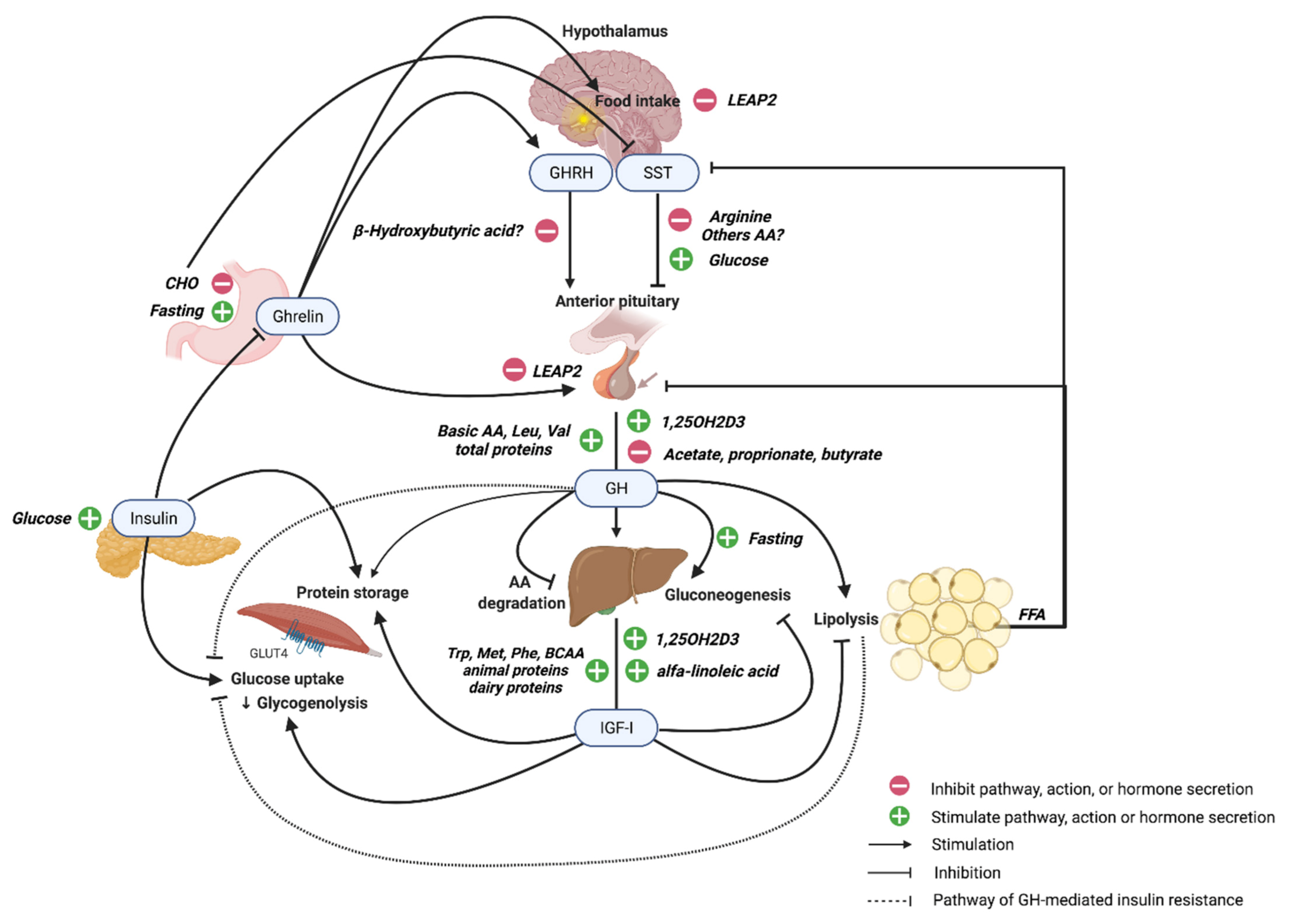
| Macronutrient | Effects on GH/IGF-I Axis | Regulation by GH/IGF-I |
|---|---|---|
| Carbohydrates | Oral glucose administration: ↓ GH (↑ hypothalamic somatostatin) [106,110,111,112] ↓ ghrelin and GH after oral glucose [114] Hypoglycemia: ↑ GH [107] | GH ↓ glucose and proteins utilization and ↑ lipid utilization [1] GH ↑ insulin resistance: ↓ insulin-mediated glucose uptake in muscle; modulates insulin signaling (PI3K activity; ↑ SOCS1, SOC3) [1,99,102,103,104] IGF-I ↑ glucose uptake, ↓ gluconeogenesis and ↓ glycogenolysis [101] GH ↑ hepatic autophagy and gluconeogenesis under fasted and fat-depleted conditions [74,89,91] |
| Fibers | ↑ GH and IGF-I [128] Mechanisms unexplored | |
| Proteins | Intravenous administration of AAs: ↑ GH [133,134] Oral administration of AAs: ↑ GH (mainly in females and youngers) [139,140,141,142,145,146] Basic AAs ↑ GH; leucine and valine less potent; isoleucine ↔ GH [134] Arginine ↑ GH (↓ somatostatin at hypothalamus) [135] Dietary tryptophan and methionine restriction: ↓ GH and IGF-I [173] Total protein: ↑ GH [147,148] Diets high in protein: ↑ GH and IGF-I [148,152] Formula milk with tryptophan and phenylalanine: ↑ IGF-I [151] High milk and dairy protein intake: ↑ IGF-I [155,156,157,158,159] Vegan vs meat-eaters and vegetarians: ↓ IGF-I and ↑ IGFBP-1/2 [153] CR and protein restriction: ↓ IGF-I [167] BCAAs: ↑ IGF-I [178] | GH/IGF-I ↓ protein breakdown and ↑ protein synthesis in muscle and other tissues [1] GH ↓ AA degradation/ureagenesis in liver [1] GH ↓ BCAAs (↑ LAT1 and uptake by muscle, ↑ gluconeogenesis) [180,181,182] IGF-I ↑ BCAAs [182] |
| Lipids | Infusion of mixed FFAs: ↓ spontaneous and GHRH-stimulated GH (by somatotropic cells or ↓ somatostatin) [52,63,191,194,195,196] Lipolysis inhibiting drugs ↑ basal and GHRH-stimulated GH [191] Diets rich in alfa-linoleic acid: ↑ IGF-I, GHR, and IGFBPs mRNA in liver; ↑ IGF-I secretion [203] Sodium salts of valerate, hexanoic, caprylic, nonanoic, and dodecanoic acids ↑ GH and PRL [204] Acetate, propionate, and butyrate ↓ GHRH, GH, PRL gene transcription in dairy cow anterior pituitary cells [207] | GH ↑ lipolysis, lipid oxidation and ketogenesis [1,185] GH regulates VLDL metabolism and availability of FFAs for peripheral tissues [188,189] GH ↑ LDL- receptor expression and HDL-cholesterol production [188,189] IGF-I ↓ plasma FFAs (↓ lipolysis) [202] |
| Micronutrient | Effects on GH/IGF-I Axis | Regulation by GH/IGF-I |
|---|---|---|
| Vitamin D | ↑ GH (binding to pituitary VDR) [210,211] ↑ Liver IGF-I and IGFBP-3 (directly; ↑ GH; ↑ Ca2+ absorption in the gut) [212,216] ↑ IGF-I in chondrocytes [215] | IGF-I: ↑ 1,25-(OH)2D3 and ↑ 24,25-(OH)2D3 (kidney, placenta) [212,218,219] |
| Vitamin A | Modulates GH gene (interaction with RXR-α in the pituitary) [220] ↑ GH (non-transcriptional effect) [222] Vitamin-A-deficient rats: ↓ GH-regulated CYP2C11, CYP4A2, IGF-I, and GH-responsiveness of the JAK-STAT system [223] | |
| Vitamin E | Low levels ↑ oxidative stress in GHD children [226] No mechanistic studies | |
| Vitamin B6 | Pyridoxal phosphate ↓ GH in acromegaly and infants [227] ↓ Cell proliferation and GH secretion [227] | |
| Vitamin B8 | Dietary restriction in mice: ↓ IGF-I availability; ↔ GH [228] | |
| Vitamin B12 | Deficiency: ↓ liver taurine; ↓ GH/IGF-I axis with GH resistance [230] | GH ↑ vitamin B12 availability [229] |
| Sodium, potassium, and water | Dehydration ↑ GH [231] Potassium depletion in animal models: ↑ intrarenal IGF-I levels, ↓ circulating and hepatic IGF-I levels [232,237,247] | IGF-I: modulation of renin release, ↓ ANP, ↑ distal tubular Na channels [232,233] GH: ↑ salt and water retention (renal tubuli) [231] GH and IGF-I: ↑ transepithelial Na transport (ENaC, cortical collecting duct) [231] GH: ↓ urinary potassium excretion [232,237,247] |
| Calcium | Basal and stimulated GH secretion depend on Ca2+ influx in pituitary cells through L-type Ca2+ channels [239,240] Pituitary extracellular Ca2+-sensing ↑ GH response to GHRH [242] ↑ PTH and Ca2+: ↓ stimulated GH secretion, ↓ IGF-I levels; tune U-shaped regulation [112,241] | GH/IGF-I: ↑ Ca2+ gut absorption by TRPV6 [231,244] IGF-I: ↑ Ca2+ reabsorption by ↑ TRPV5 in distal tubule (↑1,25-(OH)2D3) [231,245] IGF-I: absorptive hypercalciuria and ↑ fasting plasma Ca2+ in acromegalic patients (↑1,25-(OH)2D3) [231,246] |
| Phosphorus | GH ↑ renal phosphate absorption via Na-phosphate cotransport [249,250,253] IGF-I ↓ renal phosphate excretion via Na-phosphate cotransport [251,252,253] GH treatment and acromegaly: ↑ serum phosphate (↑ Klotho secretion in kidney, FGF23 resistance) [250,254,255] | |
| Magnesium | Modulates hormone-sensitive adenylate cyclase system in somatotroph cells [256] Nutritional deficiency: ↓ IGF-I; ↔ GH secretion (GH-R or post-receptor defects) [257] | GH ↓ renal Mg wasting induced by metabolic acidosis [258,259] |
| Zinc | Regulates GH signaling ability (↑ GH more stable dimeric form) [262,263] Prolongs GH binding to GHR and ↑ GH aggregation in secretory granules [263,264,265] Main determinant of IGF-I synthesis [260,261] ↑ IGF-I-anabolic effect in osteoblastic cells [261,271,273] Deficiency: ↓ body weight, IGF-I, GH and GHBP, hepatic GHR and IGF-I expression in rats [268,269] Supplementation: ↑ GH, IGF-I, IGFBP-3 levels in animal models, GHD and healthy children [260,269,270,271,272] | |
| Iron | Hemopexin is a GH-inducible gene [275] IGFBP-3 binds transferrin [276] GH treatment ↓ ferritin and ↑ serum transferrin [280] IGF-I ↑ erythropoiesis (precursors and mature erythrocytes) [277,278] | |
| Iodine | Thyroid hormones important for normal GH expression and feedback mechanisms on GHR, influencing IGF-I levels [282,283] Iodine supplementation ↑ IGF-I [282] | |
| Selenium | ↓ GH and IGF-I system; U-shaped regulation [286,287,288,289] Deficiency: growth retardation; ↓ pituitary GH; ↓ IGF-I secretion/circulating levels (direct; inflammation) [290,291] ↓ Type II deiodinase selenoenzyme activity (pituitary) [290,291] | |
| Manganese | Deficiency in animals: ↓ body weight and growth, bone abnormalities, ↓ IGF-I and insulin, ↑ GH and IGFBP-3 [296,297] Mn2+ bioavailability could affect IGF-I and insulin signaling [298] ↑ IGF-I gene expression and IGF-IR protein expression in medial basal hypothalamus [299] | |
| Copper | Deficiency: ↓ serum IGF-I; ↑ IGF-I in bones [301] Supplementation: ↑ IGF-I and IGFBP-3 (culture media of chondrocytes) [289] ↑ GHRH, GH, and ghrelin secretion; ↓ somatostatin expression [300,302] | |
| Chromium | Negligible or quite insignificant effects [303] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. https://doi.org/10.3390/cells10061376
Caputo M, Pigni S, Agosti E, Daffara T, Ferrero A, Filigheddu N, Prodam F. Regulation of GH and GH Signaling by Nutrients. Cells. 2021; 10(6):1376. https://doi.org/10.3390/cells10061376
Chicago/Turabian StyleCaputo, Marina, Stella Pigni, Emanuela Agosti, Tommaso Daffara, Alice Ferrero, Nicoletta Filigheddu, and Flavia Prodam. 2021. "Regulation of GH and GH Signaling by Nutrients" Cells 10, no. 6: 1376. https://doi.org/10.3390/cells10061376
APA StyleCaputo, M., Pigni, S., Agosti, E., Daffara, T., Ferrero, A., Filigheddu, N., & Prodam, F. (2021). Regulation of GH and GH Signaling by Nutrients. Cells, 10(6), 1376. https://doi.org/10.3390/cells10061376








