Genome-Wide Association Analysis and Molecular Marker Development for Resistance to Fusarium equiseti in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Culture and Inoculation of Fusarium equiseti
2.3. Evaluation of Soybean Resistance to Fusarium equiseti
2.4. Genotyping
2.5. Population Structure Evaluation and Linkage Disequilibrium Analysis
2.6. Genome-Wide Association Study
2.7. Identification of Candidate Genes and RT-qPCR Assay
2.8. Development of CAPS and KASP Molecular Markers
3. Results
3.1. Germplasm Evaluation of Fusarium equiseti Root Rot
3.2. Population Structure and LD Analysis
3.3. Genome-Wide Association Analysis to Identify Loci Associated with Resistance to Fusarium equiseti in Soybean
3.4. Gene-Based Association Analysis Reveals SNPs Associated with FERR Resistance
3.5. Candidate Gene Expression Analysis Under FERR Inoculation
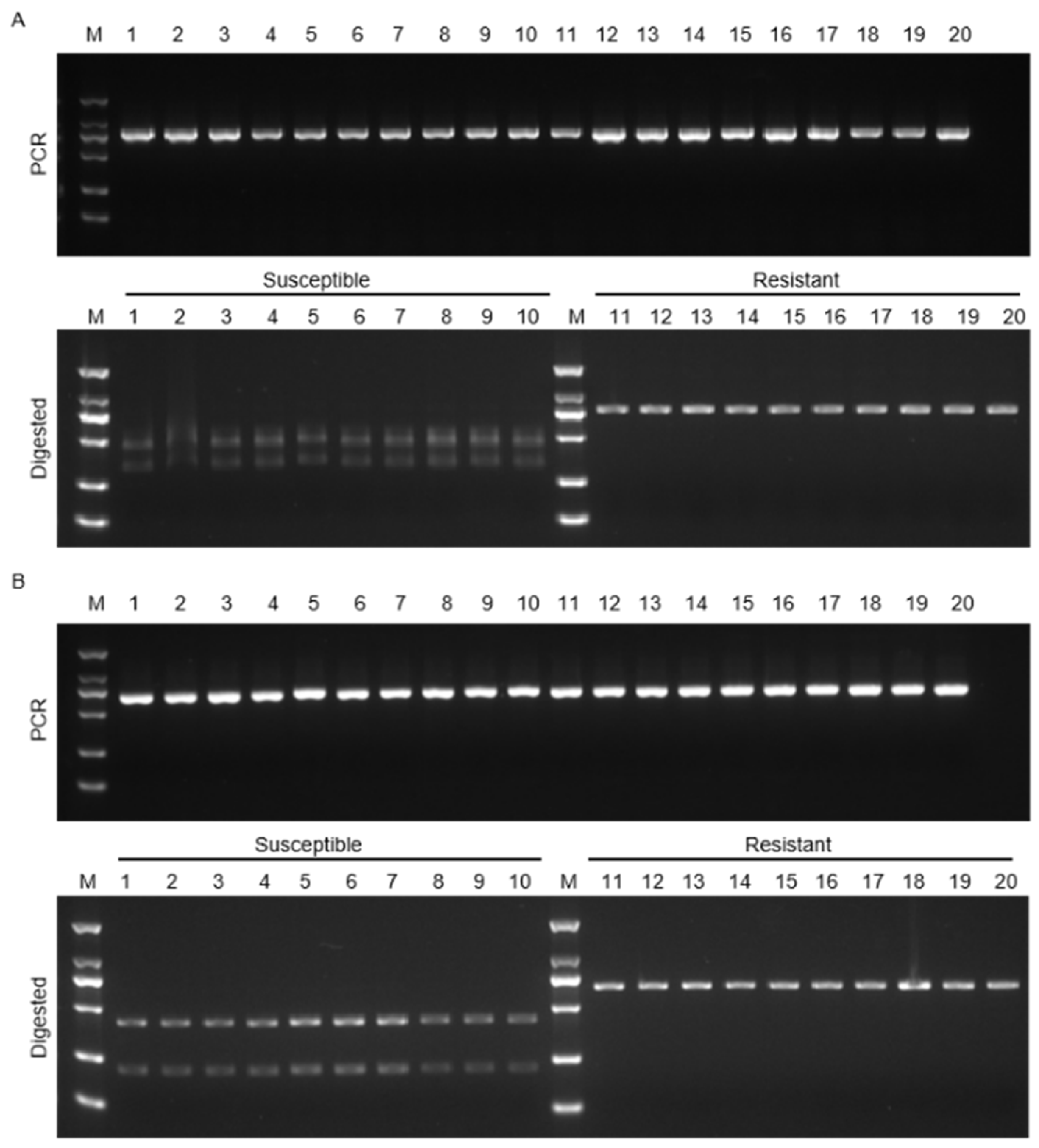
3.6. Development of CAPS Markers Associated with FERR Resistance in Soybean
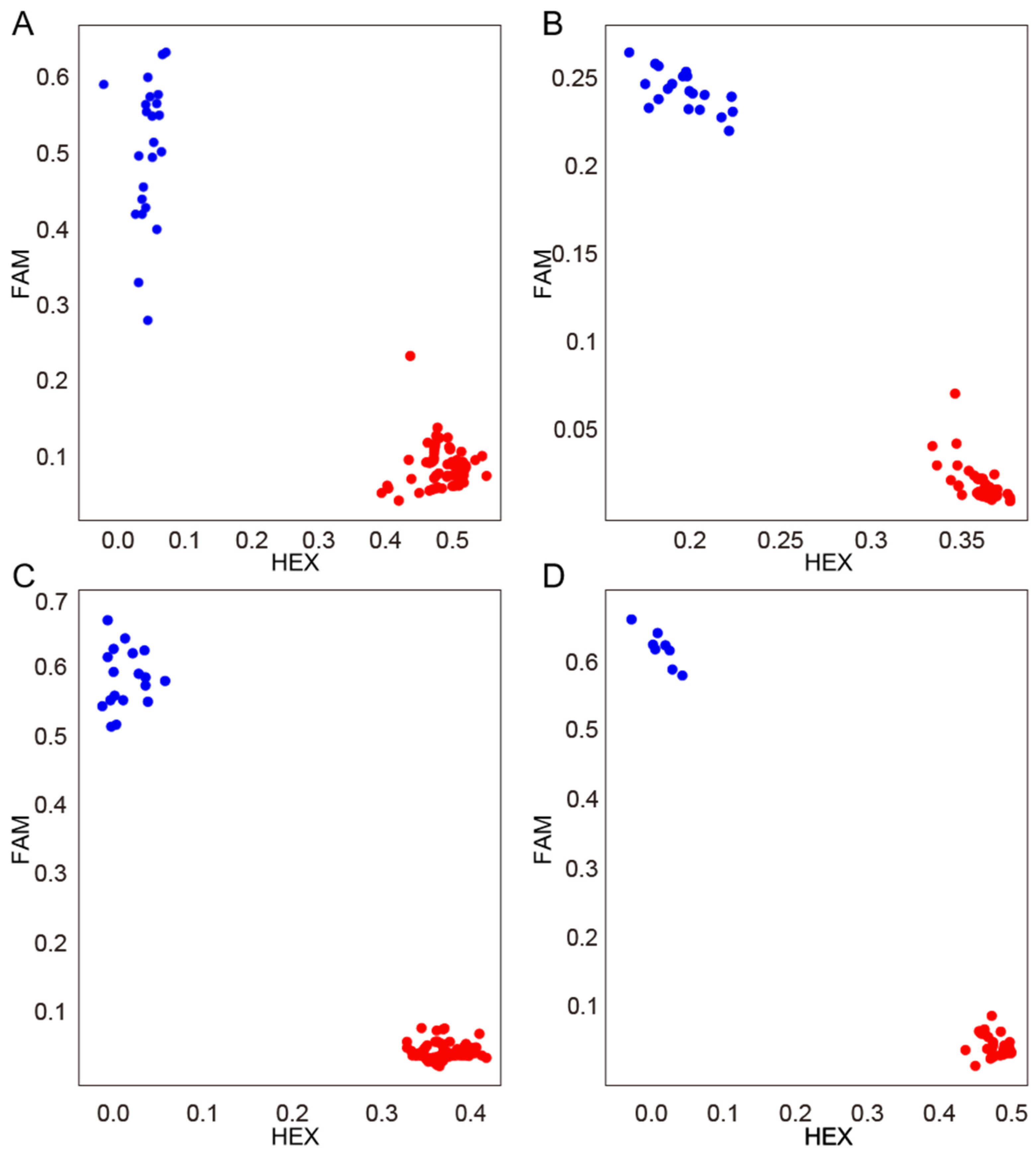
3.7. KASP Marker Development for FERR Resistance in Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Liu, Z.; Yang, Y.; Ma, Q.; Zheng, Y.; Xu, C.; Gao, X.; Gao, W.; Huang, Z.; Liu, X. Characterization of Fusarium species causing soybean root rot in Heilongjiang, China, and mechanism underlying the differences in sensitivity to DMI fungicides. Pestic. Biochem. Physiol. 2024, 200, 105828. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Liu, J.; Cui, W.; Zhao, Q.; Ren, Z.; Li, L.; Li, Y.; Sun, L.; Ding, J. Identification, Characterization, and Chemical Management of Fusarium asiaticum Causing Soybean Root Rot in Northeast China. Agronomy 2025, 15, 388. [Google Scholar] [CrossRef]
- Kuldybayev, N.M.; Dutbayev, Y.B.; Konstantinova, O.; Borodulin, D.; Yessimbekova, M.; Daugaliyeva, S.; Toishimanov, M.; Yesserkenov, A.; Bastaubaeva, S.; Temreshev, I. Identification and Pathogenicity of the Soybean Root Rot Pathogen in Arid Conditions. OnLine J. Biol. Sci. 2023, 23, 202–209. [Google Scholar] [CrossRef]
- Okello, P.N.; Mathew, F.M. Cross pathogenicity studies show South Dakota isolates of Fusarium acuminatum, F. equiseti, F. graminearum, F. oxysporum, F. proliferatum, F. solani, and F. subglutinans from either soybean or corn are pathogenic to both crops. Plant Health Prog. 2019, 20, 44–49. [Google Scholar] [CrossRef]
- Bai, L.; Li, X.; Cao, Y.; Song, Z.; Ma, K.; Fan, Y.; Ma, M. Fusarium culmorum and Fusarium equiseti causing root rot Disease on Lycium barbarum (Goji Berry) in China. Plant Dis. 2020, 104, 3066. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Sun, L.; Li, S.; Ji, P. First report of root rot of cowpea caused by Fusarium equiseti in Georgia in the United States. Plant Dis. 2017, 101, 1674. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Siddiqui, Y.; Saad, N.; Hun, T.G.; Mohd Hata, E.; Rashed, O.; Hossain, M.I. First report of Fusarium equiseti causing fruit rot disease of watermelon in Malaysia. Plant Dis. 2022, 106, 326. [Google Scholar] [CrossRef]
- Hami, A.; Rasool, R.S.; Khan, N.A.; Mansoor, S.; Mir, M.A.; Ahmed, N.; Masoodi, K.Z. Morpho-molecular identification and first report of Fusarium equiseti in causing chilli wilt from Kashmir (Northern Himalayas). Sci. Rep. 2021, 11, 3610. [Google Scholar]
- Garibaldi, A.; Gilardi, G.; Bertoldo, C.; Gullino, M.L. First report of leaf spot of rocket (Eruca sativa) caused by Fusarium equiseti in Italy. Plant Dis. 2011, 95, 1315. [Google Scholar] [CrossRef]
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Shaanker, R.U. Seed treatment with systemic fungicides: Time for review. Front. Plant Sci. 2021, 12, 654512. [Google Scholar] [CrossRef]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and prospects of genome-wide association studies in plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef]
- Kaňovská, I.; Biová, J.; Škrabišová, M. New perspectives of post-GWAS analyses: From markers to causal genes for more precise crop breeding. Curr. Opin. Plant Biol. 2024, 82, 102658. [Google Scholar] [CrossRef]
- Patel, J.; Allen, T.W.; Buckley, B.; Chen, P.; Clubb, M.; Mozzoni, L.A.; Orazaly, M.; Florez, L.; Moseley, D.; Rupe, J.C. Deciphering genetic factors contributing to enhanced resistance against Cercospora leaf blight in soybean (Glycine max L.) using GWAS analysis. Front. Genet. 2024, 15, 1377223. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Q.; Liu, Z.; Shi, X.; Wu, X.; Chen, Y.; Du, X.; Gao, Q.; He, D.; Shi, A. Genome-wide association analysis and genomic prediction of salt tolerance trait in soybean germplasm. Front. Plant Sci. 2024, 15, 1494551. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Liu, X.; Yuan, C.; Yao, T.; Li, Y.; Wang, D.; Zhao, H.; Wang, Y. Genome-wide association study on resistance of cultivated soybean to Fusarium oxysporum root rot in Northeast China. BMC Plant Biol. 2023, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.-H.; Mian, M.R.; Kang, S.-T.; Michel, A.P. Genetic mapping of the powdery mildew resistance gene in soybean PI 567301B. Theor. Appl. Genet. 2012, 125, 1159–1168. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Meng, X.; Sun, M.; Qu, S.; Liu, Y.; Li, Y.; Zhan, Y.; Teng, W.; Li, H. Genome-Wide Association Study and Marker Development for Fusarium Oxysporum Root Rot Resistance in Soybean. Int. J. Mol. Sci. 2024, 25, 12573. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Deng, W.; Liu, J.; Fang, Y.; Liu, Y.; Ma, T.; Zhang, Y.; Xue, Y.; Tang, X. Fine mapping the soybean mosaic virus resistance gene in soybean cultivar Heinong 84 and development of CAPS markers for rapid identification. Viruses 2022, 14, 2533. [Google Scholar] [CrossRef]
- Jia, Q.; Zhou, M.; Xiong, Y.; Wang, J.; Xu, D.; Zhang, H.; Liu, X.; Zhang, W.; Wang, Q.; Sun, X. Development of KASP markers assisted with soybean drought tolerance in the germination stage based on GWAS. Front. Plant Sci. 2024, 15, 1352379. [Google Scholar] [CrossRef]
- Ma, L.; Qing, C.; Zhang, M.; Zou, C.; Pan, G.; Shen, Y. GWAS with a PCA uncovers candidate genes for accumulations of microelements in maize seedlings. Physiol. Plant. 2021, 172, 2170–2180. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.; Krasynanski, S.; Kumar, S.; Thompson, W. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z. GAPIT version 3: Boosting power and accuracy for genomic association and prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef]
- Yu, Z.; Chang, F.; Lv, W.; Sharmin, R.A.; Wang, Z.; Kong, J.; Bhat, J.A.; Zhao, T. Identification of QTN and candidate gene for seed-flooding tolerance in soybean [Glycine max (L.) Merr.] using genome-wide association study (GWAS). Genes 2019, 10, 957. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, X.; Cao, G.; Wang, Y.; Li, Y.; Liu, D.; Teng, W.; Zhang, Z.; Li, D.; Qiu, L. Genetic characteristics of soybean resistance to HG type 0 and HG type 1.2. 3.5. 7 of the cyst nematode analyzed by genome-wide association mapping. BMC Genom. 2015, 16, 598. [Google Scholar] [CrossRef]
- Arias, M.M.D.; Leandro, L.F.; Munkvold, G.P. Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology 2013, 103, 822–832. [Google Scholar] [CrossRef]
- Cai, H.; Tao, N.; Guo, C. Systematic investigation of the effects of macro-elements and iron on soybean plant response to Fusarium oxysporum infection. Plant Pathol. J. 2020, 36, 398. [Google Scholar] [CrossRef]
- Lin, F.; Chhapekar, S.S.; Vieira, C.C.; Da Silva, M.P.; Rojas, A.; Lee, D.; Liu, N.; Pardo, E.M.; Lee, Y.-C.; Dong, Z. Breeding for disease resistance in soybean: A global perspective. Theor. Appl. Genet. 2022, 135, 3773–3872. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mubeen, M.; Sohail, M.A.; Solanki, M.K.; Hussain, B.; Nosheen, S.; Kashyap, B.K.; Zhou, L.; Fang, X. Root rot a silent alfalfa killer in China: Distribution, fungal, and oomycete pathogens, impact of climatic factors and its management. Front. Microbiol. 2022, 13, 961794. [Google Scholar] [CrossRef]
- Han, S.; Chen, J.; Zhao, Y.; Cai, H.; Guo, C. Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic. Biochem. Physiol. 2021, 178, 104916. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, W.; Zhang, X.; Gao, Z.; Cai, S.; Wang, S.; Li, Y. Bacillus velezensis BVE7 as a promising agent for biocontrol of soybean root rot caused by Fusarium oxysporum. Front. Microbiol. 2023, 14, 1275986. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhu, D.; Zhang, Y.; Yang, Z.; Wu, X.; Shang, J.; Yang, W.; Chang, X. Characterization of rhizosphere Pseudomonas chlororaphis IRHB3 in the reduction of Fusarium root rot and promotion of soybean growth. Biol. Control 2023, 186, 105349. [Google Scholar] [CrossRef]
- Susmitha, P.; Kumar, P.; Yadav, P.; Sahoo, S.; Kaur, G.; Pandey, M.K.; Singh, V.; Tseng, T.M.; Gangurde, S.S. Genome-wide association study as a powerful tool for dissecting competitive traits in legumes. Front. Plant Sci. 2023, 14, 1123631. [Google Scholar] [CrossRef]
- Lin, F.; Wani, S.H.; Collins, P.J.; Wen, Z.; Li, W.; Zhang, N.; McCoy, A.G.; Bi, Y.; Tan, R.; Zhang, S. QTL mapping and GWAS for identification of loci conferring partial resistance to Pythium sylvaticum in soybean (Glycine max (L.) Merr). Mol. Breed. 2020, 40, 54. [Google Scholar] [CrossRef]
- Zhao, X.; Bao, D.; Wang, W.; Zhang, C.; Jing, Y.; Jiang, H.; Qiu, L.; Li, W.; Han, Y. Loci and candidate gene identification for soybean resistance to Phytophthora root rot race 1 in combination with association and linkage mapping. Mol. Breed. 2020, 40, 100. [Google Scholar] [CrossRef]
- Tian, S.; Muneeruddin, K.; Choi, M.Y.; Tao, L.; Bhuiyan, R.H.; Ohmi, Y.; Furukawa, K.; Furukawa, K.; Boland, S.; Shaffer, S.A. Genome-wide CRISPR screens for Shiga toxins and ricin reveal Golgi proteins critical for glycosylation. PLoS Biol. 2018, 16, e2006951. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, M.; Nie, W.; Xi, Y.; Peng, L.; Zheng, Q.; Tang, K.; Satheesh, V.; Wang, Y.; Luo, J. The H3K9me2-binding protein AGDP3 limits DNA methylation and transcriptional gene silencing in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 2385–2395. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, W.; Long, F.; Cheng, S.; Yang, W.; Zhao, Y.; Zhou, D.-X. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell 2017, 29, 1088–1104. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Chen, L.; Tian, H.; Chen, C.; Fu, Y.; Du, H.; Hu, Z.; Li, R.; Du, Y. E3 ubiquitin ligase Os PIE3 destabilises the B-lectin receptor-like kinase PID2 to control blast disease resistance in rice. New Phytol. 2023, 237, 1826–1842. [Google Scholar] [CrossRef]
- Prasad, A.; Hari-Gowthem, G.; Muthamilarasan, M.; Hussain, Z.; Yadav, P.K.; Tripathi, S.; Prasad, M. Molecular characterization of SlATG18f in response to Tomato leaf curl New Delhi virus infection in tomato and development of a CAPS marker for leaf curl disease tolerance. Theor. Appl. Genet. 2021, 134, 1463–1474. [Google Scholar] [CrossRef]
- Badri Anarjan, M.; Bae, I.; Lee, S. Marker-assisted evaluation of two powdery mildew resistance candidate genes in Korean cucumber inbred lines. Agronomy 2021, 11, 2191. [Google Scholar] [CrossRef]
- Bongiorno, G.; Di Noia, A.; Ciancaleoni, S.; Marconi, G.; Cassibba, V.; Albertini, E. Development and application of a cleaved amplified polymorphic sequence marker (phyto) linked to the Pc5. 1 locus conferring resistance to Phytophthora capsici in Pepper (Capsicum annuum L.). Plants 2023, 12, 2757. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Dhungana, S.K.; Kang, B.-K.; Baek, I.-Y.; Sung, J.-S.; Ko, J.-Y.; Jung, C.-S.; Kim, K.-S.; Jun, T.-H. Development and validation of SNP and InDel markers for pod-shattering tolerance in soybean. Int. J. Mol. Sci. 2022, 23, 2382. [Google Scholar] [CrossRef] [PubMed]
- Zha, B.; Zhang, C.; Yuan, R.; Zhao, K.; Sun, J.; Liu, X.; Wang, X.; Zhang, F.; Zhang, B.; Lamlom, S.F. Integrative QTL mapping and candidate gene analysis for main stem node number in soybean. BMC Plant Biol. 2025, 25, 422. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, T.; Liu, S.; Han, J.; Wang, Y.; Zhao, X.; Li, Y.; Teng, W.; Zhan, Y.; Han, Y. QTL Detection of Salt Tolerance at Soybean Seedling Stage Based on Genome-Wide Association Analysis and Linkage Analysis. Plants 2024, 13, 2283. [Google Scholar] [CrossRef]
- Tran, D.T.; Steketee, C.J.; Boehm, J.D., Jr.; Noe, J.; Li, Z. Genome-wide association analysis pinpoints additional major genomic regions conferring resistance to soybean cyst nematode (Heterodera glycines Ichinohe). Front. Plant Sci. 2019, 10, 401. [Google Scholar] [CrossRef]





| Primer Names | Sequences(5′-3′) | Usage |
|---|---|---|
| S13_14464319-CAPS1-F | TTGATTGTAAGCAATTCAGGTCT | CAPS assay |
| S13_14464319-CAPS1-R | CTCATTTGTCTTTCAGTTGTTGG | CAPS assay |
| S15_9215524-CAPS2-F | CTAGTTGTTGCAAGTGGTGTGG | CAPS assay |
| S15_9215524-CAPS2-R | CGGCCCTCTGAAATCAAGATC | CAPS assay |
| S15_9205620-F1 | GAAGGTGACCAAGTTCATGCTCTGCAAATACTTGCCGGCACTGTCG | KASP assay |
| S15_9205620-F2 | GAAGGTGACCAAGTTCATGCTCTGCAAATACTTGCCGGCACTGTCA | KASP assay |
| S15_9205620-R | CATCTTGAACAATTGATGAGCATCAGATT | KASP assay |
| DSI | Type of Reaction | Soybean Germplasms | Percentage (%) |
|---|---|---|---|
| DI = 0 | Immune, I | 0 | 0.0 |
| 0 < DI ≤ 10 | High Resistant, HR | 17 | 4.9 |
| 10 < DI ≤ 20 | Medium Resistant, MR | 73 | 21.1 |
| 20 < DI ≤ 30 | Medium Susceptible, MS | 176 | 50.9 |
| 30 < DI ≤ 60 | Susceptible, S | 78 | 22.5 |
| DI ≥ 60 | High Susceptible, HS | 2 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Meng, X.; Han, J.; Yang, Y.; Zhu, H.; Li, Y.; Zhan, Y.; Teng, W.; Li, H.; Zhao, X. Genome-Wide Association Analysis and Molecular Marker Development for Resistance to Fusarium equiseti in Soybean. Agronomy 2025, 15, 1769. https://doi.org/10.3390/agronomy15081769
Wang Y, Meng X, Han J, Yang Y, Zhu H, Li Y, Zhan Y, Teng W, Li H, Zhao X. Genome-Wide Association Analysis and Molecular Marker Development for Resistance to Fusarium equiseti in Soybean. Agronomy. 2025; 15(8):1769. https://doi.org/10.3390/agronomy15081769
Chicago/Turabian StyleWang, Yuhe, Xiangkun Meng, Jinfeng Han, Yuming Yang, Hongjin Zhu, Yongguang Li, Yuhang Zhan, Weili Teng, Haiyan Li, and Xue Zhao. 2025. "Genome-Wide Association Analysis and Molecular Marker Development for Resistance to Fusarium equiseti in Soybean" Agronomy 15, no. 8: 1769. https://doi.org/10.3390/agronomy15081769
APA StyleWang, Y., Meng, X., Han, J., Yang, Y., Zhu, H., Li, Y., Zhan, Y., Teng, W., Li, H., & Zhao, X. (2025). Genome-Wide Association Analysis and Molecular Marker Development for Resistance to Fusarium equiseti in Soybean. Agronomy, 15(8), 1769. https://doi.org/10.3390/agronomy15081769






