Revalorization of Olive Stones from Olive Pomace: Phenolic Compounds Obtained by Microwave-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Raw Material and Pretreatment
2.3. Polyphenolic Extraction Methods
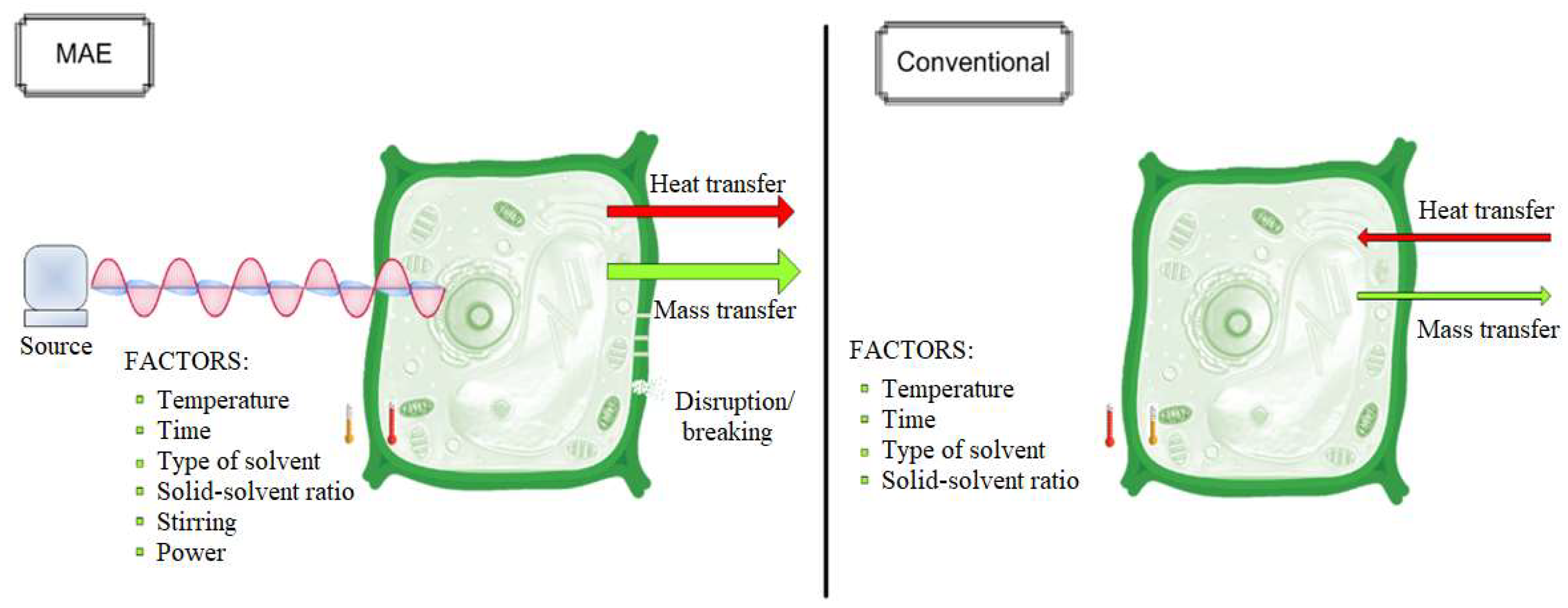
2.3.1. Microwave-Assisted Extraction (MAE)
2.3.2. Soxhlet Extraction
2.4. Total Phenolic Content
2.5. Chromatographic Analysis of Phenolic Compounds
2.6. Glucose Content
2.7. Lignin Content
2.8. Experimental Design, Modeling, and Optimization
3. Results and Discussion
3.1. Soxhlet Extraction
3.1.1. Extraction Yield and TPC with Soxhlet
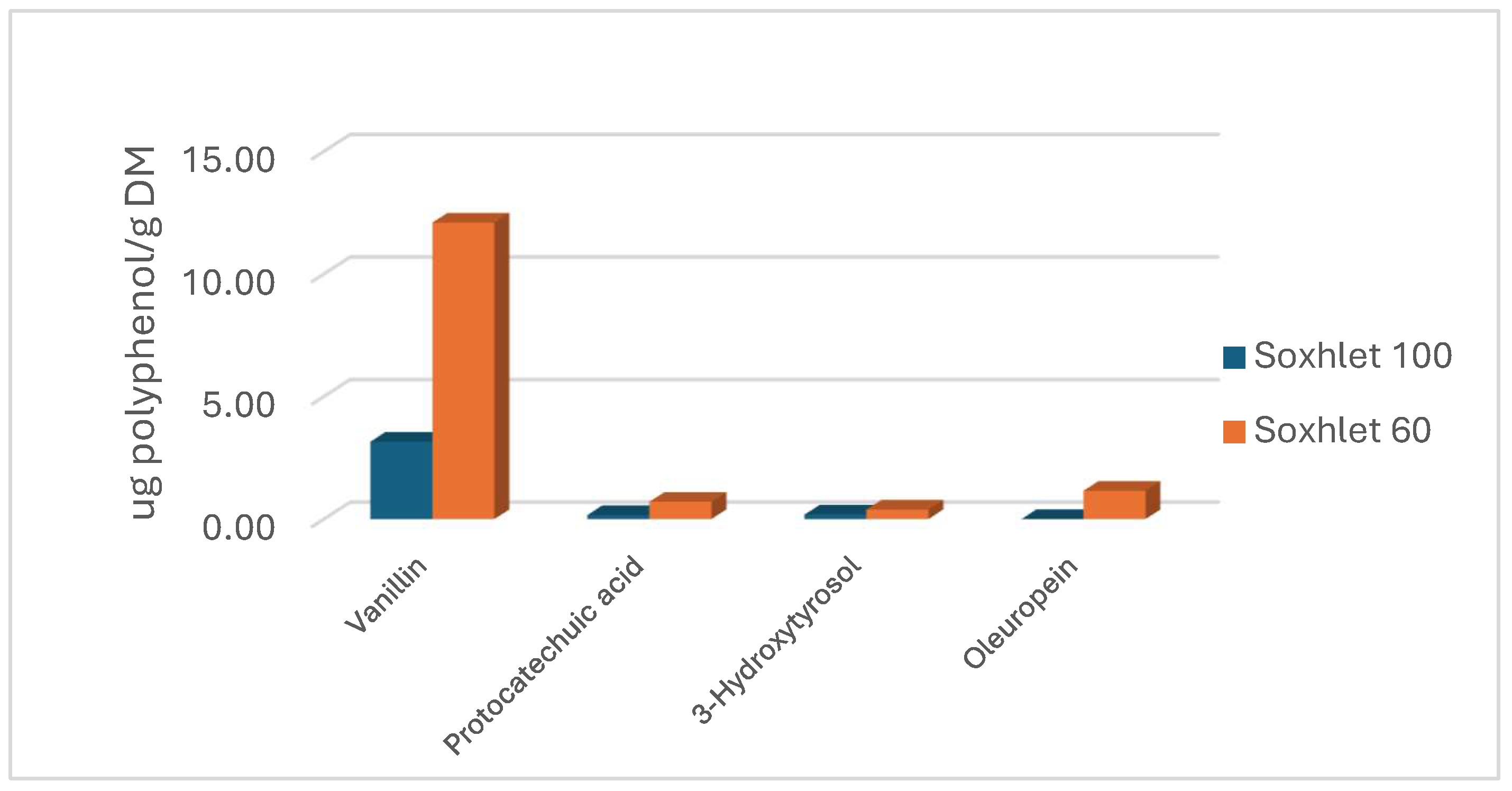
3.1.2. Phenolic Compounds with Soxhlet
3.2. MAE of Polyphenols
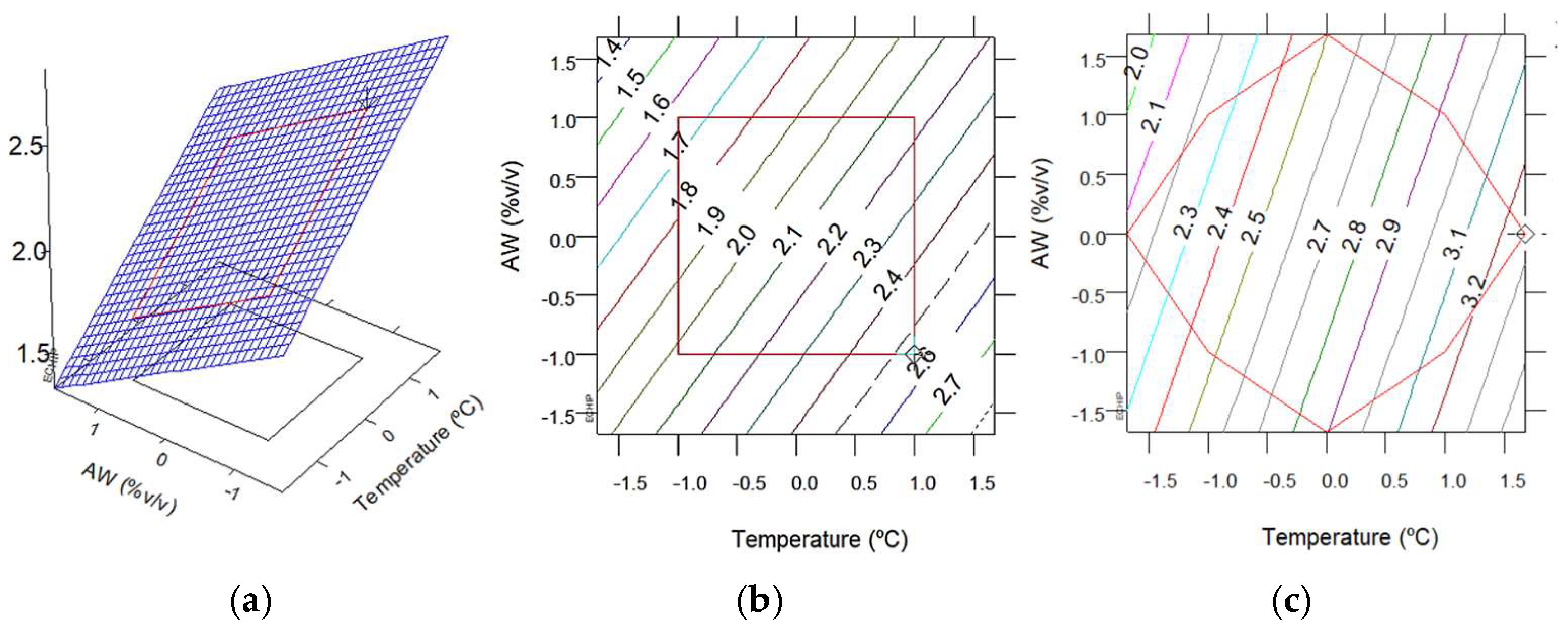
3.2.1. Extraction Yield and TPC with MAE
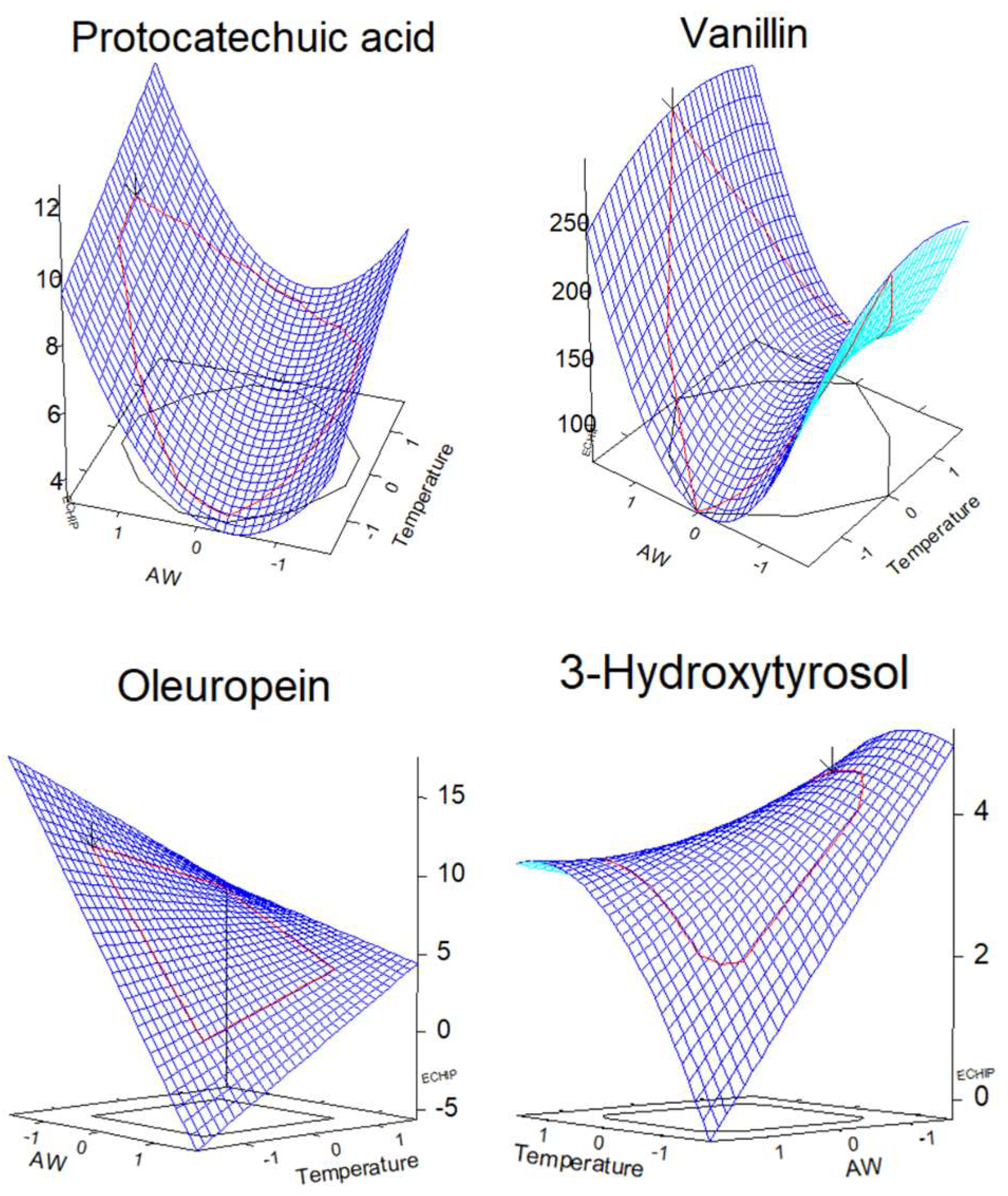
3.2.2. Phenolic Compounds with MAE
3.3. Glucose Content
3.4. Composition of Dry Extracts
3.5. Comparison Between MAE and Soxhlet Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Databases & Software|Land & Water|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/olive/en/ (accessed on 7 May 2025).
- García-Martín, J.F.; Cuevas, M.; Feng, C.H.; Álvarez-Mateos, P.; Torres-García, M.; Sánchez, S. Energetic Valorisation of Olive Biomass: Olive-Tree Pruning, Olive Stones and Pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; ISBN 9783030747688. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Segura Campos, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. ISBN 9780128147740. [Google Scholar]
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive Byproducts and Their Bioactive Compounds as a Valuable Source for Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.S.; Hu, Y.; Yong, Q. Insight in the Recent Application of Polyphenols From Biomass. Front. Bioeng. Biotechnol. 2021, 9, 753898. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Villanueva, R.; Plaza, M.; García, M.C.; Turner, C.; Marina, M.L. A Sustainable Approach for the Extraction of Cholesterol-Lowering Compounds from an Olive by-Product Based on CO2-Expanded Ethyl Acetate. Anal. Bioanal. Chem. 2019, 411, 5885–5896. [Google Scholar] [CrossRef]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-Assisted Extraction of Phenolic Compounds from Olive Leaves; a Comparison with Maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Proestos, C.; Komaitis, M. Application of Microwave-Assisted Extraction to the Fast Extraction of Plant Phenolic Compounds. LWT-Food Sci. Technol. 2008, 41, 652–659. [Google Scholar] [CrossRef]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria x Ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 2023, 16, 1030–1042. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dash, K.K. Microwave and Ultrasound Assisted Extraction of Phytocompounds from Black Jamun Pulp: Kinetic and Thermodynamics Characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102913. [Google Scholar] [CrossRef]
- Uzel, R.A. Microwave-Assisted Green Extraction Technology for Sustainable Food Processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; Kok Yeow, Y., Ed.; IntechOpen: London, UK, 2018; pp. 159–178. [Google Scholar]
- Gomez, L.; Tiwari, B.; Garcia-Vaquero, M. Emerging Extraction Techniques: Microwave-Assisted Extraction. In Sustainable Seaweed Technologies: Cultivation, Biorefinery, and Applications; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–224. ISBN 9780128179437. [Google Scholar]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of Extraction Conditions of Antioxidant Phenolic Compounds from Mashua (Tropaeolum tuberosum Ruíz & Pavón) Tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar] [CrossRef]
- Aires, A. Phenolics in Foods: Extraction, Analysis and Measurements. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; IntechOpen: London, UK, 2017; pp. 61–88. ISBN 978-953-51-5089-3. [Google Scholar]
- Das, A.J.; Khawas, P.; Seth, D.; Miyaji, T.; Deka, S.C. Optimization of the Extraction of Phenolic Compounds from Cyclosorus extensa with Solvents of Varying Polarities. Prep. Biochem. Biotechnol. 2016, 46, 755–763. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of Bioactive Components from Avocado Peels Using Microwave-Assisted Extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- Elboughdiri, N.; Ghernaout, D.; Kriaa, K.; Jamoussi, B. Enhancing the Extraction of Phenolic Compounds from Juniper Berries Using the Box-Behnken Design. ACS Omega 2020, 5, 27990–28000. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Nikafshar, S.; Master, E.R.; Nejad, M. From Acetone Fractionation to Lignin-Based Phenolic and polyurethane Resins. Ind. Crops. Prod. 2022, 178, 114604. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Sequential Solvent Fractionation of Heterogeneous Bamboo Organosolv Lignin for Value-Added Application. Sep. Purif. Technol. 2012, 101, 18–25. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Ghafoor, K.; Mohamed Ahmed, I.A.; Özcan, M.M. The Effect of Different Solvent Types and Extraction Methods on Oil Yields and Fatty Acid Composition of Safflower Seed. J. Oleo Sci. 2019, 68, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybidc-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; González-Correa, C.H. Folin-Ciocalteu Reaction Alternatives for Higher Polyphenol Quantitation in Colombian Passion Fruits. Int. J. Food Sci. 2021, 2021, 8871301. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Borja, R.; Gutiérrez-González, J.A. A Comprehensive and Critical Review of the Unstandardized Folin-Ciocalteu Assay to Determine the Total Content of Polyphenols: The Conundrum of the Experimental Factors and Method Validation. Talanta 2024, 272, 125771. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Valderrama Fernández, M.d.R. Cuantificación de Hormonas Vegetales y Estudio Metabolómico Mediante Cromatografia-Espectrometría de Masas de Alta Resolución. Aplicación a La Interacción Alfalfa-PGPR. Ph.D. Thesis, Universidad de Sevilla, Seville, Spain, 2024. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ogura, I.; Sugiyama, M.; Tai, R.; Mano, H.; Matsuzawa, T. Optimization of Microplate-Based Phenol-Sulfuric Acid Method and Application to the Multi-Sample Measurements of Cellulose Nanofibers. Anal. Biochem. 2023, 681, 115329. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate Analysis by a Phenol-Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry (TAPPI). Acid-Insoluble Lignin in Wood and Pulp (T-222-om-02); TAPPI Press: Atlanta, GA, USA, 2006; pp. 1–7. [Google Scholar]
- Nitzsche, R.; Gröngröft, A.; Köchermann, J.; Meisel, K.; Etzold, H.; Verges, M.; Leschinsky, M.; Bachmann, J.; Saake, B.; Torkler, S.; et al. Platform and Fine Chemicals from Woody Biomass: Demonstration and Assessment of a Novel Biorefinery. Biomass Convers. Biorefinery 2020, 11, 2369–2385. [Google Scholar] [CrossRef]
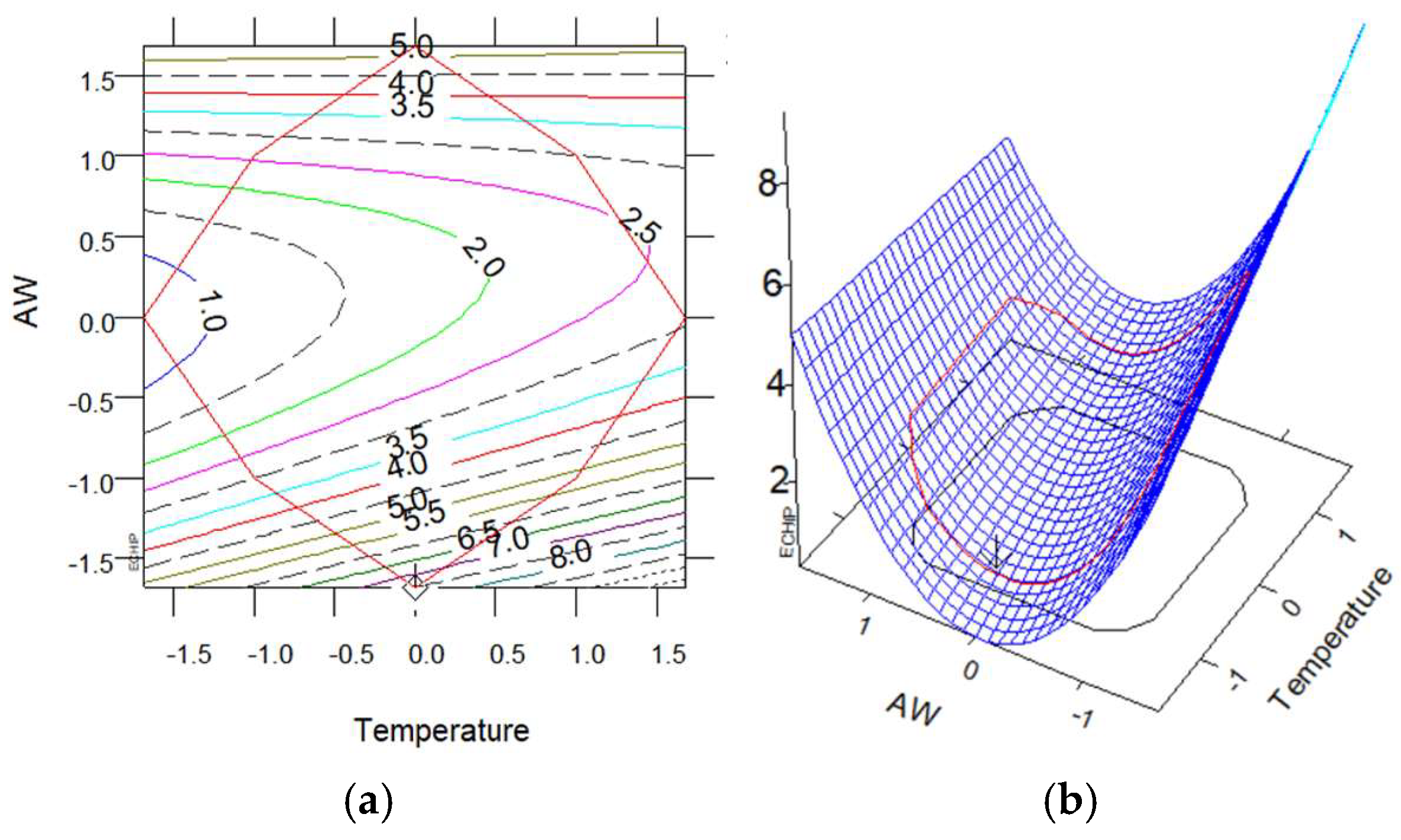
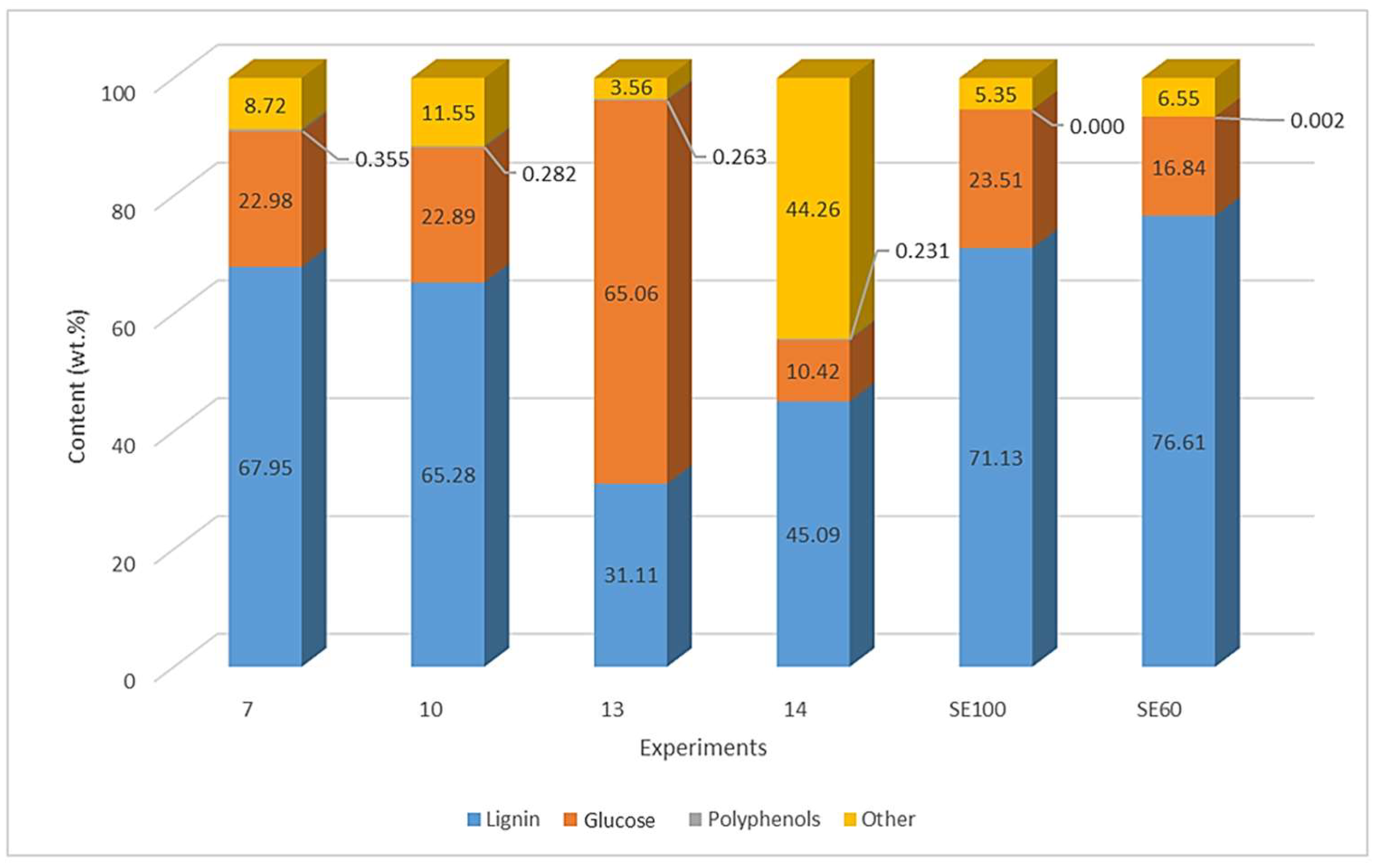
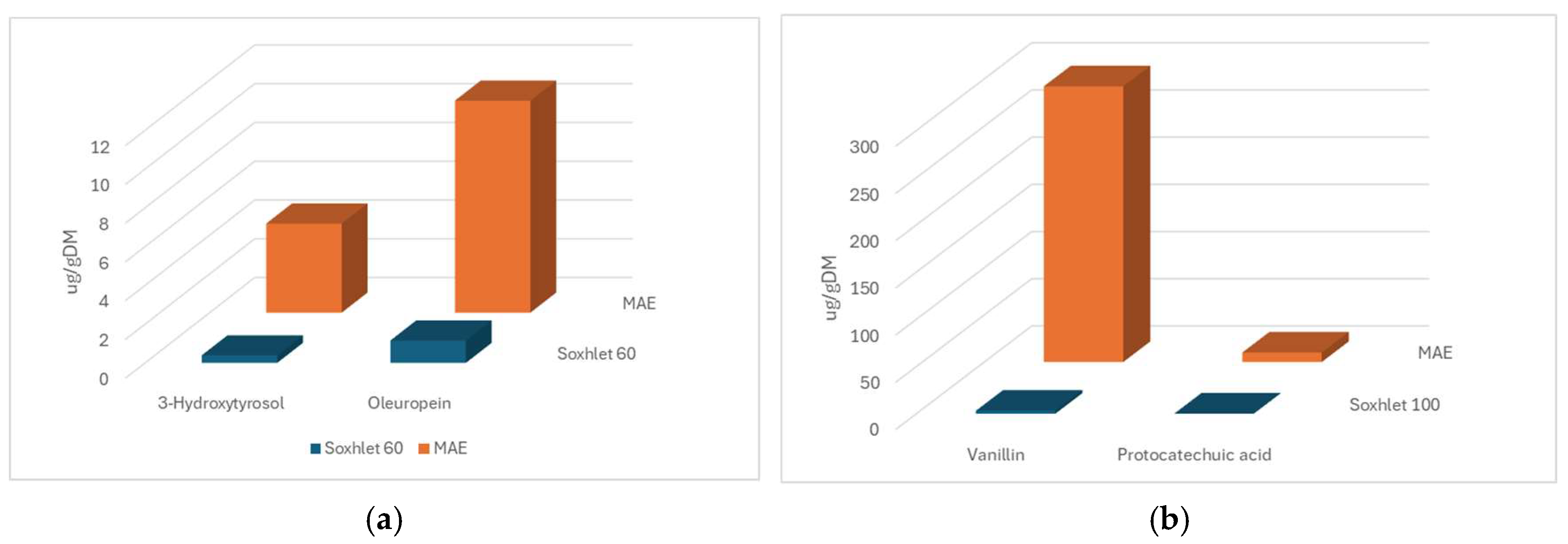
| ID | Factor (Unit) | Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| A | T (°C) | 27.5 | 45 | 60 | 75 | 92.5 |
| B | t (min) | 2 | 10 | 15 | 20 | 28 |
| C | A:W (% v/v) | 50 | 60 | 75 | 90 | 100 |
| Phenolic Compound | MAE Experiments | Soxhlet | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 60 | 100 | |
| Gallic acid 1 | A | ||||||||||||||||
| Protocatechuic acid 1 | |||||||||||||||||
| 3-Hydroxytyrosol 1 | |||||||||||||||||
| 3-O-Methylgallic acid 1 | |||||||||||||||||
| 4-Hydroxybenzoic acid 1 | |||||||||||||||||
| Chlorogenic acid 2 | A | ||||||||||||||||
| Dihydrocaffeic acid 3 | |||||||||||||||||
| 2,4-Dihydroxybenzoic acid 1 | |||||||||||||||||
| Caffeic acid 3 | |||||||||||||||||
| Vanillin 4 | |||||||||||||||||
| Phloretic acid 5 | |||||||||||||||||
| p-Coumaric acid 3 | |||||||||||||||||
| Ferulic acid 3 | |||||||||||||||||
| Salicylic acid 1 | |||||||||||||||||
| Naringin 6 | |||||||||||||||||
| Neohesperidin 6 | |||||||||||||||||
| Oleuropein 7 | A | ||||||||||||||||
| Apigenin-7-O-glucoside 6 | |||||||||||||||||
| Kaempferol-3-O-glucoside 6 | |||||||||||||||||
| Luteolin-4′-O-glucoside 6 | |||||||||||||||||
| Quercetin 6 | |||||||||||||||||
| Organic acid | |||||||||||||||||
| (L)-Malic acid | |||||||||||||||||
| Succinic acid | |||||||||||||||||
| Exp | Factors | Codified Factors | ηE 1 | TPC | Polyphenol Quantity (µg/g DM) | Glucose Quantity 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | A:W (%) | A | B | C | (wt.%) | (mg GAE/g DM) | Vanillin | Protocatechuic Acid | Hydroxytyrosol | Oleuropein | (mg Glc/g DM) | |
| 1 | 75.0 | 10 | 90 | 1 | −1 | 1 | 2.23 | 3.10 ± 0.00 | 173.93 | 5.17 | 3.62 | 4.34 | 2.31 ± 0.01 |
| 2 | 45.0 | 10 | 90 | −1 | −1 | 1 | 1.37 | 1.83 ± 0.00 | 122.50 | 3.40 | 2.22 | 1.25 | 1.35 ± 0.02 |
| 3 | 75.0 | 20 | 90 | 1 | 1 | 1 | 2.30 | 3.27 ± 0.03 | 203.49 | 9.88 | 2.44 | 8.48 | 4.84 ± 0.01 |
| 4 | 45.0 | 20 | 90 | −1 | 1 | 1 | 1.84 | 2.78 ± 0.00 | 177.55 | 9.17 | 2.15 | 2.88 | 3.46 ± 0.05 |
| 5 | 75.0 | 10 | 60 | 1 | −1 | −1 | 2.50 | 3.25 ± 0.00 | 175.31 | 5.78 | 4.28 | 9.58 | 4.24 ± 0.04 |
| 6 | 45.0 | 10 | 60 | −1 | −1 | −1 | 2.05 | 2.57 ± 0.00 | 164.88 | 6.18 | 4.99 | 12.67 | 2.30 ± 0.05 |
| 7 | 75.0 | 20 | 60 | 1 | 1 | −1 | 2.71 | 3.55 ± 0.04 | 136.22 | 8.00 | 1.92 | 3.67 | 6.23 ± 0.06 |
| 8 | 45.0 | 20 | 60 | −1 | 1 | −1 | 2.08 | 2.69 ± 0.03 | 147.82 | 5.94 | 3.75 | 5.39 | 2.26 ± 0.01 |
| 9 | 27.5 | 15 | 75 | −1.682 | 0 | 0 | 1.55 | 2.01 ± 0.00 | 63.10 | 2.24 | 1.93 | 1.58 | 1.50 ± 0.01 |
| 10 | 92.5 | 15 | 75 | 1.682 | 0 | 0 | 2.26 | 2.82 ± 0.00 | 121.91 | 7.23 | 2.02 | 4.30 | 1.88 ± 0.01 |
| 11 | 60.0 | 2 | 75 | 0 | −1.682 | 0 | 1.82 | 2.51 ± 0.00 | 108.49 | 3.58 | 3.52 | 7.93 | 0.93 ± 0.00 |
| 12 | 60.0 | 28 | 75 | 0 | 1.682 | 0 | 1.92 | 2.55 ± 0.00 | 109.55 | 4.58 | 2.61 | 6.91 | 1.92 ± 0.01 |
| 13 | 60.0 | 15 | 50 | 0 | 0 | −1.682 | 2.20 | 2.63 ± 0.02 | 212.12 | 5.22 | 3.61 | 6.51 | 8.23 ± 0.04 |
| 14 | 60.0 | 15 | 100 | 0 | 0 | 1.682 | 1.60 | 2.31 ± 0.00 | 314.70 | 10.86 | 2.79 | 1.33 | 4.75 ± 0.01 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 2.00 | 2.77 ± 0.00 | 126.76 | 4.89 | 3.73 | 4.19 | 1.95 ± 0.02 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 1.91 | 2.56 ± 0.00 | 75.99 | 2.66 | 2.95 | 3.47 | 2.83 ± 0.02 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 1.96 | 2.64 ± 0.00 | 136.56 | 4.85 | 2.89 | 4.06 | 2.20 ± 0.04 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 1.89 | 2.39 ± 0.00 | 131.09 | 4.69 | 3.01 | 3.90 | 2.00 ± 0.03 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 2.17 | 2.93 ± 0.01 | 145.41 | 5.67 | 4.30 | 4.97 | 1.90 ± 0.03 |
| 15 | 60.0 | 15 | 75 | 0 | 0 | 0 | 2.06 | 2.76 ± 0.00 | 137.6 | 4.81 | 3.26 | 4.68 | 1.33 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Rivas, A.; Álvarez-Mateos, P.; García-Martín, J.F. Revalorization of Olive Stones from Olive Pomace: Phenolic Compounds Obtained by Microwave-Assisted Extraction. Agronomy 2025, 15, 1761. https://doi.org/10.3390/agronomy15081761
Castillo-Rivas A, Álvarez-Mateos P, García-Martín JF. Revalorization of Olive Stones from Olive Pomace: Phenolic Compounds Obtained by Microwave-Assisted Extraction. Agronomy. 2025; 15(8):1761. https://doi.org/10.3390/agronomy15081761
Chicago/Turabian StyleCastillo-Rivas, Alicia, Paloma Álvarez-Mateos, and Juan Francisco García-Martín. 2025. "Revalorization of Olive Stones from Olive Pomace: Phenolic Compounds Obtained by Microwave-Assisted Extraction" Agronomy 15, no. 8: 1761. https://doi.org/10.3390/agronomy15081761
APA StyleCastillo-Rivas, A., Álvarez-Mateos, P., & García-Martín, J. F. (2025). Revalorization of Olive Stones from Olive Pomace: Phenolic Compounds Obtained by Microwave-Assisted Extraction. Agronomy, 15(8), 1761. https://doi.org/10.3390/agronomy15081761








