Zinc and Selenium Biofortification Modulates Photosynthetic Performance: A Screening of Four Brassica Microgreens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Chlorophyll a Fluorescence Measurements
2.3. Modulated 820 nm Reflection Measurements
2.4. Agronomic Traits and Photosynthetic Pigment Determination
2.5. Selenium and Zinc Content Analysis
2.6. Statistical Analysis
2.6.1. Variable Selection and Multivariate Analysis
2.6.2. Canonical Discriminant Analysis
2.6.3. Post-Hoc and Correlation Analyses
3. Results and Discussion
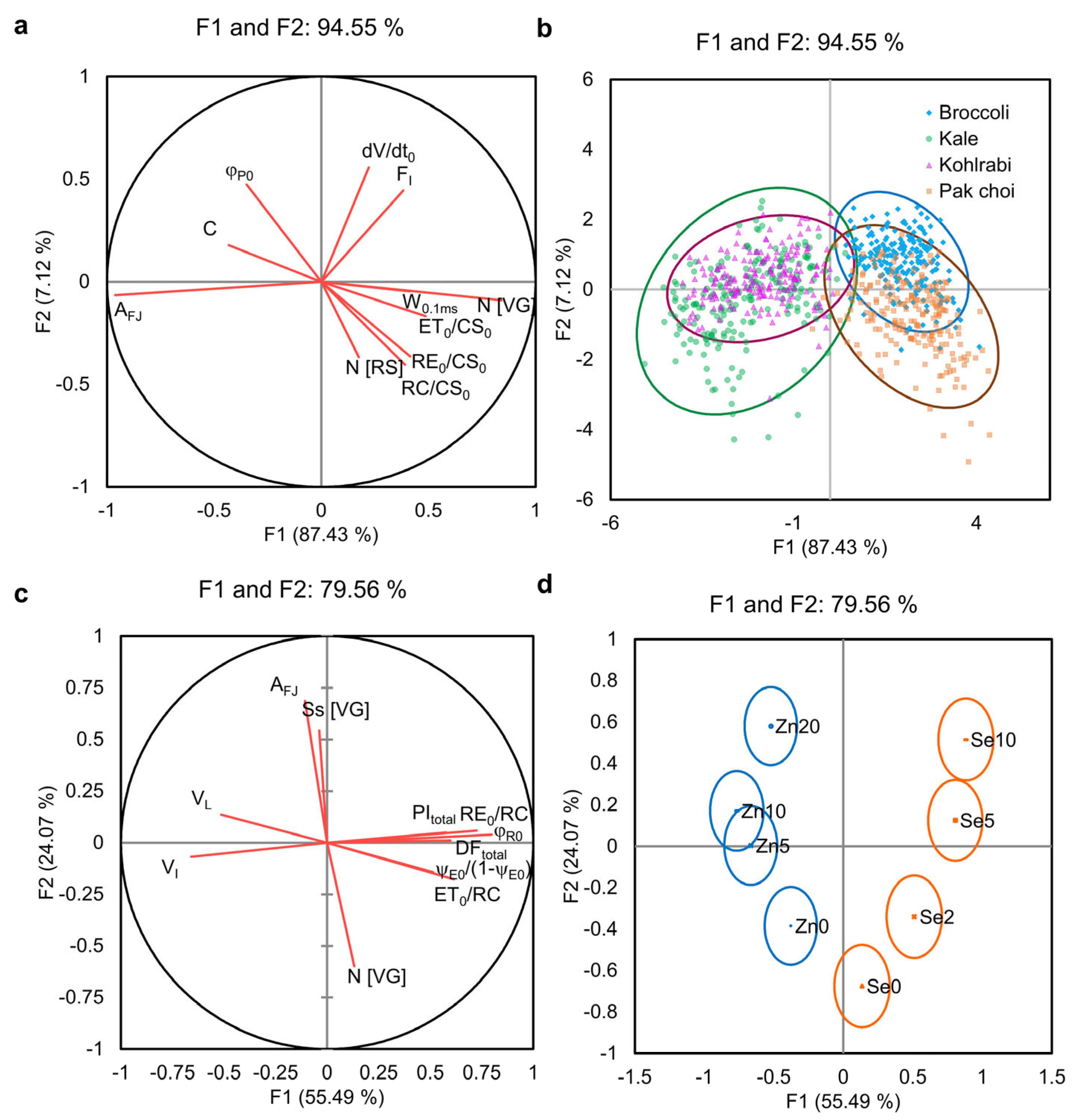
3.1. Variable Selection and Multivariate Analysis of Chlorophyll Fluorescence Parameters
3.2. Canonical Discriminant Analysis
3.3. Micronutrient Effect
3.3.1. Zinc Effect
3.3.2. Selenium Effect
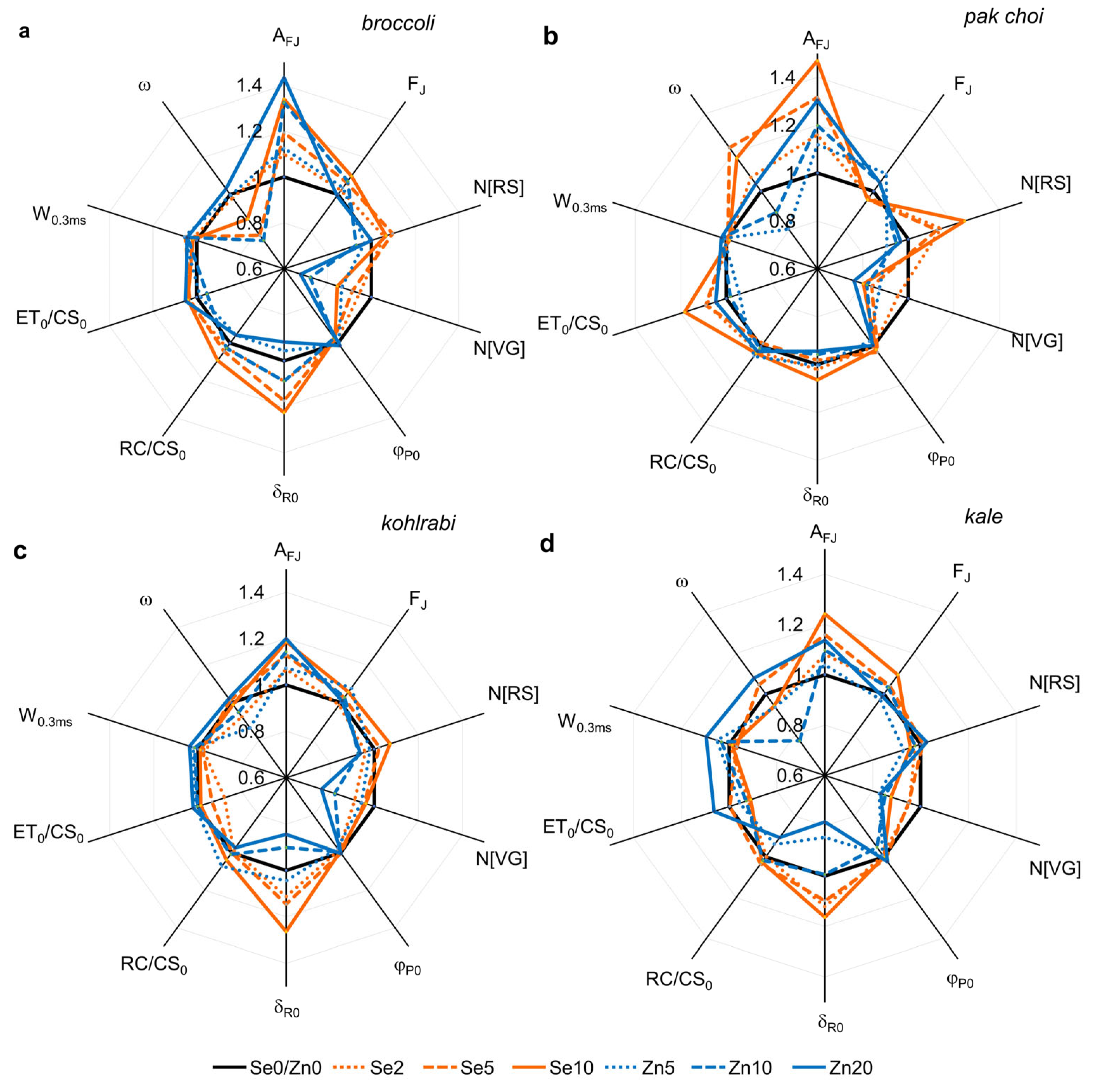
3.4. PSII Primary Photochemistry and Electron Flow
3.5. Performance Index and Driving Forces
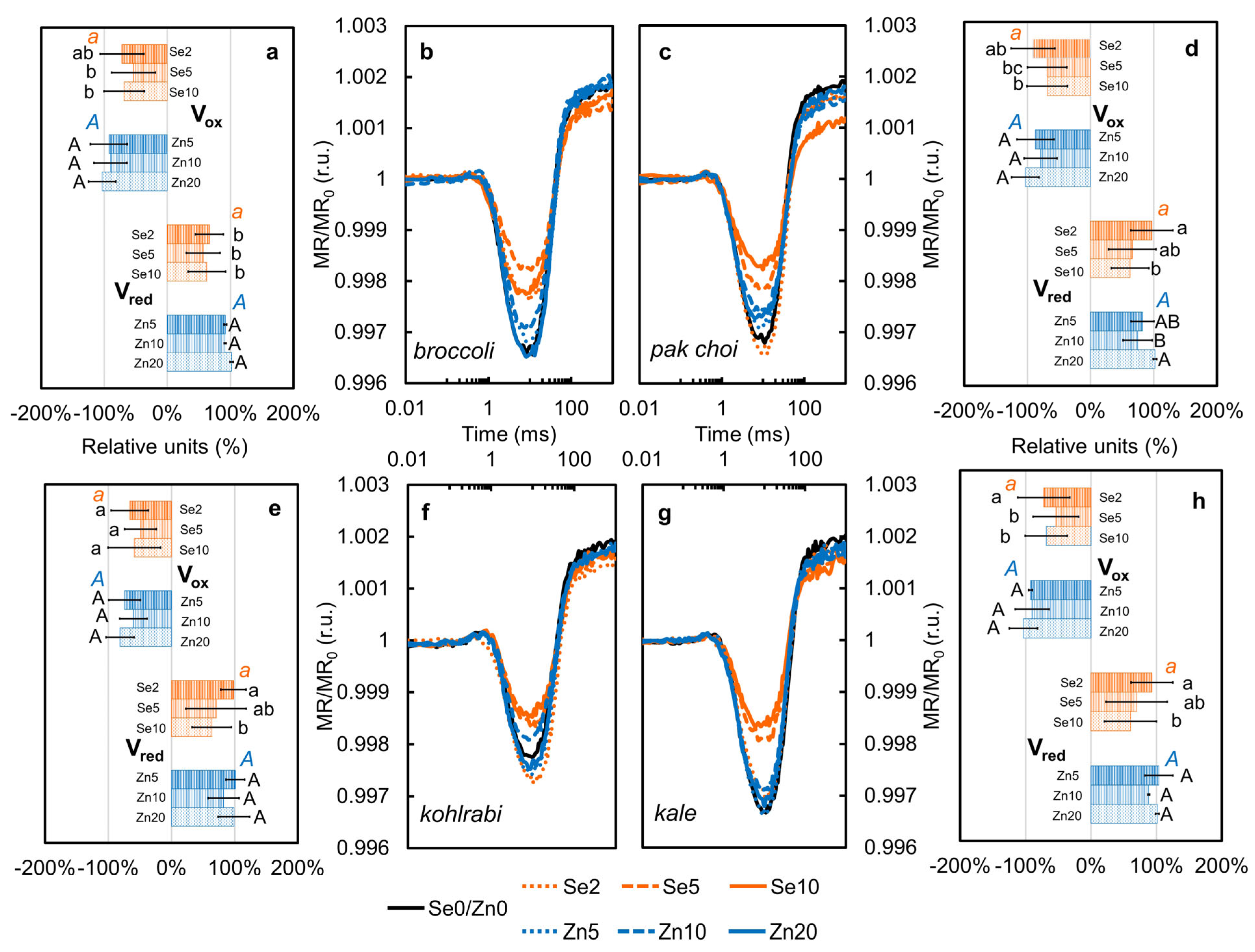
3.6. Redox Dynamics of PSI—Modulated Reflectance at 820 nm
3.7. Agronomic Traits and Pigment Ratio
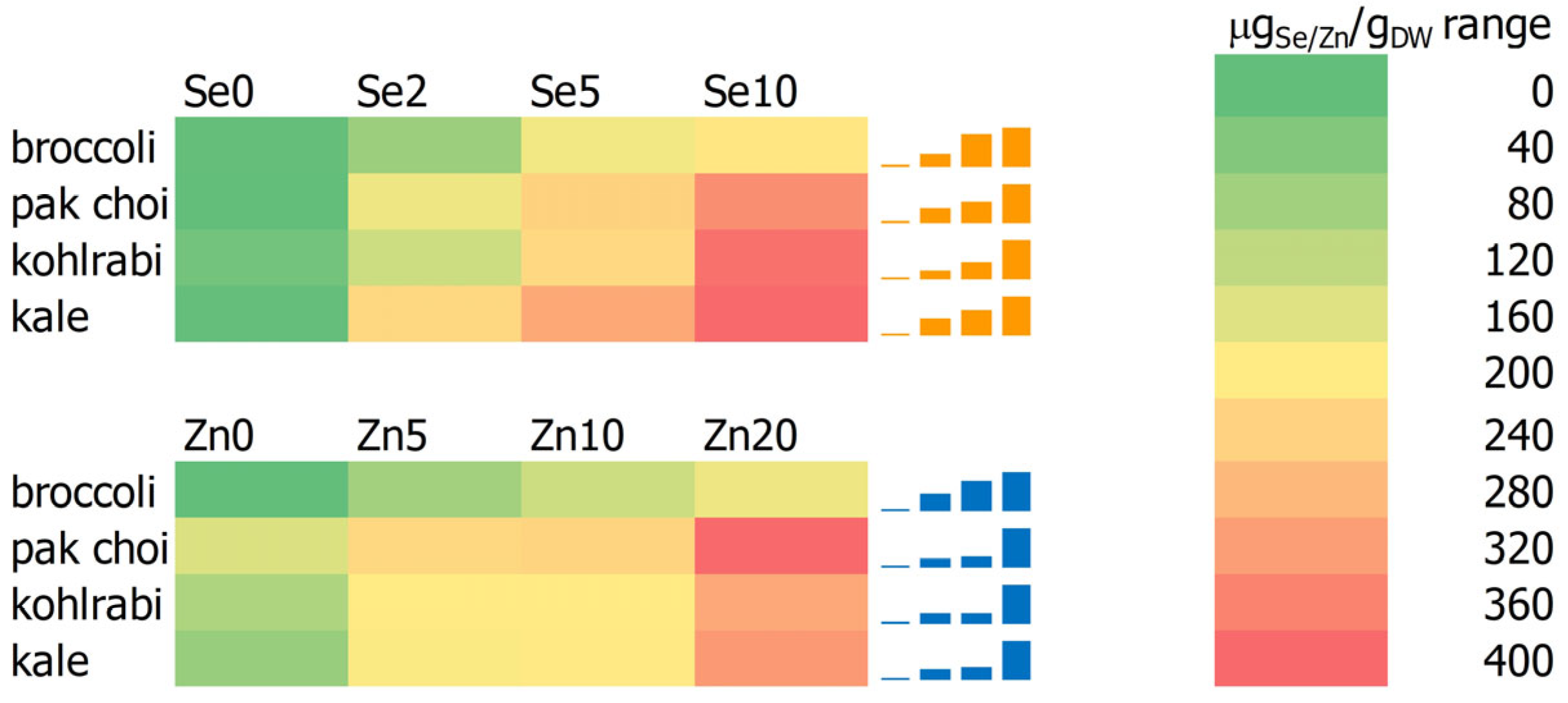
3.8. Selenium and Zinc Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic Diversity of Phytochemical Concentrations and Antioxidant Capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional Quality and Health Benefits of Microgreens, a Crop of Modern Agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Balik, S.; Elgudayem, F.; Dasgan, H.Y.; Kafkas, N.E.; Gruda, N.S. Nutritional Quality Profiles of Six Microgreens. Sci. Rep. 2025, 15, 6213. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae Microgreens: A Novel and Promissory Source of Sustainable Bioactive Compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef]
- Šola, I.; Vujčić Bok, V.; Popović, M.; Gagić, S. Phytochemical Composition and Functional Properties of Brassicaceae Microgreens: Impact of in Vitro Digestion. Int. J. Mol. Sci. 2024, 25, 11831. [Google Scholar] [CrossRef]
- Renna, M.; Stellacci, A.M.; Corbo, F.; Santamaria, P. The Use of a Nutrient Quality Score Is Effective to Assess the Overall Nutritional Value of Three Brassica Microgreens. Foods 2020, 9, 1226. [Google Scholar] [CrossRef]
- Arena, D.; Ammar, H.B.; Major, N.; Kovačević, T.K.; Ban, S.G.; Treccarichi, S.; Scalzo, R.L.; Branca, F. Light Use Efficiency of Broccoli (Brassica oleracea Var. Italica Plenck) and Rocket (Eruca sativa L.) during the Initial Plant Growth Stages. Sci. Hortic. 2024, 336, 113408. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving Nutrition through Biofortification: A Review of Evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Gödecke, T.; Stein, A.J.; Qaim, M. The Global Burden of Chronic and Hidden Hunger: Trends and Determinants. Glob. Food Secur. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, T.; Singh, S.P.; Bhardwaj, A.; Srivastava, D.; Kumar, R. Prospects of Microgreens as Budding Living Functional Food: Breeding and Biofortification through OMICS and Other Approaches for Nutritional Security. Front. Genet. 2023, 14, 1053810. [Google Scholar] [CrossRef] [PubMed]
- Szerement, J.; Szatanik–Kloc, A.; Mokrzycki, J.; Mierzwa–Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium Regulates Antioxidant, Photosynthesis, and Cell Permeability in Plants under Various Abiotic Stresses: A Review. Plants 2022, 12, 44. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, Zinc Nanoparticles and Plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Bandehagh, A.; Dehghanian, Z.; Gougerdchi, V.; Hossain, M. Selenium: A Game Changer in Plant Development, Growth, and Stress Tolerance, via the Modulation in Gene Expression and Secondary Metabolite Biosynthesis. Phyton 2023, 92, 2301. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Hawrylak–Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek–Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium Biofortification Enhances the Growth and Alters the Physiological Response of Lamb’s Lettuce Grown under High Temperature Stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic Biofortification of Significant Cereal Crops with Selenium—A Review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento–Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Sofo, A.; Moreira, I.; Gattullo, C.E.; Martins, L.L.; Mourato, M. Antioxidant Responses of Edible and Model Plant Species Subjected to Subtoxic Zinc Concentrations. J. Trace Elem. Med. Biol. 2018, 49, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El–Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef]
- Poudel, P.; Di Gioia, F.; Lambert, J.D.; Connolly, E.L. Zinc Biofortification through Seed Nutri–Priming Using Alternative Zinc Sources and Concentration Levels in Pea and Sunflower Microgreens. Front. Plant Sci. 2023, 14, 1177844. [Google Scholar] [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon–Smits, E.; Bañuelos, G.S. Selenium Cycling across Soil–Plant–Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Sulieman, S.; Mühling, K.H. Regulation of Selenium/Sulfur Interactions to Enhance Chemopreventive Effects: Lessons to Learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef]
- Tian, M.; Hui, M.; Thannhauser, T.W.; Pan, S.; Li, L. Selenium–Induced Toxicity Is Counteracted by Sulfur in Broccoli (Brassica oleracea L. Var. Italica). Front. Plant Sci. 2017, 8, 1425. [Google Scholar] [CrossRef]
- Yeasmin, M.; Lamb, D.; Choppala, G.; Rahman, M.M. Impact of Sulfur on Biofortification and Speciation of Selenium in Wheat Grain Grown in Selenium–Deficient Soils. J. Soil. Sci. Plant Nutr. 2022, 22, 3243–3253. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Hui, X.; Luo, L.; Chen, Y.; Palta, J.A.; Wang, Z. Zinc Agronomic Biofortification in Wheat and Its Drivers: A Global Meta–Analysis. Nat. Commun. 2025, 16, 3913. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-F.; Li, X.-J.; Yan, W.; Miao, Q.; Zhang, C.-Y.; Huang, M.; Sun, J.-B.; Qi, S.-J.; Ding, Z.-H.; Cui, Z.-L. Biofortification of Different Maize Cultivars with Zinc, Iron and Selenium by Foliar Fertilizer Applications. Front. Plant Sci. 2023, 14, 1144514. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Hagemeyer, J.; Poschenrieder, C.; Barceló, J. Water Relations in Heavy Metal Stressed Plants. In Heavy Metal Stress in Plants: From Molecules to Ecosystems; Springer: Berlin/Heidelberg, Germany, 1999; pp. 207–229. [Google Scholar]
- Kong, L.; Wang, M.; Bi, D. Selenium Modulates the Activities of Antioxidant Enzymes, Osmotic Homeostasis and Promotes the Growth of Sorrel Seedlings under Salt Stress. Plant Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Wang, J.; Huang, Q.; Aziz, R.; Zhou, W. Dual Behavior of Selenium: Insights into Physio–Biochemical, Anatomical and Molecular Analyses of Four Brassica napus Cultivars. Chemosphere 2019, 225, 329–341. [Google Scholar] [CrossRef]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier–Laplace, J. Effect of Selenate on Growth and Photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007, 83, 149–158. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Song, S.; Xu, F.; Pan, Y.; Wang, H. Transcriptomic and Proteomic Analyses Reveal New Insight into Chlorophyll Synthesis and Chloroplast Structure of Maize Leaves under Zinc Deficiency Stress. J. Proteom. 2019, 199, 123–134. [Google Scholar] [CrossRef]
- Dang, K.; Mu, J.; Tian, H.; Gao, D.; Zhou, H.; Guo, L.; Shao, X.; Geng, Y.; Zhang, Q. Zinc Regulation of Chlorophyll Fluorescence and Carbohydrate Metabolism in Saline–Sodic Stressed Rice Seedlings. BMC Plant Biol. 2024, 24, 464. [Google Scholar] [CrossRef] [PubMed]
- Mezeyová, I.; Hegedűsová, A.; Golian, M.; Andrejiová, A.; Šlosár, M.; Mezey, J. Influence of Microgreens Biofortification with Selenium on Their Quantitative and Qualitative Parameters. Agronomy 2022, 12, 1096. [Google Scholar] [CrossRef]
- Tavan, M.; Wee, B.; Fuentes, S.; Pang, A.; Brodie, G.; Viejo, C.G.; Gupta, D. Biofortification of Kale Microgreens with Selenate–Selenium Using Two Delivery Methods: Selenium–Rich Soilless Medium and Foliar Application. Sci. Hortic. 2024, 323, 112522. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores–Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Ramos, S.; Faquin, V.; Guilherme, L.; Castro, E.; Ávila, F.; Carvalho, G.; Bastos, C.; Oliveira, C. Selenium Biofortification and Antioxidant Activity in Lettuce Plants Fed with Selenate and Selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Zafeiriou, I.; Gasparatos, D.; Ioannou, D.; Kalderis, D.; Massas, I. Selenium Biofortification of Lettuce Plants (Lactuca sativa L.) as Affected by Se Species, Se Rate, and a Biochar Co–Application in a Calcareous Soil. Agronomy 2022, 12, 131. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli–Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; CRC Press: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli–Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Tsimilli–Michael, M. Revisiting JIP–Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Goltsev, V.; Kalaji, H.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Krüger, G.; De Villiers, M.; Strauss, A.; De Beer, M.; Van Heerden, P.; Maldonado, R.; Strasser, R. Inhibition of Photosystem II Activities in Soybean (Glycine max) Genotypes Differing in Chilling Sensitivity. South Afr. J. Bot. 2014, 95, 85–96. [Google Scholar] [CrossRef]
- van Heerden, P.D.; Tsimilli-Michael, M.; Krüger, G.H.; Strasser, R.J. Dark Chilling Effects on Soybean Genotypes during Vegetative Development: Parallel Studies of CO2 Assimilation, Chlorophyll a Fluorescence Kinetics O-J-I-P and Nitrogen Fixation. Physiol. Plant. 2003, 117, 476–491. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative Effect of Tenuazonic Acid, Diuron, Bentazone, Dibromothymoquinone and Methyl Viologen on the Kinetics of Chl a Fluorescence Rise OJIP and the MR820 Signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature Effects on Pea Plants Probed by Simultaneous Measurements of the Kinetics of Prompt Fluorescence, Delayed Fluorescence and Modulated 820 nm Reflection. PLoS ONE 2013, 8, e59433. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Fusaro, L.; Gottardini, E.; Pollastrini, M.; Goltsev, V.; Strasser, R.J.; Bussotti, F. Plant Stress Analysis: Application of Prompt, Delayed Chlorophyll Fluorescence and 820 nm Modulated Reflectance. Insights from Independent Experiments. Plant Physiol. Biochem. 2014, 85, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Fusaro, L.; Strasser, R.J.; Bussotti, F.; Manes, F. Effects of Acute O3 Stress on PSII and PSI Photochemistry of Sensitive and Resistant Snap Bean Genotypes (Phaseolus vulgaris L.), Probed by Prompt Chlorophyll “a” Fluorescence and 820 nm Modulated Reflectance. Plant Physiol. Biochem. 2015, 97, 368–377. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli–Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in Vivo Recording of Prompt and Delayed Fluorescence and 820–nm Reflection Changes during Drying and after Rehydration of the Resurrection Plant Haberlea rhodopensis. Biochim. Biophys. Acta BBA–Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Matusiewicz, H.; Sturgeon, R.E.; Berman, S.S. Trace Element Analysis of Biological Material Following Pressure Digestion with Nitric Acid–Hydrogen Peroxide and Microwave Heating. J. Anal. At. Spectrom. 1989, 4, 323–327. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Prentice Hall: Saddle River, NJ, USA, 2002. [Google Scholar]
- Stevens, J. Applied Multivariate Statistics for the Social Sciences; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2002; Volume 4. [Google Scholar]
- Ateş, C.; Kaymaz, Ö.; Kale, H.E.; Tekindal, M.A. Comparison of Test Statistics of Nonnormal and Unbalanced Samples for Multivariate Analysis of Variance in Terms of type-I Error Rates. Comput. Math. Methods Med. 2019, 2019, 2173638. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.5.1; R Core Team: Vienna, Austria, 2025.
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K. Chlorophyll Fluorescence as a Tool for Nutrient Status Identification in Rapeseed Plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of Iron, Zinc and Manganese Shortage–Induced Change on Photosynthetic Pigments, Some Osmoregulators and Chlorophyll Fluorescence Parameters in Lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, Y. Effects of Zn Deficiency and Bicarbonate on the Growth and Photosynthetic Characteristics of Four Plant Species. PLoS ONE 2017, 12, e0169812. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Goltsev, V.N.; Żuk–Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315153605. [Google Scholar]
- Feng, T.; Chen, S.S.; Gao, D.Q.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium Improves Photosynthesis and Protects Photosystem II in Pear (Pyrus bretschneideri), Grape (Vitis vinifera), and Peach (Prunus Persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Moreira, S.W.; Braga, P.C.S.; Conde, L.T.; Cipriano, R.; Falqueto, A.R.; Gontijo, A.B.P.L. Photosynthetic Apparatus Performance and Anatomical Modulations of Alcantarea imperialis (Bromeliaceae) Exposed to Selenium during in vitro Growth. Photosynthetica 2021, 59, 529–537. [Google Scholar] [CrossRef]
- Souza, A.F.C.; Martins, J.P.R.; Gontijo, A.B.P.L.; Falqueto, A.R. Selenium Improves the Transport Dynamics and Energy Conservation of the Photosynthetic Apparatus of in vitro Grown Billbergia zebrina (Bromeliaceae). Photosynthetica 2019, 57, 931–941. [Google Scholar] [CrossRef]
- Ciscato, M.; Vangronsveld, J.; Valcke, R. Effects of Heavy Metals on the Fast Chlorophyll Fluorescence Induction Kinetics of Photosystem II: A Comparative Study. Z. Für Naturforschung C 1999, 54, 735–739. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Mohanty, P. Zinc–Inhibited Electron Transport of Photosynthesis in Isolated Barley Chloroplasts. Plant Physiol. 1980, 66, 1174–1178. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Inhibition of Photosynthesis in Phaseolus Vulgaris by Treatment with Toxic Concentrations of Zinc: Effects on Electron Transport and Photophosphorylation. Physiol. Plant. 1986, 66, 717–721. [Google Scholar] [CrossRef]
- Vassilev, A.; Nikolova, A.; Koleva, L.; Lidon, F. Effects of Excess Zn on Growth and Photosynthetic Performance of Young Bean Plants. J. Phytol. 2011, 3, 58–62. [Google Scholar]
- Di Baccio, D.; Kopriva, S.; Sebastiani, L.; Rennenberg, H. Does Glutathione Metabolism Have a Role in the Defence of Poplar against Zinc Excess? New Phytol. 2005, 167, 73–80. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Q.S.; Yang, X.Q.; Sheng, Z.T.; Nan, G.N. The Alternation between PSII and PSI in Ivy (Hedera nepalensis) Demonstrated by in vivo Chlorophyll a Fluorescence and Modulated 820 nm Reflection. Plant Physiol. Biochem. 2016, 108, 499–506. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between Reactive Oxygen Species Production and Photochemistry of Photosystems I and II in Lemna gibba L. Plants under Salt Stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Srivastava, A.; Guissé, B.; Greppin, H.; Strasser, R.J. Regulation of Antenna Structure and Electron Transport in Photosystem II of Pisum sativum under Elevated Temperature Probed by the Fast Polyphasic Chlorophyll a Fluorescence Transient: OKJIP. Biochim. Biophys. Acta BBA–Bioenerg. 1997, 1320, 95–106. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli–Michael, M.; Sarin, N.B. Overexpression of γ–Tocopherol Methyl Transferase Gene in Transgenic Brassica juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta BBA–Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska–Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik–Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kalaji, H.M. Photosynthetic Responses of Sun–and Shade–Grown Barley Leaves to High Light: Is the Lower PSII Connectivity in Shade Leaves Associated with Protection against Excess of Light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Keren, N.; Liberton, M.; Pakrasi, H.B. Photochemical Competence of Assembled Photosystem II Core Complex in Cyanobacterial Plasma Membrane. J. Biol. Chem. 2005, 280, 6548–6553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katanić, Z.; Atić, L.; Ferhatović, D.; Cesar, V.; Lepeduš, H. PSII Photochemistry in Vegetative Buds and Needles of Norway Spruce (Picea abies L. Karst.) Probed by OJIP Chlorophyll a Fluorescence Measurement. Acta Biol. Hung. 2012, 63, 218–230. [Google Scholar] [CrossRef]
- Mlinarić, S.; Dunić, J.A.; Babojelić, M.S.; Cesar, V.; Lepeduš, H. Differential Accumulation of Photosynthetic Proteins Regulates Diurnal Photochemical Adjustments of PSII in Common Fig (Ficus carica L.) Leaves. J. Plant Physiol. 2017, 209, 1–10. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska, A.; Pawluśkiewicz, B.; Paunov, M.; Alexantrov, V.; Goltsev, V.; Kalaji, M. Prompt Chlorophyll a Fluorescence as a Rapid Tool for Diagnostic Changes in PSII Structure Inhibited by Salt Stress in Perennial Ryegrass. J. Photochem. Photobiol. B 2016, 157, 22–31. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of Different Metals on Photosynthesis: Cadmium and Zinc Affect Chlorophyll Fluorescence in Durum Wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Wang, H.; Jin, C. Changes of Reactive Oxygen Species and Related Enzymes in Mitochondrial Respiration During Storage of Harvested Peach Fruits. Agric. Sci. China 2011, 10, 149–158. [Google Scholar] [CrossRef]
- Todorenko, D.; Timofeev, N.; Kovalenko, I.; Kukarskikh, G.; Matorin, D.; Antal, T. Chromium Effects on Photosynthetic Electron Transport in Pea (Pisum sativum L.). Planta 2020, 251, 11. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic Performance during Leaf Expansion in Malus micromalus Probed by Chlorophyll a Fluorescence and Modulated 820 nm Reflection. J. Photochem. Photobiol. B 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Hermanns, A.S.; Zhou, X.; Xu, Q.; Tadmor, Y.; Li, L. Carotenoid Pigment Accumulation in Horticultural Plants. Hortic. Plant J. 2020, 6, 343–360. [Google Scholar] [CrossRef]
- Sun, T.; Li, L. Toward the ‘Golden’Era: The Status in Uncovering the Regulatory Control of Carotenoid Accumulation in Plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef]
- He, R.; Gao, M.; Shi, R.; Song, S.; Zhang, Y.; Su, W.; Liu, H. The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts. Molecules 2020, 25, 4788. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.; Soteriou, G.A.; Graziani, G.; De Pascale, S.; Rouphael, Y. Zinc Biofortification of Hydroponically Grown Basil: Stress Physiological Responses and Impact on Antioxidant Secondary Metabolites of Genotypic Variants. Front. Plant Sci. 2022, 13, 1049004. [Google Scholar] [CrossRef]
- Mohsenzadeh, S.; Moosavian, S.S. Zinc Sulphate and Nano–Zinc Oxide Effects on Some Physiological Parameters of Rosmarinus officinalis. Am. J. Plant Sci. 2017, 8, 2635. [Google Scholar] [CrossRef]
- Dou, L.; Tian, Z.; Zhao, Q.; Xu, M.; Zhu, Y.; Luo, X.; Qiao, X.; Ren, R.; Zhang, X.; Li, H. Transcriptomic Characterization of the Effects of Selenium on Maize Seedling Growth. Front. Plant Sci. 2021, 12, 737029. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Wu, X.; Zhang, D.; Wang, F.; Liu, K.; Lu, S. The Levels of Selenium in Tea from China and Associated Human Exposure. J. Food Compos. Anal. 2022, 110, 104567. [Google Scholar] [CrossRef]
- Viltres-Portales, M.; Sánchez-Martín, M.-J.; Llugany, M.; Boada, R.; Valiente, M. Selenium Biofortification of Microgreens: Influence on Phytochemicals, Pigments and Nutrients. Plant Physiol. Biochem. 2024, 206, 108283. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of Selenium-biofortified Microgreens from Selenium-enriched Seeds of Basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; Subcommittee on Interpretation; Uses of Dietary Reference Intakes; Subcommittee on Upper Reference Levels of Nutrients; Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 0-309-59719-6. [Google Scholar]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy Metals Health Risk Assessment for Population via Consumption of Vegetables Grown in Old Mining Area; a Case Study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef]
- Singh, V.; Garg, A.N. Availability of Essential Trace Elements in Indian Cereals, Vegetables and Spices Using INAA and the Contribution of Spices to Daily Dietary Intake. Food Chem. 2006, 94, 81–89. [Google Scholar] [CrossRef]
- Hair, J.F. Multivariate Data Analysis, 7th ed.; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]


| Root (cm) | Hypocotyl (cm) | Plant Total (cm) | DW/FW (%) | Chl a+b/Car | ||
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Broccoli | Se0 | 6.933 ± 2.517 a | 2.093 ± 0.675 a | 9.027 ± 2.454 a | 9.487 ± 0.446 d | 4.138 ± 0.031 a |
| Se2 | 6.740 ± 1.840 a | 1.820 ± 0.699 ab | 8.560 ± 2.150 a | 12.215 ± 0.271 b | 3.955 ± 0.044 b | |
| Se5 | 2.760 ± 0.702 b | 1.427 ± 0.526 b | 4.187 ± 0.959 b | 13.722 ± 0.379 a | 3.731 ± 0.062 c | |
| Se10 | 4.087 ± 1.037 b | 1.473 ± 0.317 b | 5.560 ± 1.037 b | 11.109 ± 0.678 c | 3.701 ± 0.037 c | |
| Zn0 | 3.713 ± 1.828 B | 2.113 ± 0.867 B | 5.827 ± 0.398 B | 10.494 ± 0.301 B | 4.106 ± 0.065 B | |
| Zn5 | 7.907 ± 2.970 A | 3.367 ± 0.439 A | 11.273 ± 2.865 A | 10.952 ± 0.709 AB | 4.055 ± 0.036 B | |
| Zn10 | 7.760 ± 2.611 A | 2.867 ± 0.434 A | 10.327 ± 2.749 A | 11.412 ± 0.185 A | 4.257 ± 0.027 A | |
| Zn20 | 6.167 ± 2.317 A | 1.940 ± 0.575 B | 8.107 ± 2.271 B | 10.783 ± 0.767 AB | 4.238 ± 0.063 A | |
| Pak choi | Se0 | 3.853 ± 1.548 a | 1.553 ± 0.334 a | 5.407 ± 1.697 a | 12.898 ± 0.395 b | 4.155 ± 0.017 a |
| Se2 | 8.040 ± 1.850 a | 1.400 ± 0.511 a | 4.400 ± 2.115 ab | 16.239 ± 0.494 a | 4.104 ± 0.095 a | |
| Se5 | 1.780 ± 0.592 b | 1.493 ± 0.461 a | 3.273 ± 0.631 b | 15.530 ± 0.446 a | 4.053 ± 0.215 a | |
| Se10 | 2.787 ± 0.680 ab | 1.213 ± 0.236 a | 4.000 ± 0.805 b | 12.560 ± 1.482 b | 4.165 ± 0.102 a | |
| Zn0 | 2.867 ± 1.313 B | 2.200 ± 0.393 A | 5.067 ± 1.337 B | 13.957 ± 0.421 AB | 4.209 ± 0.052 BC | |
| Zn5 | 5.533 ± 2.208 A | 20227 ± 0.349 A | 7.760 ± 2.277 A | 13.572 ± 0.599 AB | 4.239 ± 0.018 B | |
| Zn10 | 3.567 ± 2.191 A | 2.147 ± 0.242 A | 5.713 ± 2.232 B | 12.595 ± 1.301 B | 4.161 ± 0.039 C | |
| Zn20 | 3.393 ± 1.823 B | 1.653 ± 0.414 B | 5.047 ± 1.752 B | 14.527 ± 0.792 A | 4.346 ± 0.036 A | |
| Kohlrabi | Se0 | 3.893 ± 0.869 a | 1.980 ± 0.675 a | 5.873 ± 1.297 a | 13.859 ± 0.309 b | 4.067 ± 0.025 a |
| Se2 | 4.213 ± 0.966 a | 1.853 ± 0.230 ab | 6.067 ± 1.204 a | 12.885 ± 1.809 b | 4.003 ± 0.047 b | |
| Se5 | 2.100 ± 0.815 b | 1.687 ± 0.444 ab | 3.787 ± 0.823 b | 16.151 ± 0.563 a | 3.758 ± 0.044 c | |
| Se10 | 2.593 ± 1.024 b | 1.535 ± 0.223 b | 3.947 ± 1.026 b | 14.371 ± 0.930 b | 3.822 ± 0.054 c | |
| Zn0 | 2.980 ± 1.385 B | 2.253 ± 0.529 A | 5.233 ± 1.362 B | 15.502 ± 0.704 B | 4.248 ± 0.084 A | |
| Zn5 | 5.927 ± 2.514 A | 2.307 ± 0.546 A | 8.233 ± 2.661 A | 15.022 ± 0.325 B | 4.039 ± 0.041 C | |
| Zn10 | 5.120 ± 1.015 A | 2.327 ± 0.343 A | 7.447 ± 1.119 A | 13.827 ± 1.063 A | 4.130 ± 0.049 B | |
| Zn20 | 4.527 ± 2.708 AB | 1.907 ± 0.471 A | 6.433 ± 1.931 AB | 18.530 ± 2.892 B | 4.148 ± 0.036 B | |
| Kale | Se0 | 4.080 ± 1.375 ab | 1.608 ± 0.512 a | 5.760 ± 1.314 a | 12.023 ± 0.369 a | 4.142 ± 0.035 a |
| Se2 | 4.987 ± 1.529 a | 1.533 ± 0.461 a | 6.520 ± 1.500 a | 12.056 ± 0.619 a | 4.023 ± 0.018 b | |
| Se5 | 2.253 ± 0.601 c | 1.500 ± 0.492 a | 3.773 ± 0.824 b | 11.186 ± 0.277 b | 4.042 ± 0.028 b | |
| Se10 | 3.527 ± 1.102 b | 1.013 ± 0.207 b | 4.540 ± 1.079 b | 12.578 ± 0.403 a | 3.790 ± 0.039 c | |
| Zn0 | 5.573 ± 1.167 AB | 2.040 ± 0.267 A | 7.613 ± 1.157 AB | 12.968 ± 0.499 BC | 4.204 ± 0.022 B | |
| Zn5 | 6.907 ± 2.534 A | 1.193 ± 0.294 B | 8.100 ± 2.476 A | 14.014 ± 0.604 AB | 4.091 ± 0.105 BC | |
| Zn10 | 6.733 ± 1.758 A | 1.953 ± 0.323 A | 8.687 ± 1.722 A | 14.267 ± 0.996 A | 4.048 ± 0.043 C | |
| Zn20 | 4.253 ± 1.192 B | 1.730 ± 0.561 A | 5.973 ± 1.395 B | 12.178 ± 0.388 C | 4.396 ± 0.084 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šrajer Gajdošik, M.; Peršić, V.; Melnjak, A.; Ban, D.; Štolfa Čamagajevac, I.; Lončarić, Z.; Kalinić, L.; Mlinarić, S. Zinc and Selenium Biofortification Modulates Photosynthetic Performance: A Screening of Four Brassica Microgreens. Agronomy 2025, 15, 1760. https://doi.org/10.3390/agronomy15081760
Šrajer Gajdošik M, Peršić V, Melnjak A, Ban D, Štolfa Čamagajevac I, Lončarić Z, Kalinić L, Mlinarić S. Zinc and Selenium Biofortification Modulates Photosynthetic Performance: A Screening of Four Brassica Microgreens. Agronomy. 2025; 15(8):1760. https://doi.org/10.3390/agronomy15081760
Chicago/Turabian StyleŠrajer Gajdošik, Martina, Vesna Peršić, Anja Melnjak, Doria Ban, Ivna Štolfa Čamagajevac, Zdenko Lončarić, Lidija Kalinić, and Selma Mlinarić. 2025. "Zinc and Selenium Biofortification Modulates Photosynthetic Performance: A Screening of Four Brassica Microgreens" Agronomy 15, no. 8: 1760. https://doi.org/10.3390/agronomy15081760
APA StyleŠrajer Gajdošik, M., Peršić, V., Melnjak, A., Ban, D., Štolfa Čamagajevac, I., Lončarić, Z., Kalinić, L., & Mlinarić, S. (2025). Zinc and Selenium Biofortification Modulates Photosynthetic Performance: A Screening of Four Brassica Microgreens. Agronomy, 15(8), 1760. https://doi.org/10.3390/agronomy15081760








