1. Introduction
A growing number of countries are facing escalating challenges related to water scarcity, exacerbated by climate change. These impacts are recognized globally with increasing pressure on water resources becoming a key concern for both developed and developing nations [
1]. Climate change contributes to rising temperatures, higher evaporation rates, and greater variability in precipitation, all of which reduce water availability for essential uses, including agriculture, industry, and domestic consumption [
2]. As a result, water reserves, such as groundwater and reservoirs, are under greater strain, posing significant challenges for irrigation, particularly in regions reliant on water-intensive agricultural practices [
2].
Tunisia, like many other countries, is experiencing severe consequences due to water scarcity. The country has been identified as one of the most affected countries by these issues, which have profound implications for agricultural productivity and food security [
3]. In light of this pressing challenge, hydroponic farming has emerged as a promising and viable alternative. Hydroponics offers a more efficient use of water resources compared with conventional soil-based agriculture. Studies have shown that hydroponic systems can reduce water consumption by as much as 90% in some cases, making it a more sustainable solution in regions suffering from water shortages [
4]. Additionally, hydroponic farming is less dependent on climatic factors, as it does not rely on natural rainfall, making it a more stable and reliable cultivation method. It also has the added benefit of reducing the need for chemical pesticides and herbicides, thus contributing to a more environmentally friendly and sustainable form of agriculture [
4]. However, despite these advantages, hydroponic systems are not free from their own set of challenges, such as plant diseases, salinity issues, and nutrient deficiencies, all of which can limit crop productivity and the overall quality of yields.
In this context, the use of silicon has garnered attention as a highly effective solution for enhancing plant resilience to both biotic (living organism-related) and abiotic (environment-related) stresses. Silicon has been demonstrated to improve plant resistance to biotic stresses, such as pathogen infections and insect infestations, by strengthening the plant’s cell walls and activating protective biochemical pathways. For instance, silicon-enriched crops have shown enhanced resistance to fungal diseases and pests, which are common threats to crop health [
5]. Regarding abiotic stresses, silicon plays a crucial role in improving plant tolerance to environmental conditions that would otherwise reduce plant growth and productivity. This includes stresses such as drought [
6,
7,
8], salinity [
9,
10,
11], extreme temperatures [
12,
13,
14], and nutrient imbalances [
15,
16,
17], which are becoming increasingly prevalent due to climate change. Silicon enhances the plant’s ability to maintain cellular integrity and physiological functions under such stressful conditions, making it a key tool in mitigating the adverse effects of climate change on agriculture.
Recently, the integration of silicon in hydroponic systems has gained significant attention as a beneficial practice to enhance plant resilience to both biotic and abiotic stresses. This growing interest is supported by recent studies highlighting the multifaceted role of Si in hydroponics. For instance, a comprehensive review by Verma et al. [
18] discusses how Si mitigates biotic stress in plants by strengthening cell walls, inducing defense responses, and enhancing pathogen resistance. Additionally, Si supplementation in hydroponic systems has been shown to alleviate abiotic stresses such as salinity and high temperatures, by improving water relations, nutrient uptake, and photosynthetic efficiency [
19].
An important factor for the widespread adoption of silicon as a plant growth enhancer is its safety for human consumption. Silicon is a naturally occurring element that is widely distributed in the environment, and its presence in plants does not pose any harm to human health. In fact, studies have shown that silicon is beneficial for human health and does not pose any risk when incorporated into the food chain, thus making it an ideal candidate for use in crops meant for human consumption [
20]. This safety profile, combined with its positive effects on plant health and productivity, strengthens the argument for using silicon in agricultural systems, particularly in those systems aimed at producing food for human consumption.
The importance of tomatoes as one of the most widely produced fruit vegetables worldwide motivates this study. Tomatoes are not only the second most produced fruit globally, but are also among the crops that consume the most water in conventional agricultural systems (Food and Agriculture Organization of the United Nations). Given the water-intensive nature of conventional tomato cultivation, hydroponic farming presents a promising solution to reduce water consumption and improve yield, especially in water-scarce regions.
A significant research gap remains in understanding the mechanistic effects of silicon in large-scale hydroponic systems, particularly with regard to its influence on photosystem activity and its contribution to plant performance. This gap is central to our study, which aims to elucidate the effects of silicon on photosynthetic efficiency and plant growth in hydroponic tomato cultivation. We hypothesize that silicon enhances plant productivity by improving photosynthetic activity, chlorophyll biosynthesis, and the functionality of the photosystems PSII and PSI, which are essential for efficient light energy conversion and carbon fixation. These improvements are expected to lead to increased plant growth, greater yield, and enhanced resilience to environmental stresses, making Si a valuable tool for optimizing hydroponic tomato cultivation.
Thus, the novelty of our study lies in its focus on the mechanistic understanding of silicon’s role in enhancing photosynthetic processes on a large scale, which has not been sufficiently addressed in previous research.
3. Results
3.1. Morphological Aspect
As shown in
Figure 4, all plants treated with silicon exhibited improvements in both plant growth and morphological characteristics, with greener and more developed leaves and fruits compared with the control group. This improvement became evident one month after Si application and persisted until harvest, highlighting the positive effect of silicon on plant development.
3.2. Growth Parameters
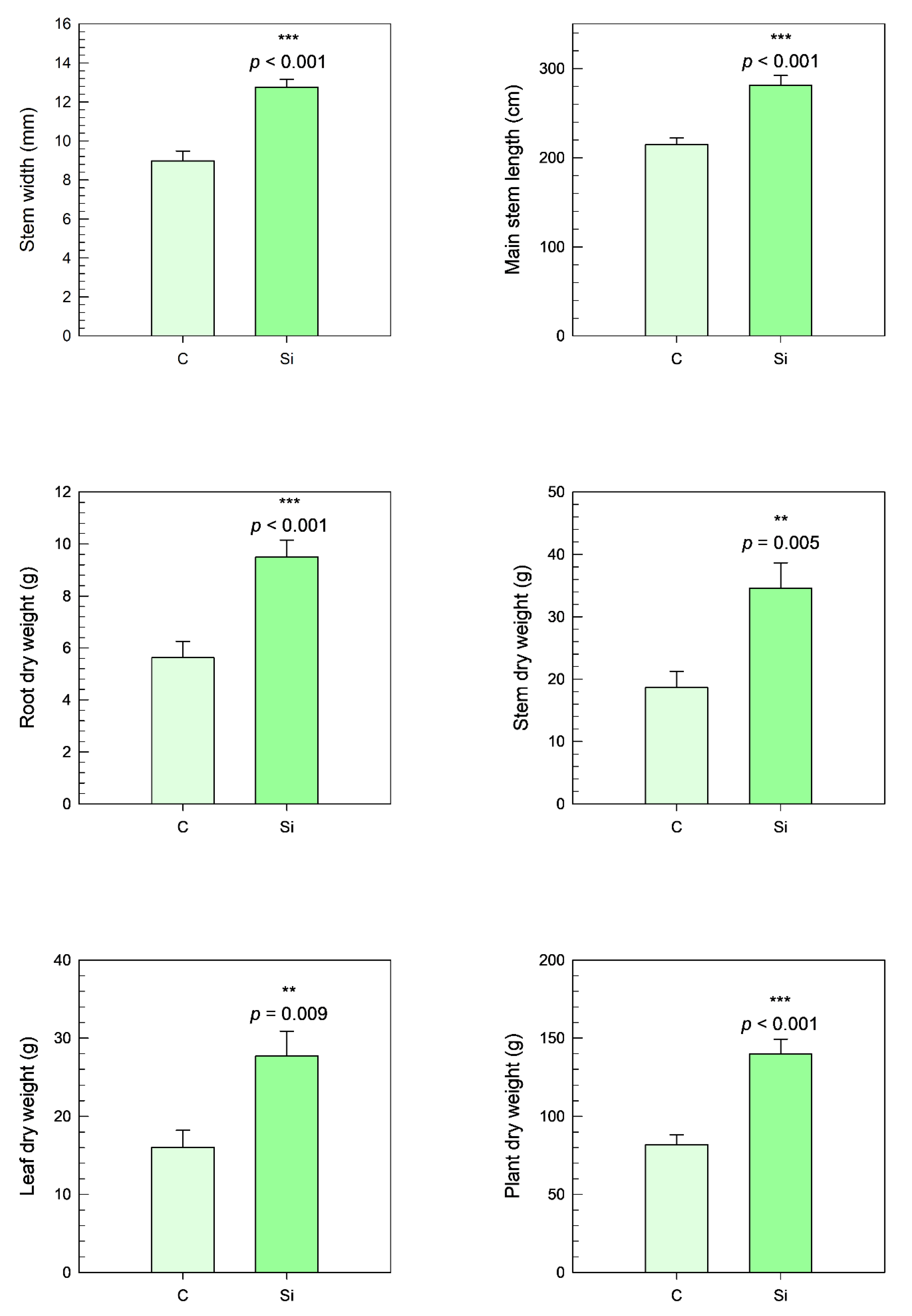
The results presented indicate that the application of a silicon foliar spray significantly enhanced plant biomass production. This effect was evident across various growth parameters, with statistical significance confirmed for all measured traits. Specifically, there was a marked increase in stem width, with a 42% improvement (
p < 0.001), and in the main stem length, which increased by 31% (
p < 0.001), compared with the control group (
Figure 5).
Furthermore, the application of silicon resulted in a substantial increase in the dry weight of the plant components. Root dry weight increased by 69% (p < 0.001), stem dry weight by 86% (p = 0.005), and leaf dry weight by 73% (p = 0.009), compared to the plants not treated with silicon. Consequently, these increases led to an overall plant dry weight enhancement of 71% (p < 0.001).
Additionally, the number of reproductive structures showed a significant improvement. The number of racemes increased by 78% (
p < 0.001), the number of flowers exhibited a 124% rise (
p < 0.001), and the number of fruits increased by 43% (
p = 0.003), relative to the control group (
Figure 6). The box plots of these parameters (
Figure 6) provide further insight into the variability and distribution of the data. For silicon-treated plants, the median, lower quartile, and upper quartile values were consistently higher than those of the control group, indicating an overall improvement in plant growth. In addition, the dispersion of data points was significantly greater for silicon-treated plants, as reflected by the longer interquartile ranges and the broader overall range of the datasets. Specifically, the distances between the ends of the whiskers were greater for the silicon-treated plants, signifying greater variation in their responses to silicon application.
The effects of silicon treatment were evaluated based on the number of fruits and flowers per raceme at various positions on the plant (
Figure 7). For the control plants (C), the number of fruits per raceme decreased as the position of the raceme moved from the base to the top of the plant. This decrease followed a clear quadratic pattern, with a strong fit to the data (
r2 = 0.91). In contrast, silicon-treated plants (Si) consistently exhibited a more gradual decline in fruit numbers with increasing raceme positions, which indicates that silicon treatment helps mitigate the reduction in fruit production, especially at higher raceme positions (
r2 = 0.82).
A similar trend was observed for the number of flowers per raceme. In control plants, the number of flowers initially increased and then decreased as the raceme position increased, peaking at an intermediate position (r2 = 0.78). However, silicon-treated plants consistently showed a more pronounced peak in flower production, with the curves for silicon-treated plants shifted toward higher raceme positions. This shift indicates that silicon treatment promotes flower production at higher raceme positions, and the number of flowers remained more consistent across raceme positions compared to the control (r2 = 0.92). The silicon treatment not only enhanced flower production but also made the distribution of flowers more stable and predictable.
The results indicate that Si foliar treatment positively influenced both fruit and flower production across various raceme positions. Specifically, silicon-treated plants maintained higher numbers of fruits and flowers at the mid-to-upper positions.
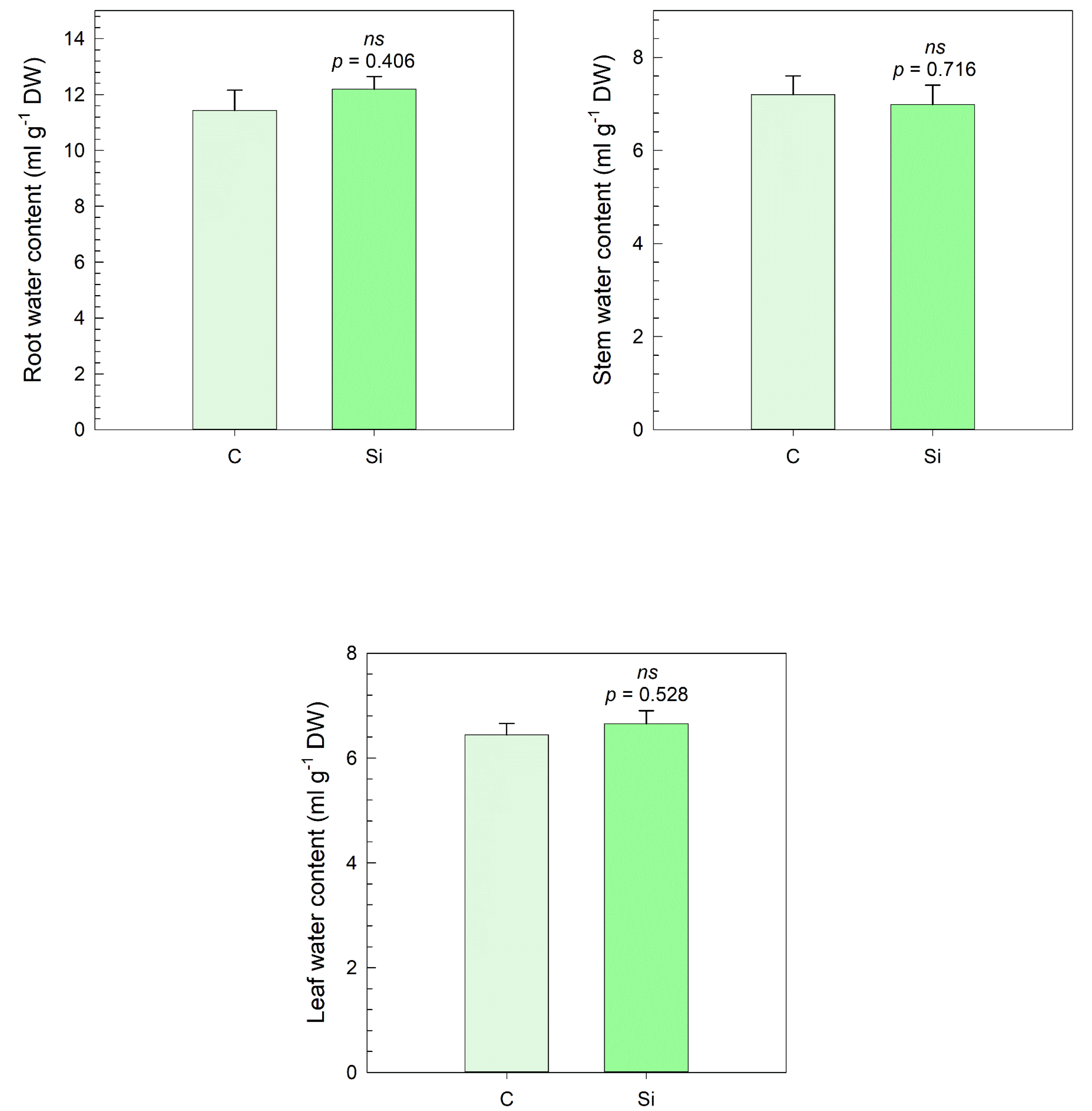
3.3. Water Content
No significant differences were observed in the water content of any plant organs between the silicon-treated plants and the control group (
Figure 8). Specifically, the water content of the root, stem, and leaf in the silicon-treated plants was similar to that of the control plants. These results suggest that silicon treatment did not significantly affect the water retention capacity in the measured plant organs.
3.4. SPAD Value and Photosynthetic Pigment Contents
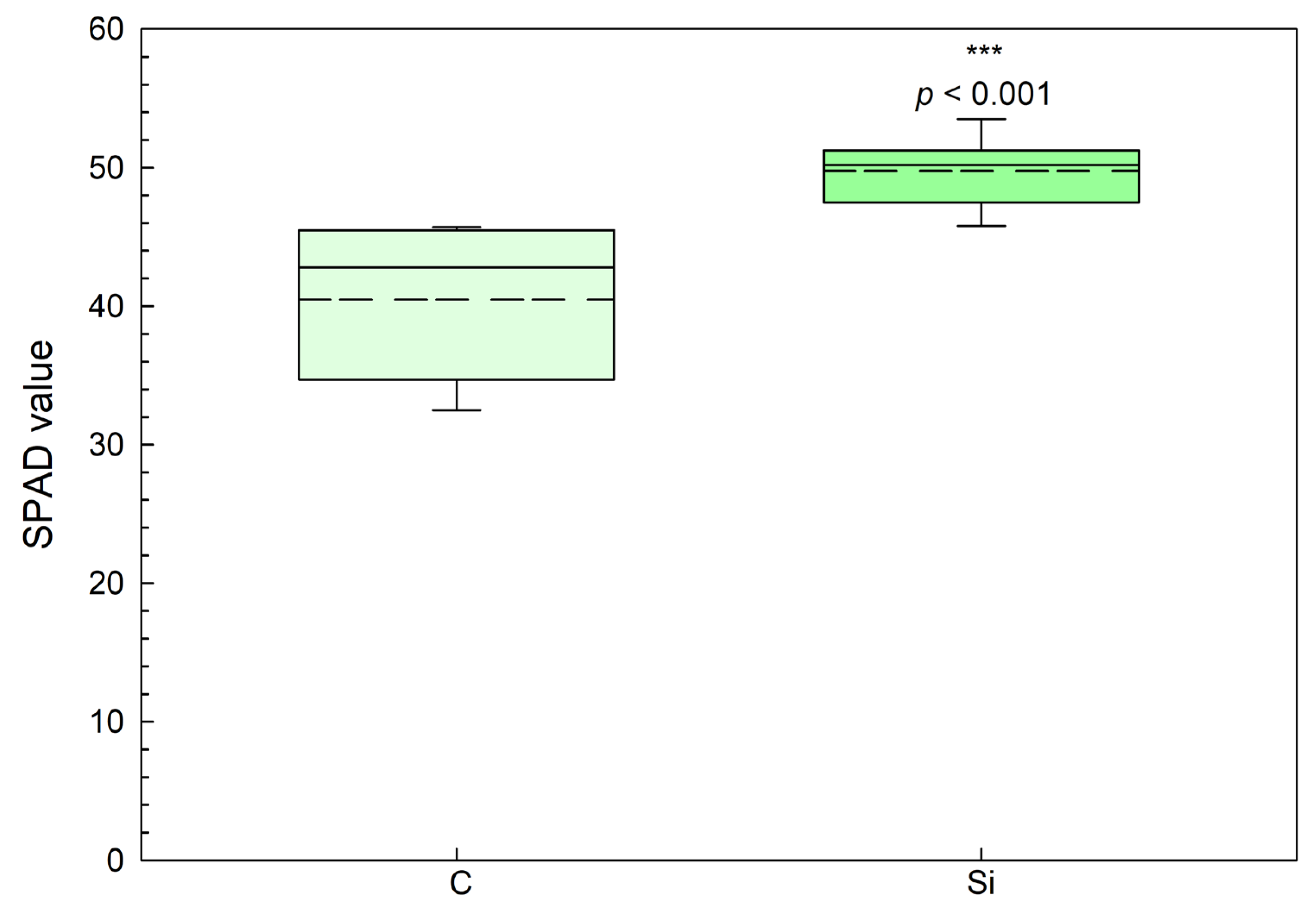
The addition of silicon resulted in a statistically significant increase in the SPAD value of the leaves (
Figure 9). Specifically, the box plots demonstrate that the median, lower quartile, and upper quartile values for the silicon-treated plants were all higher than those for the control group, with a wider overall range in the dataset for the control group. This is reflected in the longer whisker distances in the control group, indicating greater variation in SPAD values than in the silicon-treated plants. The SPAD value increased by 23% (
p < 0.001) in the silicon-treated plants compared with the control group.
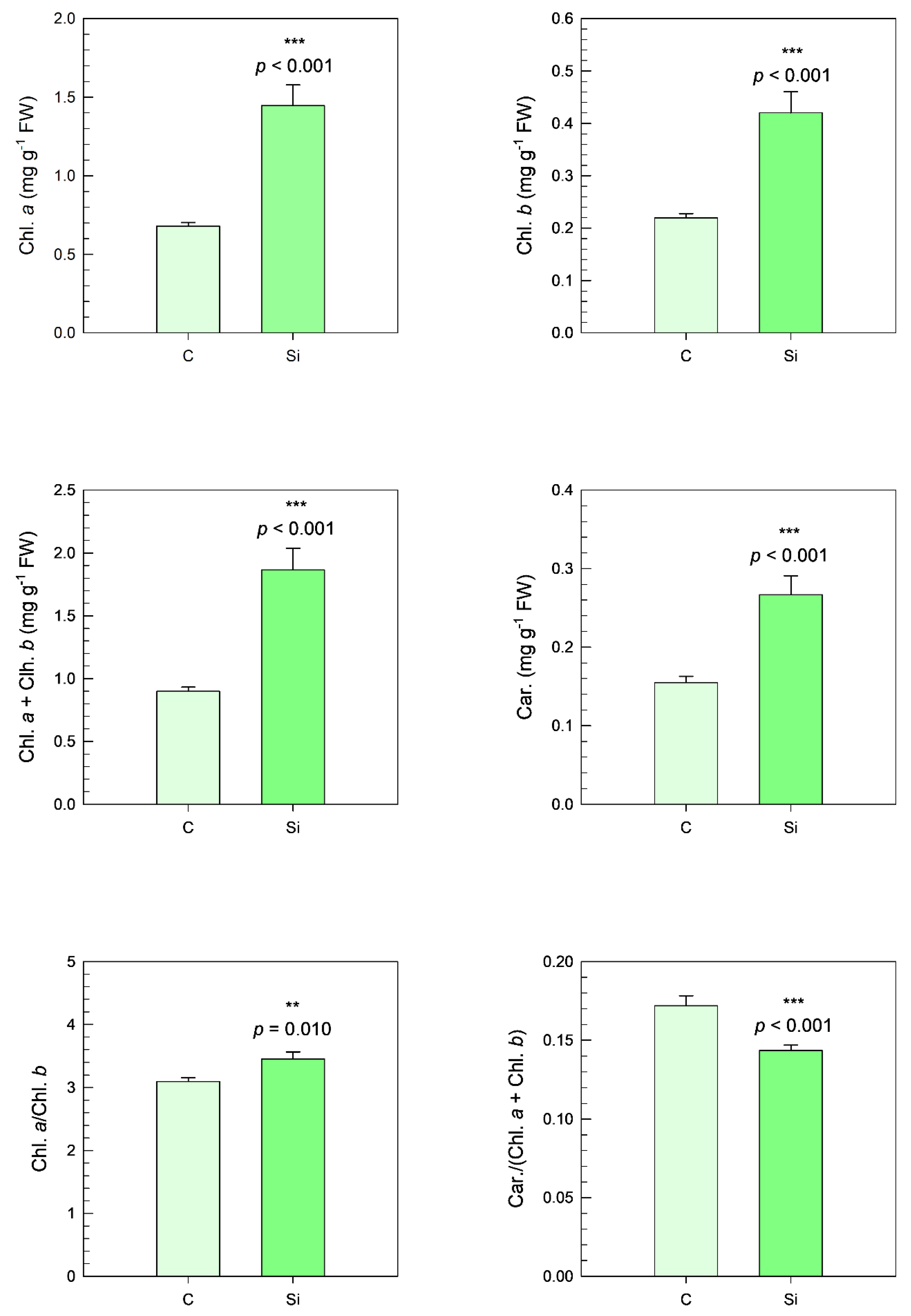
In addition to the increase in SPAD values, significant increases in the concentrations of chlorophylls (Chl.
a and Chl.
b) and total chlorophylls (Chl.
a + Chl.
b) were observed in silicon-treated plants, with increases of 113% (
p < 0.001), 91% (
p < 0.001), and 108% (
p < 0.001), respectively. A corresponding significant increase in carotenoid (Car.) content was also observed, with a 72% increase (
p < 0.001). These results indicate that silicon treatment promotes chlorophyll synthesis and enhances carotenoid production, both of which are vital components of the photosynthetic machinery (
Figure 10).
Additionally, a significant effect was detected in the Chl.
a/Chl.
b ratio (
p = 0.010), indicating a slight alteration in the relative proportions of chlorophyll a and chlorophyll b in silicon-treated plants compared with the control group. Interestingly, a marked decrease was observed in the carotenoid/chlorophyll (
a +
b) ratio in silicon-treated plants, with a reduction of 17% (
p < 0.001). This could indicate a shift in the balance between chlorophylls and carotenoids, potentially reflecting a physiological adjustment to optimize photosynthesis under the influence of silicon (
Figure 10).
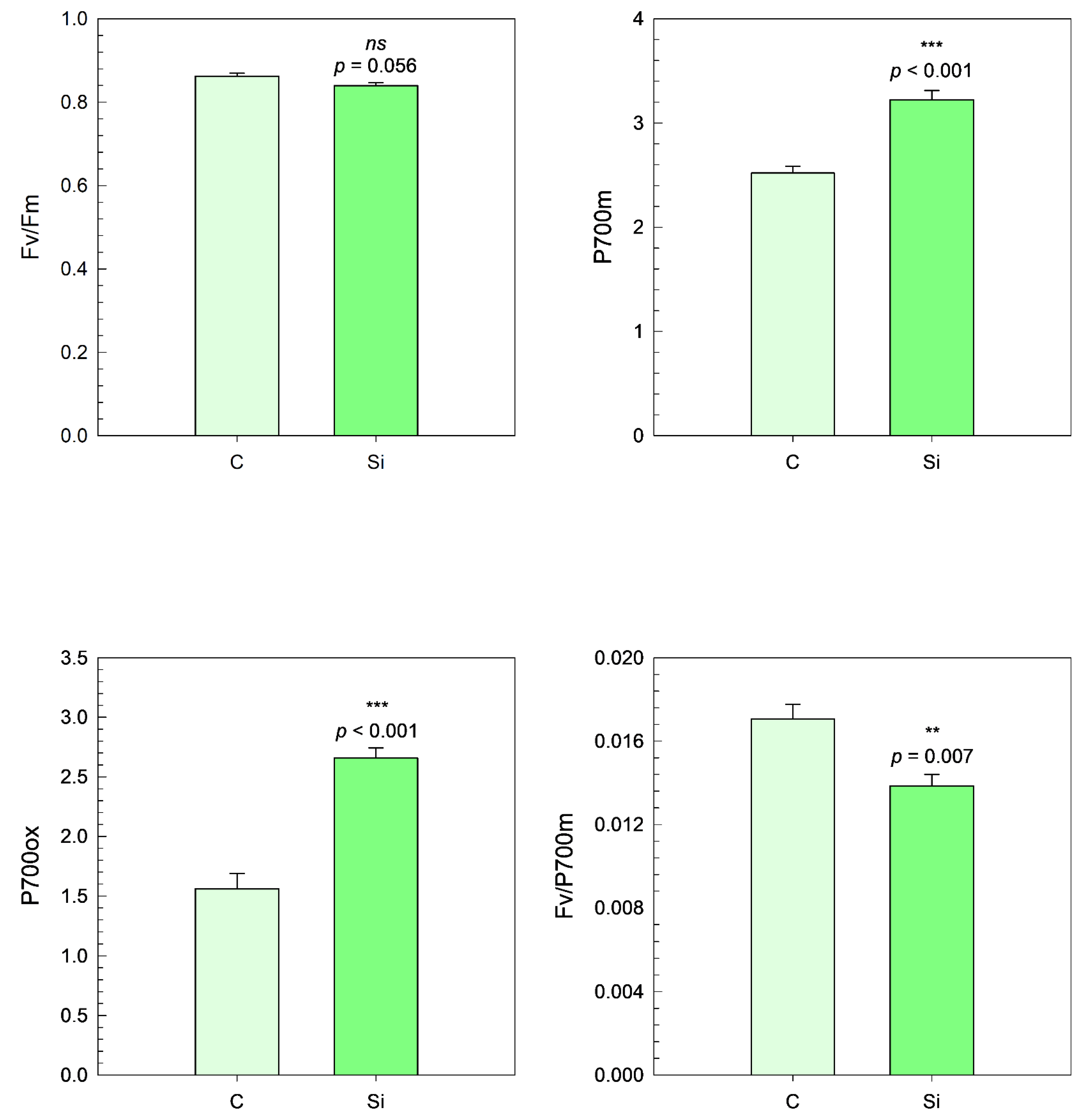
3.5. Photosystem Activities
Under dark-adapted conditions, the maximal quantum yield of PSII photochemistry (Fm) did not show a statistically significant variation between the control and the silicon-treated plants (
p > 0.05) (
Figure 11).
In contrast, significant increases were observed for PSI-related parameters. The maximal fluorescence yield of the dark-adapted sample with all PSI centers closed (P700m) increased by 28% in Si-treated samples compared to the control (p < 0.001). Similarly, the oxidized PSI signal (P700ox) increased markedly under Si treatment, corresponding to a 70% enhancement (p < 0.001).
Interestingly, the ratio between the maximal quantum yield of PSII and the maximal fluorescence yield of PSI (Fv/P700m) decreased by 19% in Si-treated plants, suggesting a relative shift in the excitation energy balance or electron transport capacities favoring PSI under these conditions (
p = 0.007) (
Figure 11).
The effects of silicon treatment on photosystem activities were assessed under varying light intensities (PAR), as shown in
Figure 12. The quantum yield of photochemical energy conversion in PSII (Y(II)) was significantly improved in Si-treated plants compared to the control, suggesting that silicon treatment enhances the overall photochemical efficiency of PSII.
In contrast, the quantum yield of regulated nonphotochemical energy dissipation in PSII (Y(NPQ)) showed a substantial increase in Si-treated plants.
Significantly, the quantum yield of nonregulated nonphotochemical energy dissipation in PSII (Y(NO)) was markedly reduced in Si-treated plants compared to the control.
Additionally, the electron transfer rate (ETR(II)) showed a considerable improvement in Si-treated plants under increasing PAR. The Si-treated plants consistently exhibited a significantly higher electron transport capacity compared to the control (
Figure 12).
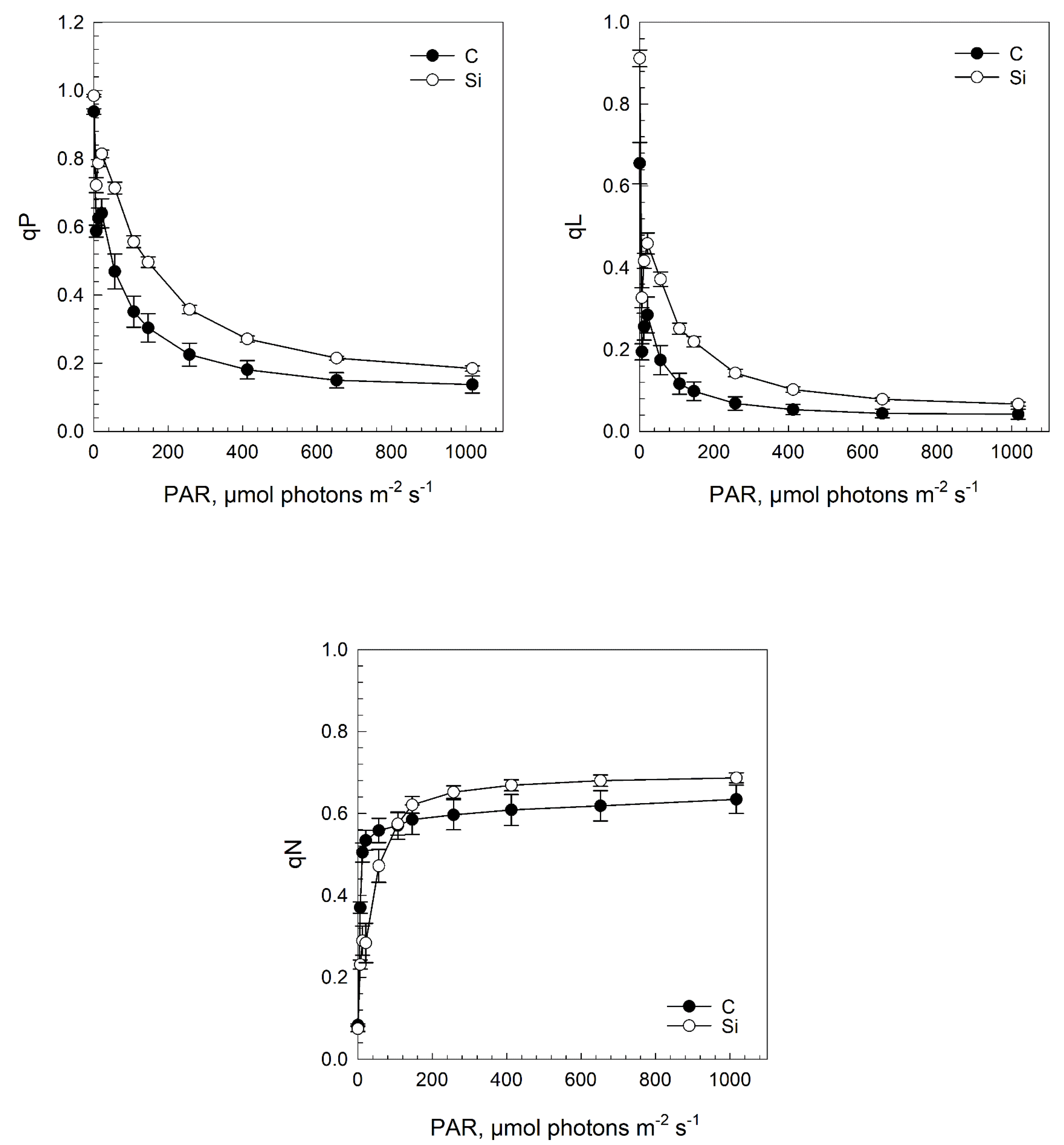
As shown in
Figure 12, the silicon treatment significantly influenced several parameters related to PSII activity and light energy dissipation.
The photochemical quenching coefficient (qP) was improved in Si-treated plants, as indicated by the higher qP values compared to the control. Similarly, the proportion of open PSII centers coefficient (qL) also increased in Si-treated plants, showing higher values compared to the control, particularly at lower PAR levels.
Interestingly, the non-photochemical light dissipation coefficient (qN) showed a significant difference between the two treatments. Si-treated plants exhibited higher qN values at low PAR. However, at higher light intensities (starting from approximately 150 µmol photons m
−2 s
−1), qN in Si-treated plants decreased significantly (
Figure 13).
The results shown in
Figure 14 indicate that the silicon treatment significantly enhanced several parameters related to PSI.
The quantum yield of photochemical energy conversion in PSI (Y(I)) was enhanced by the silicon treatment, with Si-treated plants exhibiting higher Y(I) values. However, this improvement in Y(I) was less pronounced than the increase observed for PSII (Y(II)), suggesting that silicon has a stronger enhancing effect on PSII compared to PSI.
For the quantum yield of nonphotochemical energy dissipation in reaction centers limited by the donor side (Y(ND)), the silicon treatment also led to an improvement. Si-treated plants showed higher Y(ND) values.
In contrast, the quantum yield of nonphotochemical energy dissipation in reaction centers limited by the acceptor side (Y(NA)) showed no significant change under silicon treatment.
The electron transfer rate (ETR) in PSI was also significantly improved in Si-treated plants, with higher ETR values observed at increasing light intensities.
Regarding PSI fluorescence parameters, P700m (maximal fluorescence yield of illuminated sample with all PSI centers closed) exhibited increased values in Si-treated plants. Lastly, P700ox (oxidized PSI) also showed a significant improvement with silicon treatment, with Si-treated plants exhibiting higher P700ox values at increasing PAR (
Figure 14).
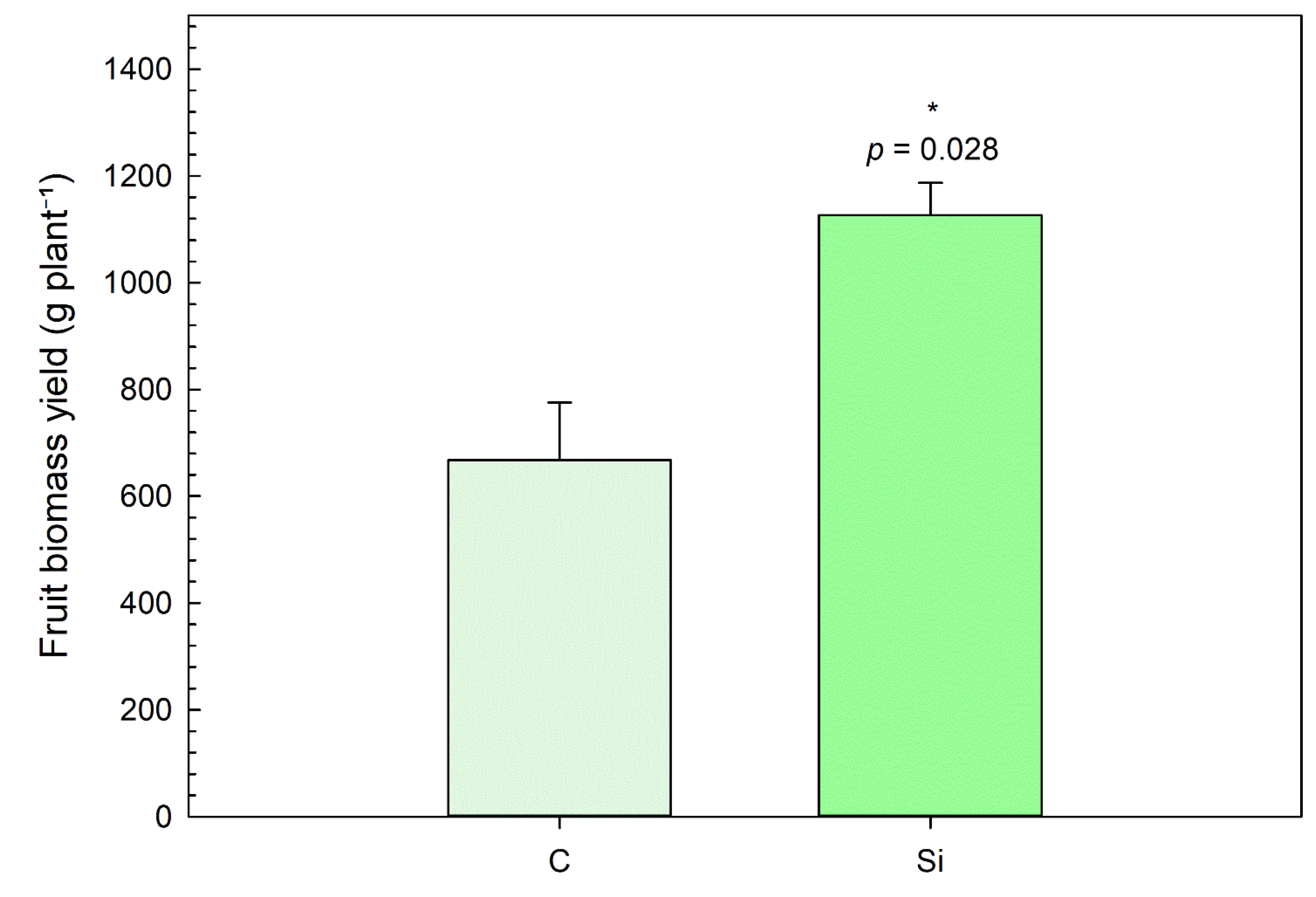
3.6. Fruit Biomass Yield
The application of silicon significantly enhanced fruit biomass yield compared to the control. As shown in
Figure 15, plants treated with Si exhibited a mean fruit biomass yield of 1126 g plant
−1, whereas control plants only reached 667 g plant
−1, corresponding to an increase of approximately 69%. Statistical analysis revealed that this difference was significant (
p = 0.028), indicating a positive impact of Si supplementation on plant productivity.
3.7. Correlation Analysis
The correlation analysis conducted in this study provided strong evidence supporting the treatment effects observed with the foliar application of silicon on various growth and physiological parameters of tomato plants in hydroponic systems. The correlations between treatments and the corresponding variables were significant, with many variables showing highly significant relationships, affirming the effectiveness of silicon application (
Table 2).
The correlation analysis revealed strong relationships between the silicon treatment and various growth parameters, including stem width, main stem length, root dry weight, stem dry weight, leaf dry weight, plant dry weight, number of racemes, flowers, and fruits. The correlations were particularly significant (ranging from 0.63 to 0.84), confirming that silicon positively impacts plant growth and fruit production.
Further, water content parameters such as root, stem, and leaf water content did not display significant correlations, indicating that the foliar application of silicon did not notably affect water retention in the plants.
However, chlorophyll-related variables, including SPAD, chlorophyll a, chlorophyll b, and carotenoids, showed strong positive correlations (ranging from 0.72 to 0.80 for silicon treatments, p < 0.001), suggesting a beneficial effect of silicon on chlorophyll synthesis and on photosynthetic efficiency.
Additionally, parameters related to photosynthetic efficiency, such as P700m and P700ox, demonstrated high correlation values with the silicon treatment (ranging from 0.79 to 0.93 for silicon treatments), confirming that silicon not only influences growth but also plays a significant role in improving photosynthetic performance. These findings were further validated by the correlation with fruit biomass yield, which showed a significant positive correlation (r = 0.70, p < 0.01), highlighting the potential of silicon to enhance yield productivity in hydroponic tomato systems.
In conclusion, the correlation analysis strongly corroborated the treatment effects observed in the study, demonstrating that foliar application of silicon effectively improves multiple growth and physiological parameters in hydroponic tomatoes, with significant effects on biomass accumulation, photosynthesis, and yield components.
3.8. Principal Component Analysis (PCA)
Principal Component Analysis (PCA) was performed to further explore the underlying structure of the dataset and to assess the relationships between the various growth and physiological variables. The PCA results, as depicted in
Figure 16, reveal the primary components that account for the variance observed in the data.
In the biplot, the red vectors represent the loadings of the variables, while the blue squares represent the treatment conditions. The clear separation of variables along distinct axes indicates that the silicon treatment influences multiple plant traits, with certain variables such as stem width, main stem length, root dry weight, and plant dry weight being closely associated with one another. The clustering of these traits along the first principal component suggests a common underlying influence, likely related to the effect of silicon on plant growth and biomass accumulation.
Figure 16 also highlights variables such as chlorophyll content (SPAD, Chl.
a, Chl.
b) and photosynthetic efficiency (P700m and P700ox), which are prominently positioned along the first principal component, indicating their strong relationship with silicon treatment and the enhancement of plant physiological processes. Furthermore, the distribution of the treatment points (blue squares) along the principal components suggests that the silicon treatment notably shifts the plant growth and physiological responses in a manner that is captured well by the first two principal components.
Overall, PCA provides a robust confirmation of the observed treatment effects, demonstrating that silicon application induces distinct and significant changes in plant morphology and physiology, as reflected in the clustering of key variables. The results support the hypothesis that silicon has a broad and positive impact on tomato plants, enhancing various traits associated with growth and yield.
4. Discussion
The present study aimed to investigate whether silicon can improve the growth and productivity of tomatoes in hydroponic systems and to explore the underlying mechanisms that might contribute to this potential enhancement.
Our findings demonstrated that Si foliar treatment significantly enhanced various growth parameters, including stem width, main stem length, and dry weight of plant components, leading to an overall increase in plant biomass. In addition, the number of reproductive structures, such as racemes, flowers, and fruits, was notably higher in Si-treated plants compared to controls.
In our study, conducted under controlled conditions in large-scale greenhouses using closed hydroponic systems, these results align with previous studies that have reported similar benefits of Si application in tomatoes. For instance, Jalali et al. [
26] observed that foliar spraying with Si improved fruit firmness, fruit volume, and yield per plant in laboratory-scale experiments. Similarly, Costan et al. [
27] reported enhanced tomato growth following Si application in hydroponic setups under controlled laboratory conditions.
The beneficial effects of silicon on plant growth can be attributed to several physiological mechanisms. Specifically, studies have demonstrated that Si promotes cell elongation, enhances cell wall extensibility, and supports stem development, all of which contribute to improved plant growth and increased biomass accumulation [
28]. Additionally, silicon has been shown to reinforce cell wall structure and optimize nutrient uptake, supporting sustained plant growth under challenging conditions. In fact, research indicates that Si deposited in cell walls enhances rigidity and stability, which in turn improves nutrient uptake and metabolism in cells [
29].
Moreover, silicon has been implicated in the regulation of phytohormone biosynthesis. It has been suggested that Si influences the synthesis and activity of plant hormones such as auxins, gibberellins, and cytokinins, which play crucial roles in cell division, elongation, and differentiation. Thus, this hormonal modulation may contribute to the observed improvements in growth and reproductive development in Si-treated plants [
30]. Recent findings have also indicated that silicon may enhance the balance between these hormones, optimizing their synergistic effects to promote growth and development. This regulatory effect could be one of the key factors driving the observed improvements in plant health and reproductive success [
31,
32].
With regard to plant hydration, while several studies have demonstrated beneficial effects of silicon on plant hydration [
6], the present study did not observe significant differences in water content between silicon-treated plants and the control group in the roots, stems, or leaves. This discrepancy may be attributed to the hydroponic cultivation system employed, which provided sufficient water availability, potentially masking the effects of silicon supplementation on water retention.
In terms of pigment biosynthesis, our results demonstrate notable increases in SPAD values and pigment content, suggesting that silicon plays a key role in modulating chlorophyll biosynthesis and prompting a reevaluation of its functional significance in plant metabolism. In fact, a growing body of evidence supports the role of Si in enhancing chlorophyll content across various plant species. For instance, it has been demonstrated that Si supplementation increases chlorophyll levels in tomato plants, a result that underscores the potential for Si to improve photosynthetic efficiency in crops [
33]. This trend is also observed in other species, such as barley [
34,
35], sea barley [
21,
36], and sorghum [
37]. Importantly, Silicon’s ability to enhance chlorophyll content is especially pronounced under conditions of abiotic stress, such as salinity, nutrient deficiency, and temperature extremes, where chlorophyll degradation frequently occurs.
The positive impact of Si on chlorophyll biosynthesis has been extensively reported [
38]. However, while the underlying mechanisms by which Si enhances chlorophyll biosynthesis have been partially explored, there is still a need for further elucidation at the molecular level [
38]. Recent research has provided deeper insights into the molecular mechanisms by which silicon influences chlorophyll biosynthesis. Studies have shown that Si interacts with secondary messengers to modulate gene expression under stress, thereby enhancing the plant’s ability to maintain chlorophyll production. Furthermore, Si has been found to protect chlorophyll pigments and reduce damage to the chloroplast structure, preserving the integrity of chlorophyll biosynthesis and improving plant resilience under adverse conditions [
38,
39].
Concerning the modulation of photosystem activities by silicon treatment, our data demonstrate a substantial enhancement in both photosystem II (PSII) and photosystem I (PSI)-related parameters. Such findings highlight the significant potential of silicon to optimize photosynthetic efficiency, which may have important implications for plant productivity. Thus, these results align with the growing body of research suggesting that silicon enhances the photosynthetic efficiency of plants, particularly under stress conditions [
35,
36,
37].
Looking more closely at the modulation of PSII activity, Si foliar treatment enhanced PSII activity, as indicated by the increased values of key parameters (Y(II), ETR(II), qP, and qL). This consistent elevation across multiple photochemical parameters underscores a robust enhancement rather than artefactual or isolated effects. This suggests that silicon treatment improves the efficiency of PSII, contributing to an overall enhancement in photosynthetic performance specifically within this system.
Additionally, silicon treatment resulted in a reduction of energy losses due to nonregulated dissipation processes (Y(NPQ) and Y(NO)), further enhancing the efficiency of the photosynthetic machinery. The observed shift from energy dissipation to photochemical utilization indicates that silicon may reprogram energy allocation mechanisms within the chloroplast. The reduction in unnecessary energy dissipation suggests that silicon helps in optimizing the plant’s energy use by reducing losses and improving energy conversion efficiency. These findings point to the role of silicon in enhancing the plant’s ability to harness absorbed light energy for photochemical processes, rather than dissipating it as heat [
35].
Regarding the regulation of PSI activity, our results demonstrate that Si foliar treatment also significantly enhanced PSI activity, as indicated by the elevated Y(I) and ETR(I) values. These increases suggest that silicon may alleviate limitations on PSI electron acceptor side capacity, thereby promoting a more efficient electron flow through the photosynthetic electron transport chain. This is particularly relevant because PSI is highly susceptible to photoinhibition under environmental stresses due to the over-reduction of its acceptor side [
40]. Moreover, the observed improvement in ETR(I) may also reflect better coordination between the light reactions and the Calvin cycle, enhancing carbon assimilation efficiency [
41].
Furthermore, the observed decrease in Y(ND) following silicon supplementation reflects enhanced regulation of donor-side electron flow in PSI, indicative of improved energy utilization efficiency. Specifically, the reduction of Y(ND) suggests that silicon mitigates donor-side over-reduction, promoting a more balanced redox state in PSI. Similar regulatory effects of silicon on PSI have been reported by Raihan et al. [
42], who demonstrated that silicon application under salt stress significantly increased the fraction of open PSI centers and minimized over-reduction [
42]. Although their observed effect was more pronounced, likely due to stress conditions, our results indicate that, even under non-stress conditions, silicon can fine-tune PSI redox poising. This donor-side modulation may reflect silicon’s broader role in stabilizing thylakoid membrane structures and optimizing electron transfer components, even in the absence of severe external stress. Previous studies suggest that silicon enhances the structural integrity of photosynthetic machinery, which may indirectly affect PSI donor-side [
36,
43].
More importantly, the present study provides novel evidence that silicon plays an active role in modulating the redox state of P700, a crucial factor for maintaining efficient electron transport and minimizing oxidative damage, especially under high light and low CO
2 conditions [
44]. Thus, the significant enhancement in P700 oxidation observed in silicon-treated plants suggests that silicon contributes directly to the stabilization of PSI reaction centers. By maintaining P700 in its oxidized state, silicon likely facilitates dissipation of excess excitation energy and minimizes the over-reduction of PSI acceptor-side components, thereby reducing ROS formation—a process in line with Kadota et al. [
45]—who demonstrated that P700 oxidation induces alternative electron flow pathways that mitigate oxidative stress [
45]. This mechanism is particularly critical under environmental stress conditions, where an imbalance between energy absorption and utilization increases ROS production and can cause photoinhibition and irreversible damage to PSI. In this way, the maintenance of P700
+ status mitigates acceptor-side over-reduction by channeling electrons into alternative flows—such as charge recombination or cyclic pathways—thus safeguarding PSI integrity [
44].
As we conclude this section on photosynthesis, it is important to mention that several studies have highlighted that Si supplementation regulates genes related to photosynthesis, thereby improving the overall photosynthetic process. However, despite this growing evidence, research on the molecular mechanisms linking Si to gene expression modulation in photosynthesis remains limited. Therefore, future studies focusing on the gene regulatory networks influenced by Si could provide deeper insights into the molecular pathways by which Si enhances photosynthetic efficiency [
38].
Having established improvements in photosynthetic efficiency and plant growth, we now focus on a critical parameter: yield. Our results demonstrated that silicon foliar application led to a 69% increase in fruit biomass yield compared to the control (
r = 0.70;
p < 0.01), along with improvements in growth parameters such as stem width, plant biomass, and reproductive structure formation. The observed increases in productivity are closely linked to enhanced photosynthetic performance, driven by silicon’s optimization of both PSII and PSI efficiency, as well as its role in boosting chlorophyll biosynthesis. These findings directly contribute to plant growth and the observed increase in fruit biomass yield. Our findings align with previous studies that highlight silicon’s role in enhancing plant productivity and stress resilience [
46,
47], reinforcing its potential to improve photosynthetic performance and crop yield.
The results effectively supported our initial hypothesis, confirming that silicon enhances photosynthetic efficiency, chlorophyll biosynthesis, and the functionality of PSII and PSI, which are essential for efficient light energy conversion and carbon fixation. These findings underscore the potential of silicon to enhance plant growth, increase yield, and improve resilience to environmental stresses in hydroponic tomato cultivation.
The schematic representation (
Figure 17) provides a comprehensive summary of the key findings, integrating the most important observations from the present study.
In conclusion, one limitation of this study is that it focuses primarily on the beneficial effects of silicon on tomato productivity, without addressing the quality of the fruits. This limitation has been explored in a separate study, the results of which will be presented in our forthcoming research.