Utilizing Environmentally Friendly Techniques for the Sustainable Control of Plant Pathogens: A Review
Abstract
1. Introduction
1.1. Importance of Plant Disease Management
1.2. Overview of Environmental Techniques for Pathogen Control
1.3. Scope and Objectives of the Review
2. Environmental Factors and Plant–Pathogen Interactions
2.1. Abiotic Factors
2.2. Biotic Factors
2.3. Impact of Climate Change on Plant Disease Dynamics
3. Plant Pathogens Control
3.1. Physical Techniques
3.1.1. Solarization and Soil Heating
3.1.2. Soil Steaming
3.1.3. Hot Water Treatment and Thermal Inactivation
3.1.4. Mulching
3.1.5. Ultraviolet (UV) Radiation Treatment
3.1.6. Practical Challenges and Limitations of Physical Techniques
3.1.7. Drawbacks or Potential Negative Impacts of Physical Techniques
3.2. Cultural and Ecological Management Techniques
3.2.1. Crop Rotation and Diversification
3.2.2. Sanitation and Residue Management
Biofumigation
Anaerobic Soil Disinfestation
Intercropping and Mixed Cropping Systems
3.3. Use of Resistant Cultivars
3.4. Soilless Culture
3.5. Biological Control Approaches
3.5.1. Microbial Inoculants as Biocontrol Agents
3.5.2. Microbial Consortia and Synergistic Effects
3.5.3. Organic Amendments and Soil Health in Disease Suppression
3.6. Challenges and Limitations of Microbiological Control
3.7. Future Directions and Innovations
3.8. Conclusions
4. Environmental Modification Techniques
4.1. Soil Moisture Management
4.2. Soil pH Adjustment
4.3. Air Circulation and Humidity Control
5. Integrated Environmentally Friendly Management
5.1. Combining Environmentally Friendly Techniques
5.2. Integration with Chemical Control Methods
6. Quantitative and Comparative Contributions of Control Diseases Strategies
7. Future Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Sundin, G.; Fuente, L.; Cubero, J.; Tatineni, S.; Brewer, M.; Zeng, Q.; Bock, C.; Cunniffe, N.; Wang, C.; et al. Key Challenges in Plant Pathology in the Next Decade. Phytopathology 2024, 114, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Senthilraja, N.; Gangwar, R.K.; Borad, C.K. Challenges and Opportunities in Plant Disease Management: A Brief Review. Madras Agric. J. 2024, 111, 1–3. [Google Scholar]
- He, D.-C.; Zhan, J.-S.; Xie, L.-H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Gill, H.K.; Aujla, I.S.; De Bellis, L.; Luvisi, A. The role of soil solarization in India: How an unnoticed practice could support pest control. Front. Plant Sci. 2017, 8, 1515. [Google Scholar] [CrossRef]
- Café-Filho, A.C.; Lopes, C.A.; Rossato, M. Management of Plant Disease Epidemics with Irrigation Practices. In Irrigation in Agroecosystems; Ondrašek, G., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 8. [Google Scholar] [CrossRef]
- Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae 2024, 10, 805. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D.L. Control of crop diseases through Integrated Crop Management to deliver climate-smart farming systems for low- and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Aytenew, M.; Wolancho, G. Effects of Organic Amendments on Soil Fertility and Environmental Quality: A Review. J. Plant Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Karlsson Green, K.; Stenberg, J.A.; Lankinen, Å. Making sense of Integrated Pest Management (IPM) in the light of evolution. Evol. Appl. 2020, 13, 1791–1805. [Google Scholar] [CrossRef]
- Gullino, M.; Pugliese, M.; Gilardi, G.; Garibaldi, A. Effect of increased CO2 and temperature on plant diseases: A critical appraisal of results obtained in studies carried out under controlled environment facilities. J. Plant Pathol. 2018, 100, 371–389. [Google Scholar] [CrossRef]
- Priya, P.; Patil, M.; Pandey, P.; Singh, A.; Babu, V.S.; Senthil-Kumar, M. Stress combinations and their interactions in plants database: A one-stop resource on combined stress responses in plants. Plant J. 2023, 116, 1097–1117. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Narisawa, K.; Shimura, M.; Usuki, F.; Fukuhara, S.; Hashiba, T. Effects of Pathogen Density, Soil Moisture, and Soil pH on Biological Control of Clubroot in Chinese Cabbage by Heteroconium chaetospira. Plant Dis. 2005, 89, 285–290. [Google Scholar] [CrossRef]
- Tripathi, R.; Tewari, R.; Singh, K.P.; Keswani, C.; Minkina, T.; Srivastava, A.K.; De Corato, U.; Sansinenea, E. Plant mineral nutrition and disease resistance: A significant linkage for sustainable crop protection. Front. Plant Sci. 2022, 13, 883970. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Stueven, M.; Pastrana, A.M.; Henry, P.M.; Dennehy, C.M.; Kirkpatrick, S.C.; Daugovish, O. The Effect of pH on Spore Germination, Growth, and Infection of Strawberry Roots by Fusarium oxysporum f. sp. fragariae, Cause of Fusarium wilt of Strawberry. Plant Dis. 2019, 103, 697–704. [Google Scholar] [CrossRef]
- Todorović, I.; Moënne-Loccoz, Y.; Raičević, V.; Jovičić-Petrović, J.; Muller, D. Microbial diversity in soils suppressive to Fusarium diseases. Front. Plant Sci. 2023, 14, 1228749. [Google Scholar] [CrossRef]
- Niekawa, E.T.G.; Simionato, A.S.; Barazetti, A.R.; Cano, B.G.; Emiliano, J.; Afonso, L.; de Lima Andreata, M.F.; Dealis, M.L.; Chryssafidis, A.L.; Andrade, G. Chapter 10—The microbial role in the control of phytopathogens—An alternative to agrochemicals. In Microbiome Stimulants for Crops; White, J., Kumar, A., Droby, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 159–177. [Google Scholar] [CrossRef]
- Yang, Y.; Singh, R.P.; Zhang, C.; You, X.; Li, Y. Chapter 23—Management of diversity and abundance of soil microorganisms to inhibit the occurrence of plant disease. In Microbial Essentialism; Pratap Singh, R., Manchanda, G., Sarsan, S., Kumar, A., Panosyan, H., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2024; pp. 519–559. [Google Scholar] [CrossRef]
- Kumar, A.P.; Murali, V. A Review on Soil and Phytomicrobiome for Plant Disease Management. Int. J. Environ. Clim. Change 2023, 13, 2890–2904. [Google Scholar]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Mikryukov, V.; Dulya, O.; Tedersoo, L.; Bahram, M.; et al. Patterns in soil microbial diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef]
- Matić, S.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emergence of leaf spot disease on leafy vegetable and ornamental crops caused by Paramyrothecium and Albifimbria species. Phytopathology 2019, 109, 1053–1061. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria leaf spot caused by Alternaria species: An emerging problem on ornamental plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Gullino, M.L.; Garibaldi, A.; Guarnaccia, V. Emerging leafy vegetable crop diseases caused by the Fusarium incarnatum-equiseti species complex. Phytopathol. Mediterr. 2020, 59, 303–317. [Google Scholar]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Trkulja, V.; Tomić, A.; Matić, S.; Trkulja, N.; Iličić, R.; Popović Milovanović, T. An Overview of the Emergence of Plant Pathogen ‘Candidatus Liberibacter solanacearum’ in Europe. Microorganisms 2023, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Caruso, A.G.; D’Errico, C.; Botto, C.S.; Noris, E.; Trkulja, V.; Panno, S.; Davino, S.; Moizio, M. Powdery mildew caused by Erysiphe corylacearum: An emerging problem on hazelnut in Italy. PLoS ONE 2024, 19, e0301941. [Google Scholar] [CrossRef]
- Tomic, A.; Trkulja, V.; Matic, S.; Trkulja, N.; Ilicic, R.; Scortichini, M.; Popovic Milovanovic, T. Net blotch (Pyrenophora teres Drechsler): An increasingly significant threat to barley production. Plant Prot. Sci. 2024, 60, 1–30. [Google Scholar] [CrossRef]
- Matić, S.; Cucu, M.A.; Garibaldi, A.; Gullino, M.L. Combined effect of CO2 and temperature on wheat powdery mildew development. Plant Pathol. J. 2018, 34, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Garibaldi, A.; Gullino, M.L. Combined and single effects of elevated CO2 and temperatures on rice bakanae disease under controlled conditions in phytotrons. Plant Pathol. 2021, 70, 815–826. [Google Scholar] [CrossRef]
- Kumar, D.; Mukhopadhyay, R. Climate change and plant pathogens: Understanding dynamics, risks and mitigation strategies. Plant Pathol. 2025, 74, 59–68. [Google Scholar] [CrossRef]
- Frías-De-León, M.; Brunner-Mendoza, C.; Reyes-Montes, M.; Duarte-Escalante, E. The Impact of Climate Change on Fungal Diseases; Springer International Publishing: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Pagliarani, C.; Moine, A.; Chitarra, W.; Nerva, L.; Catoni, M.; Tavazza, R.; Matić, S.; Vallino, M.; Secchi, F.; Noris, E. The C4 protein of tomato yellow leaf curl Sardinia virus primes drought tolerance in tomato through morphological adjustments. Hortic. Res. 2022, 9, uhac164. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Kumar, A.; Mahanta, D.; Dange, M.M.; Trivedi, A.; Nandeha, N. Global Challenges Facing Plant Pathology: A Review on Multidisciplinary Approaches to Meet the Food Security. J. Sci. Res. Rep. 2024, 30, 884–892. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Deshi, S.; Wonang, D.; Dafur, B. Control of rots and spoilage of agricultural products: A review. Int. Lett. Nat. Sci. 2014, 18, 63–72. [Google Scholar]
- Lv, H.; Fang, Z.; Yang, L.; Zhang, Y.; Wang, Y. An update on the arsenal: Mining resistance genes for disease management of Brassica crops in the genomic era. Hortic. Res. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, K.; Maesaka, M.; Murase, J.; Asakawa, S. Solarization makes a great impact on the abundance and composition of microbial communities in soil. Soil Sci. Plant Nutr. 2015, 61, 641–652. [Google Scholar] [CrossRef]
- Gullino, M.L.; Garibaldi, A.; Gamliel, A.; Katan, J. Soil Disinfestation: From Soil Treatment to Soil and Plant Health. Plant Dis. 2022, 106, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.W.; Guo, H.; Claypool, J.T.; Marshall, M.N.; Perano, K.M.; Stapleton, J.J.; VanderGheynst, J.S. Managing compost stability and amendment to soil to enhance soil heating during soil solarization. Waste Manag. 2013, 33, 1090–1096. [Google Scholar] [CrossRef][Green Version]
- Novák, V.; Hlaváčiková, H.; Novák, V.; Hlaváčiková, H. Soil temperature and heat transport in soils. In Applied Soil Hydrology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 303–318. [Google Scholar]
- Yates, S.; Ashworth, D.; Yates, M.; Luo, L. Active solarization as a nonchemical alternative to soil fumigation for controlling pests. Soil Sci. Soc. Am. J. 2011, 75, 9–16. [Google Scholar] [CrossRef]
- Gilardi, G.; Demarchi, S.; Gullino, M.L.; Garibaldi, A. Effect of Simulated Soil Solarization and Organic Amendments on Fusarium Wilt of Rocket and Basil Under Controlled Conditions. J. Phytopathol. 2014, 162, 557–566. [Google Scholar] [CrossRef]
- Elmore, C.; Stapleton, J.; Bell, C.; DeVay, J. Soil Solarization: A Non-Pesticidal Method for Controlling Diseases, Nematodes, and Weeds; University of California: Oakland, CA, USA, 1997. [Google Scholar]
- Stapleton, J.J.; DeVay, J.E. Soil solarization: A non-chemical approach for management of plant pathogens and pests. Crop Prot. 1986, 5, 190–198. [Google Scholar] [CrossRef]
- Kanaan, H.; Frenk, S.; Raviv, M.; Medina, S.; Minz, D. Long and short term effects of solarization on soil microbiome and agricultural production. Appl. Soil Ecol. 2018, 124, 54–61. [Google Scholar] [CrossRef]
- Castello, I.; D’emilio, A.; Raviv, M.; Vitale, A. Soil solarization as a sustainable solution to control tomato Pseudomonads infections in greenhouses. Agron. Sustain. Dev. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Dai, Y.; Senge, M.; Yoshiyama, K.; Zhang, P.; Zhang, F. Influencing factors, effects and development prospect of soil solarization. Rev. Agric. Sci. 2016, 4, 21–35. [Google Scholar] [CrossRef]
- Katan, J.; DeVay, J. Soil Solarization; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Gebreegziher, W.G. Agronomic use of solarization technology on soil fertility and pest management in dryland agriculture. Cogent Food Agric. 2024, 10, 2306692. [Google Scholar] [CrossRef]
- Ringselle, B.; De Cauwer, B.; Salonen, J.; Soukup, J. A Review of Non-Chemical Management of Couch Grass (Elymus repens). Agronomy 2020, 10, 1178. [Google Scholar] [CrossRef]
- Samtani, J.B.; Gilbert, C.; Weber, J.B.; Subbarao, K.V.; Goodhue, R.E.; Fennimore, S.A. Effect of Steam and Solarization Treatments on Pest Control, Strawberry Yield, and Economic Returns Relative to Methyl Bromide Fumigation. HortScience 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Luvisi, A.; Panattoni, A.; Materazzi, A. Heat treatments for sustainable control of soil viruses. Agron. Sustain. Dev. 2015, 35, 657–666. [Google Scholar] [CrossRef]
- Fennimore, S.A.; Martin, F.N.; Miller, T.C.; Broome, J.C.; Dorn, N.; Greene, I. Evaluation of a Mobile Steam Applicator for Soil Disinfestation in California Strawberry. HortScience 2014, 49, 1542–1549. [Google Scholar] [CrossRef]
- Phua, L.; Neo, S.; Khoo, G.; Yuk, H. Comparison of the efficacy of various sanitizers and hot water treatment in inactivating inoculated foodborne pathogens and natural microflora on mung bean sprouts. Food Control 2014, 42, 270–276. [Google Scholar] [CrossRef]
- Matić, S.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control 2014, 73, 59–67. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D. Hot water rinsing and brushing of fresh produce as an alternative to chemical treatment after harvest—The story behind the technology. Agronomy 2021, 11, 1653. [Google Scholar] [CrossRef]
- Forsberg, G.; Andersson, S.; Johnsson, L. Evaluation of hot, humid air seed treatment in thin layers and fluidized beds for seed pathogen sanitation/Bewertung der Saatgutbehandlung mit heißer, feuchter Luft und in einer Verwirbelungskammer zur Eliminierung von samenbürtigen Pathogenen. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 2002, 109, 357–370. [Google Scholar]
- Sultana, N.; Islam, M.; Noman, A.; Faruq, A.; Akter, N.; Islam, M. Standardization of Temperature and Duration for Hot Water Seed Treatment of Selected Vegetables. Agriculturists 2021, 19, 128–139. [Google Scholar]
- Mwando, N.; Ndlela, S.; Meyhöfer, R.; Subramanian, S.; Mohamed, S. Hot water treatment for post-harvest disinfestation of Bactrocera dorsalis (Diptera: Tephritidae) and its effect on cv. tommy atkins mango. Insects 2021, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, S.; Kang, D. Thermal and non-thermal treatment effects on Staphylococcus aureus biofilms formed at different temperatures and maturation periods. Food Res. Int. 2020, 137, 109432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hussain, I.; Azam, M.; Khan, M.; Akram, M.; Naveed, K.; Liu, H. Hot water treatment improves date drying and maintains phytochemicals and fruit quality characteristics of date palm (Phoenix dactylifera). Foods 2023, 12, 2405. [Google Scholar] [CrossRef]
- Berrios-Rodriguez, A.; Olanya, O.; Ukuku, D.; Niemira, B.; Orellana, L.; Mukhopadhyay, S.; Boyd, G. Inactivation of Listeria monocytogenes on post-harvest carrot and tomato by gamma radiation, sanitizer, biocontrol treatments and their combinations. LWT 2020, 118, 108805. [Google Scholar] [CrossRef]
- Spinks, A.; Dunstan, R.; Harrison, T.; Coombes, P.; Kuczera, G. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res. 2006, 40, 1326–1332. [Google Scholar] [CrossRef]
- Kantakhoo, J.; Imahori, Y. Antioxidative responses to pre-storage hot water treatment of red sweet pepper (Capsicum annuum L.) fruit during cold storage. Foods 2021, 10, 3031. [Google Scholar] [CrossRef]
- Haapala, T.; Palonen, P.; Korpela, A.; Ahokas, J. Feasibility of paper mulches in crop production—A review. Agric. Food Sci. 2014, 23, 60–79. [Google Scholar] [CrossRef]
- Kumar, V.; Abdul-Baki, A.; Anderson, J.D.; Mattoo, A.K. Cover Crop Residues Enhance Growth, Improve Yield, and Delay Leaf Senescence in Greenhouse-grown Tomatoes. HortScience 2005, 40, 1307–1311. [Google Scholar] [CrossRef]
- Fatima, T.; Teasdale, J.R.; Bunce, J.; Mattoo, A.K. Tomato response to legume cover crop and nitrogen: Differing enhancement patterns of fruit yield, photosynthesis and gene expression. Funct. Plant Biol. 2012, 39, 246–254. [Google Scholar] [CrossRef]
- Xu, D.; Ling, J.; Qiao, F.; Xi, P.; Zeng, Y.; Zhang, J.; Lan, C.; Jiang, Z.; Peng, A.; Li, P. Organic mulch can suppress litchi downy blight through modification of soil microbial community structure and functional potentials. BMC Microbiol. 2022, 22, 155. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Janisiewicz, W.; Takeda, F.; Glenn, D.; Camp, M.; Ii, W. Dark Period Following UV-C Treatment Enhances Killing of Botrytis cinerea Conidia and Controls Gray Mold of Strawberries. Phytopathology 2015, 106, 386–394. [Google Scholar] [CrossRef]
- Suthaparan, A.; Torre, S.; Stensvand, A.; Herrero, M.L.; Pettersen, R.; Gadoury, D.; Gislerød, H. Specific Light-Emitting Diodes Can Suppress Sporulation of Podosphaera pannosa on Greenhouse Roses. Plant Dis. 2010, 94, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, J.; Dahlquist-Willard, R.; Achmon, Y.; Marshall, M.; VanderGheynst, J.; Simmons, C. Advances in Biosolarization Technology to Improve Soil Health and Organic Control of Soilborne Pests. In Proceedings of the 2016 Organic Agriculture Research Symposium, Asilomar, CA, USA, 20–23 January 2016. [Google Scholar]
- Fennimore, S.; Goodhue, R. Soil Disinfestation with Steam: A Review of Economics, Engineering, and Soil Pest Control in California Strawberry. Int. J. Fruit Sci. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Melander, B.; Liebman, M.; Davis, A.S.; Gallandt, E.R.; Bàrberi, P.; Moonen, A.C.; Rasmussen, J.; van der Weide, R.; Vidotto, F. Non-Chemical Weed Management. In Weed Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; Chapter 9; pp. 245–270. [Google Scholar] [CrossRef]
- Noble, R.; Roberts, S.J. Eradication of plant pathogens and nematodes during composting: A review. Plant Pathol. 2004, 53, 548–568. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, D.; Thakur, A.; Bhardwaj, S.; Angurana, R.; Katoch, V.; Kapoor, D. Success Story of Arbuscular Mycorrhizal Fungi as a Bio Protectant Against Major Plant Pathogens. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Springer Nature: Singapore, 2024; pp. 321–336. [Google Scholar]
- Tyagi, A.; Raj, H. Integration of soil solarization with bio-control agents for the management of stem rot of chrysanthemum. J. Pharmacogn. Phytochem. 2021, 10, 2468–2471. [Google Scholar] [CrossRef]
- Shavnam; Raj, H. Synergistic strategies for sustainable crop protection: Harnessing soil solarization and biofumigants to combat damping-off pathogens in Solanaceous vegetable crops. J. Plant Dis. Prot. 2024, 131, 2089–2098. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Löffler, P.; Eichhöfer, S.; David, J.; Muñoz, K.; Schaumann, G.E. Are agricultural plastic covers a source of plastic debris in soil? A first screening study. Soil 2022, 8, 31–47. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Mauromicale, G.; Monaco, A.L.; Longo, A.M.G. Improved efficiency of soil solarization for growth and yield of greenhouse tomatoes. Agron. Sustain. Dev. 2010, 30, 753–761. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Barman, M.; Bera, T.; De, M.; Chatterjee, D. Agriculture: Polymers in Crop Production Mulch and Fertilizer. In Encyclopedia of Polymer Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica Seed Meal Soil Amendments Transform the Rhizosphere Microbiome and Improve Apple Production Through Resistance to Pathogen Reinfestation. Phytopathology 2015, 105, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Streck, N.A.; Schneider, F.M.; Buriol, G.A. Effect of soil solarization on thermal regime of plastic greenhouse soil. Cienc. Rural 1994, 24, 229–233. [Google Scholar] [CrossRef]
- Salman, S.R.; Mettawee, S.A.G. Soil solar-warming with different types of mulch. Arab. Univ. J. Agric. Sci. 2005, 13, 877–889. [Google Scholar]
- Shlevin, E.; Mahrer, Y.; Katan, J. Effect of moisture on thermal inactivation of soilborne pathogens under structural solarization. Phytopathology 2004, 94, 132–137. [Google Scholar] [CrossRef]
- Kim, M.; Shim, C.; Lee, J.; Wangchuk, C. Hot Water Treatment as Seed Disinfection Techniques for Organic and Eco-Friendly Environmental Agricultural Crop Cultivation. Agriculture 2022, 12, 1081. [Google Scholar] [CrossRef]
- Shekhar, M.; Shivashankar, E.; Pandey, S.K. Crop Rotation and Intercropping Techniques, 1st ed.; ND Global Publication House: Sagar, India, 2024. [Google Scholar]
- Liu, C.; Plaza-Bonilla, D.; Coulter, J.A.; Kutcher, H.R.; Beckie, H.J.; Wang, L.; Gan, Y. Diversifying crop rotations enhances agroecosystem services and resilience. Adv. Agron. 2022, 173, 299–335. [Google Scholar] [CrossRef]
- Mihrete, T.B.; Mihretu, F.B. Crop Diversification for Ensuring Sustainable Agriculture, Risk Management and Food Security. Glob. Challenges 2025, 9, 2400267. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, H.; Hussain, N.; Nasim, W.; Mubeen, M.; Ahmad, S.; Hasanuzzaman, M. Fundamentals of crop rotation in agronomic management. In Agronomic Crops; Springer: Singapore, 2019; pp. 545–559. [Google Scholar]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef]
- Cook, R.J. Management of resident plant growth-promoting rhizobacteria with the cropping system: A review of experience in the US Pacific Northwest. Eur. J. Plant Pathol. 2007, 119, 255–264. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Hubbard, J.C.; Koike, S.T. Evaluation of broccoli residue incorporation into field soil for Verticillium wilt control in cauliflower. Plant Dis. 1999, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.; Kur, A.; Bueno, E.; von Wettberg, E. Defining and improving the rotational and intercropping value of a crop using a plant–soil feedbacks approach. Crop Sci. 2020, 60, 2195–2203. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Shennan, C.; Muramoto, J.; Baird, G.; Zavatta, M.; Toyama, L.; Mazzola, M.; Koike, S.T. Anaerobic soil disinfestation (ASD): A strategy for control of soil borne diseases in strawberry production. In Proceedings of the International Symposium on Innovation in Integrated and Organic Horticulture, Avignon, France, 8–12 June 2015; Volume 1137, pp. 113–120. [Google Scholar] [CrossRef]
- Tronsmo, A.M.; Collinge, D.B.; Djurle, A.; Munk, L.; Yuen, J.; Tronsmo, A. Plant Pathology and Plant Diseases; CAB International: Boston, MA, USA, 2020. [Google Scholar]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified crop rotation: An approach for sustainable agriculture production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Rusinamhodzi, L. Crop Rotations and Residue Management in Conservation Agriculture. In Conservation Agriculture; Farooq, M., Siddique, K., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kheyrodin, H. Crop rotations for managing soil-borne plant diseases. Afr. J. Food Sci. Technol. 2011, 2, 1–9. [Google Scholar]
- Marburger, D.A.; Venkateshwaran, M.; Conley, S.P.; Esker, P.D.; Lauer, J.G.; Ané, J.M. Crop rotation and management effect on Fusarium spp. populations. Crop Sci. 2015, 55, 365–376. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Yang, X.B. Pathogenicity of Pythium populations from corn-soybean rotation fields. Plant Dis. 2000, 84, 94–99. [Google Scholar] [CrossRef]
- Peralta, A.L.; Sun, Y.; McDaniel, M.D.; Lennon, J.T. Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 2018, 9, e02235. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Zhao, J.; Zhang, J.; Cai, Z.; Huang, X. High carbon resource diversity enhances the certainty of successful plant pathogen and disease control. New Phytol. 2023, 237, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.M.; Gilbert, J.A. Microbial diversity—Exploration of natural ecosystems and microbiomes. Curr. Opin. Genet. Dev. 2015, 35, 66–72. [Google Scholar] [CrossRef]
- De Corato, U. Soil microbiota manipulation and its role in suppressing soil-borne plant pathogens in organic farming systems under the light of microbiome-assisted strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Kema, G.H.; Drenth, A.; Dita, M.; Jansen, K.; Vellema, S.; Stoorvogel, J.J. Fusarium wilt of banana, a recurring threat to global banana production. Front. Plant Sci. 2021, 11, 628888. [Google Scholar] [CrossRef]
- Siamak, S.B.; Zheng, S. Banana Fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems. Hortic. Plant J. 2018, 4, 208–218. [Google Scholar] [CrossRef]
- Viljoen, A.; Ma, L.J.; Molina, A.B. CHAPTER 8: Fusarium wilt (panama disease) and monoculture in banana production: Resurgence of a century-old disease. In Emerging Plant Diseases and Global Food Security; The American Phytopathological Society: St. Paul, MN, USA, 2020; pp. 159–184. [Google Scholar]
- CABI. Fusarium oxysporum f.sp. Cubense (Panama disease of banana). In CABI Compendium; CABI: Wallingford, UK, 2022. [Google Scholar]
- Bekele, D.; Worku, W.; Mulatu, Z.; Admasu, A.; Shimeles, F.; Dobocha, D. Evaluation of Alternative Break Crops in Rotation with Bread Wheat (Triticum aestivum L.) in South-Eastern Ethiopia. J. Aquac. Livest. Prod. 2024, 5, 1–6. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crops Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Abdulkadir, K.N. Cropping Systems Diversification as an approach to Enhancing Crop Productivity. Preprints 2023. [Google Scholar]
- Roesch-McNally, G.E.; Arbuckle, J.G.; Tyndall, J.C. Barriers to implementing climate resilient agricultural strategies: The case of crop diversification in the US Corn Belt. Glob. Environ. Change 2018, 48, 206–215. [Google Scholar] [CrossRef]
- Larkin, R. Use of crop rotations, cover crops and green manures for disease suppression in potato cropping systems. Glob. J. Agric. Innov. Res. Dev. 2021, 8, 153–168. [Google Scholar] [CrossRef]
- Morris, E.; Fletcher, R.; Veresoglou, S. Effective methods of biofumigation: A meta-analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Prasad, P.; Kumar, J.; Pandey, S. Biofumigation: Success and prospects in soilborne plant disease management. JAPSA 2015, 1, 47–59. [Google Scholar]
- Angus, J.; Gardner, P.; Kirkegaard, J.; Desmarchelier, J. Biofumigation: Isothiocyanates released from Brassica roots inhibit growth of the take-all fungus. Plant Soil 1994, 162, 107–112. [Google Scholar] [CrossRef]
- Brown, P.; Morra, M. Control of soilborne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Yang, Y.J.; Li, S.Y.; Hu, G.W.; Liao, X.J.; Hu, X.S.; Zhang, Y. Research progress on degradation pathways and products of glucosinolates. Acta Bot.-Boreali-Occident. Sin. 2011, 31, 1490–1496. [Google Scholar]
- Hanschen, F.; Winkelmann, T. Biofumigation for fighting replant disease—A review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Wang, L.L.; Jiang, H.; Qiu, Y.J.; Dong, Y.Y.; Hamouda, H.I.; Balah, M.A.; Mao, X.Z. Biochemical characterization of a novel myrosinase Rmyr from Rahnella inusitata for high-level preparation of sulforaphene and sulforaphane. J. Agric. Food Chem. 2022, 70, 2303–2311. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Miao, Z.; Guo, R.; Zhao, Z. Biofumigation for management of soilborne plant diseases. Chin. J. Biol. Control 2006, 22, 296. [Google Scholar]
- Larkin, R.; Griffin, T. Control of soilborne diseases of potato using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Yulianti, T.; Sivasithamparam, K.; Turner, D.W. Saprophytic and pathogenic behaviour of R. solani AG2-1 (ZG-5) in a soil amended with Diplotaxis tenuifolia or Brassica nigra manures and incubated at different temperatures and soil water content. Plant Soil 2007, 294, 277–289. [Google Scholar] [CrossRef]
- Fan, C.; Liu, J.; Wu, Y.; Xiong, G.; He, Y. Screening of several plants suppressing soil borne plant fungi by biofumigation. J. Yunnan Agric. Univ. 2007, 22, 654–658. [Google Scholar] [CrossRef]
- Sihag, M.; Kumar, V.; Rana, M.; Srivastava, S.; Singh, S. Biofumigation: Prospects for control of soil borne plant diseases. J. Biopestic. 2022, 15, 136–149. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Wang, Y.; Lu, X.; Wang, P.; Su, Z.; Zhang, X.; Ma, P. Control efficacy of broccoli residues on cotton Verticillium wilt and its effect on soil bacterial community at different growth stages. Sci. Agric. Sin. 2019, 52, 4505–4517. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, Z.; Wang, G.; Ma, Y. Integration of Pseudomonas aeruginosa with biofumigation to control phytophthora blight of pepper. Jiangsu J. Agric. Sci. 2015, 31, 290–297. [Google Scholar] [CrossRef]
- Walker, B.A.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Soil microbial community dynamics during ryegrass green manuring and brassica biofumigation. Appl. Soil Ecol. 2022, 179, 104600. [Google Scholar] [CrossRef]
- Walker, B.A.; Powell, S.M.; Tegg, R.S.; Doyle, R.B.; Hunt, I.G.; Wilson, C.R. Ten years of green manuring and biofumigation alters soil characteristics and microbiota. Appl. Soil Ecol. 2023, 187, 104836. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Fang, W.; Li, Y.; Yan, D.; Cao, A.; Wang, Q. A review of biofumigation effects with plant materials. New Plant Prot. 2024, 1, e21. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, R.; Zhang, C.; Yao, X.; Yang, Z.; Li, S.; Liu, T.; Zheng, C.; Wang, X.; Xu, N. Changes in soil microbial diversity and control of Fusarium oxysporum in continuous cropping cucumber greenhouses following biofumigation. Emir. J. Food Agric. 2018, 30, 644–653. [Google Scholar] [CrossRef]
- Sennett, L.; Goyer, C.; Burton, D.; Zebarth, B.; Whitney, S. Chemical fumigation and biofumigation alter soil bacterial community diversity and composition. FEMS Microbiol. Ecol. 2022, 98, fiac026. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, J.; Kirkegaard, J. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J. Biofumigation potential of brassicas: II. Effect of environment and ontogeny on glucosinolate production and implications for screening. Plant Soil 1998, 201, 91–101. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Chen, D.; Zebarth, B.; Goyer, C.; Comeau, L.; Nahar, K.; Dixon, T. Effect of biofumigation on population densities of Pratylenchus spp. and Verticillium spp. and potato yield in Eastern Canada. Am. J. Potato Res. 2022, 99, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Zhang, D.; Fang, W.; Song, Z.; Ren, L.; Li, Q.; Li, W.; Wang, Q.; Yan, D.; Li, Y.; et al. Progresses and challenges in soil-borne disease prevention and control technology. Plant Prot. 2023, 49, 260–269. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Shinmura, A. Principle and effect of soil sterilization methods by reducing the redox potential of soil. PSJ Soilborne Dis. Workshop Rep. 2004, 22, 2–12, (In Japanese with English Summary). [Google Scholar]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. An assessment of the modulation of the population dynamics of pathogenic Fusarium oxysporum f. sp. lycopersici in the tomato rhizosphere by means of the application of Bacillus subtilis QST 713, Trichoderma sp. TW2 and two composts. Biol. Control 2020, 142, 104158. [Google Scholar] [CrossRef]
- Gilardi, G.; Pugliese, M.; Gullino, M.; Garibaldi, A. Evaluation of different carbon sources for anaerobic soil disinfestation against Rhizoctonia solani on lettuce in controlled production systems. Phytopathol. Mediterr. 2020, 59, 77–96. [Google Scholar] [CrossRef]
- Lopes, E.A.; Canedo, E.J.; Gomes, V.A.; Vieira, B.S.; Parreira, D.F.; Neves, W.S. Anaerobic soil disinfestation for the management of soilborne pathogens: A review. Appl. Soil Ecol. 2022, 174, 104408. [Google Scholar] [CrossRef]
- Goud, J.; Termorshuizen, A.; Blok, W.; van Bruggen, A. Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis. 2004, 88, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Rosskopf, E.; Burelle, N.; Hong, J.; Butler, D.; Noling, J.; He, Z.; Booker, B.; Sances, F. Comparison of anaerobic soil disinfestation and drip-applied organic acids for raised-bed specialty crop production in Florida. Acta Hortic. 2014, 1044, 221–228. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Lamers, J.; Mazzola, M.; Rosskopf, E.; Kokalis-Burelle, N.; Momma, N.; Butler, D.; Kobara, Y. Anaerobic soil disinfestation for soil borne disease control in strawberry and vegetable systems: Current knowledge and future directions. Acta Hortic. 2014, 1044, 165–175. [Google Scholar] [CrossRef]
- Shrestha, U.; Augé, R.M.; Butler, D.M. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Guo, H.; Di Gioia, F.; Zhao, X.; Ozores-Hampton, M.; Swisher, M.; Hong, J.; Rosskopf, E. Optimizing anaerobic soil disinfestation for fresh market tomato production: Nematode and weed control, yield, and fruit quality. Sci. Hortic. 2017, 218, 105–116. [Google Scholar] [CrossRef]
- Rosskopf, E.; Serrano-Pérez, P.; Hong, J.; Shrestha, U.; Rodríguez-Molina, M.; Martin, K.; Butler, D. Anaerobic soil disinfestation and soilborne pest management. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Springer: Berlin/Heidelberg, Germany, 2015; pp. 277–305. [Google Scholar]
- Shrestha, U.; Dee, M.E.; Ownley, B.H.; Butler, D.M. Anaerobic soil disinfestation reduces germination and affects colonization of Sclerotium rolfsii sclerotia. Phytopathology 2018, 108, 342–351. [Google Scholar] [CrossRef]
- Strauss, S.L.; Kluepfel, D.A. Anaerobic soil disinfestation: A chemical-independent approach to pre-plant control of plant pathogens. J. Integr. Agric. 2015, 14, 2309–2318. [Google Scholar] [CrossRef]
- Ueki, A.; Kaku, N.; Ueki, K. Role of anaerobic bacteria in biological soil disinfestation for elimination of soil-borne plant pathogens in agriculture. Appl. Microbiol. Biotechnol. 2018, 102, 6309–6318. [Google Scholar] [CrossRef]
- Di Gioia, F.; Ozores-Hampton, M.; Hong, J.; Kokalis-Burelle, N.; Albano, J.; Zhao, X.; Rosskopf, E. The effects of anaerobic soil disinfestation on weed and nematode control, fruit yield, and quality of Florida fresh-market tomato. HortScience 2016, 51, 703–711. [Google Scholar] [CrossRef]
- Stremińska, M.A.; Runia, W.T.; Termorshuizen, A.J.; Feil, H.; Van Der Wurff, A.W.G. Anaerobic soil disinfestation in microcosms of two sandy soils. Commun. Agric. Appl. Biol. Sci. 2014, 79, 15–18. [Google Scholar]
- Hewavitharana, S.; Klarer, E.; Reed, A.; Leisso, R.; Poirier, B.; Honaas, L.; Mazzola, M. Temporal dynamics of the soil metabolome and microbiome during simulated anaerobic soil disinfestation. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef] [PubMed]
- Thaning, C.; Gerhardson, B. Reduced sclerotial soil-longevity by whole-crop amendment and plastic covering/Feldversuche zum Einfluss einer Bodenabdeckung mit Kunststoffplane und zur Einarbeitung von Grünmasse auf die Überdauerung von Sklerotien. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 2001, 143–151. [Google Scholar]
- Gholami, M.; Khakvar, R.; Niknam, G. Introduction of some new endophytic bacteria from Bacillus and Streptomyces genera as successful biocontrol agents against Sclerotium rolfsii. Arch. Phytopathol. Plant Prot. 2014, 47, 122–130. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Mazzola, M.; Muramoto, J.; Shennan, C. Anaerobic disinfestation induced changes to the soil microbiome, disease incidence and strawberry fruit yields in California field trials. Appl. Soil Ecol. 2018, 127, 74–86. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Wang, F.; Cai, Z. Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Appl. Microbiol. Biotechnol. 2016, 100, 5581–5593. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.; Wang, Y.; Liu, Z.; Cai, T.; Zhu, D.; Duan, G. Effects of reductive soil disinfestation on potential pathogens and antibiotic resistance genes in soil. J. Environ. Sci. 2025, 150, 373–384. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Xu, R.; Song, J.; Wei, X.; Liu, X.; Wang, Y. Effects of anaerobic soil disinfestation on antibiotics, human pathogenic bacteria, and their associated antibiotic resistance genes in soil. Appl. Soil Ecol. 2024, 195, 105266. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y. The Function of Root Exudates in the Root Colonization by Beneficial Soil Rhizobacteria. Biology 2024, 13, 95. [Google Scholar] [CrossRef]
- Yu, R.P.; Dresbøll, D.B.; Finckh, M.R.; Justes, E.; van der Werf, W.; Fletcher, A.; Carlsson, G.; Li, L. Intercropping: Ecosystem functioning and sustainable agriculture. Plant Soil 2025, 506, 1–6. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Keshavarz Afshar, R.; Liu, X.; Herbert, S.J. Understanding intercropping to improve agricultural resiliency and environmental sustainability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–256. [Google Scholar]
- Boudreau, M.A. Diseases in intercropping systems. Annu. Rev. Phytopathol. 2013, 51, 499–519. [Google Scholar] [CrossRef]
- Civitello, D.J.; Cohen, J.; Fatima, H.; Halstead, N.T.; Liriano, J.; McMahon, T.A.; Ortega, C.N.; Sauer, E.L.; Sehgal, T.; Young, S.; et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 2015, 112, 8667–8671. [Google Scholar] [CrossRef]
- Chadfield, V.G.A.; Hartley, S.E.; Redeker, K.R. Associational resistance through intercropping reduces yield losses to soil-borne pests and diseases. New Phytol. 2022, 235, 2393–2405. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Fan, J.; Wang, Y.; Li, Y.; Chen, J.; Fan, J.; Yang, S.; Hu, L.; Leung, H.; et al. Genetic diversity and disease control in rice. Nature 2000, 406, 718–722. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defence. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Cipollini, D.; Morris, K.; Gurusinghe, S.; Weston, L.A. Ecological realism and rigor in the study of plant-plant allelopathic interactions. Plant Soil 2023, 489, 1–39. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Zampieri, E.; Sillo, F.; Metelli, G.; Cucu, M.A.; Montesano, V.; Quagliata, G.; Philipp, L.; Brescia, F.; Conte, A.; Giovannini, L.; et al. Insights into the influence of intercropping and arbuscular mycorrhizal inoculation on two modern durum wheat cultivars and their associated microbiota. Biol. Fertil. Soils 2025, 61, 85–107. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, M.; Zhai, Z. Soil organic carbon, carbon fractions, and microbial community under various organic amendments. Sci. Rep. 2024, 14, 25431. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Y.; Tang, L.; Zheng, Y.; Makowski, D.; Yu, Y.; Zhang, F.; van der Werf, W. Intercropping cereals with faba bean reduces plant disease incidence regardless of fertilizer input; a meta-analysis. Eur. J. Plant Pathol. 2019, 154, 931–942. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Alarcón-Segura, V.; Grass, I.; Breustedt, G.; Rohlfs, M.; Tscharntke, T. Strip intercropping of wheat and oilseed rape enhances biodiversity and biological pest control in a conventionally managed farm scenario. J. Appl. Ecol. 2022, 59, 1513–1523. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Sairam, M. Intercropping—A low input agricultural strategy for food and environmental security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.E.; Anderson, P.; Carlsson, G.; Friberg, H.; Larsson, M.C.; Wallenhammar, A.C.; Lundin, O. The potential of intercropping for multifunctional crop protection in oilseed rape (Brassica napus L.). Front. Agron. 2021, 3, 782686. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Bedoussac, L.; Justes, E. The efficiency of a durum wheat-winter pea intercrop to improve yield and wheat grain protein concentration depends on N availability during early growth. Plant Soil 2010, 330, 19–35. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Z.G.; Bao, X.G.; Sun, J.H.; Yang, S.C.; Wang, P.; Li, L. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 2021, 4, 943–950. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, Y.; Li, X.; Qin, H.; Wang, J.; Zhang, S.; Li, X.F. Increased overyielding probability and yield stability from a 5-year cotton-based intercropping. Eur. J. Agron. 2024, 156, 127145. [Google Scholar] [CrossRef]
- Martin-Guay, M.O.; Paquette, A.; Dupras, J.; Rivest, D. The new green revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; van Der Werf, W. Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv. Agron. 2020, 160, 1–50. [Google Scholar] [CrossRef]
- Carolan, K.; Helps, J.; van den Berg, F.; Bain, R.; Paveley, N.; van den Bosch, F. Extending the durability of cultivar resistance by limiting epidemic growth rates. Proc. Biol. Sci. 2017, 284, 20170828. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Rutkoski, J. Advances and challenges in genomic selection for disease resistance. Annu. Rev. Phytopathol. 2016, 54, 79–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Lubberstedt, T.; Xu, M. The genetic and molecular basis of plant resistance to pathogens. J. Genet. Genom. 2013, 40, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ragimekula, N.; Varadarajula, N.N.; Mallapuram, S.P.; Gangimeni, G.; Reddy, R.K.; Kondreddy, H.R. Marker assisted selection in disease resistance breeding. J. Plant Breed. Genet. 2013, 1, 90–109. [Google Scholar]
- Pathania, A.; Rialch, N.; Sharma, P.N. Marker-assisted selection in disease resistance breeding: A boon to enhance agriculture production. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 187–213. [Google Scholar] [CrossRef]
- Tomar, S.S. Breeding Strategies for Stem Rust Resistance in Wheat. Ph.D. Dissertation, Cornell University, Ithaca, NY, USA, 2011. [Google Scholar]
- Thakur, R.P.; Rai, K.N.; Khairwal, I.S.; Mahala, R.S. Strategy for downy mildew resistance breeding in pearl millet in India. J. SAT Agric. Res. 2008, 6, 1–11. [Google Scholar]
- Legg, J.P.; Thresh, J.M. Cassava mosaic virus disease in East Africa: A dynamic disease in a changing environment. Virus Res. 2000, 71, 135–149. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Hayes, R.J.; Vallad, G.E.; Qin, Q.M.; Grube, R.C.; Subbarao, K.V. Variation for resistance to Verticillium wilt in lettuce (Lactuca sativa L.). Plant Dis. 2007, 91, 439–445. [Google Scholar] [CrossRef]
- Kim, S.G.; Hur, O.S.; Ro, N.Y.; Ko, H.C.; Rhee, J.H.; Sung, J.S.; Baek, H.J. Evaluation of resistance to Ralstonia solanacearum in tomato genetic resources at seedling stage. Plant Pathol. J. 2016, 32, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chitwood-Brown, J.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Breeding for resistance to Fusarium wilt of tomato: A review. Genes 2021, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mena, S.; Rubiales, D.; González, M. Identification of Sources of Resistance to Aphanomyces Root Rot in Pisum. Plants 2024, 13, 2454. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; Lu, J.; Sánchez-Martín, J.; Wulff, B.B.H. Resistance that stacks up: Engineering rust and mildew disease control in the cereal crops wheat and barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef]
- Joobeur, T.; King, J.J.; Nolin, S.J.; Thomas, C.E.; Dean, R.A. The fusarium wilt resistance locus Fom-2 of melon contains a single resistance gene with complex features. Plant J. 2004, 39, 283–297. [Google Scholar] [CrossRef]
- de Vallavieille-Pope, C. Management of disease resistance diversity of cultivars of a species in single fields: Controlling epidemics. Comptes Rendus Biol. 2004, 327, 611–620. [Google Scholar] [CrossRef]
- Brown, J.K.M. Durable resistance of crops to disease: A Darwinian perspective. Annu. Rev. Phytopathol. 2015, 53, 513–539. [Google Scholar] [CrossRef]
- Brun, H.; Chèvre, A.M.; Fitt, B.D.L.; Powers, S.; Besnard, A.L.; Ermel, M.; Huteau, V.; Marquer, B.; Eber, F.; Renard, M.; et al. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010, 185, 285–299. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Singh, R.P.; Wagoire, W.W.; Payne, T.S. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 2000, 84, 203. [Google Scholar] [CrossRef]
- Mundt, C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Chen, X.; Zhou, J.M. From plant immunity to crop disease resistance. J. Genet. Genom. 2022, 49, 693–703. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Newman, T.E.; Thomas, W.J.W.; Batley, J.; Edwards, D. The complex relationship between disease resistance and yield in crops. Plant Biotechnol. J. 2024, 22, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hao, Z.; Ning, Y.; He, Z. Revisiting growth–defence trade-offs and breeding strategies in crops. Plant Biotechnol. J. 2024, 22, 1198–1205. [Google Scholar] [CrossRef]
- Khan, A.H.; Hassan, M.; Khan, M.N. Conventional plant breeding program for disease resistance. In Plant Disease Management Strategies for Sustainable Agriculture Through Traditional and Modern Approaches; Springer: Berlin/Heidelberg, Germany, 2020; pp. 27–51. [Google Scholar] [CrossRef]
- Trudgill, D.L. Resistance to and tolerance of plant parasitic nematodes in plants. Annu. Rev. Phytopathol. 1991, 29, 167–192. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Sakr, N. Durable genetic plant resistance: A key to sustainable pathogen management. Open Agric. J. 2023, 17, e187433152306220. [Google Scholar] [CrossRef]
- Stam, R.; McDonald, B.A. When resistance gene pyramids are not durable—The role of pathogen diversity. Mol. Plant Pathol. 2018, 19, 521. [Google Scholar] [CrossRef]
- Sharma, H.C.; Ortiz, R. Host plant resistance to insects: An eco-friendly approach for pest management and environment conservation. J. Environ. Biol. 2002, 23, 111–135. [Google Scholar]
- Paul, M.; Mahla, J.S.; Upadhyay, D.K.; Das, D.; Wankhade, M.; Kumar, M.; Lallawmkimi, M.C. Integration of Genetic Resistance Mechanisms in Sustainable Crop Breeding Programs-A Review. J. Adv. Biol. Biotechnol. 2025, 28, 193–211. [Google Scholar] [CrossRef]
- Boiteux, L.S.; de Noronha Fonseca, M.E.; Vieira, J.V.; de Cássia Pereira-Carvalho, R. Breeding for resistance to viral diseases. In Plant Breeding for Biotic Stress Resistance; Springer: Berlin/Heidelberg, Germany, 2012; pp. 57–79. [Google Scholar]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Walkey, D.G. Control through resistant cultivars. In Applied Plant Virology; Springer: Dordrecht, The Netherlands, 1991; pp. 244–269. [Google Scholar] [CrossRef]
- Ntui, V.O.; Tripathi, J.N.; Kariuki, S.M.; Tripathi, L. Cassava molecular genetics and genomics for enhanced resistance to diseases and pests. Mol. Plant Pathol. 2024, 25, e13402. [Google Scholar] [CrossRef]
- Yuvashree, B.; Johnson, I.; Karthikeyan, M.; Anandham, R. Downy mildew of millets—An overview. Plant Sci. Today 2024, 11. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Sinha, S. Natural and engineered resistance: Implications for managing the cassava mosaic disease. In Geminivirus: Detection, Diagnosis and Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–548. [Google Scholar]
- Agrawal, M.K.; Fageria, M.S.; Dhaka, R.S. Breeding for multiple disease resistance in vegetables: A review. Agric. Rev. 2000, 21, 125–128. [Google Scholar]
- Stuthman, D.D.; Leonard, K.J.; Miller-Garvin, J. Breeding crops for durable resistance to disease. Adv. Agron. 2007, 95, 319–367. [Google Scholar] [CrossRef]
- Wiesner-Hanks, T.; Nelson, R. Multiple disease resistance in plants. Annu. Rev. Phytopathol. 2016, 54, 229–252. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.P.; Caixeta, E.T.; Moreira, K.F.; de Oliveira, A.C.B.; de Freitas, K.N.P.; Pereira, A.A.; Rosado, R.D.S.; Zambolim, L.; Cruz, C.D. Marker-assisted pyramiding of multiple disease resistance genes in coffee genotypes (Coffea arabica). Agronomy 2021, 11, 1763. [Google Scholar] [CrossRef]
- Kumar, A.; Jindal, S.K.; Dhaliwal, M.S.; Sharma, A.; Kaur, S.; Jain, S. Development of multiple disease resistant tomato lines through marker assisted breeding and their evaluation for horticultural traits. Indian Phytopathol. 2022, 75, 47–55. [Google Scholar] [CrossRef]
- Tiwari, S.; Tomar, R.S.; Chand, S.; Singh, N.K. Combining multiple rust resistance genes by phenotypic and marker assisted selection in wheat (Triticum aestivum L.). Indian J. Genet. Plant Breed. 2014, 74, 181–188. [Google Scholar] [CrossRef]
- Milczarek, D.; Plich, J.; Tatarowska, B.; Flis, B. Early selection of potato clones with resistance genes: The relationship between combined resistance and agronomical characteristics. Breed. Sci. 2017, 67, 416–420. [Google Scholar] [CrossRef][Green Version]
- Motsnyi, I.I.; Molodchenkova, O.O.; Nargan, T.P.; Nakonechnyy, M.Y.; Mishchenko, I.A.; Lyfenko, S.P.; Mishchenko, L.T. Impact of Alien Genes on Disease Resistance, Drought Tolerance, and Agronomic Traits in Winter Wheat Commercial Varieties. Open Agric. J. 2022, 16. [Google Scholar] [CrossRef]
- Wind, J.J. Balancing trait improvement with tradeoff side-effects using genome editing technology. In A Roadmap for Plant Genome Editing; Springer Nature: Cham, Switzerland, 2024; pp. 69–77. [Google Scholar]
- Avelino, J.; Ten Hoopen, G.M.; DeClerck, F.A. Ecological mechanisms for pest and disease control in coffee and cacao agroecosystems of the Neotropics. In Ecosystem Services from Agriculture and Agroforestry; Routledge: Londen, UK, 2012; pp. 91–117. [Google Scholar]
- Djian-Caporalino, C.; Palloix, A.; Fazari, A.; Marteu, N.; Barbary, A.; Abad, P.; Sage-Palloix, A.M.; Mateille, T.; Risso, S.; Lanza, R.; et al. Pyramiding, alternating or mixing: Comparative performances of deployment strategies of nematode resistance genes to promote plant resistance efficiency and durability. BMC Plant Biol. 2014, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Jomanga, K.E.; Lucas, S.S.; Mgenzi, A.R.; Gaudence, M.; Kiurugo, R.F.S.; Biseko, E. Review on broken-down resistance to diseases and its management; the hidden challenge in breeding and production of banana and plantains in developing countries. Int. J. Curr. Sci. Res. Rev. 2022, 5, 3939–3953. [Google Scholar] [CrossRef]
- Taylor, N.P.; Cunniffe, N.J. Modelling quantitative fungicide resistance and breakdown of resistant cultivars: Designing integrated disease management strategies for Septoria of winter wheat. PLoS Comput. Biol. 2023, 19, e1010969. [Google Scholar] [CrossRef]
- Akem, C.; Ceccarelli, S.; Erskine, W.; Lenné, J. Using genetic diversity for disease resistance in agricultural production. Outlook Agric. 2000, 29, 25–30. [Google Scholar] [CrossRef]
- Chakravarthy, A.K.; Jose Luis, E.V.; Onkara Naik, S.; Rajkumar, B. Economic and ecological values of resistant plants. In Experimental Techniques in Host-Plant Resistance; Springer: Singapore, 2019; pp. 253–263. [Google Scholar]
- Vanloqueren, G.; Baret, P.V. Why are ecological, low-input, multi-resistant wheat cultivars slow to develop commercially? A Belgian agricultural ‘lock-in’ case study. Ecol. Econ. 2008, 66, 436–446. [Google Scholar] [CrossRef]
- van Hove, L.; Gillund, F. Is it only the regulatory status? Broadening the debate on cisgenic plants. Environ. Sci. Eur. 2017, 29, 22. [Google Scholar] [CrossRef]
- van Hove, L.; Gillund, F. Is It Only the Regulatory Status? Broadening the Debate on Cisgenic Plants. In Cisgenic Crops: Safety, Legal and Social Issues; Chaurasia, A., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 269–288. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. An overview of soil and soilless cultivation techniques—Chances, challenges and the neglected question of sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Gullino, M.L.; Gilardi, G.; Garibaldi, A. Emerging soilborne diseases of horticultural crops and new trends in their management. Acta Hortic. 2010, 883, 37–47. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Bihari, C.; Ahamad, S.; Kumar, M.; Kumar, A.; Kamboj, A.D.; Singh, S.; Srivastava, V.; Gautam, P. Innovative Soilless Culture Techniques for Horticultural Crops: A Comprehensive Review. Int. J. Environ. Clim. Change 2023, 13, 4071–4084. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a Circular Economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Postma, J. The status of biological control of plant diseases in soilless cultivation. In Recent Developments in Management of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–146. [Google Scholar] [CrossRef]
- Vallance, J.; Déniel, F.; Floch, G.; Guérin-Dubrana, L.; Blancard, D.; Rey, P. Pathogenic and beneficial microorganisms in soilless cultures. Agron. Sustain. Dev. 2011, 31, 191–203. [Google Scholar] [CrossRef]
- Calvo-Bado, L.A.; Petch, G.; Parsons, N.R.; Morgan, J.A.W.; Pettitt, T.R.; Whipps, J.M. Microbial community responses associated with the development of oomycete plant pathogens on tomato roots in soilless growing systems. J. Appl. Microbiol. 2006, 100, 1194–1207. [Google Scholar] [CrossRef]
- Schnitzler, W. Pest and disease management of soilless culture. Acta Hortic. 2004, 648, 191–203. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- van Os, E.A.; Postma, J.; Pettitt, T.; Wohanka, W. Microbial optimisation in soilless cultivation, a replacement for methyl bromide. Acta Hortic. 2004, 635, 47–58. [Google Scholar] [CrossRef]
- Minuto, A.; Gullino, M.L.; Garibaldi, A. Disinfection of nutrient solution in closed soilless systems: Results in Italy. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, San Diego, CA, USA, 3–6 November 2003; pp. 37-1–37-4. [Google Scholar]
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. Agric. Eng. Int. Cigr J. Sci. Res. Dev. 2003, 1, 80–86. [Google Scholar]
- Khan, P.; Bora, L.C.; Borah, P.K.; Bora, P.; Talukdar, K. Efficacy of microbial consortia against bacterial wilt caused by Ralstonia solanacearum in hydroponically grown lettuce plant. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3046–3055. [Google Scholar] [CrossRef]
- Arumugam, T.; Sandeep, G.; Maheswari, M.U. Soilless farming of vegetable crops: An overview. Pharma Innov. J. 2021, 10, 773–785. [Google Scholar]
- Rajesh, E.; Basheer, S.; Baskar, K. Hydroponics soilless smart farming in improving productivity of crop using Intelligent Smart Systems. In Proceedings of the 2023 3rd International Conference on Innovative Practices in Technology and Management (ICIPTM), Uttar Pradesh, India, 22–24 February 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Wohanka, W. Root zone microbiology of soilless cropping systems. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–194. [Google Scholar]
- Feng, W.; Nukaya, A.; Satou, M.; Fukuta, N.; Ishiguro, Y.; Suga, H.; Kageyama, K. Use of LAMP detection to identify potential contamination sources of plant-pathogenic Pythium species in hydroponic culture systems of tomato and eustoma. Plant Dis. 2018, 102, 1357–1364. [Google Scholar] [CrossRef]
- Van Os, E.A. Disease Management IN Soilless Culture Systems. Acta Hortic. 2010, 385–393. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, Á. Optimization of nutrition in soilless systems: A review. Adv. Bot. Res. 2010, 53, 193–245. [Google Scholar] [CrossRef]
- Postma, J.; Os, E.V.; Bonants, P.J.M. Pathogen Detection and Management Strategies in Soilless Plant Growing Systems; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Zheng, Y. Advances in Understanding Plant Root Behaviour and Rootzone Management in Soilless Culture Systems; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 23–44. [Google Scholar] [CrossRef]
- Ally, N.M.; Neetoo, H.; Ranghoo-Sanmukhiya, V.M.; Coutinho, T.A. Greenhouse-grown tomatoes: Microbial diseases and their control methods: A review. Int. J. Phytopathol. 2023, 12, 99–127. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Gullino, M.L. Effect of potassium silicate and electrical conductivity in reducing powdery mildew of hydroponically grown tomato. Phytopathol. Mediterr. 2011, 50, 192–202. [Google Scholar]
- Gamliel, A. Soil and substrate health. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2020; pp. 355–383. [Google Scholar] [CrossRef]
- McGovern, R.J.; McSorley, R. Physical methods of soil sterilization for disease management including soil solarization. In Environmentally Safe Approaches to Crop Disease Control; CRC Press: Boca Raton, FL, USA, 2018; pp. 283–314. [Google Scholar]
- Cuervo, B.; Flórez, W.J.; González, V.J. Aspects to consider for optimizing a substrate culture system with drainage recycling. Agron. Colomb. 2012, 30, 379–387. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Pandey, K. Nutrient Management Strategies for Water and Nutrient Saving in Substrate Soilless Culture Under Protected Cultivation. In Artificial Intelligence and Smart Agriculture: Technology and Applications; Springer Nature: Singapore, 2024; pp. 369–386. [Google Scholar] [CrossRef]
- Voogt, W.; Bar-Yosef, B. Water and nutrient management and crops response to nutrient solution recycling in soilless growing systems in greenhouses. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–507. [Google Scholar]
- Guy, O.; Dai, N.; Cohen, S.; Bustan, A. Winter Strawberries Production in Greenhouse Soilless Culture under an Arid Climate–Cultivars, Phenology, Physiology, and Consequent Practices. In Recent Studies on Strawberries; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Jensen, M.H. Controlled Environment agriculture in deserts, tropics and temperate regions—A World Review. In Proceedings of the International Symposium on Design and Environmental Control of Tropical and Subtropical Greenhouses, Taichung, Taiwan, 15–18 April 2001; Volume 578, pp. 19–25. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M. Controlled environment ecosystem: A plant growth system to combat climate change through soilless culture. Crop Des. 2024, 3, 100044. [Google Scholar] [CrossRef]
- Wilkinson, A.; Gerlach, C.; Karlsson, M.; Penn, H. Controlled environment agriculture and containerized food production in northern North America. J. Agric. Food Syst. Community Dev. 2021, 10, 127–142. [Google Scholar] [CrossRef]
- Conner, D.S.; Christy, R.D. Consumer preferences for organic standards: Guiding demand-expansion strategies for organic food. J. Food Distrib. Res. 2002, 33, 46–51. [Google Scholar]
- Fahlevi, M.; Dandi, M.; Matroji, F.J.; Asetya, D.R. How Do Consumer Awareness, Health Consciousness, and Environmental Concern Drive the Willingness to Buy Hydroponic Vegetables? Proc. Iop Conf. Ser. Earth Environ. Sci. 2024, 1324, 012131. [Google Scholar] [CrossRef]
- Martini, M.; Fedi, A.; Murphy, B.; Dean, M.; Loera, B. More than organic: Consumer expectations of sustainability and quality. Evidences from a qualitative study in Italy. J. Food Prod. Mark. 2024, 30, 1–15. [Google Scholar] [CrossRef]
- Spendrup, S.; Bergstrand, K.J.; Thörning, R.; Hultberg, M. Consumer attitudes towards hydroponic cultivation of vegetables–Specifically exploring the impact of the fertilisation strategy (using mineral origin or food waste as fertilisers). Food Qual. Prefer. 2024, 113, 105085. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pool, J.A.; Calderón-Pérez, B.; Ruiz-Medrano, R.; Ortiz-Castro, R.; Xoconostle-Cazares, B. Bacillus strains as effective biocontrol agents against phytopathogenic bacteria and promoters of plant growth. Microb. Ecol. 2024, 87, 76. [Google Scholar] [CrossRef]
- Pellegrini, M.; Djebaili, R.; Pagnani, G.; Spera, D.M.; Del Gallo, M. Plant growth-promoting bacterial consortia render biological control of plant pathogens: A review. In Microorganisms for Sustainability; Springer Nature: Singapore, 2023; pp. 57–74. [Google Scholar]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting bacterial genera as biocontrol agents: Mechanisms, interactions and applications in sustainable agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Petkova, M.; Marcheva, M.; Petrova, A.L.; Slavova, V.; Shilev, S. Plant growth-promoting and biocontrol characteristics of four Bacillus strains and evaluation of their effects on wheat (Tr. aestivum L.). Int. J. Plant Biol. 2024, 16, 1. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant growth-promoting activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato and taro pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Ursan, M.; Boiu-Sicuia, O.A.; Voaides, C.; Stan, V.; Bubueanu, C.; Cornea, C.P. The potential of new Streptomyces isolates as biocontrol agents against Fusarium spp. In Proceedings of the “Agriculture for Life, Life for Agriculture” Conference Proceedings, Bucharest, Romania, 7–9 June 2018; Volume 1, pp. 594–600. [Google Scholar]
- Alharbi, A.A. Efficacy of the bacterium Lysobacter enzymogenes strain ch3B10 as a new biocontrol agent on the pathogenic fungi Alternaria solani and Fusarium oxysporum under laboratory conditions. Egypt. J. Biol. Pest Control 2022, 32, 70. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Sarrocco, S. Biological disease control by beneficial (micro) organisms: Selected breakthroughs in the past 50 years. Phytopathology 2023, 113, 732–740. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Ruan, S.; Nzabanita, C.; Wang, Y.; Guo, L. A mycovirus VIGS vector confers hypovirulence to a plant pathogenic fungus to control wheat FHB. Adv. Sci. 2023, 10, e2302606. [Google Scholar] [CrossRef]
- Bilgili, A. The effectiveness of arbuscular mycorrhizal fungal species (Funneliformis mosseae, Rhizophagus intraradices, and Claroideoglomus etunicatum) in the biocontrol of root and crown rot pathogens, Fusarium solani and Fusarium mixture in pepper. PeerJ 2025, 13, e18438. [Google Scholar] [CrossRef] [PubMed]
- Ravensberg, W.J. Registration of microbial pest control agents and products and other related regulations. In A Roadmap to the Successful Development and Commercialization of Microbial Pest Control Products for Control of Arthropods; Springer: Dordrecht, The Netherlands, 2011; pp. 171–233. [Google Scholar]
- Veerabhadraswamy, A.L.; Garampalli, R.H. Effect of arbuscular mycorrhizal fungi in the management of black bundle disease of maize caused by Cephalosporium acremonium. Sci. Res. Report. 2011, 1, 96–100. [Google Scholar]
- Zampieri, E.; Cucu, M.A.; Franchi, E.; Fusini, D.; Pietrini, I.; Centritto, M.; Balestrini, R. Characterization of Different Soil Bacterial Strains and Assessment of Their Impact on the Growth of Triticum turgidum spp. durum and Lens culinaris spp. culinaris. Curr. Microbiol. 2025, 82, 199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Khan, M.H.; Yuan, Z.; Hussain, S.; Cao, H.; Liu, Y. Response of soil microbiome structure and its network profiles to four soil amendments in monocropping strawberry greenhouse. PLoS ONE 2021, 16, e0245180. [Google Scholar] [CrossRef] [PubMed]
- Tomada, S. Underexplored Microbial Species in the Pipeline for the Development of Biopesticides. In Microbial Biocontrol Agents: Developing Effective Biopesticides; CABI: Wallingford, UK, 2022; pp. 202–225. [Google Scholar]
- Haritha, D.; Faiz Ahmed, M.; Bala, S.; Choudhury, D. Eco-friendly plant based on botanical pesticides. Plant Arch. 2021, 21, 2197–2204. [Google Scholar] [CrossRef]
- Lone, S.A.; Malik, A.; Padaria, J.C. Applications of Bacillus thuringiensis for Prevention of Environmental Deterioration. In Environmental Deterioration and Human Health; Springer: Dordrecht, Tthe Netherlands, 2014; pp. 73–95. [Google Scholar]
- Gašparovski, J. Effect of microbial biocontrol agents for plant diseases on soil microbiome. Biljn. Lek. 2021, 49, 170–177. [Google Scholar] [CrossRef]
- Patil, P.; Behera, S.K.; Raghu, S.; Annamalai, M. Biological control of insect pests in vegetable crops: An Eco-friendly approach. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1358–1373. [Google Scholar] [CrossRef]
- Vlaiculescu, A.; Varrone, C. Sustainable and eco-friendly alternatives to reduce the use of pesticides. In Pesticides in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–364. [Google Scholar]
- Hannusch, D.J.; Boland, G.J. Interactions of air temperature, relative humidity and biological control agents on grey mold of bean. Eur. J. Plant Pathol. 1996, 102, 133–142. [Google Scholar] [CrossRef]
- Fedele, G.; González-Domínguez, E.; Rossi, V. Influence of environment on the biocontrol of Botrytis cinerea: A systematic literature review. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–82. [Google Scholar]
- Bode, R.F.; Cervantez, O. Weather patterns determine success rates of two biocontrol agents on Cytisus scoparius in the USA. Entomol. Exp. Appl. 2024, 172, 1024–1032. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A. Climate change and resilience of biological control agents. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–93. [Google Scholar]
- Magan, N. Importance of ecological windows for efficacy of biocontrol agents. In Progress in Biological Control; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–14. [Google Scholar]
- Cray, J.A.; Connor, M.C.; Stevenson, A.; Houghton, J.D.R.; Rangel, D.E.N.; Cooke, L.R.; Hallsworth, J.E. Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb. Biotechnol. 2016, 9, 330–354. [Google Scholar] [CrossRef]
- Xu, X.M.; Jeger, M.J. Combined use of two biocontrol agents with different biocontrol mechanisms most likely results in less than expected efficacy in controlling foliar pathogens under fluctuating conditions: A modeling study. Phytopathology 2013, 103, 108–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimopoulou, A.; Theologidis, I.; Varympopi, A.; Papafotis, D.; Mermigka, G.; Tzima, A.; Panopoulos, N.J.; Skandalis, N. Shifting perspectives of translational research in bio-bactericides: Reviewing the Bacillus amyloliquefaciens paradigm. Biology 2021, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Elango, S.; Shahni, Y.S.; Padamini, R.; Hazarika, S.; Wongamthing, R.; Oraon, S.; Panigrahi, C.K.; Kumar, A.; Thangaraj, R. Harnessing microbial antagonists for biological control of plant pathogens: A global perspective. Microbiol. Res. J. Int. 2024, 34, 1–17. [Google Scholar] [CrossRef]
- Gerbore, J.; Benhamou, N.; Vallance, J.; Le Floch, G.; Grizard, D.; Regnault-Roger, C.; Rey, P. Biological control of plant pathogens: Advantages and limitations seen through the case study of Pythium oligandrum. Environ. Sci. Pollut. Res. Int. 2014, 21, 4847–4860. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, P. Mechanisms of action of bacterial biological control agents. In Biological Management of Diseases of Crops; Springer: Dordrecht, The Netherlands, 2013; pp. 295–429. [Google Scholar]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challenges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Montoya-Martínez, A.C.; Ruiz, V.V.; Chávez-Luzanía, R.A.; Villa-Rodríguez, E.D.; Villalobos, S.d.L.S. Biological control agents for mitigating plant diseases. In New Insights, Trends, and Challenges in the Development and Applications of Microbial Inoculants in Agriculture; Elsevier: Amsterdam, The Netherlands, 2024; pp. 27–35. [Google Scholar]
- Dan Jensen, F.; Lumsden, R.D. Biological control of soilborne pathogens. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 319–337. [Google Scholar]
- Iftikhar, Y.; Sajid, A.; Shakeel, Q.; Ahmad, Z.; Ul Haq, Z. Biological antagonism: A safe and sustainable way to manage plant diseases. In Sustainability in Plant and Crop Protection; Springer International Publishing: Cham, Switzerland, 2020; pp. 83–109. [Google Scholar]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Angelopoulou, D.J.; Naska, E.J.; Paplomatas, E.J.; Tjamos, S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014, 63, 1062–1069. [Google Scholar] [CrossRef]
- Alderley, C.L.; Greenrod, S.T.E.; Friman, V.P. Plant pathogenic bacterium can rapidly evolve tolerance to an antimicrobial plant allelochemical. Evol. Appl. 2022, 15, 735–750. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Clough, S.E.; Elphinstone, J.G.; Friman, V.P. Plant pathogenic bacterium Ralstonia solanacearum can rapidly evolve tolerance to antimicrobials produced by Pseudomonas biocontrol bacteria. J. Evol. Biol. 2024, 37, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I.; García-Arenal, F. Tolerance to plant pathogens: Theory and experimental evidence. Int. J. Mol. Sci. 2018, 19, 810. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Matić, S.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Influence of different biological control agents and compost on total and nitrification-driven microbial communities at rhizosphere and soil level in a lettuce - Fusarium oxysporum f. sp. lactucae pathosystem. J. Appl. Microbiol. 2019, 126, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Ferrocino, I.; Gullino, M.L. Effects of biocontrol agents and compost against the Phytophthora capsici of zucchini and their impact on the rhizosphere microbiota. Appl. Soil Ecol. 2020, 154, 103659. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mukherjee, A.; Ganguli, S.; Chakraborti, A.; Roy, S.; Choudhury, S.S.; Subramaniyan, V.; Kumarasamy, V.; Sayed, A.A.; El-Demerdash, F.M.; et al. Marvels of Bacilli in soil amendment for plant-growth promotion toward sustainable development having futuristic socio-economic implications. Front. Microbiol. 2023, 14, 1293302. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bharat, N.; Thakur, A. Role of Plant Growth-Promoting Rhizobacteria (PGPR) and Bio-Control Agents (BCAs) in Crop Production. Int. J. Econ. Plants 2020, 7, 144–150. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Gaur, A.; Bhatti, L.; Khatak, S. Interaction between Plants and Microorganisms: Microbial Biological Control Agents against Plant Pathogens. Res. J. Biotechnol. 2023, 18, 134–144. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Diyarza-Sandoval, N.A.; Guevara-Avendaño, E.; Meza-Contreras, J.J.; Reverchon, F. Plant growth-promoting microorganisms from native plants: An untapped resource of biocontrol and biofertilizer agents. In Biocontrol Agents for Improved Agriculture; Academic Press: Cambridge, MA, USA, 2024; pp. 29–66. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275. [Google Scholar] [CrossRef]
- Joshi, M.D.; Srivastava, A.K.; Ashaq, M.; Jaggi, S.; Gupta, P.; Hasan, W.; Gupta, S. Biocontrol Agents and Plant Protection. Uttar Pradesh J. Zool. 2024, 45, 109–131. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Bacillus thuringiensis based biopesticides for integrated crop management. In Biopesticides; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–6. [Google Scholar]
- Tiwari, A.K. Advances in biological control strategies for sustainable pest management. Uttar Pradesh J. Zool. 2024, 45, 214–232. [Google Scholar] [CrossRef]
- Tjamos, E.C.; Tjamos, S.E.; Antoniou, P.P. Biological management of plant diseases: Highlights on research and application. J. Plant Pathol. 2010, S17–S21. [Google Scholar]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Gusain, Y.S.; Kumar, V.; Sharma, A.K. Disease management through biological control agents: An eco-friendly and cost effective approach for sustainable agriculture-A Review. Agric. Rev. 2015, 36, 37. [Google Scholar] [CrossRef]
- Wong, C.K.F. Bioencapsulation of biocontrol agents as a management strategy for plant pathogens. In Microorganisms for Sustainability; Springer Nature: Singapore, 2023; pp. 339–358. [Google Scholar]
- Slininger, P.J.; Schisler, D.A. Biological control agents for suppression of post-harvest diseases of potatoes: Strategies on discovery and development. In Fungicides for Plant and Animal Diseases; InTech: London, UK, 2012; pp. 141–166. [Google Scholar] [CrossRef]
- Lange, L. Microbes and Microbial Products in Plant Protection. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 1992; pp. 252–270. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar]
- Gómez-Ramos, M.D.M.; Nannou, C.; Martínez Bueno, M.J.; Goday, A.; Murcia-Morales, M.; Ferrer, C.; Fernández-Alba, A.R. Pesticide residues evaluation of organic crops. A critical appraisal. Food Chem. X 2020, 5, 100079. [Google Scholar] [CrossRef]
- Walker, K.; Mendelsohn, M.; Matten, S.; Alphin, M.; Ave, D. The Role of Microbial Bt Products in U.S. Crop Protection. J. New Seeds 2003, 5, 31–51. [Google Scholar] [CrossRef]
- Lovell-Read, F.A.; Parnell, S.; Cunniffe, N.J.; Thompson, R.N. Using ’sentinel’ plants to improve early detection of invasive plant pathogens. PLoS Comput. Biol. 2023, 19, e1010884. [Google Scholar] [CrossRef]
- Pesti, R.; Kontra, L.; Paul, K.; Vass, I.; Csorba, T.; Havelda, Z.; Várallyay, É. Differential gene expression and physiological changes during acute or persistent plant virus interactions may contribute to viral symptom differences. PLoS ONE 2019, 14, e0216618. [Google Scholar] [CrossRef]
- Ward, M. Action against pest spread—The case for retrospective analysis with a focus on timing. Food Secur. 2016, 8, 77–81. [Google Scholar] [CrossRef]
- Marchand, P. The regulatory obstacles to bio control agents from Directive (EC) No 128/2009. Regsci 2024, 12. [Google Scholar] [CrossRef]
- Kiewnick, S. Practicalities of developing and registering microbial biologicalcontrol agents. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2007, 11. [Google Scholar] [CrossRef]
- Stewart, A. Commercial biocontrol—Reality or fantasy? Australas. Plant Pathol. 2001, 30, 127–131. [Google Scholar] [CrossRef]
- Strauch, O.; Strasser, H.; Hauschild, R.; Ehlers, R.U. Proposals for bacterial and fungal biocontrol agents. In Regulation of Biological Control Agents; Springer: Dordrecht, The Netherlands, 2011; pp. 267–288. [Google Scholar] [CrossRef]
- Das, M.M.; Abdulhameed, S. Agro-processing residues for the production of fungal bio-control agents. In Applied Environmental Science and Engineering for a Sustainable Future; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–126. [Google Scholar]
- Hao, Y.; Pan, X.; You, J.; Li, G.; Xu, M.; Rao, Z. Microbial production of branched chain amino acids: Advances and perspectives. Bioresour. Technol. 2024, 397, 130502. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Haridas, M.; Leza, H.; Abdulhameed, S. Customization of Bioreactor Technology for On-Site Production of Biocontrol Agents; Academic Press: Cambridge, MA, USA, 2022; pp. 23–43. [Google Scholar] [CrossRef]
- Ramanujam, B.; Prasad, R.D.; Sriram, S.; Rangeswaran, R. Mass production, formulation, quality control and delivery of Trichoderma for plant disease management. J. Plant Prot. Sci. 2010, 2, 1–8. [Google Scholar]
- Fravel, D.R.; Rhodes, D.J.; Larkin, R.P. Production and commercialization of biocontrol products. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 365–376. [Google Scholar]
- García-Bayona, L.; Comstock, L. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, G.M.; Jackson, M.A.; Behle, R.W.; Kobori, N.N.; Júnior, Í.D. Improved shelf life of dried Beauveria bassiana blastospores using convective drying and active packaging processes. Appl. Microbiol. Biotechnol. 2016, 100, 8359–8370. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Fulwider, V.M.; Duong, A.; Duffy, M.; Cafmeyer, J.; Ducceschi, S.; Hinton, C. Assessment of long-term stability of encapsulated agricultural biologicals in lipid-coated alginate beads. ACS Agric. Sci. Technol. 2023, 3, 389–398. [Google Scholar] [CrossRef]
- Llorens, E.; Agustí-Brisach, C. Biocontrol of plant diseases by means of antagonist microorganisms, biostimulants and induced resistance as alternatives to chemicals. Plants 2022, 11, 3521. [Google Scholar] [CrossRef]
- Fravel, D.R.; Keinath, A.P. Biocontrol of soilborne plant pathogens with fungi. In The Rhizosphere and Plant Growth; Springer: Dordrecht, The Netherlands, 1991; pp. 237–243. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, W. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Kumar, S.; Tiwari, R.K.S.; Nirmalkar, V.K.; Dayasagar; Singh, Y. Ecological niches of Bacillus species and their multifaceted mechanisms in combatting plant pathogens. J. Sci. Res. Rep. 2024, 30, 514–525. [Google Scholar] [CrossRef]
- Fedele, G.; Bove, F.; González-Domínguez, E.; Rossi, V. A generic model accounting for the interactions among pathogens, host plants, biocontrol agents, and the environment, with parametrization for Botrytis cinerea on grapevines. Agronomy 2020, 10, 222. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. ECOLOGY. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Srivastava, R.; Khalid, A.; Singh, U.S.; Sharma, A.K. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control 2010, 53, 24–31. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world - bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C.; Graber, E.R. Biochar effects on the abundance, activity and diversity of the soil biota. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 327–389. [Google Scholar]
- El-Masry, M.; Khalil, A.; Hassouna, M.; Ibrahim, H. In situ and in vivo suppressiveeffect of agricultural composts and their water extracts on some phytopathogenic fungi. World J. Microbiol. Biotechnol. 2002, 18, 551–558. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Omokaro, G.O.; Osarhiemen, I.O.; Idama, V.; Airueghian, E.O.; West, S.T.; Igbigbi, F.E.; Nnake, D.C.; Obolokor, E.; Ahmed, A.; Omoshie, V.O. The role of organic amendments and their impact on soil restoration: A review. Asian J. Environ. Ecol. 2024, 23, 41–52. [Google Scholar] [CrossRef]
- Singh, S.; Devi, N.B.; Divakaran, D.; Kumar, A.; Singh, S.; Tyagi, G.; Nath, A.; Kumar, A. Soil fertility management: Role of organic amendments and bio-fertilizers: A review. Int. J. Res. Agron. 2024, 7, 766–772. [Google Scholar] [CrossRef]
- Lazarovits, G. Management of soil-borne plant pathogens with organic soil amendments: A disease control strategy salvaged from the past. Can. J. Plant Pathol. 2001, 23, 1–7. [Google Scholar] [CrossRef]
- Bellini, A.; Ferrocino, I.; Cucu, M.A.; Pugliese, M.; Garibaldi, A.; Gullino, M.L. A Compost Treatment Acts as a Suppressive Agent in Phytophthora capsici–Cucurbita pepo Pathosystem by Modifying the Rhizosphere Microbiota. Front. Plant Sci. 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, A.O.; Babalola, O.O.; Venturi, V.; Ayilara, M.S.; Adeleke, B.S.; Amoo, A.E.; Sobowale, A.A.; Fadiji, A.E.; Glick, B.R. Plant disease management: Leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 2021, 12, 700507. [Google Scholar] [CrossRef]
- Andreo-Jimenez, B.; Schilder, M.T.; Nijhuis, E.H.; Te Beest, D.E.; Bloem, J.; Visser, J.H.M.; van Os, G.; Brolsma, K.; de Boer, W.; Postma, J. Chitin- and keratin-rich soil amendments suppress Rhizoctonia solani disease via changes to the soil microbial community. Appl. Environ. Microbiol. 2021, 87, e00318-21. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Liu, H.; Xu, Z.; Li, R.; Shen, Q.; Salles, J.F. Soil microbiome manipulation triggers direct and possible indirect suppression against Ralstonia solanacearum and Fusarium oxysporum. NPJ Biofilms Microbiomes 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao Salles, J. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Liao, H.; Li, Y.; Yao, H. Biochar amendment stimulates utilization of plant-derived carbon by soil bacteria in an intercropping system. Front. Microbiol. 2019, 10, 1361. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–379. [Google Scholar]
- Hussain, M.I.; Khan, Z.I.; Akhter, P.; Al-Hemaid, F.M.; Al-Hashimi, A.; Elshikh, M.S.; Ahmad, K.; Yang, H.H. Potential of organic amendments for heavy metal contamination in soil–coriander system: Environmental fate and associated ecological risk. Sustainability 2022, 14, 11374. [Google Scholar] [CrossRef]
- Obriot, F.; Stauffer, M.; Goubard Delaunay, Y.; Cheviron, N.; Peres, G.; Eden, M.; Revallier, A.; Vieublé, L.; Houot, S. Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agric. Ecosyst. Environ. 2016, 232, 165–178. [Google Scholar] [CrossRef]
- Rosa, D.; Petruccelli, V.; Iacobbi, M.C.; Brasili, E.; Badiali, C.; Pasqua, G.; Di Palma, L. Functionalized biochar from waste as a slow-release nutrient source: Application on tomato plants. Heliyon 2024, 10, e29455. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Neeraj, A.; Siddiqui, M.A.; Singh, V. Nutrient management technologies and the role of organic matrix-based slow-release biofertilizers for agricultural sustainability: A review. Agric. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Neethu, C.B.; Vardhanan, Y.S. Development of slow-release fertilizer from animal origin wastes: Sustainable organic agriculural perspective. Curr. Agric. Res. J. 2023, 11, 69–77. [Google Scholar] [CrossRef]
- Noble, R. Risks and benefits of soil amendment with composts in relation to plant pathogens. Australas. Plant Pathol. 2011, 40, 157–167. [Google Scholar] [CrossRef]
- Dey, A.; Srivastava, P.C.; Pachauri, S.P.; Shukla, A.K. Release kinetics of some nutrients from a sandy loam soil treated with different organic amendments. Commun. Soil Sci. Plant Anal. 2019, 50, 2718–2732. [Google Scholar] [CrossRef]
- Rosskopf, E.; Gioia, F.; Hong, J.; Pisani, C.; Kokalis-Burelle, N. Organic amendments for pathogen and nematode control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, N.; Gutiérrez-Barranquero, J.; Vicente, A.; Cazorla, F. Enhancing soil quality and plant health through suppressive organic amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic amendments modulate soil Microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef]
- Pugliese, M.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. Organic amendments and soil suppressiveness: Results with vegetable and ornamental crops. In Soil Biology; Springer International Publishing: Cham, Switzerland, 2015; pp. 495–509. [Google Scholar]
- Sloot, M.; Maerowitz-McMahan, S.; Postma, J.; Limpens, J.; De Deyn, G.B. Soil-borne disease suppressiveness after short and long term application of fermented, composted or fresh organic amendment treatments in arable soils. Appl. Soil Ecol. 2024, 195, 105268. [Google Scholar] [CrossRef]
- Senesi, N.; Plaza, C. Role of Humification Processes in Recycling Organic Wastes of Various Nature and Sources as Soil Amendments. Clean-Soil Air Water 2007, 35, 26–41. [Google Scholar] [CrossRef]
- Calleja-Cervantes, M.E.; Fernández-González, A.J.; Irigoyen, I.; Fernández-López, M.; Aparicio-Tejo, P.M.; Menéndez, S. Thirteen years of continued application of composted organic wastes in a vineyard modify soil quality characteristics. Soil Biol. Biochem. 2015, 90, 241–254. [Google Scholar] [CrossRef]
- Brichi, L.; Fernandes, J.V.M.; Silva, B.M.; Vizú, J.d.F.; Junior, J.N.G.; Cherubin, M.R. Organic residues and their impact on soil health, crop production and sustainable agriculture: A review including bibliographic analysis. Soil Use Manag. 2023, 39, 686–706. [Google Scholar] [CrossRef]
- Jiang, S.; Dai, G.; Rashid, M.S.; Zhang, J.; Lin, H.; Shu, Y. Effects of BC on metal uptake by crops (availability) and the vertical migration behavior in soil: A 3-year field experiments of crop rotation. Chemosphere 2024, 350, 141075. [Google Scholar] [CrossRef] [PubMed]
- Devi, G. Impact of organic amendments on Entomopathogenic nematodes: An overview. Int. J. Plant Soil Sci. 2024, 36, 461–467. [Google Scholar] [CrossRef]
- Reddy, P.P. Crop residue management and organic amendments. In Agro-Ecological Approaches to Pest Management for Sustainable Agriculture; Springer: Singapore, 2017; pp. 29–41. [Google Scholar]
- Usman, A.; Siddiqui, M.A. Integrated management of phytonematodes by the application of organic amendment and ploughing under field conditions. Arch. Phytopathol. Plant Prot. 2012, 45, 2437–2444. [Google Scholar] [CrossRef]
- Bollen, G.J.; Volker, D.; Wijnen, A.P. Inactivation of soil-borne plant pathogens during small-scale composting of crop residues. Neth. J. Plant Pathol. 1989, 95, 19–30. [Google Scholar] [CrossRef]
- Manga, M.; Muoghalu, C.C.; Acheng, P.O. Inactivation of faecal pathogens during faecal sludge composting: A systematic review. Environ. Technol. Rev. 2023, 12, 150–174. [Google Scholar] [CrossRef]
- Logo, A.; Boppré, B.; Fuchs, J.; Maurhofer, M.; Oberhänsli, T.; Thürig, B.; Widmer, F.; Flury, P.; Mayerhofer, J. Comprehensive analysis of 37 composts: Microbial indicators for soilborne disease suppression in three plant-pathogen systems. bioRxiv 2025. [Google Scholar] [CrossRef]
- Zaccardelli, M.; De Nicola, F.; Villecco, D.; Scotti, R. The development and suppressive activity of soil microbial communities under compost amendment. J. Soil Sci. Plant Nutr. 2013, 13, 730–742. [Google Scholar] [CrossRef]
- Bezanson, G.S.; Ells, T.C.; Prange, R.K. Effect of composting on microbial contamination and quality of fresh fruits and vegetables—A mini-review. Acta Hortic. 2014, 1018, 631–638. [Google Scholar] [CrossRef]
- Churchill, D.B.; Alderman, S.C.; Mueller-Warrant, G.W.; Elliott, L.F.; Bilsland, D.M. Survival of weed seeds and seed pathogen propagates in composted grass seed straw. Appl. Eng. Agric. 1995, 12, 57–63. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Grebus, M.E. Status of biological control of plant diseases with composts. Compost Sci. Util. 1994, 2, 6–12. [Google Scholar] [CrossRef]
- Neher, D.A.; Weicht, T.R.; Dunseith, P. Compost for management of weed seeds, pathogen, and early blight on brassicas in organic farmer fields. Agroecol. Sustain. Food Syst. 2015, 39, 3–18. [Google Scholar] [CrossRef]
- Vinnerås, B.; Agostini, F.; Jönsson, H. Sanitation by composting. In Microbes at Work; Springer: Berlin/Heidelberg, Germany, 2010; pp. 171–191. [Google Scholar]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease suppressive soils: New insights from the soil microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Aioub, A.A.A.; Ghosh, S.; AL-Farga, A.; Khan, A.N.; Bibi, R.; Elwakeel, A.M.; Nawaz, A.; Sherif, N.T.; Elmasry, S.A.; Ammar, E.E. Back to the origins: Biopesticides as promising alternatives to conventional agrochemicals. Eur. J. Plant Pathol. 2024, 169, 697–713. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Kusstatscher, P.; Cernava, T.; Abdelfattah, A.; Gokul, J.; Korsten, L.; Berg, G. Microbiome approaches provide the key to biologically control postharvest pathogens and storability of fruits and vegetables. FEMS Microbiol. Ecol. 2020, 96. [Google Scholar] [CrossRef]
- Rivas-García, T.; González-Estrada, R.; Chiquito-Contreras, R.; Reyes-Pérez, J.; González-Salas, U.; Hernández-Montiel, L.; Murillo-Amador, B. Biocontrol of phytopathogens under aquaponics systems. Water 2020, 12, 2061. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soilborne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Charkowski, A.; Sharma, K.; Parker, M.; Secor, G.; Elphinstone, J. Bacterial Diseases of Potato. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Puig-Sirera, A.; Provenzano, G.; González-Altozano, P.; Intrigliolo, D.; Rallo, G. Irrigation water saving strategies in Citrus orchards: Analysis of the combined effects of timing and severity of soil water deficit. Agric. Water Manag. 2021, 248, 106773. [Google Scholar] [CrossRef]
- Imbernón-Mulero, A.; Maestre-Valero, J.; Martínez-Alvarez, V.; García-García, F.; Jódar-Conesa, F.; Gallego-Elvira, B. Evaluation of an autonomous smart system for optimal management of fertigation with variable sources of irrigation water. Front. Plant Sci. 2023, 14, 1149956. [Google Scholar] [CrossRef]
- Maurya, S.; Dubey, S.; Kumari, R.; Verma, R. Management tactics for fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici (Sacc.): A review. Management 2019, 4, 1–7. [Google Scholar]
- Kulkarni, S. Sustainable Soil Conservation and Management: Principles, Issues, and Strategies. In Field Practices for Wastewater Use in Agriculture; Apple Academic Press: Cambridge, MA, USA, 2020; pp. 133–178. [Google Scholar]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Probiotic Bacteria, Anaerobic Soil Disinfestation, and Mustard Cover Crop Biofumigation Suppress Soilborne Disease and Increase Yield of Strawberry in a Perennial Organic Production System. Plant Dis. 2023, 107, 2490–2499. [Google Scholar] [CrossRef]
- Msimbira, L.; Smith, D. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Janati, W.; Mikou, K.; El Ghadraoui, L.; Errachidi, F. Growth stimulation of two legumes (Vicia faba and Pisum sativum) using phosphate-solubilizing bacteria inoculation. Front. Microbiol. 2023, 14, 1212702. [Google Scholar] [CrossRef] [PubMed]
- Sommermann, L.; Babin, D.; Behr, J.; Chowdhury, S.; Sandmann, M.; Windisch, S.; Grosch, R. Long-term fertilization strategy impacts Rhizoctonia solani–microbe interactions in soil and rhizosphere and defense responses in lettuce. Microorganisms 2022, 10, 1717. [Google Scholar] [CrossRef]
- Geoffrey, R. Pests and pathogens. Veg. Brassicas Relat. Crucif. 2024, 40, 295. [Google Scholar]
- Pahalvi, H.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A. Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers; Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–20. [Google Scholar]
- Qi, J.; Fu, D.; Wang, X.; Zhang, F.; Ma, C. The effect of alfalfa cultivation on improving physicochemical properties of soil microorganisms community structure of grey desert soil. Sci. Rep. 2023, 13, 13747. [Google Scholar] [CrossRef]
- Chai, A.; Yuan, L.; Li, X.; Li, L.; Shi, Y.; Xie, X.; Li, B. Effect of temperature and humidity on dynamics and transmission of Pseudomonas amygdali pv. lachrymans aerosols. Front. Plant Sci. 2023, 14, 1087496. [Google Scholar] [CrossRef]
- Gachomo, E.; Jimenez-Lopez, J.; Kayodé, A.; Baba-Moussa, L.; Kotchoni, S. Control of Major Diseases in Horticulture. In Fungicides for Plant and Animal Diseases; IntechOpen: London, UK, 2012. [Google Scholar]
- Kuroyanagi, T.; Yoshikoshi, H.; Kinoshita, T.; Kawashima, H. Use of air circulation to reduce wet leaves under high humidity conditions. Environ. Control Biol. 2014, 51, 215–220. [Google Scholar] [CrossRef]
- Autio, W.; Cooley, D. Summer pruning of apple: Impacts on disease management. Adv. Hortic. Sci. 2011, 25, 199–204. [Google Scholar]
- Daunde, A.; Baghele, R.; Khandare, V. Management of prevalent diseases of cucumber (Cucumis sativus) through integrated approach. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3022–3028. [Google Scholar] [CrossRef]
- Lecoq, L.; Flick, D.; Laguerre, O. Study of the water evaporation rate on stainless steel plate in controlled conditions. Int. J. Therm. Sci. 2017, 111, 450–462. [Google Scholar] [CrossRef]
- Baptista, F.; Bailey, B.; Meneses, J. Development of a warning system for controlling Botrytis cinerea in unheated tomato greenhouses. In Proceedings of the International Symposium on High Technology for Greenhouse Systems: GreenSys2009, Québec, QC, Canada, 14–19 June 2009; pp. 1263–1269. [Google Scholar]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in European agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Leoni, C.; Rossing, W.; van Bruggen, A.H.C. CHAPTER 4.2: Crop Rotation. In Plant Diseases and Their Management in Organic Agriculture; IPM, The American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 127–140. [Google Scholar] [CrossRef]
- Zhu, S.; Morel, J.B. Molecular mechanisms underlying microbial disease control in intercropping. Mol. Plant. Microbe. Interact. 2019, 32, 20–24. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Wang, C. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Rajput, P.; Choudhary, R.; Yadav, S.; Yadav, S.K.; Rajput, V.D.; Minkina, T.; Ercisli, S.; Matić, S. Multifaceted Characteristics of Biochar and Its Implementation in Environmental Management in a Sustainable Way. Environ. Qual. Manag. 2024, 34, e22305. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species–opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Murray-Watson, R.E.; Cunniffe, N.J. How the epidemiology of disease-resistant and disease-tolerant varieties affects grower behaviour. J. R. Soc. Interface 2022, 19, 20220517. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.H. Bacterial disease management: Challenges, experience, innovation and future prospects: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Hooks, C.R.; Fereres, A. Protecting crops from non-persistently aphid-transmitted viruses: A review on the use of barrier plants as a management tool. Virus Res. 2006, 120, 1–16. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Trivellone, V.; Krüger, K. The biology and ecology of Leafhopper transmission of phytoplasmas. In Phytoplasmas: Plant Pathogenic Bacteria—II; Springer: Singapore, 2019; pp. 27–51. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Niño-liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef]
- Katan, J. Solar heating (solarization) of soil for control of soilborne pests. Annu. Rev. Phytopathol. 1981, 19, 211–236. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Antón-Domínguez, B.I.; López-Moral, A.; Romero-Salguero, F.J.; Trapero, A.; Trapero, C.; Agustí-Brisach, C. Bioprotection of Olive Trees Against Verticillium Wilt by Pomegranate and Carob Extracts. Plant Dis. 2024, 108, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]

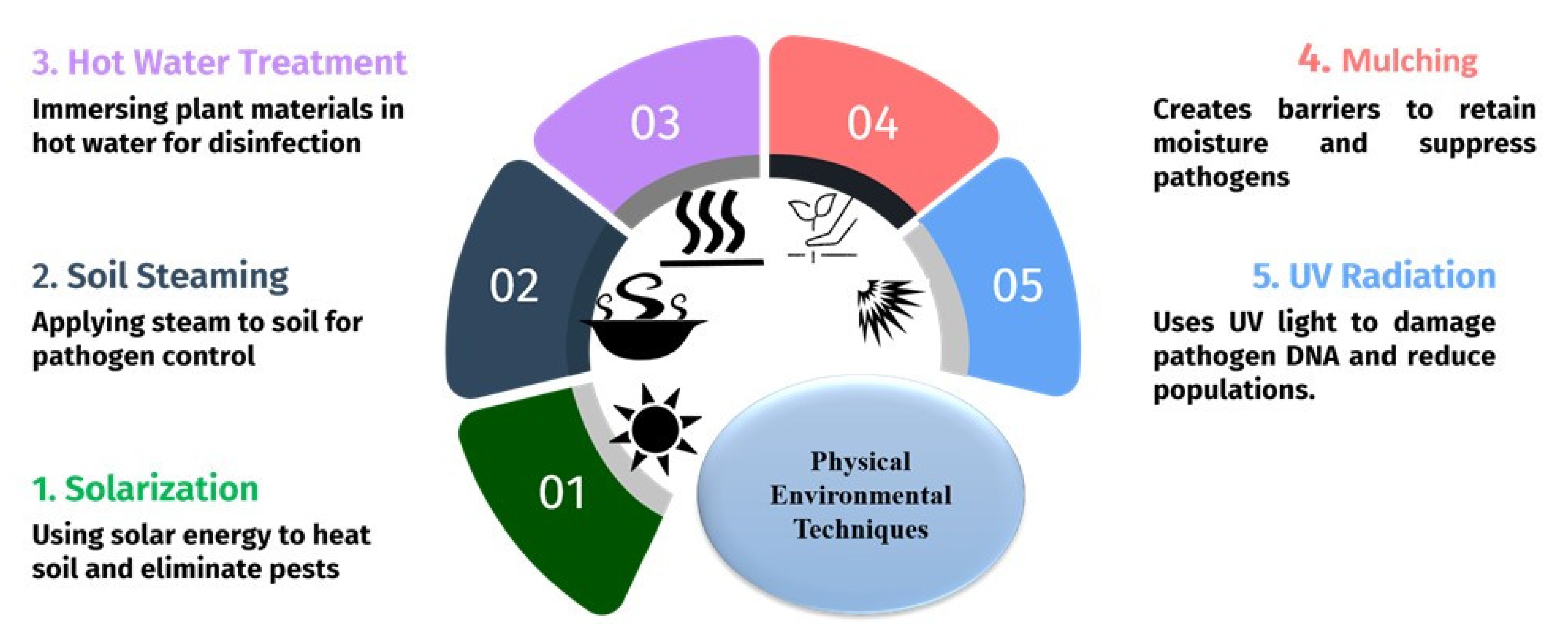


| Advantages | Disadvantages/Limitations |
|---|---|
| Significantly reduces inoculum build-up of many soil-borne pathogens by breaking the host cycle (e.g., less Verticillium wilt and common scab under rotations) [95] | Ineffective against pathogens with broad host ranges or long-lived resting spores that survive between crops (e.g., Sclerotinia spp., Verticillium in short rotations) [95] |
| Can lower disease pressure in subsequent crops, often translating to yield benefits and reduced need for chemical controls [95] | Requires availability of suitable alternative crops; may not be economically attractive if break crops have lower market value or if land is limited [115] |
| Improves overall soil health and microbial diversity, which can induce suppressiveness to pathogens over time [101,116] | Not a rapid solution—benefits accrue over multiple seasons, while some diseases might still occur at low levels, necessitating additional measures for full control [117] |
| Suited to organic and low-input systems as a cost-effective, non-chemical disease management strategy [102] | Limited applicability in perennial cropping systems and in regions with highly specialized monocultures; logistical and management challenges in diversifying crop rotations in intensive farming [118,119] |
| Can be combined with cover crops or biofumigant crops to enhance pathogen suppression (e.g., mustard cover crops release biocidal compounds) [120] | Does little to prevent airborne or polycyclic foliar diseases that re-infest from outside sources each season [101] |
| Advantages | Disadvantages/Limitations |
|---|---|
| Reduces disease incidence in many systems by diluting host presence and impeding pathogen spread (e.g., 73% of studies show lowered foliar disease in intercrops) [172] | Added management complexity—requires coordinating different crops’ agronomy (sowing, harvest, fertilization) and often complicates mechanized farming operations [185] |
| Can target multiple pest types simultaneously: certain intercrops suppress diseases, insect pests, and weeds at once, offering holistic protection [186,187] | Risk of competition between intercrop components for light, nutrients, or water, which can reduce the yield of the main crop if not properly managed [188] |
| Often highly effective against foliar fungal diseases due to altered microclimate and physical barrier effects—e.g., significantly lower rust and leaf spot severity in cereal-legume mixtures [172] | If both crops share a susceptibility to a pathogen, disease can spread on both (poor intercrop pairing can inadvertently increase total host area for a pathogen). Proper selection of complementary crops is essential [172] |
| Provides greater yield, stability and resilience: even if one crop is hit by disease, the other crop can still yield, buffering total production [175,189,190] | Adoption barriers in large-scale conventional farming—market logistics for multiple crops and lack of compatible machinery make farmers hesitant to adopt intercropping widely [191] |
| Low input cost strategy suitable for resource-poor farmers (no need for purchased inputs), and compatible with organic farming principle [179,192] | Requires knowledge and careful planning. Site-specific research and farmer training are needed to implement successful intercropping designs that truly confer disease protection without undue trade-offs [193] |
| Advantages | Disadvantages/Limitations |
|---|---|
| Can provide high efficacy disease control with little to no additional cost or effort during the season—the resistance is built into the plant (e.g., resistant varieties often suffer minimal yield loss under heavy disease pressure) [217,218] | Pathogen populations can evolve to overcome genetic resistance, especially single-gene (vertical) resistances, leading to potential breakdown of control within a few seasons if not managed [210,219,220,221] |
| Environmentally friendly and compatible with all farming systems (conventional and organic)—reduces the need for chemical fungicides and other inputs [101,218,222,223] | Breeding and delivering resistant varieties take time and resources. There can be a lag in availability for certain crops or emerging diseases, and minor crops may lack resistant options due to limited breeding programs [224,225,226] |
| Often the most cost-effective strategy: farmers typically pay the same (or only slightly more) for resistant seed, but avoid major losses—e.g., virus-resistant cassava and downy mildew-resistant millet dramatically improved yields for smallholders [227,228,229] | Resistance is usually disease-specific—a variety resistant to one pathogen may still require management of other diseases. No cultivar is immune to all threats, so farmers may need multiple resistant varieties or other controls for a full protection spectrum [230,231,232] |
| Can be combined (pyramided) to address multiple diseases in one cultivar (e.g., modern tomato hybrids with resistance to wilt, nematodes, virus, etc.), thereby simplifying disease management for growers [233,234,235] | Potential trade-offs: in some cases, resistance genes might be linked with less desirable agronomic traits, possibly affecting yield or quality. Farmers might be hesitant to adopt a new resistant variety if it differs in taste or market preferences from traditional ones [215,236,237,238] |
| Proven track record of preventing crop epidemics and stabilizing production—for example, rust-resistant wheat and coffee leaf rust-resistant coffee varieties have largely averted what would have been severe losses [239] | Over-reliance on resistance alone can be risky; if resistance breaks, growers can be left with little defense; best practices require integrating resistant cultivars with other strategies (rotation, crop diversification, etc.) to hedge against resistance breakdown [223,240,241,242] |
| Readily used in both high-input and low-input systems: high-input agriculture uses resistance to reduce spray programs, while low-input farmers rely on resistant landraces or improved varieties as a primary defense when chemicals are unaffordable [243,244,245] | Regulatory and acceptance issues for certain types of resistance—transgenic or gene-edited disease-resistant crops (e.g., virus-resistant papaya, late blight-resistant GM potato) could vastly improve control, but public concerns and regulations may restrict their deployment, delaying benefits to farmers (GMO debates) [246,247] |
| Advantages | Disadvantages/Limitations |
|---|---|
| Virtually eliminates many soil-borne disease problems by removing the crop from native soil (e.g., no build-up of wilt fungi or nematodes in a hydroponic system) [36,251,260,261] | High initial investment and technical expertise required—not economically feasible for low-value crops or many smallholders; primarily used in capital-intensive operations [250] |
| Allows continuous cropping without rotation or fallow, since clean substrate can be used each cycle; no need for soil fumigation or sterilization chemicals [257,262,263] | Pathogens can still enter via contaminated transplants or water and then spread rapidly in recirculating systems (e.g., Pythium root rot in hydroponics); demands rigorous sanitation and monitoring [264,265,266] |
| Highly controlled root environment—optimal nutrition and moisture can strengthen plant defenses and reduce stress-related disease susceptibility. Also easier to treat the root zone (e.g., sterilizing nutrient solution) if a pathogen is detected [264,267,268,269] | Does not inherently stop foliar diseases—greenhouse crops still face risks like powdery mildew or gray mold, requiring separate control measures. Dense plantings and stable environments can even favor some airborne pathogens if climate control falters [253,260,270,271]) |
| Substrates (rockwool, coir, etc.) are typically pathogen-free to start, and can be steam-sterilized or replaced, preventing carryover of inoculum between crops. This resets the disease cycle in a way not possible in field soil [150,249,260,272,273] | Waste management and sustainability concerns—spent substrate and nutrient runoff must be handled properly. Closed systems need careful management to avoid phytotoxic accumulation or algal growth, which can indirectly introduce pathogens [274,275,276,277] |
| Proven success in greenhouse vegetable and berry production: dramatic reductions in soil disease losses and consistent high yields (e.g., substrate-grown strawberries largely avoid soil wilt issues, enabling production in infested areas) [251,278] | Limited scope of use—mostly confined to high-value crops and controlled environments. Not a practical solution for broad-acre field crops or resource-limited farming communities without significant support and infrastructure [279,280,281] |
| Reduces reliance on chemical pesticides for soil pests, aligning with stricter environmental regulations (no need for methyl bromide, etc.), and can be combined with biological control in the nutrient solution to suppress any pathogen that appears (e.g., beneficial microbes in hydroponics) [253,254] | Some organic and consumer market barriers: hydroponic produce may not be accepted as “organic” under certain standards, and consumers might perceive soilless produce differently, which can influence growers’ decisions in niche markets [282,283,284,285] |
| Aspect | Advantages | Limitations |
|---|---|---|
| Environmental impact | Eco-friendly, reduces chemical pesticide use [303,304,305,306,307] | May be less effective in certain environmental conditions [308,309,310,311,312,313,314] |
| Specificity | Often targets specific pathogens, preserving beneficial microbiota [289,315,316,317,318] | Narrow spectrum may require multiple agents for broad protection [319,320] |
| Resistance management | Lower risk of resistance development compared to chemicals [321,322,323,324] | Pathogens may still develop tolerance over time [325,326,327,328] |
| Soil and plant health | Improves soil microbiome and plant growth (some PGPRs) [147,179,300,329,330,331,332,333] | May compete with native microbes, affecting performance [289,319,334,335] |
| Sustainability | Suitable for organic and integrated pest management (IPM) systems [320,336,337,338,339,340,341] | Requires more complex management and monitoring [317,320,342,343,344] |
| Residues | No harmful residues on crops [345,346,347,348] | Slower action may not be suitable for acute outbreaks [314,349,350,351] |
| Regulatory approval | Increasing support in regulations and organic standards [352] | Registration and commercialization can be lengthy and costly [298,353,354,355] |
| Production and storage | Can be mass-produced using fermentation technologies [356,357,358,359] | Shelf-life and formulation stability may be challenging [360,361,362,363] |
| Mode of action | Diverse mechanisms (competition, antibiosis, parasitism, induced resistance) [320,364] | Mechanisms not always fully understood; variable efficacy in the field [314,365,366,367,368] |
| Aspect | Advantages | Limitations |
|---|---|---|
| Soil health | Improves soil structure, fertility, and microbial activity [9,147,329,330,376,377,378] | Effects may vary, depending on soil type and amendment composition [147,329,330,379,380] |
| Microbial activity | Stimulates beneficial microbes that suppress pathogens [381,382,383,384,385] | May also induce heavy metal contamination and stimulate some pathogens if not properly managed [386,387,388] |
| Nutrient supply | Provides slow-release nutrients to plants [389,390,391] | Nutrient release is less predictable than synthetic fertilizers [392,393,394] |
| Disease suppression | Enhances natural suppressiveness (e.g., via microbial antagonists or competition) [373,395,396] | Inconsistent results, depending on quality, type, and application method [397,398] |
| Sustainability | Recycles organic waste; environmentally friendly [330,399,400] | Requires large quantities for field-scale use [394] |
| Residue-free | No harmful chemical residues on crops [401,402] | May take longer to see effects compared to synthetic treatments [379,396] |
| Compatibility with IPM | Suitable for organic farming and integrated pest management [403,404,405] | Not a stand-alone solution for severe disease outbreaks [382,397] |
| Cost | Low-cost and locally available materials can be used [147,380] | Transport, processing, and application can be labor-intensive and costly [379,394] |
| Pathogen inactivation | Composting can inactivate some soil-borne pathogens [406,407,408,409] | If not properly composted, may introduce new pathogens or weed seeds [410,411,412,413,414] |
| Control Strategy | Documented Effectiveness | References/Notes |
|---|---|---|
| Use of resistant cultivars | Up to 100% protection in certain crops (e.g., virus-resistant cassava, mildew-resistant millet) | Demonstrated high effectiveness and cost-efficiency, especially in smallholder systems |
| Crop rotation | Reduces soil-borne pathogens significantly, though context-dependent | Fusarium in bananas needs >5 years fallow to reduce pressure |
| Intercropping | Improves plant diversity, enhances microbial competition, can significantly inhibit pathogen dissemination | Effective but highly variable; depends on crop and pathogen combination |
| Biofumigation | Suppression rates > 60%; Indian mustard can inhibit up to 80–100% of potato pathogens in vitro | Black mustard reduced R. solani by 75% for up to 6 months |
| Organic amendments | Improves suppressiveness, soil fertility, microbial diversity; effectiveness variable (25–75%) | Compost, biochar, manure, etc. influence microbial balance and resilience |
| Biological control agents (BCAs) | Variable; some BCAs reduce disease severity by 30–80% under favorable conditions | B. subtilis, Trichoderma spp., Pseudomonas spp. affected by humidity/soil temp |
| Soil solarization/steaming | Up to 90–100% pathogen reduction if temperature maintained >45–50 °C for several days | Less effective in temperate climates without sustained heat |
| Hot water treatment (HWT) | Effective for seed/propagation material sterilization (e.g., eliminating Clavibacter, Ralstonia spp.) | Temperature/time must be optimized to avoid plant damage (e.g., 50 °C for 20–30 min) |
| Air circulation and humidity control | 30–40% reduction in fungal disease incidence in greenhouse settings with proper ventilation | e.g., 40% drop in Botrytis incidence in geraniums |
| Soilless culture | Removes soil-borne pathogen pressure almost entirely, if managed correctly | High initial cost; widely used in high-value horticulture |
| Integrated techniques (IEPM) | Highest resilience and suppression across multiple pathogens (no exact %, but synergistic benefit emphasized) | Particularly effective when combining resistance, rotation, and BCA |
| Strategy | Effectiveness (%) | References |
|---|---|---|
| Physical techniques (e.g., heat treatment) | 39 (post-harvest control)–70 | [459,460] |
| Biological control (e.g., Trichoderma spp.) | 50–80 | [461] |
| Pomegranate and carob extracts | 77 | [462] |
| Cultural and ecological management | 30–60 | [463] |
| Integrated eco-friendly components | 75 | [464] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucu, M.A.; Choudhary, R.; Trkulja, V.; Garg, S.; Matić, S. Utilizing Environmentally Friendly Techniques for the Sustainable Control of Plant Pathogens: A Review. Agronomy 2025, 15, 1551. https://doi.org/10.3390/agronomy15071551
Cucu MA, Choudhary R, Trkulja V, Garg S, Matić S. Utilizing Environmentally Friendly Techniques for the Sustainable Control of Plant Pathogens: A Review. Agronomy. 2025; 15(7):1551. https://doi.org/10.3390/agronomy15071551
Chicago/Turabian StyleCucu, Maria Alexandra, Ravish Choudhary, Vojislav Trkulja, Shivani Garg, and Slavica Matić. 2025. "Utilizing Environmentally Friendly Techniques for the Sustainable Control of Plant Pathogens: A Review" Agronomy 15, no. 7: 1551. https://doi.org/10.3390/agronomy15071551
APA StyleCucu, M. A., Choudhary, R., Trkulja, V., Garg, S., & Matić, S. (2025). Utilizing Environmentally Friendly Techniques for the Sustainable Control of Plant Pathogens: A Review. Agronomy, 15(7), 1551. https://doi.org/10.3390/agronomy15071551








