From Coconut Waste to Circular Plant Factories with Artificial Light: Renewable Substrate-Enhanced Crop Yield and Energy Efficiency
Abstract
1. Introduction
| Substrates | Origin | Advantages | Disadvantages |
|---|---|---|---|
| Rockwool | Melted silicates at 1500–2000 °C | Light volume weight, high total pore space, ease of handling, totally inert, nutrition can be carefully controlled. | Disposal problems, energy consumed during manufacture |
| Vermiculite | Mg, Al, and Fe silicate sieved and heated to 1000 °C | Light volume weight, high nutrient-holding ability, good water-holding ability, good pH buffering capacity, good aeration due to high pore space. | Compacts when too wet, energy-consuming product, expensive |
| Perlite | Siliceous volcanic mineral sieved and heated to 1000 °C | Light volume weight, sterile, neutral in pH (6.5–7.5), no decay, sufficient total pore space. | Energy-consuming product, expensive |
| Coconut coir | By-product of fiber coconut processing | Physical stability, light weight, good air content due to high total pore space and high water-holding capacity, sub-acid–neutral pH (5–6.8) | May contain high salt levels, energy consumption during transport |
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Treatments and Substrate Physical Properties
2.2. Measurement of Plant Morphology and Growth Characteristics
2.3. Measurement of Pigment Content
2.4. Measurement of Soluble Sugar
2.5. Measurement of Soluble Protein
2.6. Measurement of Nitrate Content
2.7. Measurement of Vitamin C
2.8. Measurement of Antioxidant Content and Antioxidant Activity
2.9. Measurement of Energy Consumption Indicators
2.10. Statistical Analysis
3. Results
3.1. Plant Morphology and Growth Characteristics
3.2. Substrate Physical Properties
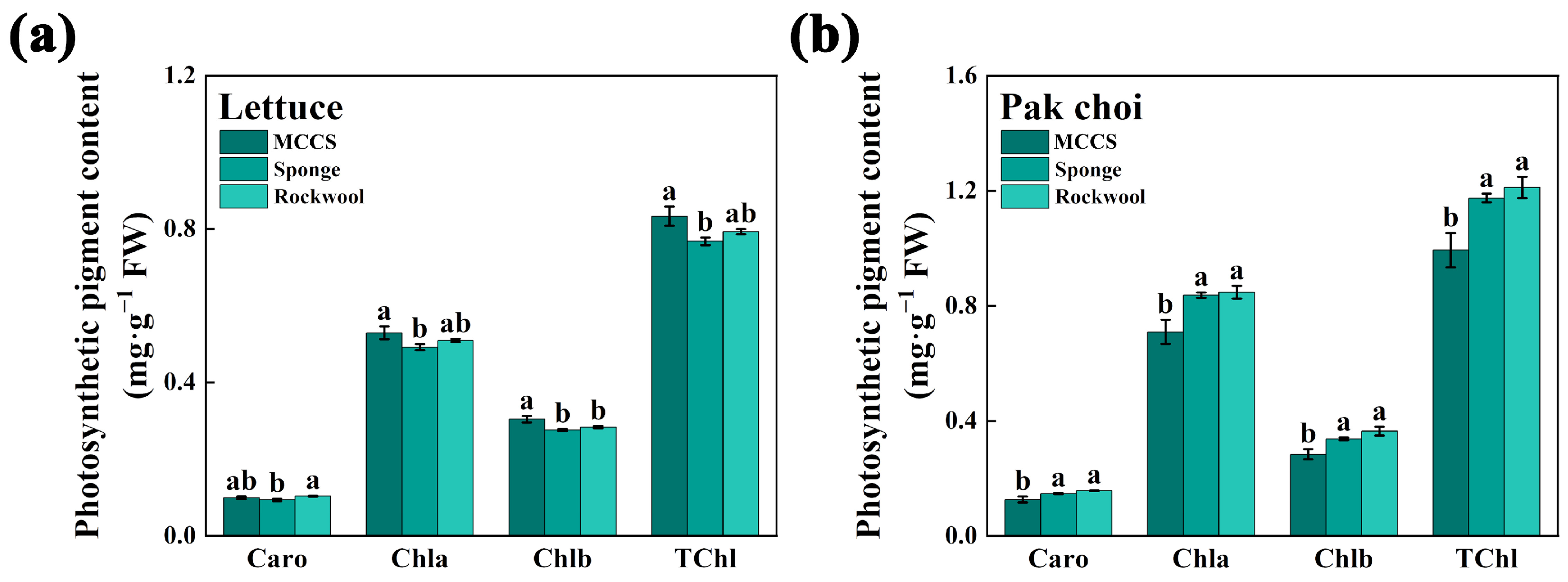
3.3. Photosynthetic Pigment Content
3.4. Contents of Soluble Sugars, Soluble Protein, Nitrates, and Vitamin C
3.5. Antioxidant Content and Antioxidant Activity
3.6. Energy Consumption Indicators
3.7. Multivariate Analysis of Variance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pradhan, P.; Callaghan, M.; Hu, Y.; Dahal, K.; Hunecke, C.; Reusswig, F.; Lotze-Campen, H.; Kropp, J.P. A Systematic Review Highlights That There Are Multiple Benefits of Urban Agriculture besides Food. Glob. Food Secur. 2023, 38, 100700. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Chen, X.; Wang, Y.; Niu, B.; Liu, D.L.; He, J.; Pulatov, B.; Hassan, I.; Meng, Q. Impact of Climate Change and Planting Date Shifts on Growth and Yields of Double Cropping Rice in Southeastern China in Future. Agric. Syst. 2023, 205, 103581. [Google Scholar] [CrossRef]
- Kozai, T. Plant Factories with Artificial Lighting (PFALs): Benefits, Problems, and Challenges. In Smart Plant Factory; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 15–29. ISBN 9789811310645. [Google Scholar]
- Kozai, T.; Niu, G.; Takagaki, M. (Eds.) Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press: San Diego, CA, USA, 2020; ISBN 978-0-12-816691-8. [Google Scholar]
- Song, S.; Ong, E.J.K.; Lee, A.M.J.; Chew, F.T. How Crop Breeding Programs Can Improve Plant Factories’ Business and Environmental Sustainability: Insights from a Farm Level Analysis. Sustain. Prod. Consum. 2024, 44, 298–311. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, H.-R.; Oh, S.; Yu, K.-H. Analysis on the Economic Feasibility of a Plant Factory Combined with Architectural Technology for Energy Performance Improvement. Agriculture 2022, 12, 684. [Google Scholar] [CrossRef]
- Kozai, T. (Ed.) Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; ISBN 9789811310645. [Google Scholar]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional Quality, Mineral and Antioxidant Content in Lettuce Affected by Interaction of Light Intensity and Nutrient Solution Concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Hu, Y.; He, R.; Ju, J.; Zhang, S.; He, X.; Li, Y.; Liu, X.; Liu, H. Effects of Substituting B with FR and UVA at Different Growth Stages on the Growth and Quality of Lettuce. Agronomy 2023, 13, 2547. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, S.; Hu, Y.; Zhang, M.; He, R.; Li, Y.; Liu, X.; Liu, H. Effects of Supplemental Red and Far-Red Light at Different Growth Stages on the Growth and Nutritional Properties of Lettuce. Agronomy 2024, 14, 55. [Google Scholar] [CrossRef]
- Dsouza, A.; Newman, L.; Graham, T.; Fraser, E.D.G. Exploring the Landscape of Controlled Environment Agriculture Research: A Systematic Scoping Review of Trends and Topics. Agric. Syst. 2023, 209, 103673. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Lidón, A. Analysis of Two Biochars and One Hydrochar from Different Feedstock: Focus Set on Environmental, Nutritional and Horticultural Considerations. J. Clean. Prod. 2015, 86, 40–48. [Google Scholar] [CrossRef]
- Patil, S.T.; Kadam, U.S.; Mane, M.S.; Mahale, D.M.; Dhekale, J.S. Hydroponic Growth Media (Substrate): A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 106–113. [Google Scholar] [CrossRef]
- Nerlich, A.; Karlowsky, S.; Schwarz, D.; Förster, N.; Dannehl, D. Soilless Tomato Production: Effects of Hemp Fiber and Rock Wool Growing Media on Yield, Secondary Metabolites, Substrate Characteristics and Greenhouse Gas Emissions. Horticulturae 2022, 8, 272. [Google Scholar] [CrossRef]
- Qaryouti, M.; Osman, M.; Alharbi, A.; Voogt, W.; Abdelaziz, M.E. Using Date Palm Waste as an Alternative for Rockwool: Sweet Pepper Performance under Both Soilless Culture Substrates. Plants 2023, 13, 44. [Google Scholar] [CrossRef]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. Soilless Culture: Theory and Practice, 2nd ed.; Academic Press: London, UK, 2019; ISBN 978-0-444-63696-6. [Google Scholar]
- Vinci, G.; Rapa, M. Hydroponic Cultivation: Life Cycle Assessment of Substrate Choice. Br. Food J. 2019, 121, 1801–1812. [Google Scholar] [CrossRef]
- Jia, X.; Ma, P.; Wei, C.-I.; Wang, Q. Chitin and Chitosan: Pioneering Sustainable Substrates for next-Generation Soilless Vertical Farming. Trends Food Sci. Technol. 2024, 150, 104599. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Z.; Luo, Y. Advances and Emerging Trends in Cultivation Substrates for Growing Sprouts and Microgreens toward Safe and Sustainable Agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Both, A.K.; Choudhry, D.; Cheung, C.L. Eco-Friendly Fabrication of Coco Coir Composites for Hydroponic Cultivation: A Green Chemistry Approach. New J. Chem. 2023, 47, 5488–5497. [Google Scholar] [CrossRef]
- Both, A.K.; Linderman, J.A.; Madireddy, G.; Helle, M.A.; Cheung, C.L. Valorization of Coco Coir into Biocomposite Materials through Water-Based Chemistry. Ind. Crops Prod. 2022, 178, 114563. [Google Scholar] [CrossRef]
- Tuckeldoe, R.B.; Maluleke, M.K.; Adriaanse, P. The Effect of Coconut Coir Substrate on the Yield and Nutritional Quality of Sweet Peppers (Capsicum annuum) Varieties. Sci. Rep. 2023, 13, 2742. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Microgreen Vegetables’ Production Can Be Optimized by Combining the Substrate and Nutrient Solution in a PFAL. Sci. Hortic. 2024, 333, 113277. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Terrones, L.A.H.; D’Almeida, J.R.M. Mechanical Performance of Coir Fiber/Polyester Composites. Polym. Test. 2008, 27, 591–595. [Google Scholar] [CrossRef]
- FAO. Coconut—Tree of Life. 2020. Available online: http://www.fao.org/3/y3612e/y3612e03.htm (accessed on 1 February 2025).
- Teh, C.B.S.; Talib, J. Soil and Plant Analyses. In Soil Physics Analyses; Universiti Putra Malaysia Press: Selangor, Malaysia, 2006; Volume 1. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kohyama, K.; Nishinari, K. Effect of Soluble Sugars on Gelatinization and Retrogradation of Sweet Potato Starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of Antioxidant Activity and Antioxidant Compounds of Some Edible Plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal Dynamics of Total Flavonoid Contents and Antioxidant Activity of Dryopteris Erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol Inhibition of Lipid Peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Gruda, N. Application of Soilless Culture Technologies in the Modern Greenhouse Industry—A Review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- He, X.; He, R.; Li, Y.; Liu, K.; Tan, J.; Chen, Y.; Liu, X.; Liu, H. Effect of Ratios of Red and White Light on the Growth and Quality of Pak Choi. Agronomy 2022, 12, 2322. [Google Scholar] [CrossRef]
- Liu, K.; He, R.; He, X.; Tan, J.; Chen, Y.; Li, Y.; Liu, R.; Huang, Y.; Liu, H. Speed Breeding Scheme of Hot Pepper through Light Environment Modification. Sustainability 2022, 14, 12225. [Google Scholar] [CrossRef]
- He, L.; Ding, X.; Jin, H.; Zhang, H.; Cui, J.; Chu, J.; Li, R.; Zhou, Q.; Yu, J. Comparison of Rockwool and Coir for Greenhouse Cucumber Production: Chemical Element, Plant Growth, and Fruit Quality. Heliyon 2022, 8, e10930. [Google Scholar] [CrossRef]
- Gruda, N. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Zhao, R.; Sofkova-Bobcheva, S.; Cartmill, D.L.; Hardy, D.; Zernack, A. Comparative Evaluation of Pumice as a Soilless Substrate for Indoor Rubus idaeus L. Cultivation. N. Z. J. Crop Hortic. Sci. 2024, 52, 280–297. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L. Addition of Coco Coir and Rice Hull Ash Improves the Quality of Seedling Substrate Based on Green Waste Compost for Cucurbitaceae Vegetable Seedlings. J. Mater. Cycles Waste Manag. 2024, 26, 562–577. [Google Scholar] [CrossRef]
- Martins, T.; Machado, R.; Alves-Pereira, I.; Ferreira, R.; Gruda, N. Coir-Based Growing Media with Municipal Compost and Biochar and Their Impacts on Growth and Some Quality Parameters in Lettuce Seedlings. Horticulturae 2023, 9, 105. [Google Scholar] [CrossRef]
- Adak, N.; Tozlu, I.; Gubbuk, H. Influence of Different Soilless Substrates to Morpho-Physiological Characteristics and Yield Relations in Strawberries. Erwerbs-Obstbau 2018, 60, 341–348. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Lorente, B.; Sánchez-Blanco, M.J.; Ortuño, M.F.; Nortes, P.A.; Alarcón, J.J. Influence of Mixed Substrate and Arbuscular Mycorrhizal Fungi on Photosynthetic Efficiency, Nutrient and Water Status and Yield in Tomato Plants Irrigated with Saline Reclaimed Waters. Water 2020, 12, 438. [Google Scholar] [CrossRef]
- Sarkar, M.D.; Rahman, M.J.; Uddain, J.; Quamruzzaman, M.; Azad, M.O.K.; Rahman, M.H.; Islam, M.J.; Rahman, M.S.; Choi, K.-Y.; Naznin, M.T. Estimation of Yield, Photosynthetic Rate, Biochemical, and Nutritional Content of Red Leaf Lettuce (Lactuca sativa L.) Grown in Organic Substrates. Plants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Aali, N.; Alemzadeh Ansari, N.; Zahedi, S.M. Development of Sustainable Strawberry Production in Closed Cultivation Systems: Effects of Bagasse Biochar on Morphological and Physiological Attributes, Yield and Autotoxic Changes. J. Environ. Manage. 2024, 371, 123100. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.A.; Ilari, A.; Olivi, L.; Zucchi, P.; Osti, L.; Mezzetti, B.; Duca, D. Environmental Sustainability and Quality Assessment of New Raspberry Genotypes Cultivated in a Soilless System. Sustain. Prod. Consum. 2025, 54, 502–515. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Ju, J.; Hu, Y.; Liu, X.; He, R.; Song, J.; Huang, Y.; Liu, H. The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory. Agronomy 2024, 15, 70. [Google Scholar] [CrossRef]
- Ruett, J.; Abdelshafy, A.; Walther, G. Using Miscanthus and Biochar as Sustainable Substrates in Horticulture: An Economic and Carbon Footprint Assessment of Their Primary and Cascading Value Chains. Sustain. Prod. Consum. 2024, 49, 163–178. [Google Scholar] [CrossRef]







| Substrates | TPS (%) | AV (%) | WHC (%) | BD (g·cm−3) | EC (μS·cm−1) | pH |
|---|---|---|---|---|---|---|
| MCCS | 44.71 ± 0.61 c | 35.09 ± 0.89 c | 9.62 ± 0.56 b | 0.1344 ± 0.0096 a | 170.50 ± 1.36 a | 6.30 ± 0.03 b |
| Sponge | 79.26 ± 0.91 a | 75.38 ± 0.91 a | 3.88 ± 0.24 c | 0.0229 ± 0.0003 c | 8.16 ± 0.05 c | 7.92 ± 0.08 a |
| Rockwool | 62.53 ± 0.97 b | 46.71 ± 0.85 b | 15.82 ± 0.61 a | 0.0548 ± 0.0030 b | 19.59 ± 0.10 b | 7.93 ± 0.03 a |
| Crop Species | Substrates | PAR (W·m−2) | DLI (mol·m−2·d−1) | Eunit (Wh·g−1·m−2) |
|---|---|---|---|---|
| Lettuce | MCCS | 0.058 | 9.0 | 0.2665 ± 0.0099 c |
| Sponge | 0.058 | 9.0 | 0.3088 ± 0.0162 b | |
| Rockwool | 0.058 | 9.0 | 0.3461 ± 0.0087 a | |
| Pak choi | MCCS | 0.058 | 9.0 | 0.2312 ± 0.0148 b |
| Sponge | 0.058 | 9.0 | 0.3913 ± 0.0298 a | |
| Rockwool | 0.058 | 9.0 | 0.2520 ± 0.0076 b |
| Source of Variance | SFW | RFW | SDW | RDW | RL | LA | LN | CW | Chla | Chlb | Caro | TChl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crop species (C) | ** | NS | *** | * | *** | *** | *** | *** | *** | *** | *** | *** |
| Substrates (S) | *** | ** | NS | ** | *** | ** | ** | *** | * | * | ** | * |
| C*S | * | NS | NS | NS | NS | NS | ** | * | ** | *** | * | ** |
| Source of Variance | SSs | SP | Nitrates | VC | TP | TF | FRAP | DPPH |
|---|---|---|---|---|---|---|---|---|
| Crop species(C) | *** | *** | *** | *** | *** | NS | *** | *** |
| Substrates (S) | NS | *** | NS | *** | NS | ** | NS | NS |
| Organs (O) | * | *** | *** | *** | *** | *** | *** | *** |
| C*S | *** | ** | NS | *** | NS | *** | * | *** |
| S*O | * | *** | * | *** | NS | *** | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.; Zhang, Y.; Yu, Y.; Zhang, M.; Hu, Y.; Liu, X.; Yang, X.; Song, J.; Liu, H. From Coconut Waste to Circular Plant Factories with Artificial Light: Renewable Substrate-Enhanced Crop Yield and Energy Efficiency. Agronomy 2025, 15, 1929. https://doi.org/10.3390/agronomy15081929
Ju J, Zhang Y, Yu Y, Zhang M, Hu Y, Liu X, Yang X, Song J, Liu H. From Coconut Waste to Circular Plant Factories with Artificial Light: Renewable Substrate-Enhanced Crop Yield and Energy Efficiency. Agronomy. 2025; 15(8):1929. https://doi.org/10.3390/agronomy15081929
Chicago/Turabian StyleJu, Jun, Yingjun Zhang, Yangyue Yu, Minggui Zhang, Youzhi Hu, Xiaojuan Liu, Xiaolong Yang, Jiali Song, and Houcheng Liu. 2025. "From Coconut Waste to Circular Plant Factories with Artificial Light: Renewable Substrate-Enhanced Crop Yield and Energy Efficiency" Agronomy 15, no. 8: 1929. https://doi.org/10.3390/agronomy15081929
APA StyleJu, J., Zhang, Y., Yu, Y., Zhang, M., Hu, Y., Liu, X., Yang, X., Song, J., & Liu, H. (2025). From Coconut Waste to Circular Plant Factories with Artificial Light: Renewable Substrate-Enhanced Crop Yield and Energy Efficiency. Agronomy, 15(8), 1929. https://doi.org/10.3390/agronomy15081929








