Species-Specific Chemotactic Responses of Entomopathogenic and Slug-Parasitic Nematodes to Cannabinoids from Cannabis sativa L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Maintenance of Parasitic Nematodes
2.2. Tested Cannabinoids
2.3. Chemotaxis Assay
2.4. Statistical Analysis
3. Results
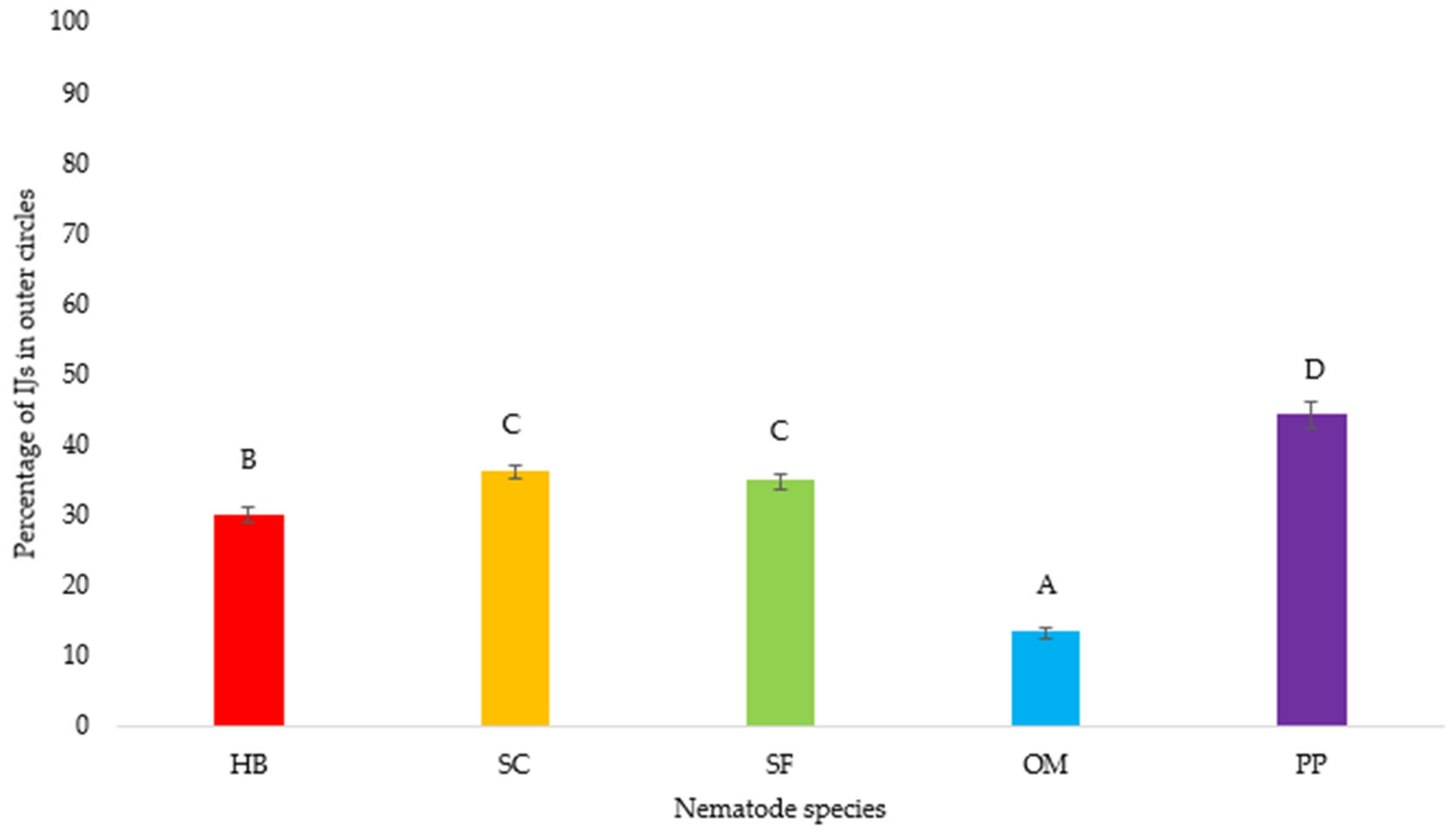
3.1. Nematode Motility
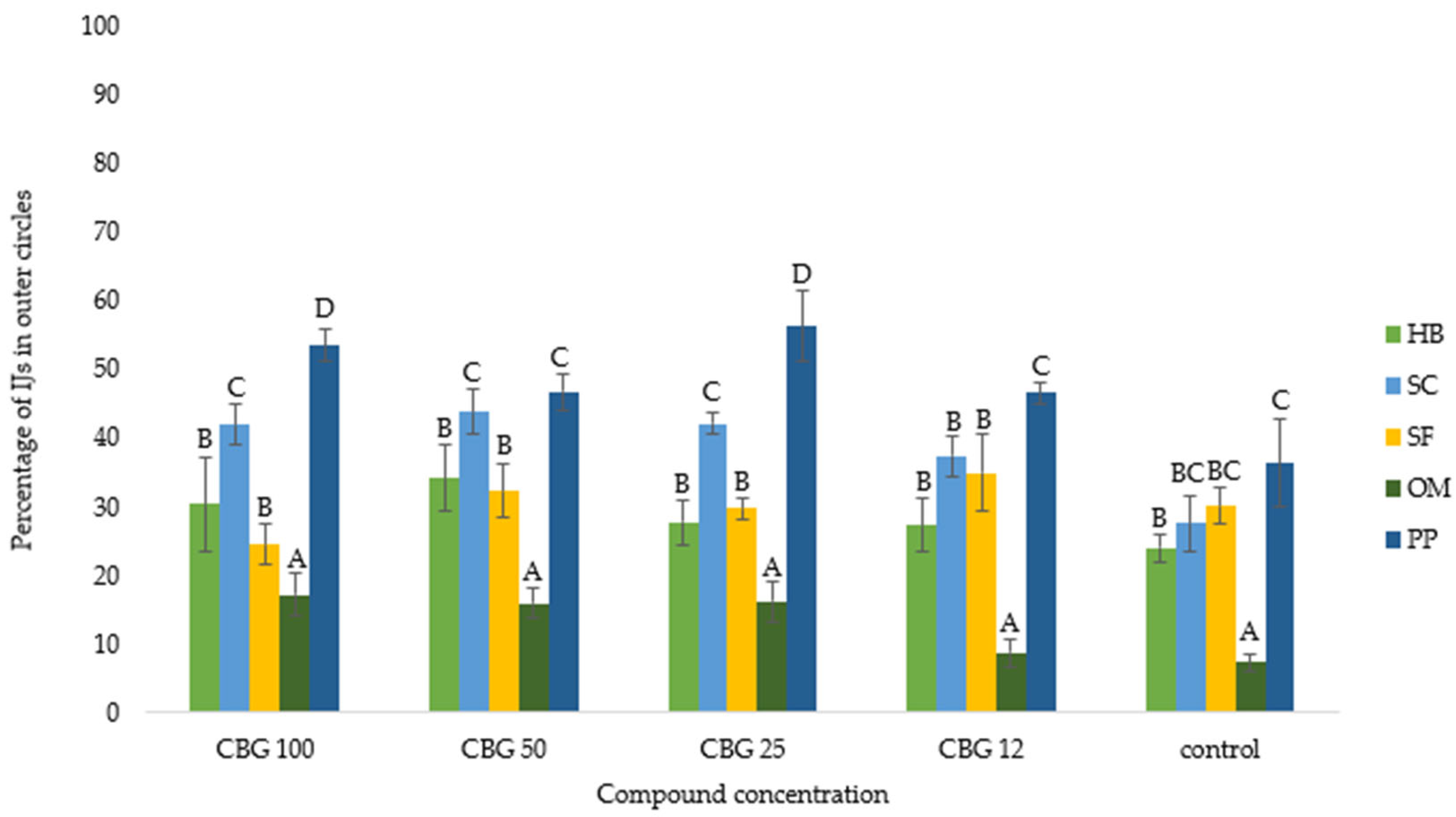
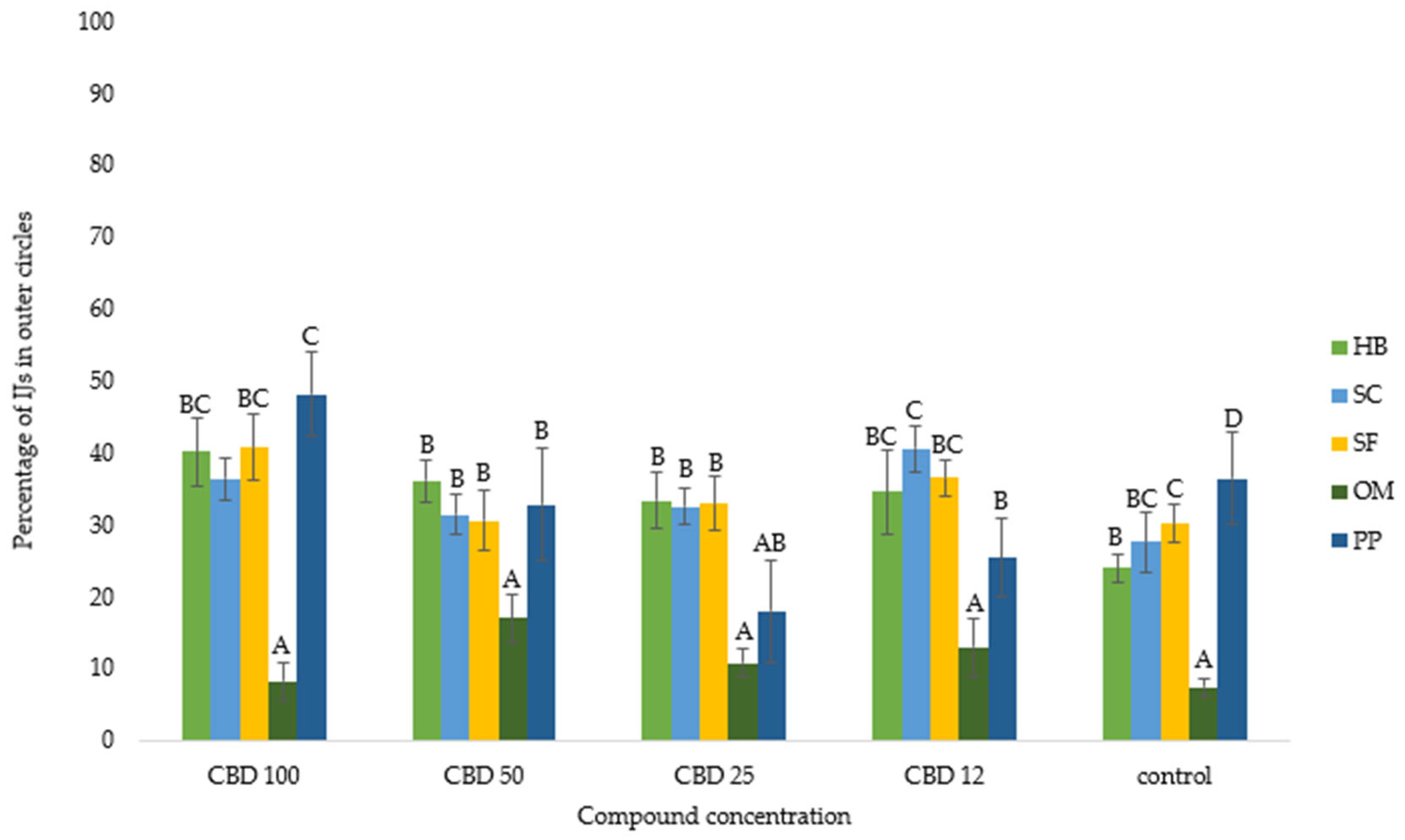
3.2. Chemotaxis Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes, Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Hayo, M.G.; van der Werf, H.M.G. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- FAOSTAT. Pesticides Use. 2024. Available online: http://www.fao.org/faostat/en/#data (accessed on 5 April 2025).
- Rao, M.S.; Umamaheswari, R.; Priti, K.; Rajinikanth, R.; Grace, G.N.; Kamalnath, M.; Prabu, P.; Kumar, R.M.; Chaya, M.K. Role of biopesticides in the management of nematodes and associated diseases in horticultural crops. In Plant, Soil and Microbes, Volume 1: Implications in Crop Science; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 117–148. [Google Scholar] [CrossRef]
- Mukhtar, T.; Kayani, M.Z.; Hussain, M.A. Nematicidal activities of Cannabis sativa L. and Zanthoxylum alatum Roxb. against Meloidogyne incognita. Ind. Crops Prod. 2013, 42, 447–453. [Google Scholar] [CrossRef]
- Pavela, R.; Canale, A.; Mehlhorn, H.; Benelli, G. Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: A review. Res. Vet. Sci. 2016, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Sheikh, Z. A review of Cannabis sativa-based insecticides, miticides, and repellents. J. Entomol. Zool. Stud. 2018, 6, 1288–1299. [Google Scholar]
- Górski, R.; Sobieralski, K.; Siwulski, M. The effect of hemp essential oil on mortality of Aulacorthum solani Kalt. and Tetranychus urticae Koch. Ecol. Chem. Eng. S 2016, 23, 505–511. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef]
- Regulation (EU) 2021/2115 of the European Parliament and of the Council of 2 December 2021 establishing rules on support for strategic plans to be drawn up by Member States under the common agricultural policy (CAP Strategic Plans) and financed by the European Agricultural Guarantee Fund (EAGF) and by the European Agricultural Fund for Rural Development (EAFRD) and repealing Regulations (EU) No 1305/2013 and (EU) No 1307/2013. Available online: https://eur-lex.europa.eu/eli/reg/2021/2115/oj/eng (accessed on 10 April 2025).
- Janatova, A.; Frankova, A.; Tlustoš, P.; Hamouz, K.; Božik, M.; Klouček, P. Yield and cannabinoid contents in different cannabis (Cannabis sativa L.) genotypes for medical use. Ind. Crops Prod. 2018, 112, 363–367. [Google Scholar] [CrossRef]
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic Nematodes. Curr. Biol. 2012, 22, R430–R431. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 2010, 36, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the intraguild competition between Oscheius spp. nematodes and entomopathogenic nematodes: Implications for their natural distribution in Swiss agricultural soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Trdan, S.; Tóth, T.; Ádám, S.; Lakatos, T.; Majić, I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy 2023, 13, 1386. [Google Scholar] [CrossRef]
- Wilson, M.J.; Rae, R. Phasmarhabditis hermaphrodita as a Control Agent for Slugs. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 509–521. [Google Scholar]
- Lewis, E.E. Behavioural Ecology. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 205–223. [Google Scholar]
- Campbell, J.F.; Lewis, E.E.; Stock, S.P.; Nadler, S.; Kaya, H.K. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 2003, 35, 142–145. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Ádám, S.; Trdan, S.; Majić, I.; Lakatos, T. Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots. Agronomy 2025, 15, 664. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S.; Yonesi, M. Chemotactic Responses of Slug-Parasitic Nematodes to Potato-Tuber-Emitted Volatile Organic Compounds. Agronomy 2025, 15, 951. [Google Scholar] [CrossRef]
- Laznik, Ž.; Košir, I.J.; Košmelj, K.; Murovec, J.; Jagodič, A.; Trdan, S.; Kocjan Ačko, D.; Flajšman, M. Effect of Cannabis sativa L. root, leaf and inflorescence ethanol extracts on the chemotrophic response of entomopathogenic nematodes. Plant Soil 2020, 455, 367–379. [Google Scholar] [CrossRef]
- O’Halloran, D.M.; Burnell, A.M. An Investigation of Chemotaxis in the Insect Parasitic Nematode Heterorhabditis bacteriophora. Parasitology 2003, 127, 375–385. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory Neurons with Overlapping Functions Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef]
- Hiltpold, I.; Hibbard, B.E.; French, B.W.; Turlings, T.C.J. Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant Soil 2012, 358, 11–25. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]


| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 39,465.0 | 4 | 137.6 | 0.0001 |
| Cannabinoids (C) | 1702.1 | 2 | 11.9 | 0.0001 |
| Cannabinoid concentration (Cc) | 5157.1 | 4 | 18.0 | 0.0001 |
| Temporal replication | 933.1 | 9 | 1.4 | 0.1627 |
| Spatial replication | 210.0 | 2 | 1.5 | 0.2330 |
| S × C | 6057.9 | 8 | 10.6 | 0.0001 |
| S × Cc | 1166.1 | 16 | 1.0 | 0.4374 |
| C × Cc | 1262.7 | 8 | 2.2 | 0.0280 |
| S × C × Cc | 6014.1 | 32 | 2.6 | 0.0001 |
| Residual | 21,220.5 | 296 | ||
| Total (Corrected) | 83,188.6 | 381 |
| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 0.76 | 4 | 38.78 | 0.0001 |
| Cannabinoids (C) | 2.10 | 2 | 214.29 | 0.0001 |
| Cannabinoid concentration (Cc) | 0.27 | 4 | 13.78 | 0.0001 |
| Temporal replication | 0.03 | 9 | 0.67 | 0.7350 |
| Spatial replication | 0.02 | 2 | 2.04 | 0.1310 |
| S × C | 1.25 | 8 | 31.88 | 0.0001 |
| S × Cc | 0.37 | 16 | 4.71 | 0.0001 |
| C × Cc | 0.64 | 8 | 16.33 | 0.0001 |
| S × C × Cc | 0.71 | 32 | 4.53 | 0.0001 |
| Residual | 1.45 | 296 | ||
| Total (Corrected) | 7.58 | 381 |
| THC 100% | THC 50% | THC 25% | THC 12% | Control | |
|---|---|---|---|---|---|
| HB | 0.13 ± 0.04 Bb | 0.16 ± 0.05 Bb | 0.12 ± 0.04 Bb | 0.10 ± 0.03 BCb | −0.01 ± 0.03 ABa |
| OM | 0.01 ± 0.01 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa |
| PP | 0.09 ± 0.01 Bb | 0.38 ± 0.04 Cd | 0.15 ± 0.02 Bc | 0.13 ± 0.01 Cc | 0.00 ± 0.03 ABa |
| SC | 0.24 ± 0.05 Cd | 0.15 ± 0.02 Bc | 0.12 ± 0.02 Bbc | 0.07 ± 0.03 Bb | 0.01 ± 0.02 ABa |
| SF | 0.26 ± 0.05 Cc | 0.15 ± 0.02 Bb | 0.13 ± 0.02 Bb | 0.13 ± 0.04 Cab | 0.05 ± 0.04 Ba |
| CBG 100% | CBG 50% | CBG 25% | CBG 12% | Control | |
| HB | 0.20 ± 0.05 Db | 0.17 ± 0.03 Cb | 0.04 ± 0.05 ABa | −0.03 ± 0.05 Aa | −0.01 ± 0.03 ABa |
| OM | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa | −0.01 ± 0.03 Aa | 0.00 ± 0.00 Aa |
| PP | 0.11 ± 0.03 Cb | 0.09 ± 0.02 Bb | 0.18 ± 0.03 Cc | 0.19 ± 0.03 Cc | 0.00 ± 0.03 ABa |
| SC | 0.05 ± 0.04 Bb | 0.04 ± 0.06 ABab | 0.13 ± 0.04 BCb | −0.06 ± 0.06 Aa | 0.01 ± 0.02 ABab |
| SF | 0.12 ± 0.03 CDab | 0.15 ± 0.04 BCb | 0.14 ± 0.03 Cb | 0.13 ± 0.01 Bb | 0.05 ± 0.04 Ba |
| CBD 100% | CBD 50% | CBD 25% | CBD 12% | Control | |
| HB | −0.14 ± 0.04 Ba | −0.12 ± 0.03 Aa | −0.17 ± 0.04 Ba | −0.16 ± 0.02 Ba | −0.01 ± 0.03 ABb |
| OM | 0.01 ± 0.01 Ca | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Da | −0.02 ± 0.02 Da | 0.00 ± 0.00 Aa |
| PP | 0.16 ± 0.05 Dc | 0.16 ± 0.02 Cc | 0.06 ± 0.01 Cb | 0.06 ± 0.03 Cb | 0.00 ± 0.03 ABa |
| SC | −0.11 ± 0.01 Bc | −0.15 ± 0.02 Ab | −0.22 ± 0.03 ABa | −0.27 ± 0.02 Aa | 0.01 ± 0.02 ABd |
| SF | −0.24 ± 0.04 Aa | −0.16 ± 0.03 Ab | −0.28 ± 0.05 Aa | −0.30 ± 0.02 Aa | 0.05 ± 0.04 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flajšman, M.; Trdan, S.; Laznik, Ž. Species-Specific Chemotactic Responses of Entomopathogenic and Slug-Parasitic Nematodes to Cannabinoids from Cannabis sativa L. Agronomy 2025, 15, 1469. https://doi.org/10.3390/agronomy15061469
Flajšman M, Trdan S, Laznik Ž. Species-Specific Chemotactic Responses of Entomopathogenic and Slug-Parasitic Nematodes to Cannabinoids from Cannabis sativa L. Agronomy. 2025; 15(6):1469. https://doi.org/10.3390/agronomy15061469
Chicago/Turabian StyleFlajšman, Marko, Stanislav Trdan, and Žiga Laznik. 2025. "Species-Specific Chemotactic Responses of Entomopathogenic and Slug-Parasitic Nematodes to Cannabinoids from Cannabis sativa L." Agronomy 15, no. 6: 1469. https://doi.org/10.3390/agronomy15061469
APA StyleFlajšman, M., Trdan, S., & Laznik, Ž. (2025). Species-Specific Chemotactic Responses of Entomopathogenic and Slug-Parasitic Nematodes to Cannabinoids from Cannabis sativa L. Agronomy, 15(6), 1469. https://doi.org/10.3390/agronomy15061469








