Repair Effects of Scenedesmus obliquus on Cucumber Seedlings Under Saline–Alkali Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Experimental Methods
3. Results
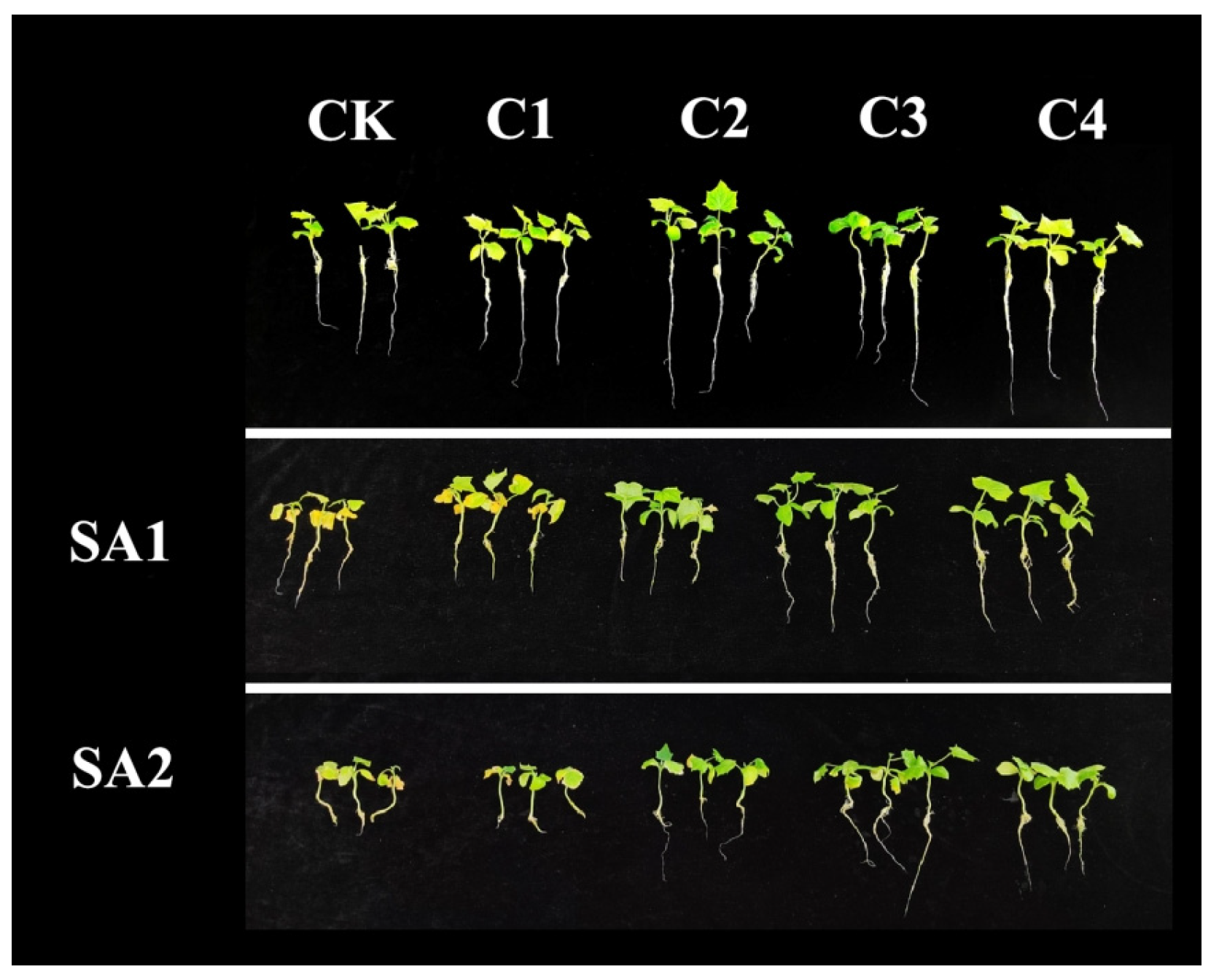
3.1. Effect of Scenedesmus obliquus Addition on Plant Height and Fresh Weight of Cucumber Seedlings Under Saline–Alkali Stress
3.2. Effects of Scenedesmus obliquus on Antioxidant Enzyme Activities in Cucumber Seedlings Under Saline–Alkali Stress
3.3. Effects of Scenedesmus obliquus on MDA Content in Cucumber Seedlings Under Saline–Alkali Stress
3.4. Effects of Scenedesmus obliquus on Photosynthetic Pigment Content in Cucumber Seedlings Under Saline–Alkali Stress
3.5. Effects of Scenedesmus obliquus on Microstructure of Cucumber Seedlings Under Saline–Alkali Stress
3.5.1. Effects of Scenedesmus obliquus on Microstructure of Cucumber Seedling Leaves Under Saline–Alkali Stress
3.5.2. Effects of Scenedesmus obliquus on the Microstructure of Cucumber Seedling Stem Under Saline–Alkali Stress
3.5.3. Effects of Scenedesmus obliquus on the Microstructure of Cucumber Seedling Roots Under Saline–Alkali Stress
3.6. Effects of Scenedesmus obliquus on the Ultrastructure of Cucumber Seedlings Under Saline–Alkali Stress
3.6.1. Effects of Scenedesmus obliquus on the Ultrastructure of Mesophyll Cells in Cucumber Seedlings Under Saline–Alkali Stress
3.6.2. Effects of Scenedesmus obliquus on the Ultrastructure of Cucumber Seedling Stems Under Saline–Alkali Stress
3.6.3. Effects of Scenedesmus obliquus on the Ultrastructure of Cucumber Seedling Roots Under Saline–Alkali Stress
4. Discussion
4.1. Effects of Scenedesmus obliquus on the Growth and Development of Cucumber Seedlings Under Saline–Alkali Stress
4.2. Effects of Scenedesmus obliquus on Physiological Indices of Cucumber Seedlings Under Saline–Alkali Stress
4.3. Effects of Scenedesmus obliquus on Microstructure and Ultrastructure of Cucumber Seedling Roots and Stems Under Saline–Alkali Stress
4.4. Effects of Scenedesmus obliquus on Microstructure, Ultrastructure, and Physiology of Cucumber Seedling Leaves Under Saline–Alkali Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Yu, J.; Hou, D.; Yue, H.; Zhang, D.; Li, Y.; Lyu, J.; Jin, L.; Jin, N. Response of soil microbial community diversity to continuous cucumber cropping in facilities along the Yellow River irrigation area. PLoS ONE 2023, 18, e0289772. [Google Scholar] [CrossRef]
- Zhao, W.; Ban, Y.; Su, Z.; Li, S.; Liu, X.; Guo, Q.; Ma, P. Colonization ability of Bacillus subtilis NCD-2 in different crops and its effect on rhizosphere microorganisms. Microorganisms 2023, 11, 776. [Google Scholar] [CrossRef]
- Li, C.; Zeng, Q.; Han, Y.; Zhou, X.; Xu, H. Effects of Bacillus subtilis on cucumber seedling growth and photosynthetic system under different potassium ion levels. Biology 2024, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Urón, P.; Glick, B.R.; Giachini, A.; Rossi, M.J. Genomic analysis of the 1-aminocyclopropane-1-carboxylate deaminase-producing Pseudomonas thivervalensis SC5 reveals its multifaceted roles in soil and in beneficial interactions with plants. Front. Microbiol. 2021, 12, 752288. [Google Scholar] [CrossRef]
- Liu, D.; Dong, S.; Bo, K.; Miao, H.; Li, C.; Zhang, Y.; Zhang, S.; Gu, X. Identification of QTLs controlling salt tolerance in cucumber (Cucumis sativus L.) seedlings. Plants 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Yang, X.X.; Zhang, L.; Zhang, J.; Du, B.; Yao, L.; Li, X.T.; Guo, C. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep. 2020, 39, 997–1011. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of research on the physiology and molecular regulation of sorghum growth under salt stress by gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Du, Y.; Feng, N.; Zheng, D.; Zhou, H.; Huo, J. Exogenous Uniconazole promotes physiological metabolism and grain yield of rice under salt stress. Front. Plant Sci. 2024, 15, 1459121. [Google Scholar] [CrossRef]
- Othman, Y.A.; Hani, M.B.; Ayad, J.Y.; St Hilaire, R. Salinity level influenced morpho-physiology and nutrient uptake of young citrus rootstocks. Heliyon 2023, 9, e13336. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, J.; Peng, Y.; Tan, Z.; Li, L.; Yu, L.; Jin, C.; Fang, S.; Lu, S.; Guo, L.; et al. Genome-wide association studies of salt tolerance at seed germination and seedling stages in Brassica napus. Front. Plant Sci. 2022, 12, 772708. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yu, L.; Gu, Y.; Zhang, L.; Wang, J.; Qiu, L. Overexpression of the Purple Perilla (Perilla frutescens (L.)) FAD3a gene enhances salt tolerance in soybean. Int. J. Mol. Sci. 2023, 24, 10533. [Google Scholar] [CrossRef]
- Liu, H.; Chong, P.; Liu, Z.; Bao, X.; Tan, B. Exogenous hydrogen sulfide improves salt stress tolerance of Reaumuria soongorica seedlings by regulating active oxygen metabolism. PeerJ 2023, 11, e15881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.; Liu, H.; Han, H.; Zhuang, H.; Wang, J.; Yang, T.; Wang, H.; Qin, Y. Comparative proteomic analysis for revealing the advantage mechanisms of salt-tolerant tomato (Solanum lycoperscium). PeerJ 2022, 10, e12955. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, F.; Ma, Y.; Dang, H.; Hu, X. Transcription factor SlAREB1 is involved in the antioxidant regulation under saline–alkaline stress in tomato. Antioxidants 2022, 11, 1673. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, C.; Li, W.; Hu, L.; Fu, X.; Hu, B.; Liao, Y.; Xiang, Z.; Jiang, H.; Huang, W.; et al. OsCSLD4 confers salt-alkali tolerance by regulating gene expressions in photosynthesis and carbohydrate biosynthesis pathways, cell wall hemicellulose accumulation and physio-biochemical adaptability in rice. Plant Stress 2024, 14, 100604. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Shao, Q.; Wang, Q.; Wang, S.; Yu, R.; Dong, S.; Xin, Z.; Xiao, H.; Cheng, J. Photosynthetic Performance and Heterogeneous Anatomical Structure in Prunus humilis under Saline–Alkaline Stress. Agriculture 2024, 14, 1606. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, C. Intraspecific differences in plant functional traits are related to urban atmospheric particulate matter. BMC Plant Biol. 2021, 21, 430. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Mostofa, M.G. Mechanistic insight into salt tolerance of Acacia auriculiformis: The importance of ion selectivity, osmoprotection, tissue tolerance, and Na+ exclusion. Front. Plant Sci. 2017, 8, 155. [Google Scholar] [CrossRef]
- Yeo, A.R.; Kramer, D.; Liuchli, A.; Gullasch, J. Ion distribution in salt-stressed mature Zea mays roots in relation to ultrastructure and retention of sodium. J. Exp. Bot. 1977, 28, 17–29. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Z.; Yin, L.; Chen, S.; Cai, Z.; Geng, X.; Wang, D. Physiological basis and differentially expressed genes in the salt tolerance mechanism of Thalassia hemprichii. Front. Plant Sci. 2022, 13, 975251. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Fan, Y.; Zhou, H.; Pu, X. Genome-wide identification and expression analysis of the TCP transcription factor family and its response to abiotic stress in rapeseed (Brassica napus L.). 3 Biotech 2025, 15, 119. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Wang, Y. Effects of salt stress on salt-repellent and salt-secreting characteristics of two apple rootstocks. Plants 2024, 13, 1046. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ranathunge, K.; Nayak, S.; Schreiber, L.; Mathew, M.K. Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 4215–4228. [Google Scholar] [CrossRef]
- Mitsuya, S.; Takeoka, Y.; Miyake, H. Effects of sodium chloride on foliar ultrastructure of sweet potato (Ipomoea batatas Lam.) plantlets grown under light and dark conditions In Vitro. J. Plant Physiol. 2000, 157, 661–667. [Google Scholar] [CrossRef]
- Mutale-Joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef]
- Nisha, R.; Kiran, B.; Kaushik, A.; Kaushik, C.P. Bioremediation of salt affected soils using cyanobacteria in terms of physical structure, nutrient status and microbial activity. Int. J. Environ. Sci. Technol. 2018, 15, 571–580. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Qiu, P.; Li, J.; Zhang, L.; Chen, K.; Shao, J.; Zheng, B.; Yuan, H.; Qi, J.; Yue, L.; Hu, Q.; et al. Polyethyleneimine-coated MXene quantum dots improve cotton tolerance to Verticillium dahliae by maintaining ROS homeostasis. Nat. Commun. 2023, 14, 7392. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, G.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzym. 1995, 2, 764–775. [Google Scholar]
- Cubas, C.; Lobo, M.G.; González, M. Optimization of the extraction of chlorophylls in green beans (Phaseolus vulgaris L.) by N, N-dimethylformamide using response surface methodology. J. Food Compos. Anal. 2008, 21, 125–133. [Google Scholar] [CrossRef]
- Dong, R.; Wang, X.; Li, Y.; Zhang, H.; Li, X.; Song, J.; Chang, F.; Feng, W.; Pang, H.; Wang, J. Soil bacterial diversity and community structure of Suaeda glauca vegetation in the Hetao Irrigation District, Inner Mongolia, China. Front. Microbiol. 2024, 15, 1358783. [Google Scholar] [CrossRef]
- He, X.; Wan, Z.; Jin, N.; Jin, L.; Zhang, G.; Lyu, J.; Liu, Z.; Luo, S.; Yu, J. Enhancement of cucumber resistance under salt stress by 2, 4-epibrassinolide lactones. Front. Plant Sci. 2022, 13, 1023178. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Tang, Z.; Yu, J.; Wang, G.; An, W.; Zhang, Y.; Yang, Q. Effects of exogenous melatonin on the growth and photosynthetic characteristics of tomato seedlings under saline-alkali stress. Sci. Rep. 2025, 15, 5172. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, H.; Yu, Y.; Gao, D.; Leng, C.; Zhang, S.; Yan, P. MWCNTs Alleviated saline-alkali stress by optimizing photosynthesis and sucrose metabolism in rice seedling. Plant Signal. Behav. 2023, 18, 2283357. [Google Scholar] [CrossRef]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar. Drugs 2016, 14, 191. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Feng, N.; Zheng, D.; Liu, M.; Zhou, H.; Deng, P.; Wang, Y.; Zhao, H. Combined transcriptomic and metabolomic analysis of alginate oligosaccharides alleviating salt stress in rice seedlings. BMC Plant Biol. 2023, 23, 455. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant potential of Scenedesmus obliquus grown in brewery wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef]
- Spain, O.; Plohn, M.; Funk, C. The cell wall of green microalgae and its role inheavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Abideen, Z.; Ansari, R.; Hasnain, M.; Flowers, T.J.; Koyro, H.W.; El-Keblawy, A.; Abouleish, M.; Khan, M.A. Potential use of saline resources for biofuel production using halophytes and marine algae: Prospects and pitfalls. Front. Plant Sci. 2023, 14, 1026063. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, X.T.; Wang, F.; Shao, Y.; Zhang, A.; Chang, W. The effects of chilling stress on antioxidant enzymes activities and proline, malondialdehyde, soluble sugar contents in three Paphiopedilum species. Russ. J. Plant Physiol. 2023, 70, 61. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Chong, X.; Zhou, T.; Lu, X.; Wang, X.; Zheng, B. Transcriptome Analysis of the Regulatory Mechanisms of Holly (Ilex dabieshanensis) under Salt Stress Conditions. Plants 2024, 13, 1638. [Google Scholar] [CrossRef] [PubMed]
- Kollmen, J.; Strieth, D. The beneficial effects of cyanobacterial co-culture on plant growth. Life 2022, 12, 223. [Google Scholar] [CrossRef]
- El Arroussi, H.; El Baouchi, A.; Benhima, R.; Bendaou, N.; Smouni, A.; Wahby, I. Halophilic microalgae Dunaliella salina extracts improve seed germination and seedling growth of Triticum aestivum L. under salt stress. Acta Hortic. 2016, 1148, 13–26. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X. Impact of the accumulation and adhesion of released oxygen during Scenedesmus obliquus photosynthesis on biofilm formation and growth. Bioresour. Technol. 2017, 244, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.; Usman, Z.; Azam, A.; Abbas, H.; Mehmood, A.; Ahmad, K.S. Invasive success of star weed (Parthenium hysterophorus L.) through alteration in structural and functional peculiarities. PeerJ 2023, 11, e16609. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, J.; Kang, J.; Kang, S. Trade-Offs Between Hydraulic Efficiency and Safety in Cotton (Gossypium hirsutum L.) Stems Under Elevated CO2 and Salt Stress. Plants 2025, 14, 298. [Google Scholar] [CrossRef]
- Banna, E.F.M.; Mosa, A. Exogenous application of proline mitigates deteriorative effects of salinity stress in NFT closed-loop system: An ultrastructural and physio-biochemical investigation on hydroponically grown tomato (Solanum lycopersicon L.). Sci. Hortic. 2024, 330, 113061. [Google Scholar] [CrossRef]
- Pan, X.; Xiang, L.; Hu, X.; Ren, W.; Zhang, L.; Ni, X. Effects of exogenous spermidine on mitochondrial function of tomato seedling roots under salinity-alkalinity stress. J. Appl. Ecol. 2016, 27, 491–498. [Google Scholar]
- Wang, J.; Ao, M.; Ma, A.; Yu, J.; Guo, P.; Huang, S.; Peng, X.; Yun, D.; Xu, Z.A. Mitochondrial Localized Chaperone Regulator OsBAG6 Functions in Saline-Alkaline Stress Tolerance in Rice. Rice 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Wu, Y.; Xing, D.; Javed, Q.; Ullah, I. Photosynthetic response of two okra cultivars under salt stress and re-watering. J. Plant Interact. 2017, 12, 67–77. [Google Scholar] [CrossRef]
- Sellami, S.; Le Hir, R.; Thorpe, M.R.; Aubry, E.; Wolff, N.; Vilaine, F.; Brini, F.; Dinant, S. Arabidopsis natural accessions display adaptations in inflorescence growth and vascular anatomy to withstand high salinity during reproductive growth. Plants 2019, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guo, S.; Dai, L.; Mi, L.; Li, W.; Xing, J.; Hu, Z.; Wu, W.; Duan, Z.; Li, B.; et al. Tubulin participates in establishing protoxylem vessel reinforcement patterns and hydraulic conductivity in maize. Plant Physiol. 2024, 196, 931–947. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plantphysiology Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Ali, B.; Ren, X.; Chen, X.; Li, Q.; Saqib, M.; Ahmad, N. Recent progress in understandingsalinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiol. Biochem. 2021, 160, 239–256. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.; Hussain, Q.; Asim, M.; Iqbal, A.; Ren, X.; Shah, S.; Chen, K.; Shi, Y. Application of 2, 4-epibrassinolide improves drought tolerance in tobacco through physiological and biochemical mechanisms. Biology 2022, 11, 1192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Linyerera, S.M.; Magwanga, R.O.; Hou, Y.; Wang, Y.; Xu, Y.; Khan, A.; Yu, S.; Zhou, Z.; et al. Overexpression and knockdown of cotton GhdadD gene reveals its drought and salt stress tolerance role. iScience 2024, 27, 108664. [Google Scholar] [CrossRef]
- Guo, S.; Lv, L.; Zhao, Y.; Wang, J.; Lu, X.; Zhang, M.; Wang, R.; Zhang, Y.; Guo, X. Using high-throughput phenotyping analysis to decipher the phenotypic components and genetic architecture of maize seedling salt tolerance. Genes 2023, 14, 1771. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and MetabolomicProfiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; El-Naggar, N.E.; Karim-Eldeen, M.A.; Alamer, K.H.; Saleh, M.A.; Al Masoudi, L.M.; Sharaf, E.M.; El-Azeem, R.M.A. Promoting effect of soluble polysaccharides extracted from Ulva spp. on Zea mays L. growth. Molecules 2022, 27, 1394. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gao, L.; Shen, G.; Yang, X.; Li, M. The role of adsorption in microalgae biological desalination: Salt removal from brackish water using Scenedesmus obliquus. Desalination 2020, 493, 114616. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisersin Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]










| Treatment Group | NaCl (mmol·L−1) | NaHCO3 (mmol·L−1) | Concentration of Scenedesmus obliquus (g·L−1) |
|---|---|---|---|
| CK | 0 | 0 | 0 |
| C1 | 0.25 | ||
| C2 | 0.5 | ||
| C3 | 0.75 | ||
| C4 | 1 | ||
| SA1 CK | 20 | 40 | 0 |
| SA1 C1 | 0.25 | ||
| SA1 C2 | 0.5 | ||
| SA1 C3 | 0.75 | ||
| SA1 C4 | 1 | ||
| SA2 CK | 30 | 60 | 0 |
| SA2 C1 | 0.25 | ||
| SA2 C2 | 0.5 | ||
| SA2 C3 | 0.75 | ||
| SA2 C4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Dong, Y.; Jin, X.; Liu, Y.; Yue, Z.; Li, W. Repair Effects of Scenedesmus obliquus on Cucumber Seedlings Under Saline–Alkali Stress. Agronomy 2025, 15, 1468. https://doi.org/10.3390/agronomy15061468
Liu Z, Dong Y, Jin X, Liu Y, Yue Z, Li W. Repair Effects of Scenedesmus obliquus on Cucumber Seedlings Under Saline–Alkali Stress. Agronomy. 2025; 15(6):1468. https://doi.org/10.3390/agronomy15061468
Chicago/Turabian StyleLiu, Zhao, Yanlong Dong, Xiaoxia Jin, Yan Liu, Zhonghui Yue, and Wei Li. 2025. "Repair Effects of Scenedesmus obliquus on Cucumber Seedlings Under Saline–Alkali Stress" Agronomy 15, no. 6: 1468. https://doi.org/10.3390/agronomy15061468
APA StyleLiu, Z., Dong, Y., Jin, X., Liu, Y., Yue, Z., & Li, W. (2025). Repair Effects of Scenedesmus obliquus on Cucumber Seedlings Under Saline–Alkali Stress. Agronomy, 15(6), 1468. https://doi.org/10.3390/agronomy15061468





