Abstract
Soil-borne pathogens can severely reduce vegetable crop output and quality. A disease complex may develop when many soil-borne pathogens attack a crop simultaneously, which can cause more damage. The soil-borne fungus Fusarium oxysporum (Fo) and the nematode Meloidogyne incognita (Mi) significantly reduce global tomato (Solanum lycopersicum L.) yields. After a soil-borne pathogenic infection, plants undergo numerous changes. Therefore, we conducted the present study to examine the impact of soil-borne pathogens Fo and Mi on the growth, physiology, biochemical, and root morphology of tomato cultivars Zhongza 09 (ZZ09) and Gailiang Maofen 802 (GLMFA and GLMFB) at 10, 20, and 30 days after-inoculation (DAI). The present study revealed that combined infections adversely damaged plant growth, photosynthetic pigmentation, gas exchange, biochemistry, and root morphology. The plant growth reduction in GLMFA and GLMFB was greater than in ZZ09. The chlorophyll content and photosynthetic indices declined dramatically; however, ZZ09 declined less than GLMFA and GLMFB plants. In GLMFA and GLMFB plants, the combined infection of Fo and Mi lowered plant-defense-related antioxidant activity compared to their single infection or control. ZZ09’s antioxidants were greatly up-regulated, indicating pathogen tolerance. ZZ09 had significantly lower gall and wilt disease indices than GLMFA and GLMFB. Moreover, the microscopic examination of roots showed that Fo and Mi infection damaged GLMFA and GLMFB more than ZZ09 plants. Thus, combined infection induced severe root damage, reduced plant growth, reduced antioxidants, and increased reactive oxygen species (ROS) production compared to single inoculation. However, the ZZ09 cultivar exhibited significantly stronger tolerance to combined infection.
1. Introduction
Soil-borne plant pathogens have the potential to dramatically impair vegetable crop quality and yield [1,2]. These pathogens pose a serious challenge for agricultural production because they often remain in the soil for an extended period of time, and each vegetable may be vulnerable to many species [3]. A disease complex may develop when many soil-borne pathogens attack a crop at the same time, which can cause more damage [4]. These pathogens present in the soil cause several diseases that are difficult to predict, identify, and diagnose [2]. Furthermore, the soil ecosystem is very intricate, posing difficulty in comprehending each aspect of diseases produced by soil-borne pathogens [4].
Fusarium Link, 1809 is a widespread genus of imperfect fungus that is recognized for its various species that serve as significant pathogens for plants [5]. Fusarium species are categorized as diverse soil-borne pathogens [6]. Numerous studies have demonstrated that Fusarium species can thrive on both living and dead plants, as well as other organic substances like animal remains [7]. Additionally, evidence suggests that Fusarium conidia are water-borne and can become air-borne when dried or dehydrated, while F. chlamydospores are soil-borne [8]. One of the most prevalent soil-borne plant pathogens of the genus Fusarium is F. oxysporum Snyder and Hansen, 1940, which affects over 120 different plants species [9]. According to Fernandes and Ghag [10], the symptoms often include root rot, and infected plants display stunted growth. Fusarium oxysporum has emerged as the most prevalent pathogen in a number of crops, including tomato (Solanum lycopersicum), banana (Musa acuminata), soybean (Glycine max), cotton (Gossypium herbaceum), and melon (Cucumis melo), either as detrimental pathogens or as prospective pathogens in the coming years [11]. Fusarium oxysporum is well-known for producing root rot and tomato crown diseases, which result in significant output losses in tomato production [12].
Root-knot nematodes (RKN, Meloidogyne Göldi, 1887) are among the most detrimental pathogens and may infect more than 3000 different plant species [13]. These nematodes are economically significant parasites and are among the most destructive pathogens of vegetables and other crops [14]. Managing RKNs is quite challenging because they are soil-borne pathogens that may infect a wide range of host plants [15]. Juveniles enter the root tips of the host plant and migrate to the vascular cylinder, where they produce their feeding areas known as “giant cells” [16]. Meloidogyne incognita (Kofoid and White, 1919), Chitwood 1949 is one of the most prevalent RKN species [17]. Meloidogyne incognita is a stationary, obligate endoparasite, responsible for the development of an abnormal root system that is characterized by the formation of galls [18]. This causes root deformation, defoliation, leaf wilting, and dwarfing in plants [19]. Additionally, infected plants exhibit indications of nutrient deficiency, especially nitrogen. Meloidogyne incognita is the most common RKN species that infects tomato plants, impairs their ability to absorb water and nutrients, and increases the risk of other microbial diseases [20]. As a result, tomato production and quality are reduced, resulting in substantial economic losses [21].
The combined infection of two soil-borne pathogens, F. oxysporum (Fo) and M. incognita (Mi), resulted in common foliar symptoms such as chlorosis, wilting, necrosis, and premature leaf drop in tomato plants [22]. These symptoms were primarily caused by disrupted mineral balance and impaired water absorption due to the infection of plant roots [23,24]. Additionally, the dual infection increased the plants’ susceptibility to other pathogens [25]. Previous studies have shown that Meloidogyne spp. exacerbates the severity of F. oxysporum infection in tomatoes, leading to more severe disease outcomes [26,27,28].
A variety of changes occur in plant bodies after soil-borne pathogenic infection [29]. In response to pathogens, the protective defense mechanism begins with local cell wall remodeling, followed by the accumulation of callose within infected cells [30]. When pathogens attack plant tissue, they cause an oxidative burst that generates reactive oxygen species (ROS) such as malondialdehyde (MDA), hydrogen peroxide (H2O2), and other chemicals [31]. Infected plants trigger the activation of many antioxidant enzymes to counteract the detrimental impacts of ROS [32]. Antioxidant enzymes are capable of neutralizing free radicals as they oxidize cellular components [33]. Antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and others [34]. Superoxide dismutase (SOD) is an important part of plants’ natural defense systems against deteriorating free radicals and ROS caused by both abiotic and biotic stress [35]. Catalase (CAT) is a key antioxidant enzyme that influences plant growth and tolerance to stress [36]. It further serves a crucial role in plant defense, stress responses, and maintaining the balance of redox in the cells [37]. To reduce the impact of ROS, resistant host plants frequently generate a higher quantity of biochemical substances (e.g., antioxidant enzymes) and photosynthetic pigments (e.g., chlorophyll) than susceptible host plants [38].
Therefore, it is crucial to develop techniques to manage soil-borne plant diseases and eradicate their prevalence and severity to ensure sustainable agricultural output [39]. The use of biofumigants, anaerobic soil disinfestation, crop rotation, soil solarization, and resistant cultivars are some environmentally benign practices [1]. The selection of resistant cultivars is an effective management strategy for controlling soil-borne diseases [3]. Recent decades have seen the development of many commercial hybrid-resistant cultivars, and managing soil-borne diseases through host resistance may provide an easy and effective approach for controlling Fusarium wilt disease in tomato plants [40]. However, the simultaneous presence of fungi and plant-parasitic nematodes reduces the efficacy of resistant cultivars. Therefore, we conducted the current research to investigate the impact of either a single or combined infection of soil-borne pathogens (M. incognita and F. oxysporum) on tomato cultivars. The main purpose of this study was to investigate how two types of tomato hybrid cultivars, Zhongza 09 (ZZ09) and Gailiang Maofen 802 (GLMFA and GLMFB), respond to two soil-borne pathogens in terms of disease severity, physiological and biochemical changes, and root morphology.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
The tested tomato (Solanum lycopersicum) varieties Gailiang Maofen 802 (non-fuzzy plant or without hairs (GLMFA) and fuzzy plant or with hairs (GLMFB)) were purchased from Xi’an Hangfeng Seed Industry (Xi’an, China) Co., Ltd., and Zhongza 09 (ZZ09) was purchased from Zhongshu Seed Industry Technology (Beijing, China) Co., Ltd. Tomato seeds were soaked in clean water for 2 h at room temperature, then sterilized in a 1.0% NaOCl solution for 15 min. Afterward, seeds were immediately rinsed with sterilized water to remove any NaOCl residue and placed in a Petri dish to accelerate germination (28 °C, 2 days). The germinated seeds were transferred to sterilized substrate (Nanning Kelv Agricultural Resources (Nanning, China) Co., Ltd., organic matter + humus ≥ 55%, total porosity 70–80%, pH 5.5–8.0) in 72-hole plastic seedling trays, placed in a light incubator (28 °C, 16 h light, 8 h dark) for cultivation, and watered daily. When the tomato plants reached the three-leaf stage, those with the same height were selected, transplanted into pots, and watered with an appropriate amount of water every 2 days.
2.2. Nematodes Inoculum
The RKN M. incognita was obtained from the nematology laboratory at Guangxi University. The RKN was multiplied using a tomato plant as a host. About 500 newly hatched second-stage juveniles (J2) of M. incognita were inoculated into the soil around the roots of tomato plants. After 35 days, the roots were removed and the soil adhering to the root surface was rinsed with running water. Subsequently, the roots were submerged in a solution of 2% NaClO and vigorously agitated for 30 s. After that, the mixture was passed through a series of sieves with mesh sizes of 100, 325, and 500. The eggs were collected through a 500-mesh sieve and then thoroughly rinsed with running tap water to eliminate any remaining NaOCl residues. Subsequently, the eggs were allowed to hatch in accordance with the Baermann–Funnel method. A substantial number of freshly emerged J2s were collected and counted using a stereomicroscope (OLYMPUS SZX2-ILLT, Tokyo, Japan). The concentration of J2s in the resulting suspension was adjusted to 500 J2/mL for future usage [41].
2.3. Fungus Inoculum
The F. oxysporum strain (accession number: MN240928) was kept in the nematology laboratory of Guangxi University. For activation, a small amount of F. oxysporum was cultured in PDA-filled cavities and placed in an incubator at 28 °C for 3–5 days. The young hyphae at the edge of the colony were scraped and put into 100 mL of PDB liquid medium and cultured at 160 rpm on a shaker (MQD-S2R, Minquan Instrument Co., Ltd., Shanghai, China) at 28 °C for 3 days. After that, the suspension was filtered through three layers of lens-cleaning paper to obtain a spore suspension, and the concentration of spores was measured with a hemocytometer chamber (Yancheng Cordial Medlab. Co., Ltd., Yancheng, China). The spore suspension was adjusted to 1 × 108 CFU/mL [42].
2.4. Pot Experiment
The pot experiment was carried out from November 2021 to January 2022. The present experiments consisted of four treatments: CK (control), Fo (inoculation with F. oxysporum only), Mi (inoculation with M. incognita only), and Fo+Mi (inoculation with both F. oxysporum and M. incognita). Three holes were formed in the rhizospheric area of each plant, and 1 × 108 CFU mL−1 chlamydospores of F. oxysporum and 1500 J2s of M. incognita were inoculated into different treatments. Each treatment was replicated five times.
2.4.1. Determination of Leaf Photosynthetic Parameters
The photosynthetic parameters of tomato leaves in a pot experiment were measured. The evaluation period for measuring the photosynthetic parameters lasted 3 h, from 08:30 to 11:30. The parameters assessed included the net photosynthetic rate (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), intercellular CO2 partial pressure (Pci), total CO2 conductance (GTC), and total water vapor conductance (GTW). The environmental parameter settings were airflow rate of 500 μmols−1, temperature of the leaf chamber of 28 °C, light quantum density of 1000 μmol m−2s−1, relative humidity of 65%, CO2 concentration of 400 μmol mol−1, fan speed of 1000 rpm, and the blade to be tested was measured with a ruler. Moreover, a CCM-200 plus chlorophyll content meter (Opti-Sciences Inc., Hudson, NY, USA) was used to measure the chlorophyll content of the fourth functional leaf below the growth point [43].
2.4.2. Determination of Stress-Resistant Physiological Indicators of Leaves
Three tomato plants were randomly selected from each treatment group at 10, 20, and 30 days after inoculation (DAI). The fresh, mature, fully expanded leaves from the lower, middle, and upper portions of the plant (0.5 g) were promptly frozen with liquid nitrogen and stored at −80 °C for the extraction of crude enzyme solution. All enzyme extraction processes were conducted at 4 °C. The malondialdehyde (MDA) content was determined using the thiobarbituric acid (TBA) method, the peroxidase (POD) activity was determined by the guaiacol method, the catalase (CAT) activity was determined by decomposition of H2O2, and the superoxide dismutase (SOD) activity was determined by measuring its ability to inhibit photochemical reduction of nitro blue tetrazolium chloride (NBT) [44].
In order to find the proline content, 0.25 g of leaves was grinded in 5 mL of 3% sulfosalicylic acid, and then the mixture was centrifuged at 8000 rpm for 5 min at 25 °C. A solution of ninhydrin, deionized water, and glacial acetic acid was combined in equal volumes with plant extract. After that, the mixture was heated in a boiling water bath for one hour at 100 °C. Before adding toluene, the liquid was promptly cooled to stop the reaction. The mixture was agitated vigorously using a vortex shaker for 10–20 s, after which it was left undisturbed to settle. The spectrophotometer was used to measure the absorbance of the topmost layer of the mixture at a wavelength of 520 nm [45].
2.4.3. Determination of Plant Growth
The initial height of plants was measured on the day of inoculation of pathogens. At 10, 20, and 30 DAI (days after inoculum), the final height of plants was measured. Using the following formula, the rate of plant height increase was measured:
Among them, h0 represents the initial plant height of tomato, and h1 represents the plant height of tomato at each measurement time.
Furthermore, three plants were randomly selected from each treatment group at 10, 20, and 30 DAI. The selected plants were uprooted, and the roots were washed with running tap water. After cleaning, the surface water of the roots was dried with absorbent paper, and the fresh weight of the roots was measured.
2.4.4. Fusarium Wilt Disease Index and Root Gall Index
The disease index of Fusarium wilt (Disease index, DI) and root gall index (Gall index, GI) were determined at 10, 20, and 30 DAI. The Fusarium wilt disease index was graded according to Ma et al. [46]: grade 0 (asymptomatic or healthy plant); grade 1 (one or two leaves turn yellow and fall off); grade 2 (three or four true leaves turn yellow and wilt and fall off); grade 3 (five or six true leaves turn yellow, or the true leaves wilt and fall off); grade 4 (severe wilting of the whole plant); and grade 5 (the whole plant dies).
The galls on the root were graded according to Zeck [47]: grade 0 (no gall); grade 1 (few small galls); grade 2 (several small galls on root system); grade 3 (several small galls of which some are grown together); grade 4 (several small and some large galls); grade 5 (25% of roots severely galled); grade 6 (50% of roots severely galled); grade 7 (75% of roots severely galled); grade 8 (90% of roots severely galled, only a few healthy roots); grade 9 (no healthy roots, resulting in plant death).
The disease severity index (DI) and gall index (GI) were calculated following the formula given by Purwati and Hidayah [48]:
where the No. of diseased plants at each level refers to the count of plants categorized by their disease or gall severity based on Zeck’s scale. The Representative level is the numerical severity value assigned to each grade (e.g., grade 0 = 0, grade 1 = 1, and so on). The Σ (sum) represents the total of all severity level values across all plants. The total No. of Plants is the overall number of plants assessed in the experiment. The Highest Representative Level refers to the highest severity grade used in the assessment, which is grade 9 in this case, representing the most severe condition.
2.4.5. Determination of Stem Browning Rate and Histopathology of Root
At 10, 20, and 30 DAI, five plants were randomly selected. The lowered stems (cotyledons and the first part near the ground) were intercepted. The stem between leaves was sectioned by the frozen section method, and the browning area of vascular bundles and the area of vascular bundles in plants treated with Fo and Fo+Mi were measured under a microscope (Axio Imager Z2, Zeiss), and the browning rate of cross-section of vascular bundles was calculated by using the following formula:
To determine the impact of F. oxysporum and M. incognita on tomato roots, five plants were selected at random from the Fo+Mi treatment at 10, 20, and 30 DAI. The roots were trimmed and washed with water. After that, the surfaces of the roots were dried with sterilized absorbent paper and fixed in a formalin–acetoethanol (FAA) solution. The paraffin sections were cut and observed under microscope.
2.5. Statistical Analysis
The one-way ANOVA was performed under a completely randomized experimental design. This design allowed for the comparison of means across different treatment groups, with LSD (p < 0.05) used to determine significance differences between treatments. The statistical analysis was carried out using IBM SPSS software (version 25.0). The graphs were created using Sigma Plot (version 10.0).
3. Results
3.1. Effects of Combined Infection on Photosynthetic Parameters of Tomato Leaves
Figure 1 shows the chlorophyll content of tomato treatment groups at 10, 20, and 30 days after inoculation (DAI). Throughout the observation period, chlorophyll content increased in all treatments. However, the reduction in chlorophyll content was lesser in the Fo+Mi treatment of ZZ09 plants compared to GLMFA and GLMFB plants. At 10 DAI, the chlorophyll content of the Fo, Mi, and Fo+Mi treatments of ZZ09 decreased by 21.51%, 15.02%, and 16.42%, respectively. Similarly, the Fo and Fo+Mi treatments of GLMFA plants declined by 18.64% and 22.32% compared to the control. However, no significant difference was observed between the Mi treatment and the control group. The Fo+Mi treatment of GLMFB plants exhibited a reduction of 25.06% compared to the control. There were no notable differences observed between the other treatments and the control (Figure 1A). At 20 DAI, the chlorophyll contents in the Fo, Mi, and Fo+Mi treatments of ZZ09 exhibited substantial reductions of 24.38%, 20.97%, and 30.55%, respectively. The chlorophyll content of GLMFA plants decreased significantly by 33.28%, 36.12%, and 39.88%, respectively. In the case of GLMFA plants, the chlorophyll content in the Mi and Fo+Mi treatments reduced by 24.64% and 36.76%, respectively. There was no significant difference in chlorophyll contents between the Fo treatment and the control group (Figure 1B). At 30 DAI, the chlorophyll content of the Fo+Mi treatment of ZZ09 was reduced by 11.27% compared to the control. No significant difference was found between the Fo and Mi treatments. In contrast, the chlorophyll content of GLMFA plants in the Fo, Mi, and Fo+Mi treatments decreased by 31.34%, 13.59%, and 29.47%, respectively. Similarly, the chlorophyll content in the Fo+Mi treatment of GLMFB plants decreased significantly by 27.40% compared to the control. However, no significant difference was observed between the other two treatments and the control group (Figure 1C). In addition, the chlorophyll content of ZZ09 increased by 60.14% within 10-30 days after Fo+Mi treatment, whereas it increased by 50.45% in GLMFA and 43.14% in GLMFB. Thus, reduction in chlorophyll content in GLMFB was larger in the combined infection of F. oxysporum and M. incognita compared to ZZ09.
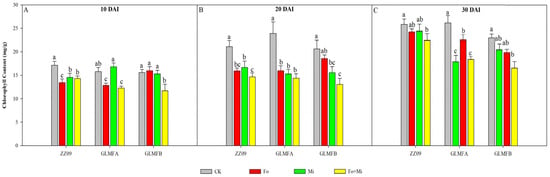
Figure 1.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on chlorophyll content at 10 DAI. (B) The effects of F. oxysporum and M. incognita on chlorophyll content at 20 DAI. (C) The effects of F. oxysporum and M. incognita on chlorophyll content at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); CK (non-inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
As shown in Figure 2, the Tr value initially increased, then decreased by 30 DAI. At 10 DAI, no significant variation was observed (Figure 2A). At 20 DAI, Tr values for ZZ09 in the Fo and Mi treatments decreased by 19.66% and 13.68%, respectively. GLMFA and GLMFB showed decreases of 21.19%, 31.36%, and 19.78%, 13.19%, respectively. In the Fo+Mi treatment, Tr values dropped by 26.50% for ZZ09, 37.29% for GLMFA, and 56.04% for GLMFB (Figure 2B). At 30 DAI, ZZ09 and GLMFB in the Fo+Mi treatment decreased significantly by 36.07% and 75.00%, respectively, while GLMFA showed no significant change (Figure 2C).
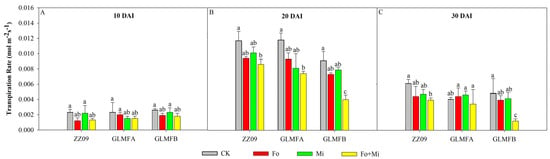
Figure 2.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on transpiration rates (Tr) at 10 DAI. (B) The effects of F. oxysporum and M. incognita on transpiration rates (Tr) at 20 DAI. (C) The effects of F. oxysporum and M. incognita on transpiration rates (Tr) at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); CK (non-inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
After pathogen inoculation, the Pn values of ZZ09, GLMFA, and GLMFB exhibited various degrees of reduction (Figure 3). In comparison to the control, the Pn values of the Fo+Mi treatment in ZZ09 and GLMFA plants exhibited a decrease of 35.89% and 38.06%, respectively, at 10 DAI. Additionally, the Pn values of GLMFB plants were reduced by 31.43% (Figure 3A). At 20 DAI, the Pn value of the Fo+Mi treatment in GLMFA plants exhibited a substantial reduction of 33.17%, but there was no significant difference for ZZ09 and GLMFB (Figure 3B). At 30 DAI, the Pn value of the Fo+Mi treatment in GLMFA was only 3.813 µmol m−2s−1, which was significantly reduced by 59.29% compared with the control, while the Pn value of the Fo+Mi treatment in ZZ09 and GLMFB showed significant difference from the control (Figure 3C).
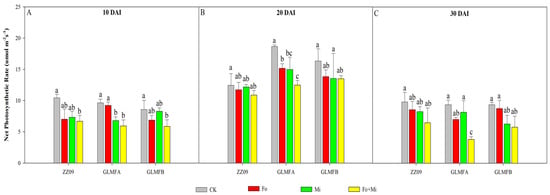
Figure 3.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on net photosynthesis rate (Pn) at 10 DAI. (B) The effects of F. oxysporum and M. incognita on net photosynthesis rate (Pn) at 20 DAI. (C) The effects of F. oxysporum and M. incognita on net photosynthesis rate (Pn) at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
Figure 4 illustrates the intercellular CO2 concentration (Ci) of various treatment plants at each stage. Within thirty days of inoculation, the Ci value underwent a synergistic change, and the reduction in Ci values was more pronounced than that of ZZ09. At 10 DAI, the Ci levels in ZZ09 treatments showed no significant difference. However, the Ci levels in the treatments containing Fo+Mi decreased by 42.95%, while in Fo and Mi treatments decreased by 36.22% and 20.63%, respectively, in the GLMFA plants (Figure 4A). At 20 DAI, the Ci values of the Mi treatment were significantly lower than those of the CK treatment; however, there was no significant difference between the other treatments and CK (Figure 4B). At 30 DAI, the Ci values of ZZ09, GLMFA, and GLMFB plants treated with Fo+Mi increased by 1.69%, 1.93%, and 8.11%, respectively. However, these increases were not statistically significant from one another (Figure 4C).
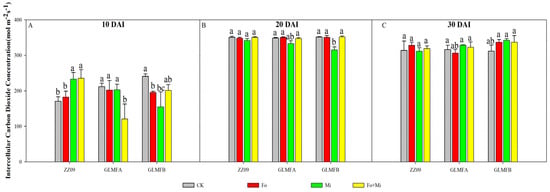
Figure 4.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on intracellular CO2 concentrations (Ci) at 10 DAI. (B) The effects of F. oxysporum and M. incognita on intracellular CO2 concentrations (Ci) at 20 DAI. (C) The effects of F. oxysporum and M. incognita on intracellular CO2 concentrations (Ci) at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
The changes in intercellular CO2 partial pressure (Pci) for each treatment followed a similar pattern to the Ci value (Figure 5). At 10 DAI, the Pci value of the Fo+Mi treatment in ZZ09 showed a significant increase of 37.96% compared to the control. The Fo+Mi treatment of the GLMFA plants exhibited a significant decrease of 42.83%, while the Fo and Mi treatments of the GLMFB plants showed significant decreases of 19.59% and 36.20%, respectively. However, Figure 5A showed no significant difference between the control and the Fo+Mi treatment. At 20 DAI, the GLMFB plant Mi treatment significantly reduced its Pci value by 10.26% compared to the control. There were no significant differences between the other treatments and the control (Figure 5B). Figure 5C showed no statistically significant variation in the Pci values of plants subjected to different treatments at 30 DAI.
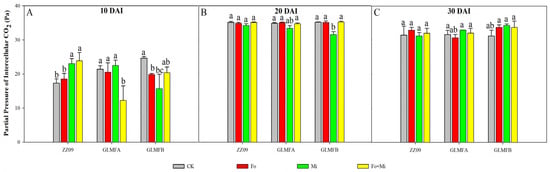
Figure 5.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on partial pressures of intercellular CO2 (Pci) at 10 DAI. (B) The effects of F. oxysporum and M. incognita on partial pressures of intercellular CO2 (Pci) at 20 DAI. (C) The effects of F. oxysporum and M. incognita on partial pressures of intercellular CO2 (Pci) at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
Figure 6 displays the measurement results of the total CO2 conductance (GTC) and total water conductance (GTW) of tomato leaves. Within 10–30 DAI, the GTC and GTW values of ZZ09 treated with Fo+Mi decreased by 85.19% and 79.41%, respectively, while GLMFA and GLMFB values decreased less (66.67% and 66.13%, 68.89% and 69.44%, respectively). ZZ09 showed greater stomatal closure than GLMFA and GLMFB after combined infection. At 10 DAI, the GTC values of ZZ09 treated with Fo and Mi increased significantly compared to the control, while no significant differences were observed between the Fo+Mi treatments of GLMFA and GLMFB and their respective controls (Figure 6A).
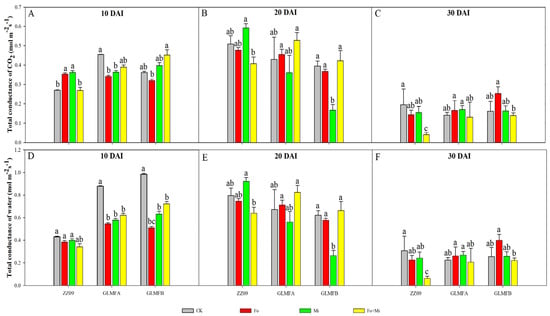
Figure 6.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on total CO2 conductance (GTC) at 10 DAI. (B) The effects of F. oxysporum and M. incognita on total CO2 conductance (GTC) at 20 DAI. (C) The effects of F. oxysporum and M. incognita on total CO2 conductance (GTC) at 30 DAI. (D) The effects of F. oxysporum and M. incognita on total water conductance (GTW) at 10 DAI. (E) The effects of F. oxysporum and M. incognita on total water conductance (GTW) at 20 DAI. (F) The effects of F. oxysporum and M. incognita on total water conductance (GTW) at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
At 10 DAI, the GTW values of the GLMFA plants and the GLMFB plants treated with Fo+Mi were significantly lower than those of the control by 29.55% and 26.53%, respectively (Figure 6D). At 20 DAI, the GTC and GTW values for ZZ09 treated with Fo+Mi decreased by 19.09% and 19.59%, respectively, relative to the control. In contrast, GLMFA plants exhibited increases of 23.26% and 22.39%, while GLMFB plants showed increases of 5.00% and 6.45%. However, these increases were not statistically significant when compared to the control (Figure 6B,E). At 30 DAI, ZZ09 showed significant reductions in both GTC and GTW, while GLMFA and GLMFB had no significant changes (Figure 6C,F).
3.2. Effects of Combined Infection on Stress-Resistant Physiological Indicators of Leaves
The SOD activity increased significantly after infection with M. incognita and F. oxysporum at 30 DAI. At 10 DAI, the SOD activity in the Fo, Mi, and Fo+Mi treatments of ZZ09 was 124.94, 124.20, and 128.64 U·g−1, respectively. For the GLMFA plants, the SOD activity was 138.32, 130.34, and 125.44 U·g−1 for the Fo, Mi, and Fo+Mi treatments, respectively. In GLMFB plants, the SOD activity was 120.42, 111.07, and 117.75 U·g−1 for the Fo, Mi, and Fo+Mi treatments, respectively. The SOD activity in the control plants of ZZ09, GLMFA, and GLMFB was 151.13, 167.75, and 158.34 U·g−1, respectively (Figure 7A). At 20 DAI, ZZ09 treated with Fo had SOD activity of 167.03 U·g−1, similar to the control (155.95 U·g−1), while Mi and Fo+Mi treatments showed slight increases (6.75% and 5.78%) but were not significant. GLMFA and GLMFB plants had no significant differences in SOD activity between treatments and controls (Figure 7B). At 30 DAI, SOD activity in ZZ09 treated with Fo, Mi, and Fo+Mi was 176.75, 171.16, and 170.37 U·g−1, respectively, showing significant increases compared to the control (151.13 U·g−1), while no significant changes were observed in GLMFA and GLMFB (Figure 7C).
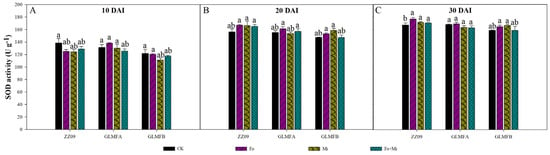
Figure 7.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on SOD activity at 10 DAI. (B) The effects of F. oxysporum and M. incognita on SOD activity at 20 DAI. (C) The effects of F. oxysporum and M. incognita on SOD activity at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
Fusarium oxysporum and M. incognita significantly increased the POD activity of ZZ09. At 10 DAI, the POD activity of GLMFB plants treated with Fo and Mi was 1133.33 U·g−1, and Fo+Mi was 1533.33 U·g−1, with no significant difference from the control (1433.33 U·g−1). GLMFA plants showed a significant decrease in POD activity in Fo and Mi treatments (22.35% and 36.47%, respectively), while Fo treatment in GLMFB increased POD activity by 43.48%, and Mi decreased by 34.78% (Figure 8A). At 20 DAI, the POD activities of ZZ09 treated with Fo, Mi, and Fo+Mi were 3366.67, 2566.67, and 4700 U·g−1, respectively, which were significantly increased by 215.62%, 140.62%, and 340.62% compared with the control (1066.67 U·g−1), respectively. The POD activities of Fo and Fo+Mi treatments of GLMFA plants were 5000.00 and 4666.67 U·g−1, respectively, which were significantly increased by 134.38% and 118.75% compared with the control (2133.33 U·g−1). However, there was no significant difference between the POD activity of the GLMFB plants and the control (Figure 8B). At 30 DAI, the POD activity of ZZ09 treated with Fo+Mi was 5066.67 U·g−1, which was significantly increased by 50.49% compared with the control. Also, the POD activities of GLMFA and GLMFB plants treated with Fo+Mi were 5766.67 and 6333.33 U·g−1, which were 19.31% and 2.70% higher than the control, but significantly not different (Figure 8C).
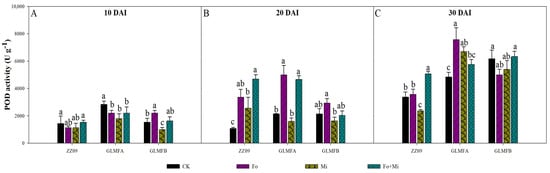
Figure 8.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on POD activity at 10 DAI. (B) The effects of F. oxysporum and M. incognita on POD activity at 20 DAI. (C) The effects of F. oxysporum and M. incognita on POD activity at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
At 10 DAI, CAT activity in ZZ09 treated with Fo, Mi, and Fo+Mi was 83.33, 100, and 50 U·g−1, respectively, compared to 33.33 U·g−1 in the control. The Fo+Mi treatment significantly increased activity by 150.02% and 200.03%. For GLMFA, CAT activities were 100, 66.67, and 83.33 U·g−1, with no significant differences from the control (66.67 U·g−1) (Figure 9A). At 20 DAI, the CAT activities of ZZ09 treated with Fo, Mi, and Fo+Mi were 283.33, 316.67, and 250.00 U·g−1, respectively, while the control was 166.67 U·g−1. There was no significant difference. The CAT activities of Fo and Mi-treated GLMFA plants were 116.67 and 66.67 U·g−1, respectively, which were significantly reduced by 53.33% and 73.33% compared with the control (250 U·g−1), while there was no significant difference between the Fo+Mi-treated and the control plants. The CAT activities of the GLMFB treatments, Fo and Mi, were both 133.33 U·g−1, while the Fo+Mi treatment was 200 U·g−1. These were compared to the control, which was 150 U·g−1, but there was no significant difference between them (Figure 9B). At 30 DAI, CAT activities in ZZ09 treated with Fo and Mi were 300 and 250 U·g−1, respectively, with no significant difference compared to the control (183.33 U·g−1). For GLMFA, the Fo treatment showed a significant increase of 366.67% compared to the control (50 U·g−1), while no significant differences were observed between the other treatments and controls (Figure 9C).
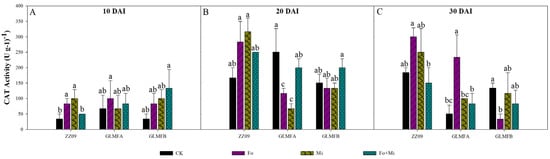
Figure 9.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on CAT activity at 10 DAI. (B) The effects of F. oxysporum and M. incognita on CAT activity at 20 DAI. (C) The effects of F. oxysporum and M. incognita on CAT activity at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
The MDA content remained high early in pathogen infection but decreased later. At 10 DAI, MDA content in ZZ09 treated with Fo, Mi, and Fo+Mi was 0.0077, 0.0102, and 0.0104 µmol·g−1, respectively, with no significant difference compared to the control (0.0081 µmol·g−1). Similarly, no significant differences were observed in GLMFA and GLMFB plants (Figure 10A). At 20 DAI, no significant differences in MDA content were found in ZZ09 and GLMFB plants treated with Fo+Mi compared to the control. However, MDA in GLMFA treated with Fo+Mi increased by 44.68% (Figure 10B). At 30 DAI, MDA in ZZ09 treated with Fo, Mi, and Fo+Mi decreased significantly by 42.50%, 37.50%, and 60.00%, respectively, compared to the control. In GLMFA, Fo+Mi decreased MDA by 34.85%, while no significant difference was observed in GLMFB treated with Fo+Mi (Figure 10C).
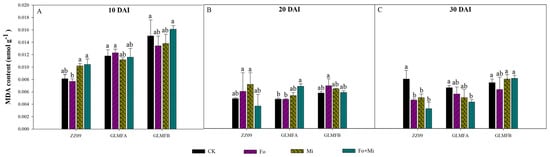
Figure 10.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on MDA content at 10 DAI. (B) The effects of F. oxysporum and M. incognita on MDA content at 20 DAI. (C) The effects of F. oxysporum and M. incognita on MDA content at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
Figure 11 shows the proline content of different tomato treatments’ leaves at different stages. At 10 DAI, the proline contents of leaves treated with Fo, Mi, and Fo+Mi in ZZ09 were 73.00, 269.47, and 71.54 µg·g−1, respectively, as compared with the control (154.47 µg·g−1), which decreased significantly by 52.74% and 53.69%, respectively, and the Mi treatment significantly increased by 74.45%. In GLMFA, the proline content of the Fo+Mi treatment was significantly decreased by 38.92% compared with the control (110.30 µg·g−1), while there was no significant difference between the Fo and Mi treatments and the control. The proline content of the GLMFB plants’ control was 28.61 µg·g−1, which was at a low level, but the proline contents of the Fo, Mi, and Fo+Mi treatments were 55.09, 43.84, and 51.22 µg·g−1, which were significantly increased by 92.45%, 53.23%, and 79.03% (Figure 11A). At 20 DAI, ZZ09 treated with Fo+Mi showed a significant decrease of 59.28%, while GLMFA treatments increased proline by 92.45%, 53.23%, and 79.03%. GLMFB treated with Fo and Mi had significant increases (108.85% and 125.83%), but no difference in Fo+Mi (Figure 11B). At 30 DAI, proline in ZZ09 treated with Fo was significantly reduced by 42.14%, while no significant difference was observed in Mi and Fo+Mi. In GLMFA, proline content was significantly decreased by 70.63% and 71.36% for Fo and Mi treatments, while Fo+Mi had no significant change. GLMFB showed significant increases in proline for Fo and Mi treatments, but no significant difference for Fo+Mi (Figure 11C).
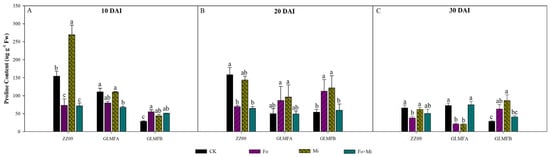
Figure 11.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on proline content at 10 DAI. (B) The effects of F. oxysporum and M. incognita on proline content at 20 DAI. (C) The effects of Fusarium oxysporum and Meloidogyne incognita on proline content at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non−fuzzy plants (GLMFB); CK (non−inoculated control); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significance difference between treatments according to LSD at the p < 0.05 level.
3.3. The Effect of Combined Infection on the Growth Rate of Tomato Plant Height
Table 1 shows the plant height growth rate for different treatments over various periods. At 10 DAI, ZZ09, GLMFA, and GLMFB plants treated with Fo+Mi showed significant reductions in growth rate by 30.58%, 16.00%, and 14.04%, respectively, compared to the control. At 20 DAI, ZZ09 showed significant decreases in growth rate up to 25.35%, 26.67%, and 43.78% for Fo, Mi, and Fo+Mi, respectively, while GLMFA showed reductions of 12.62%, 7.22%, and 24.05%. Fo+Mi treatment resulted in the smallest growth rate for both ZZ09 and GLMFA. GLMFB plants showed the highest growth with the Mi treatment (193.37%, with no significant difference) and the lowest with the Fo treatment (152.24%, significantly reduced by 19.66%), while Fo+Mi reduced growth by 18.42%. At 30 DAI, ZZ09 and GLMFA plants treated with Fo, Mi, and Fo+Mi showed significant decreases in plant height, with Fo+Mi treatments showing the largest reductions of 35.49% and 37.71%. Similarly, GLMFB plants treated with Fo and Fo+Mi had significant growth reductions of 24.39% and 35.02%, while Mi treatment had no significant difference from the control.

Table 1.
The effects of Fusarium oxysporum and Meloidogyne incognita on plant height rate of tomatoes at different inoculation times.
3.4. Effect of Compound Infection on Root Fresh Weight
After inoculation with F. oxysporum and M. incognita, the fresh root weight of ZZ09, GLMFA, and GLMFB plants significantly decreased. Table 2 shows the fresh root weight at 10, 20, and 30 DAI for each treatment. At 10 DAI, ZZ09 treated with Fo, Mi, and Fo+Mi had reductions of 68.95%, 52.71%, and 30.80%, respectively, compared to the control. GLMFA plants treated with Mi and GLMFB plants treated with Fo and Mi showed no significant reductions, but Fo and Fo+Mi treatments in GLMFA, and Fo+Mi treatment in GLMFB, caused reductions of 41.00%, 45.98%, and 49.05%, respectively. At 20 DAI, ZZ09 showed a slight decrease in root weight with Fo and Mi treatments, while Fo+Mi caused a 58.70% reduction. GLMFA showed no significant change in the Fo+Mi treatment, but Fo treatment reduced root weight by 67.27%, and Mi treatment increased it by 87.27%. GLMFB showed a significant decrease in root weight with all treatments (Fo: 65.06%, Mi: 50.00%, Fo+Mi: 44.58%). At 30 DAI, ZZ09 treated with Fo and Mi had increases in root weight by 28.17% and 29.26%, respectively, while Fo+Mi showed no significant difference. GLMFA treated with Fo+Mi had a significant increase of 22.68%, while GLMFB showed a significant decrease of 35.83% with Fo+Mi treatment.

Table 2.
The effects of Fusarium oxysporum and Meloidogyne incognita on fresh root weight of tomatoes at different inoculation times.
3.5. Effect of Compound Infection on the Number of Galls and Eggs
The effect of soil-borne pathogen infection on the number of galls and eggs is displayed in Table 3. In the Mi treatment, the number of galls in ZZ09, GLMFA, and GLMFB plants was 398.67, 599.63, and 275.11, and the number of eggs was 128,000.00, 154,000.00, and 10,666.70, respectively. The number of galls in ZZ09 decreased by 33.51% and increased by 44.91% compared with that in the GLMFA plants. There was no significant difference in the number of eggs between ZZ09, GLMFA, and GLMFB plants. In the Fo+Mi treatment, the number of galls in ZZ09, GLMFA, and GLMFB plants was 122.59, 194.38, and 215.94, and the number of eggs of ZZ09 was 51,777.78, 47,444.44, and 36,777.78, respectively. The number of galls in ZZ09 was significantly reduced by 36.93% and 43.23%, respectively, compared with that of GLMFA and GLMFB plants. Similarly, the number of eggs in ZZ09 was not significantly different compared with those of GLMFA and GLMFB plants. In addition, compared with the same variety of the Mi treatment, the number of galls in ZZ09 and Fo+Mi treatment decreased significantly by 69.25% and 67.58%, respectively, and the number of galls of Fo+Mi treatment decreased by 21.51% but not significantly. The number of galls treated with Fo+Mi in ZZ09, GLMFA, and GLMFB plants resulted in a significant reduction of egg mass by 59.55%, 69.19%, and 65.52%, respectively.

Table 3.
The effects of Fusarium oxysporum and Meloidogyne incognita alone or combined infection on root system at 30 DAI.
3.6. Effect of Compound Infection on Gall Index and Disease Index of Fusarium Wilt
The results of the gall index and wilt disease index of plants under different tomato treatments are summarized in Table 4. The gall index and disease index of ZZ09 were significantly lower than those of GLMFA and GLMFB plants. The Mi treatments for GLMFA and GLMFB were 53.74 and 55.67, respectively, whereas the Fo+Mi treatments were 48.83 and 45.47. There was no significant difference in the gall index between the two tomato treatments. However, the Mi treatment for ZZ09 had a significantly lower gall index than those of GLMFA and GLMFB, decreasing by 20.38% and 23.14%, respectively. Similarly, the Fo+Mi treatment of ZZ09 was significantly lower than that in GLMFA and GLMFB, with decreases of 36.62% and 31.93%, respectively. The Fo treatment of ZZ09 (31.79) was also significantly lower than GLMFA (48.52) and GLMFB (42.61), and the Fo+Mi treatment (45.45) was lower than GLMFA (63.23) and GLMFB (58.94), showing reductions of 28.12% and 22.89%, respectively.

Table 4.
The effects of Fusarium oxysporum and Meloidogyne incognita on gall index (GI) and disease index (DI) at 30 DAI.
Furthermore, when the two pathogens co-infected (Fo+Mi) the ZZ09, GLMFA, and GLMFB plants, the gall index decreased by 26.67%, 9.14%, and 18.32%, respectively, compared to a single infection with M. incognita and F. oxysporum. In contrast, co-infection increased the Fusarium wilt disease index by 42.97%, 30.32%, and 38.32% in ZZ09, GLMFA, and GLMFB plants, respectively, compared to a single infection.
3.7. Effect of Combined Infection on the Development of Tomato Stem Lesions
The lesion length of ZZ09 treated with Fo+Mi increased by 14.60% from 10 to 30 DAI, while GLMFA showed a 343.24% increase (Figure 12). At 10 DAI, the rate of stem lesions in GLMFA and GLMFB plants treated with Fo+Mi averaged 25%, compared to 16% in ZZ09. GLMFA had lesion lengths of 1.53 cm (Fo) and 1.64 cm (Fo+Mi), while ZZ09 had lesion lengths of 1.07 cm (Fo) and 1.57 cm (Fo+Mi). GLMFB plants had significantly higher lesion lengths (2.78 cm) with Fo+Mi (Figure 12A). At 20 DAI, the rate of stem lesions in ZZ09, GLMFA, and GLMFB plants treated with Fo was 8%, 5%, and 5%, respectively, with lesion lengths of 0.30 cm, 0.60 cm, and 0.30 cm. Moreover, the rate of stem lesion percentage in these plants treated with Fo+Mi was 12%, 10%, and 10%, and the stem lesion lengths were 1.37 cm, 0.37 cm, and 1.80 cm. In other words, the co-infection significantly increased ZZ09’s lesion length by 72.99% compared to the GLMFA plants, while there was no significant difference from the GLMFB (Figure 12B). At 30 DAI, the stem lesion rate for GLMFB treated with Fo and Fo+Mi was 15% and 20%, with lesion lengths of 0.67 cm and 2.20 cm. In ZZ09, the rates were 12% and 16%, with lesion lengths of 0.57 cm and 1.53 cm. There was no significant difference between ZZ09 and GLMFB, but GLMFA had a 62.00% higher lesion length (1.50 cm) than ZZ09 (0.57 cm) with Fo treatment (Figure 12C).
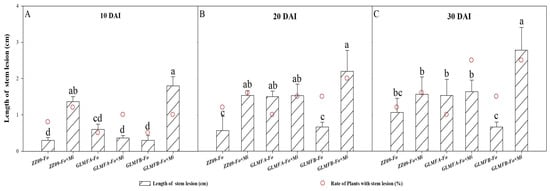
Figure 12.
(A) The effects of Fusarium oxysporum and Meloidogyne incognita on stem lesion at 10 DAI. (B) The effects of F. oxysporum and M. incognita on stem lesion at 20 DAI. (C) The effects of F. oxysporum and M. incognita on stem lesion at 30 DAI. The empty circles with red borders in the graphs represent the rate of stem lesion. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); Fo (plants were infected with F. oxysporum only); Mi (plants were infected with M. incognita only); Fo+Mi (plants were infected with both F. oxysporum and M. incognita). The data represent mean ± standard error. The same letters on bars indicate the non-significant difference between treatments according to LSD at the p < 0.05 level.
Table 5 shows the longitudinal browning length in the stems of ZZ09, GLMFA, and GLMFB plants treated with Fo and Fo+Mi. At different stages, there was no significant difference in the length of browning between the Fo+Mi and Fo treatments. At 10 DAI, the browning length of ZZ09 under Fo+Mi treatment increased by 19.77% compared with Fo treatment but was not significantly different. However, the browning length of GLMFA plants treated with Fo+Mi increased significantly by 164.25% as compared to the Fo treatment. In addition, in the Fo treatment, the browning length of the GLMFA plants was significantly increased by 28.49% compared with ZZ09, and in the Fo+Mi treatment, the browning length of the GLMFA plants was significantly increased by 183.50% compared with ZZ09. At 20 DAI, the Fo+Mi treatment significantly increased browning lengths in GLMFA (276.23%) and GLMFB (111.07%) compared to ZZ09. The browning lengths in GLMFA and GLMFB were also significantly higher than ZZ09, with increases of 102.19% and 284.67%, respectively. At 30 DAI, the Fo and Fo+Mi treatments of the GLMFA significantly increased by 146.51% and 152.67%, respectively, compared with the same treatment in Zhongza 09. In contrast, the Fo and Fo+Mi treatments of the GLMFB plant significantly increased by 331.01% and 111.07% compared with those of Zhongza 09, respectively.

Table 5.
The effects of Fusarium oxysporum and Meloidogyne incognita on browning length (cm) of infected tomato stem at 10, 20, and 30 DAI.
3.8. Histopathological Changes of Tomato Roots in Different Stages of Root-Knot Nematode and Fusarium Oxysporum Co-Infection
At 10 DAI, the cortical cells containing hyphae in the roots of ZZ09, GLMFA, and GLMFB plants treated with Fo+Mi were significantly increased, but the cortical cells of the GLMFA and GLMFB roots were more shrunken than those of ZZ09 (Figure 13A,D,G). The phloem of ZZ09 and GLMFA contained a small number of hyphae in the vascular bundle, while the pericycle cells of GLMFB contained the mycelial segments. The phloem cells of the three tomatoes were not shrunken or damaged due to hyphal infection, except for the deformity due to the development of gall cells (GCs) (Figure 13B,E,H). At 10 DAI, the cross-sectional area of giant cells (GCs) in the roots of all three tomatoes increased, severely displacing the xylem in the vascular bundles, making it difficult to distinguish the primary xylem. The root-knot sections showed that the enlarged GCs occupied most of the positions in the vascular bundles, squeezing the phloem, xylem, and parenchyma cells, causing vessel structure damage (Figure 13C,F,I). Moreover, Table 6 shows the morphological measurements of intra-root cells of ZZ09, GLMFA, and GLMFB treated with Fo+Mi at 10 DAI. The GC area of ZZ09 increased by 64.93% and 125.52% compared with that of GLMFA and GLMFB, respectively. The number of nuclei was 1.63 and 2.58 times higher than that of GLMFA and GLMFB, respectively, but there was no significant difference. In addition, the cortical cell area of ZZ09 was 11.64% higher than that of GLMFA plants, but only 2.01% lower than GLMFB plants, with no significant difference between them.
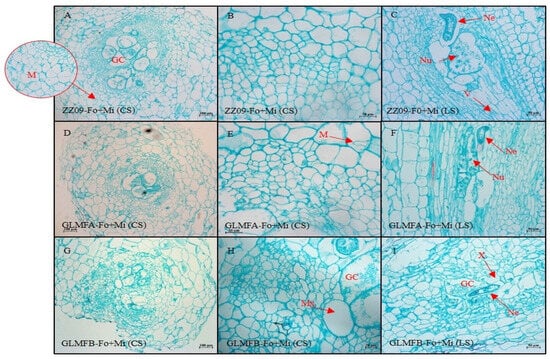
Figure 13.
(A–C) The cross-sectional and longitudinal sections of the ZZ09 cultivar showing the effects of combined infection by Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 10 DAI. (D–F) The cross-sectional and longitudinal sections of the GLMFA cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 10 DAI. (G–I) The cross-sectional and longitudinal sections of the GLMFB cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 10 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); Fo+Mi (plants were infected with both F. oxysporum and M. incognita); Cross-section (CS); longitudinal section (LS); giant cell (GC); mycelium (M); nematode (Ne); nuclei of giant cell (Nu); metaxylem (Mx); xylem (X); vessel (V).

Table 6.
The effects combined infection of Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 10 DAI.
At 20 DAI, some cortical cells in ZZ09 showed necrosis, and the non-necrotic cells shrank severely, with hyphal segments visible (Figure 14A,C). The GLMFB root’s endothelial cells showed no significant change compared to GLMFA. In GLMFA, the xylem and vessels contained hyphae in clusters, with some locations turning brown (Figure 14E), and a few pericycle and phloem cells showed browning (Figure 14F). In contrast, we found only a few mycelial segments in the abnormal xylem of ZZ09 and GLMFB plants, without any serious blockage or browning. During this period, the number of GCs containing mycelial segments in GLMFB roots began to increase, while a small number of mycelial segments were observed in individual GCs of ZZ09 (Figure 14B,I). Moreover, no hyphal invasion was observed in the root-knot nematodes in different tomato varieties (Figure 14B,H). Furthermore, Table 7 shows the morphological measurements of intra-root cells of ZZ09, GLMFA, and GLMFB treated with Fo+Mi at 20 DAI. The GC area of the Fo+Mi treatment of ZZ09 was reduced by 23.43% compared to GLMFA plants and increased by 20.55% compared to GLMFB plants, but there was no significant difference. Similarly, there was no significant difference in the number of nuclei in the GCs of the three tomato varieties observed. The cortical area of ZZ09 increased by 83.84% as compared to GLMFA and decreased by 31.87% compared to GLMFB.
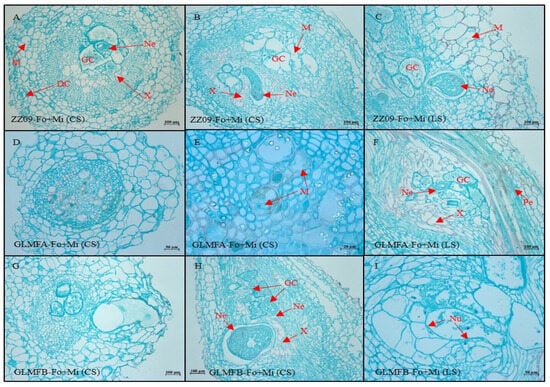
Figure 14.
(A–C) The cross-sectional and longitudinal sections of the ZZ09 cultivar showing the effects of combined infection by Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 20 DAI. (D–F) The cross-sectional and longitudinal sections of the GLMFA cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 20 DAI. (G–I) The cross-sectional and longitudinal sections of the GLMFB cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 20 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); Fo+Mi (plants were infected with both F. oxysporum and M. incognita); Cross-section (CS); longitudinal section (LS); giant cell (GC); mycelium (M); nematode (Ne); nuclei of giant cell (Nu); xylem (X); pericycl (Pe); death cell (DC).

Table 7.
The effects combined infection of Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 20 DAI.
Figure 15 shows the root cell morphology of different tomato cultivars treated with Fo+Mi at 30 DAI. No significant differences were observed in the skin cell morphology or mycelium distribution between ZZ09, GLMFA, and GLMFB. Moreover, the distribution of mycelium in the cortex of different varieties was similar. A large number of hyphae were seen in the xylem and GC of ZZ09 in the vascular bundles (Figure 15A,B). The longitudinal sections of ZZ09 showed that there was also hyphal distribution in the vessel, especially in the position where the threads were missing (Figure 15C). In GLMFA, mycelial segments were distributed around the nematode body but not within it (Figure 15E,F). In GLMFB, mycelium distribution remained similar to earlier stages, with no hyphae found in the ducts (Figure 15H,I). Moreover, the morphological indices of root cells of different tomato plants treated with Fo+Mi were observed at 30 DAI, and the results are shown in Table 8. The GC area of ZZ09 decreased by 15.88% compared with that of the GLMFA, and increased by 2.38% compared with the GLMFB, but neither was significant. Similarly, the number of nuclei of ZZ09 was 0.45 and 0.60 times less than GLMFA and GLMFB, respectively, but there was no significant difference. The cortical cell area of ZZ09 was significantly increased by 94.68% and 83.65% compared with that of GLMFA and GLMFB, respectively.
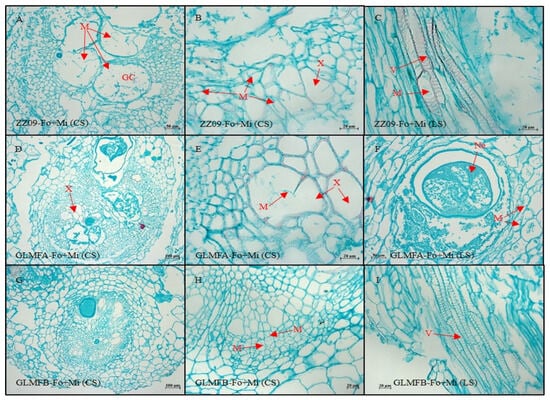
Figure 15.
(A–C) The cross-sectional and longitudinal sections of the ZZ09 cultivar showing the effects of combined infection by Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 30 DAI. (D–F) The cross-sectional and longitudinal sections of the GLMFA cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 30 DAI. (G–I) The cross-sectional and longitudinal sections of the GLMFB cultivar showing the effects of combined infection by F. oxysporum and M. incognita on root cell morphology at 30 DAI. ZZ09 (Zhongza 09 cultivar); Gailiang Maofen 802 fuzzy plants (GLMFA); Gailiang Maofen 802 non-fuzzy plants (GLMFB); Fo+Mi (plants were infected with both F. oxysporum and M. incognita); Cross-section (CS); longitudinal section (LS); giant cell (GC); mycelium (M); nematode (Ne); xylem (X); vessel (V).

Table 8.
The effects combined infection of Fusarium oxysporum and Meloidogyne incognita on root cell morphology at 30 DAI.
4. Discussion
Selecting resistant plant varieties is an effective strategy for managing soil-borne diseases. This study examines the physiological and biochemical changes in ZZ09, GLMFA, and GLMFB tomato varieties under combined infection by F. oxysporum and M. incognita, revealing their resistance mechanisms. Previous studies show that both F. oxysporum and M. incognita significantly reduce tomato plant height. Research also indicates that combined infections by soil-borne pathogens cause more severe damage than single infections, highlighting the importance of addressing these issues in crop management. In our experiment, the plant height growth rate of ZZ09, GLMFA, and GLMFB plants in Fo, Mi, and Fo+Mi treatments decreased compared to the control at 10, 20, and 30 DAI. Moreover, the growth rate of plant height in the Fo+Mi treatment decreased significantly, indicating that the combined infection of F. oxysporum and M. incognita severely inhibited plant height. The reduction in plant growth in GLMFA and GLMFB was greater compared to ZZ09, reflecting that ZZ09 had a stronger tolerance to combined infection.
Photosynthesis is essential for plant energy and defense, with photosynthetic parameters reflecting a plant’s tolerance to stresses. Studies show that combined infections of F. oxysporum and M. incognita reduce chlorophyll content and photosynthesis. Our results align with those of Meena et al. [49] and Ramalingam [50], demonstrating that the combined infection of F. oxysporum and M. incognita significantly impacts the photosynthetic traits of plants such as Dianthus caryophyllus and tomato. Similarly, the present study showed that the net photosynthetic rate (Pn) in ZZ09, GLMFA, and GLMFB plants was significantly reduced, at 10 DAI under combined infection. At 20 and 30 DAI, GLMFA showed greater reductions in Pn, while ZZ09 exhibited no significant difference, suggesting higher resistance to the pathogens. These findings highlight the varying tolerance of plant varieties to combined pathogen stress, which is important for developing resistant cultivars. Leaf stomatal closure is a plant’s first line of defense against pathogens [51]. The previous study showed that the photosynthetic efficiency of susceptible varieties decreased, but there was no significant change in resistant varieties due to the difference in stomatal regulation between resistant and susceptible wheat varieties [52]. The concentration of CO2 and water (GTC and GTW) in leaves reflects the degree of stomatal opening and closing. At 30 DAI, the current study found that the GTC and GTW values of ZZ09 with Fo+Mi treatment were significantly reduced by 80.00% and 77.42%, respectively. The combined infection of F. oxysporum and M. incognita resulted in a lower stomatal opening of ZZ09 compared to GLMFA and GLMFB, potentially due to the activation of ZZ09’s defense mechanism.
The plants reduce water loss by closing stomata and decreasing the transpiration rate (Tr) under stress [53]. The present study also showed that the Tr of plants was directly proportional to the degree of disease and inversely proportional to the degree of resistance. Our results indicate that the Tr values of different tomato varieties and treatment plants showed a trend of first increasing and then decreasing. The reduction in the Tr value in ZZ09 plants was significantly less than that in GLMFA and GLMFB plants. It shows that ZZ09 has a higher degree of resistance to the combined infection of F. oxysporum and M. incognita than GLMFA and GLMFB plants.
Reactive oxygen species (ROS) are produced by plants when they are subjected to certain stresses [54]. ROS-induced damage is a major factor contributing to harm in plants under biotic stresses such as soil-borne pathogens [35]. Several antioxidant enzymes are activated in in infected plants to counteract the detrimental effects of ROS [55]. Feng et al. [56] reported that the activities of SOD, CAT, POD, and PPO were significantly increased in ginseng after F. oxysporum infection. This phenomenon is considered one of the defensive effects of ginseng against F. oxysporum [56]. In the present study, the SOD activity of the GLMFA and GLMFB plants was not significant compared with the control, and we speculated that the ability of the GLMFA and GLMFB to synthesize SOD during the compound infection was lower and the damage was greater.
Plant disease resistance positively correlates with POD’s ability to scavenge excess ROS in plants [57]. In the present study, Fo+Mi treatment increased POD activity in ZZ09 plants at 20 and 30 DAI, while it reduced POD in GLMFA plants at 10 DAI and had no significant effect on GLMFB plants. ZZ09 demonstrated sustained and substantial POD synthesis during compound infection, indicating a stronger resistance to the combined infection compared to GLMFA and GLMFB.
When a pathogen infects plant cells, they produce more ROS; however, excessive ROS can cause lipid peroxidation in the cell membrane, leading to MDA production and plant damage [55]. The present study revealed that ZZ09 has the ability to eliminate the burst of reactive oxygen species, resulting in a low level of MDA content in the plant, thereby reducing pathogen infection damage compared to GLMFA and GLMFB.
Proline is an important osmoregulatory substance that helps plants retain water under stress [58]. The present study showed that the proline content in ZZ09 decreased significantly under Fo+Mi treatment, while GLMFA and GLMFB showed no significant change. We speculate that the compound infection may have a greater impact on the GLMFA and GLMFB compared to ZZ09. Therefore, they maintain a certain level of proline content to maintain the progress of life activities, and the specific reasons require further research.
This study evaluated the gall index and the Fusarium wilt index of various tomato varieties, either alone or in combination. The gall index and the Fusarium wilt index of ZZ09 plants were significantly lower than those of GLMFA and GLMFB plants, demonstrating that ZZ09 has a higher degree of resistance to the combined infection of the two pathogens. In the experiment, we noticed that the GLMFA plants’ leaves became more affected after inoculation with the pathogen. The chlorophyll content directly reflects the degree of leaf yellowing. In a previous study, Hashem et al. [59] reported that the chlorophyll content of tomatoes inoculated with F. oxysporum (F) was significantly lower than that of healthy plants (CK), while the chlorophyll content of tomatoes inoculated with arbuscular mycorrhizal fungi (AMF) was not significantly different from that of CK, but the chlorophyll content of tomatoes inoculated with F. oxysporum+arbuscular mycorrhizal fungi (F+AMF) was significantly higher than that of F but still significantly lower than CK, proving that arbuscular mycorrhizal fungi have a preventive effect on tomato Fusarium wilt. Our study showed that the chlorophyll content of the three tomatoes was significantly decreased in Fo+Mi treatment, and the decrease in ZZ09 was significantly lower than that of GLMFA and GLMFB plants. During the observation period, the inhibition of chlorophyll synthesis in GLMFA and GLMFB plants was greater than that of ZZ09, indicating that ZZ09 was significantly less affected by the combined infection of F. oxysporum and M. incognita.
Fusarium wilt-infected stems showed browning of the vascular bundles [60]. According to some studies, Fusarium wilt disease grades are based on the length of the stem divided by the length of the browning of vascular bundles [61,62]. The severity of the disease is directly related to the length of the browning of vascular bundles in tomato stems after being infected with F. oxysporum [63]. Our investigation demonstrated that the rate of browning in ZZ09 plants treated with Fo+Mi was much lower than GLMFA and GLMFB plants. The ZZ09 plants showed better tolerance when exposed to F. oxysporum alone or in combination with M. incognita.
Studies at the histopathological level have elucidated the process of host colonization and infection by F. oxysporum or M. incognita alone, but there are few reports on the combined infection of these two pathogens. The present study observed the root paraffin sections of different tomato varieties treated with Fo+Mi under a microscope and clarified the pathological differences between the interaction of the two pathogens in the tomato roots of ZZ09, GLMFA, and GLMFB. This study found that there was no significant difference between the roots of ZZ09, GLMFA, and GLMFB in terms of the average area and number of giant cell (GC) nuclei. Our results are in line with those of Wang et al. [64], who found that both resistant and susceptible varieties of tobacco produced GCs in the roots after inoculation with M. incognita, but there was no significant difference in the number, size, or number of nuclei in the GCs. Moreover, the plants’ resistance against pathogens can manifest in multiple aspects, such as inhibiting pathogen invasion, feeding, and development [65]. The present study observed no giant cell disintegration or nematode death in tomato roots within 30 days of pathogen inoculation, indicating certain limitations in the evaluation of giant cell development due to differences in the resistance of ZZ09, GLMFA, and GLMFB to combined infection. The F. oxysporum infection process can be divided into two stages: the first stage involves the attachment of hyphae to the root surface, followed by subsequent colonization in the epidermis, cortex, endothelial layer tissues, and vascular bundles [9]. The second stage involves fungal invasion into the xylem vessels and development upwards along the vessels, ultimately leading to colonization throughout the entire vascular system [66]. In another previous study, Zhang et al. [67] reported that the degree of plant resistance to Fusarium wilt was closely related to the distribution of xylem, the number of vessels, and the thickness of the cell wall in the vascular bundles. Moreover, compared with susceptible varieties, the distribution of vessels in resistant varieties was more dispersed, and the vascular bundles were smaller. In the histopathological analysis of F. oxysporum and M. incognita compound infections, this study recorded the infection dynamics of F. oxysporum in the roots and its impact on cells in the roots and observed the infection of F. oxysporum in the roots of different varieties during the compound infection. The GLMFA plant exhibited an earlier distribution of mycelium in the endothelial layer and vascular bundle as compared to ZZ09. In addition, mycelium initially infected the xylem of the GLMFA plant, and its reproduction rate in the cortex and vascular bundle also occurred earlier. Moreover, the cortical cells of the GLMFB showed a large area of shrinkage and even necrosis, as compared to ZZ09. The current study demonstrated that F. oxysporum and M. incognita infections caused more severe damage to GLMFA and GLMFB plants compared to ZZ09 plants.
5. Conclusions
Based on the findings of the current research study, we conclude that the combined infection of F. oxysporum and M. incognita notably impacted the growth and physiological responses of tomato plants, with ZZ09 showing superior resistance compared to GLMFA and GLMFB. ZZ09 exhibited a smaller reduction in chlorophyll content, more stable photosynthetic activity, and a stronger antioxidant response, indicating better tolerance to the pathogens. Additionally, disease indices such as Fusarium wilt and gall index were significantly lower in ZZ09, further confirming its higher resistance. This research highlights the potential of ZZ09 as a candidate for breeding more resilient tomato cultivars. The current work lays the groundwork for the development of efficient techniques to improve the quality of future breeding programs, aiming to generate commercially viable resistant cultivars. Additionally, it provides valuable insights for the formulation of pesticides to effectively control these two soil-borne diseases simultaneously. However, more studies are required to clarify the molecular understanding of the relationship between F. oxysporum and M. incognita.
Author Contributions
Conceptualization, A.S. and H.W.; methodology, A.S., Y.M., B.C. and Y.N.; software, A.S.; validation, A.S. and B.C.; formal analysis, A.S. and Y.N.; investigation, A.S. and B.C.; resources, H.W.; data curation, A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.S., Y.N. and H.W.; visualization, A.S.; supervision, H.W.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (32160627), Guangxi Innovation Team of National Modern Agricultural Technology System (nycytxgxcxtd-2023-10-03), the Guangxi Natural Science Foundation (2020GXNSFDA297003) and the Special Funds for Guiding Local Scientific and Technological Development by the Central Government of China (Hezhou) (ZY2023005).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Singh, S.; Bhoi, T.K.; Majhi, P.K.; Vyas, V.; Singh, I.; Khan, I.; Rathi, A. Emergent tools and techniques in diagnosis of soil-borne phytopathogens. In Detection, Diagnosis and Management of Soil-Borne Phytopathogens; Singh, U.B., Kumar, R., Singh, H.B., Eds.; Springer: Singapore, 2023; pp. 41–66. [Google Scholar] [CrossRef]
- Colla, P.; Gilardi, G.; Gullino, M.L. A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica 2012, 40, 515–523. [Google Scholar] [CrossRef]
- Stirling, G.; Hayden, H.; Pattison, T.; Stirling, M. Soil Health, Soil Biology, Soilborne Diseases and Sustainable Agriculture: A Guide; Csiro Publishing: Oakland, CA, USA, 2016; p. 280. [Google Scholar] [CrossRef]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, ecological characteristics and identification of some problematic phytopathogenic Fusarium in soil: A review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Saremi, H.; Okhovvat, S.; Ashrafi, S. Fusarium diseases as the main soil borne fungal pathogen on plants and their control management with soil solarization in Iran. Afr. J. Biotechnol. 2011, 10, 18391–18398. [Google Scholar] [CrossRef]
- Timmusk, S.; Nevo, E.; Ayele, F.; Noe, S.; Niinemets, Ü. Fighting Fusarium pathogens in the era of climate change: A conceptual approach. Pathogens 2020, 9, 419. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathol. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311. [Google Scholar] [CrossRef]
- Fernandes, L.B.; Ghag, S.B. Molecular insights into the jasmonate signaling and associated defense responses against wilt caused by Fusarium oxysporum. Plant Physiol. Biochem. 2022, 174, 22–34. [Google Scholar] [CrossRef]
- Cai, H.; Yu, N.; Liu, Y.; Wei, X.; Guo, C. Meta-analysis of fungal plant pathogen Fusarium oxysporum infection-related gene profiles using transcriptome datasets. Front. Microbiol. 2022, 13, 970477. [Google Scholar] [CrossRef]
- De Lamo, F.J.; Takken, F.L. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 500488. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Xuan, Y.; Chen, L.; Duan, Y. Review article: Meloidogyne incognita (root-knot nemtaode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Tapia-Vázquez, I.; Montoya-Martínez, A.C.; De los Santos-Villalobos, S.; Ek-Ramos, M.J.; Montesinos-Matías, R.; Martínez-Anaya, C. Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. World J. Microbiol. Biotechnol. 2022, 38, 26. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Zhang, M.; Yang, R.; Liu, D.; Zhu, X.; Liu, X.; Fan, H.; Duan, Y.; Wang, Y. Analysis of gene expression in cucumber roots interacting with Penicillium chrysogenum strain Snef1216 through seed coating, which induced resistance to Meloidogyne incognita. Nematology 2021, 24, 121–135. [Google Scholar] [CrossRef]
- Jagdale, S.; Rao, U.; Giri, A.P. Effectors of root-knot nematodes: An arsenal for successful parasitism. Front. Plant Sci. 2021, 12, 800030. [Google Scholar] [CrossRef]
- Ploeg, A.T.; Edwards, S. Host status of melon, carrot, and Meloidogyne incognita-susceptible and-resistant cotton, cowpea, pepper, and tomato for M. floridensis from California. J. Nematol. 2024, 56, e2023. [Google Scholar] [CrossRef]
- Cabianca, A.; Ruthes, A.C.; Pawlowski, K.; Dahlin, P. Tomato sterol 22-desaturase gene CYP710A11: Its roles in Meloidogyne incognita infection and plant stigmasterol alteration. Int. J. Mol. Sci. 2022, 23, 15111. [Google Scholar] [CrossRef]
- Patil, J.; Yadav, S.; Kumar, A. Management of root-knot nematode, Meloidogyne incognita and soil borne fungus, Fusarium oxysporum in cucumber using three bioagents under polyhouse conditions. Saudi J. Biol. Sci. 2021, 28, 7006–7011. [Google Scholar] [CrossRef]
- Rutter, W.B.; Franco, J.; Gleason, C. Rooting out the mechanisms of root-knot nematode–plant interactions. Annu. Rev. Phytopathol. 2022, 60, 43–76. [Google Scholar] [CrossRef]
- Caccia, M.; Marro, N.; Novák, V.; Ráez, J.A.L.; Castillo, P.; Janoušková, M. Divergent colonization traits, convergent benefits: Different species of arbuscular mycorrhizal fungi alleviate Meloidogyne incognita damage in tomato. Mycorrhiza 2024, 34, 145–158. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Belal, E.B.; El-Khateeb, N.M.; Shreef, B.A. Utilizing bio-synthesis of nanomaterials as biological agents for controlling soil-borne diseases in pepper plants: Root-knot nematodes and root rot fungus. BMC Plant Biol. 2024, 24, 110. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Riad, S.N.; Abo-Elyousr, K.A.; Nashwa, S.M.; Khalil Bagy, H.M.; Abdelrazek, S.; Abdellatif, A.A. Unraveling the Mysteries of Mycorrhiza-Plant Interactions: Mechanisms of Protection and Ecological Factors Influencing Symbioses. In Mycorrhizal Symbiosis and Agroecosystem Restoration; Ansari, R.A., Rizvi, R., Mahmood, I., Eds.; Springer: Singapore, 2024; pp. 197–226. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Mukhopadhyay, A.; Paul, S.; De, N.; Singh, R.K. Urea and Trichoderma harzianum Loaded Clay-Polymer Composite in Conjunction with Farmyard Manure Sustains Soil Moisture and Nitrogen Availability to Manage Fusarium-Meloidogyne Disease Complex in Lentil (Lens culinaris). Commun. Soil Sci. Plant Anal. 2024, 55, 1071–1091. [Google Scholar] [CrossRef]
- Yaseen, I.; Mukhtar, T.; Kim, H.-T.; Arshad, B. Interactive effects of Meloidogyne incognita and Fusarium oxysporum f. sp. vasinfectum on okra cultivars. Bragantia 2024, 83, e20230266. [Google Scholar] [CrossRef]
- Kassie, Y.G. Status of root-knot nematode (Meloidogyne Species) and Fusarium wilt (Fusarium oxysporum) disease complex on tomato (Solanum lycopersicum L.) in the central Rift Valley, Ethiopia. Agric. Sci. 2019, 10, 1090. [Google Scholar] [CrossRef]
- Maqsood, A.; Wu, H.; Kamran, M.; Altaf, H.; Mustafa, A.; Ahmar, S.; Hong, N.T.T.; Tariq, K.; He, Q.; Chen, J.-T. Variations in growth, physiology, and antioxidative defense responses of two tomato (Solanum lycopersicum L.) cultivars after co-infection of Fusarium oxysporum and Meloidogyne Incognita. Agronomy 2020, 10, 159. [Google Scholar] [CrossRef]
- Kassie, Y.G.; Ebrahim, A.S.; Mohamed, M.Y. Interaction effect between Meloidogyne incognita and Fusarium oxysporum f. sp. lycopersici on selected tomato (Solanum lycopersicum L.) genotypes. Afr. J. Agric. Res. 2020, 15, 330–342. [Google Scholar] [CrossRef]
- Lucas, P. Diseases caused by soil-borne pathogens. In The Epidemiology of Plant Diseases; Cooke, B., Jones, D., Kaye, B., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 373–386. [Google Scholar] [CrossRef]
- Lorrai, R.; Ferrari, S. Host cell wall damage during pathogen infection: Mechanisms of perception and role in plant-pathogen interactions. Plants 2021, 10, 399. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A.; Mallick, N. Biochemical characterization of oxidative burst during interaction between Solanum lycopersicum and Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 2008, 72, 56–61. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Cheng, Y.; Aslam, M.; Jakada, B.H.; Wai, M.H.; Ye, K.; He, X.; Luo, T.; Ye, L.; Dong, C. ROS and oxidative response systems in plants under biotic and abiotic stresses: Revisiting the crucial role of phosphite triggered plants defense response. Front. Microbiol. 2021, 12, 631318. [Google Scholar] [CrossRef]
- Bajaj, S.; Singh, S.; Sharma, P. Chapter 19—Role of antioxidants in neutralizing oxidative stress. In Nutraceutical Fruits and Foods for Neurodegenerative Disorders; Keservani, R.K., Kesharwani, R.K., Emerald, M., Sharma, A.K., Eds.; Academic Press: London, UK, 2024; pp. 353–378. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Biswas, K.; Adhikari, S.; Tarafdar, A.; Kumar, R.; Saha, S.; Ghosh, P. Reactive Oxygen Species and Antioxidant Defence Systems in Plants: Role and Crosstalk Under Biotic Stress. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 265–292. [Google Scholar] [CrossRef]
- Baker, A.; Lin, C.-C.; Lett, C.; Karpinska, B.; Wright, M.H.; Foyer, C.H. Catalase: A critical node in the regulation of cell fate. Free Radic. Biol. Med. 2023, 199, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Chellemi, D.; Gamliel, A.; Katan, J.; Subbarao, K. Development and deployment of systems-based approaches for the management of soilborne plant pathogens. Phytopathology 2016, 106, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef]
- Sikandar, A.; Gao, F.; Mo, Y.; Chen, Q.; Ullah, R.M.; Wu, H. Efficacy of Aspergillus tubingensis GX3′ Fermentation against Meloidogyne enterolobii in Tomato (Solanum lycopersicum L.). Plants 2023, 12, 2724. [Google Scholar] [CrossRef]
- Mo, Y.X.; Kan, Y.Z.; Jia, L.M.; Cao, X.T.; Sikandar, A.; Wu, H.Y. Characterization and Effect of a Nematophagous Fungus Talaromyces cystophila sp. nov. for the Biological Control of Corn Cyst Nematode. Phytopathology 2024, 114, 618–629. [Google Scholar] [CrossRef]
- Chen, B.; Sikandar, A.; Ahmad, S.; Luo, M.; Wu, H. Meloidogyne graminicola’s Effect on Growth Performance of Rice under Low Population Density. Agronomy 2022, 12, 587. [Google Scholar] [CrossRef]
- Sikandar, A.; Wu, F.; He, H.; Ullah, R.M.; Wu, H. Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii. Plants 2024, 13, 293. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for Determination of Proline in Plants; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 317–331. [Google Scholar]
- Ma, X.; Qiao, J.; Ma, R. Effect of Phellodendron chinense extraction on inhibiting postharvest pathogens (Alternatia alternata and Penicillium expansum) of cherry tomato. Food Ferment. Ind. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Zeck, W. Ein bonitierungsschema zur feldauswertung von wurzelgallenbefall. Pflanzenschutz–Nachrichten Bayer 1971, 24, 144–147. [Google Scholar]
- Purwati, R.D.; Hidayah, N. Inoculation methods and conidial densities of Fusarium oxysporum f. sp. cubense in Abaca. HAYATI J. Biosci. 2008, 15, 1–7. [Google Scholar] [CrossRef]
- Meena, K.S.; Ramyabharathi, S.; Raguchander, T.; Jonathan, E. Interaction of Meloidogyne incognita and Fusarium oxysporum in carnation and physiological changes induced in plants due to the interaction. SAARC J. Agric. 2016, 14, 59–69. [Google Scholar]
- Ramalingam, K. Exploring the disease severity by the interaction of fusarium wilt and root knot nematode in tomato. Int. J. Fauna Biol. Stud. 2019, 6, 1–5. [Google Scholar]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Chen, W.; Liu, T.; Zhong, S.; Ma, L.; Zhang, M.; Zhang, H.; Yu, D.; Luo, P. Wheat resistance to fusarium head blight is associated with changes in photosynthetic parameters. Plant Dis. 2016, 100, 847–852. [Google Scholar] [CrossRef]
- Mishra, R.; Shteinberg, M.; Shkolnik, D.; Anfoka, G.; Czosnek, H.; Gorovits, R. Interplay between abiotic (drought) and biotic (virus) stresses in tomato plants. Mol. Plant Pathol. 2022, 23, 475–488. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Suman, S.; Bagal, D.; Jain, D.; Ragini, S.; Singh, I.K.; Singh, A. Chapter 14—Biotic stresses on plants: Reactive oxygen species generation and antioxidant mechanism. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 381–411. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, G.; Sun, R.; Wang, J.; Sun, T.; Xing, S.; Lian, W.; Zhao, Y. Dynamic changes in cell wall-degrading enzymes and physio-biochemistry of ginseng in response to Fusarium oxysporum infection. Eur. J. Plant Pathol. 2023, 165, 569–578. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, J.; Gu, X.; Zhao, L.; Li, B.; Wang, K.; Yang, Q.; Zhang, H. Pichia caribbica improves disease resistance of cherry tomatoes by regulating ROS metabolism. Biol. Cont. 2022, 169, 104870. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhen, B.; Lv, M.; Zhou, X.; Yong, B.; Niu, Q.; Yang, S. Proline spray relieves the adverse effects of rought on wheat flag leaf function. Plants 2024, 13, 957. [Google Scholar] [CrossRef]
- Hashem, A.; Akhter, A.; Alqarawi, A.A.; Singh, G.; Almutairi, K.F.; Abd_Allah, E.F. Mycorrhizal fungi induced activation of tomato defense system mitigates Fusarium wilt stress. Saudi J. Biol. Sci. 2021, 28, 5442–5450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, S.; Jiang, J.; Gao, C.; Qi, X.; Zhang, L.; Sun, S.; Dai, Y.; Fan, X. Synchronized efficacy and mechanism of alkaline fertilizer and biocontrol fungi for Fusarium oxysporum f. sp. cubense tropical race 4. J. Fungi 2022, 8, 261. [Google Scholar] [CrossRef]
- Li, Y.-T.; Hwang, S.-G.; Huang, Y.-M.; Huang, C.-H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Huang, C.H.; Tsai, R.T.; Vallad, G.E. Development of a TaqMan Real-Time Polymerase Chain Reaction Assay for Detection and Quantification of Fusarium oxysporum f. sp. lycopersici in Soil. J. Phytopathol. 2016, 164, 455–463. [Google Scholar] [CrossRef]
- Makhlouf, A.H.; Ammar, M.; Selim, M. Biological, histological and pathological studies of tomato wilt disease caused by Fusarium oxysporum f. sp. Lycopersici. J. Plant Prot. Pathol. 2021, 12, 615–626. [Google Scholar] [CrossRef]
- Wang, G.; Shen, Y.; Wang, J.; Wang, R. Histopathological changes of different tobacco varieties infected by Meloidogyne incognita. J. Henan Agric. Sci. 2006, 2, 61–63. [Google Scholar]
- Patel, S.; Saraf, M. Interaction of root colonizing biocontrol agents demonstrates the antagonistic effect against Fusarium oxysporum f. sp. lycopersici on tomato. Eur. J. Plant Pathol. 2017, 149, 425–433. [Google Scholar] [CrossRef]
- Zvirin, T.; Herman, R.; Brotman, Y.; Denisov, Y.; Belausov, E.; Freeman, S.; Perl-Treves, R. Differential colonization and defence responses of resistant and susceptible melon lines infected by Fusarium oxysporum race 1·2. Plant Pathol. 2010, 59, 576–585. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Liu, G.; Yao, X.; Li, P.; Yang, X. Characterization of the watermelon seedling infection process by Fusarium oxysporum f. sp. niveum. Plant Pathol. 2015, 64, 1076–1084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).