Postharvest Application of Abscisic Acid and Methyl Jasmonate on Fruit Quality of ‘Red Zaosu’ Pear
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Treatments
2.3. Color Properties and Texture
2.4. Total Soluble Solids (TSSs), Titratable Acid (TA) and Sugar Contents
2.5. Total Anthocyanin and Total Chlorophyll Contents
2.6. Total Phenolic and Total Flavonoid Contents
2.7. MDA Content and the Activities of SOD, POD, CAT and APX
2.8. Statistical Analysis
3. Results
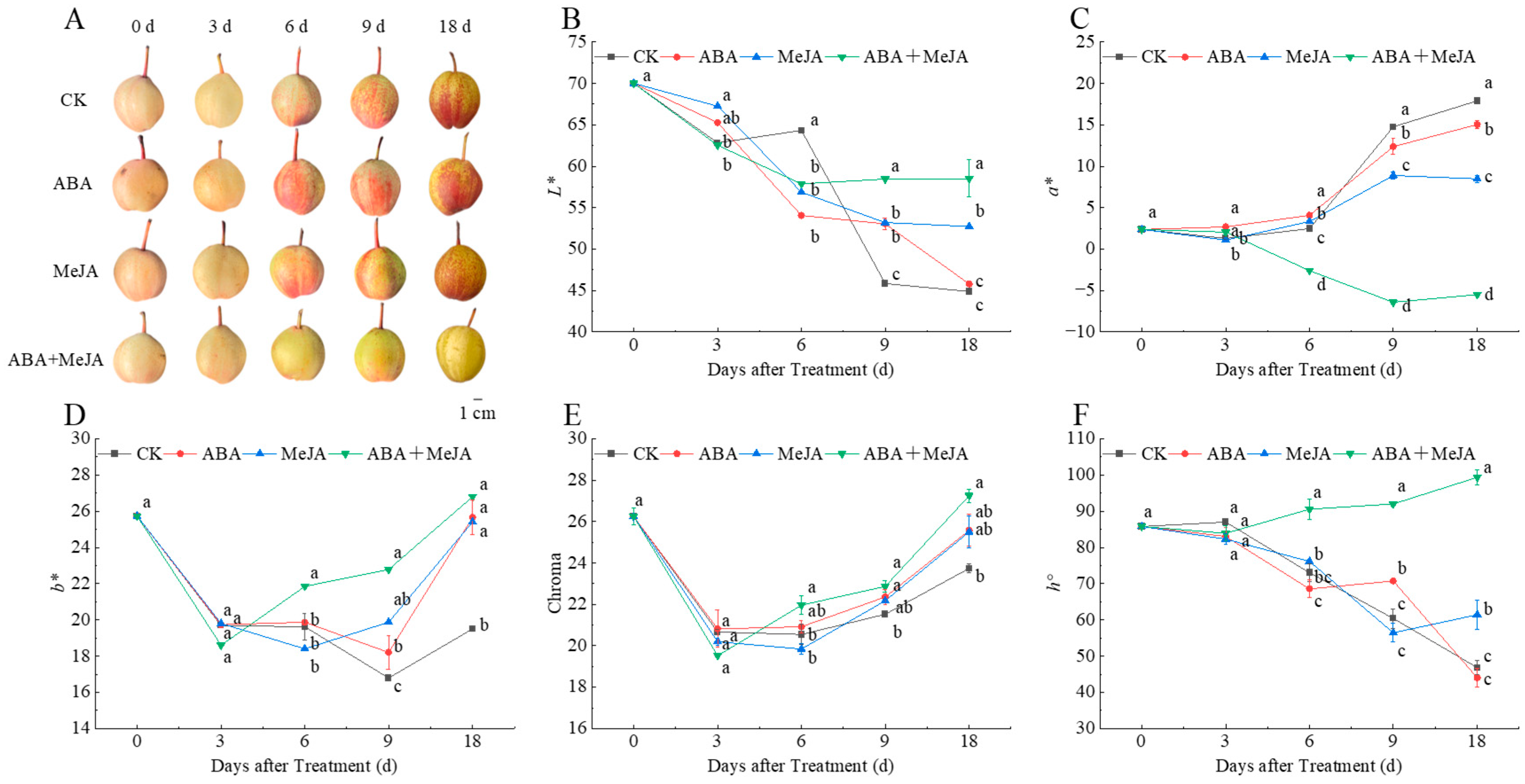
3.1. Effect of Different Treatments on Coloration of ‘Red Zaosu’ Pears
3.2. Effects of Different Treatments on Total Anthocyanin and Chlorophyll Contents of ‘Red Zaosu’ Pears
3.3. Effects of Different Treatments on Fruit Texture of ‘Red Zaosu’ Pears
3.4. Effects of Different Treatments on TSS, TA and Sugar Compositions of ‘Red Zaosu’ Pears
3.5. Effects of Different Treatments on Total Phenol and Flavonoid Contents of ‘Red Zaosu’ Pears
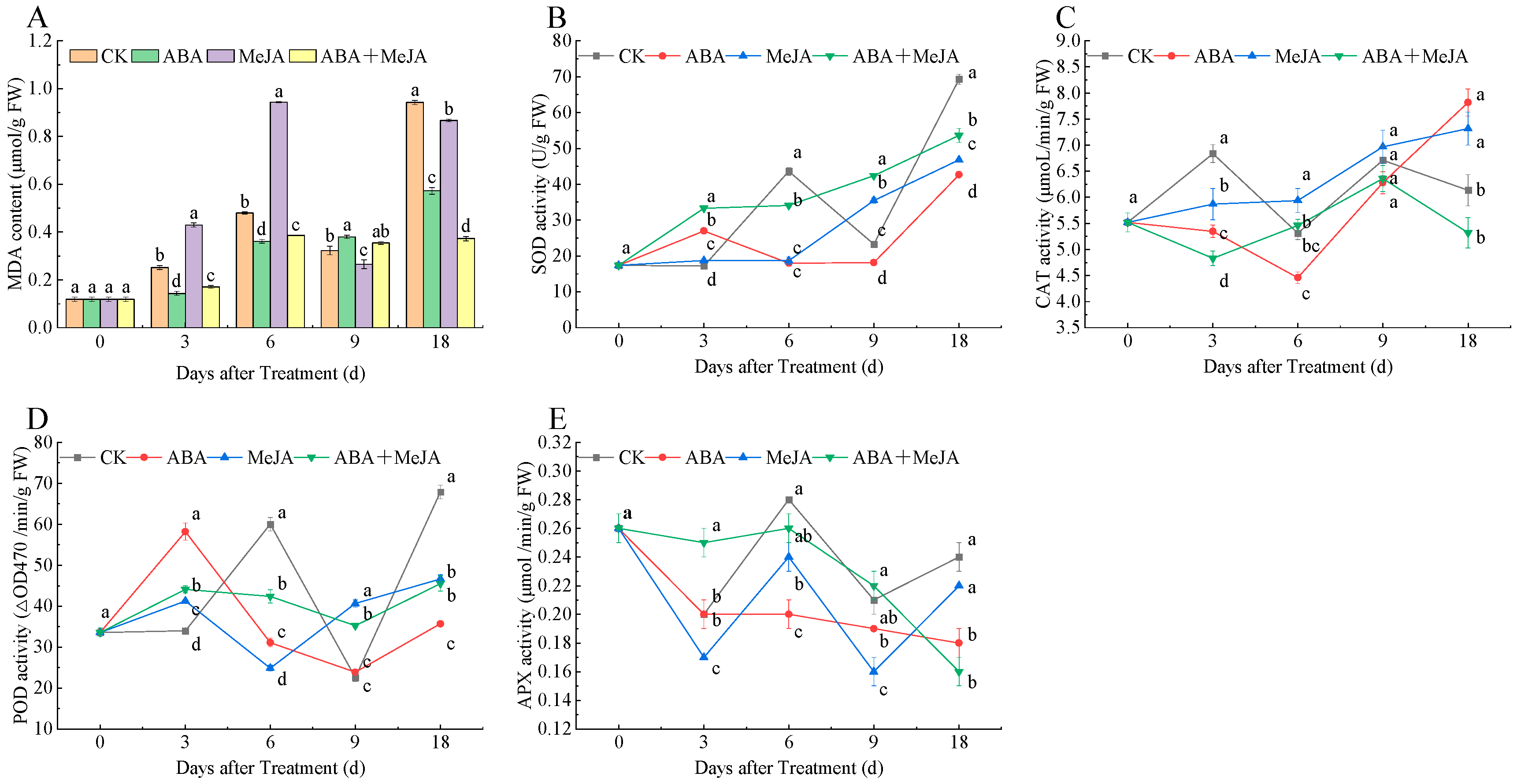
3.6. Effects of Different Treatments on MDA Content and Activities of SOD, CAT, APX, POD of ‘Red Zaosu’ Pears
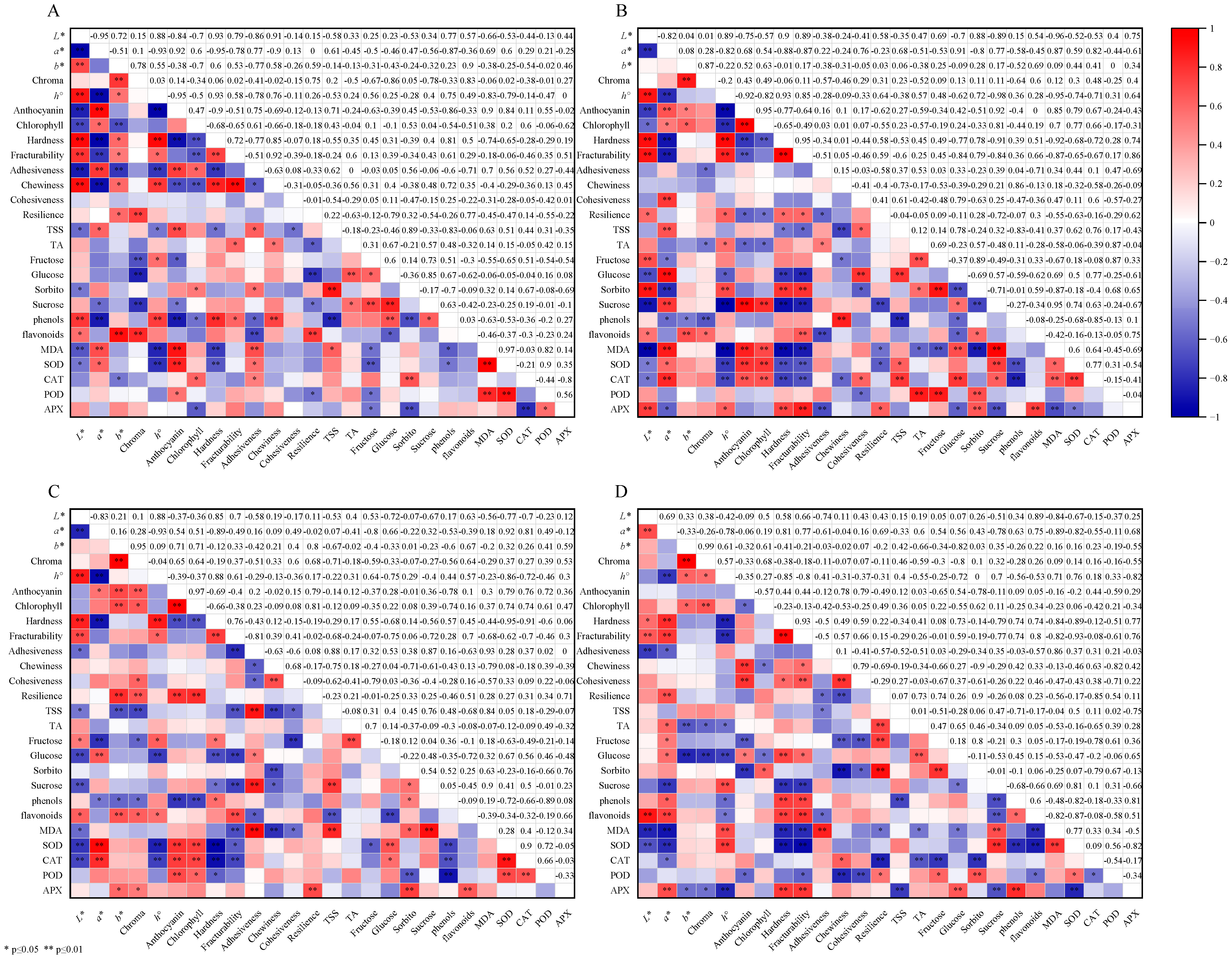
3.7. Correlation Analysis of Different Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.Q.; Tian, Y.L.; Wang, L.M.; Geng, G.M.; Zhao, W.J.; Hu, B.S.; Zhao, Y.F. Fire blight disease, a fast-approaching threat to apple and pear production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Staff, F.C. China: Fresh Deciduous Fruit Annual; USDA: Washington, DC, USA, 2023.
- Zheng, S.; Xu, R.; Wei, J.; Tian, J.; He, Q.; Zhang, F.; Li, J.; Wu, B.; Guan, J. Nitric oxide effects on postharvest and Alternaria-infected pear fruit. Postharvest Biol. Technol. 2023, 195, 112118. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. R. Soc. A 2014, 372, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Grimplet, J.; David, K.; Castellarin, S.D.; Terol, J.; Wong, D.C.J.; Luo, Z.; Schaffer, R.; Celton, J.-M.; Talon, M.; et al. Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Sci. 2018, 276, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.-P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Cao, X.; Sun, H.; Wang, X.; Li, W.; Wang, X. ABA signaling mediates 5-aminolevulinic acid-induced anthocyanin biosynthesis in red pear fruits. Sci. Hortic. 2022, 304, 111290. [Google Scholar] [CrossRef]
- Kondo, S.; Tsukada, N.; Niimi, Y.; Seto, H. Interactions between Jasmonates and Abscisic Acid in Apple Fruit, and Stimulative Effect of Jasmonates on Anthocyanin Accumulation. J. Jpn. Soc. Hortic. Sci. 2001, 70, 546–552. [Google Scholar] [CrossRef]
- Jia, H.F.; Chai, Y.M.; Li, C.L.; Lu, D.; Luo, J.J.; Qin, L.; Shen, Y.Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Chen, X.; Gong, X.; Wang, N.; Ma, L.; Qiu, Y.; Wang, Y.; Feng, S. Effects of methyl jasmonate and abscisic acid on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2017, 130, 227–237. [Google Scholar] [CrossRef]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A.; Afrifa-Yamoah, E.; Vithana, M.D.K. Preharvest methyl jasmonate application regulates ripening, colour development and improves phytochemical quality of fruits: A review. Sci. Hortic. 2025, 339, 113909. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Han, C.; Duan, R.; Yang, J.; Xue, H. Effects of Methyl Jasmonate on Fruit Coloration and Quality Improvement in Pears (Pyrus bretschneideri). Agronomy 2023, 13, 2409. [Google Scholar] [CrossRef]
- Vithana, M.D.K.; Singh, Z.; Ul Hasan, M. Pre- and Post-harvest Elicitation with Methyl Jasmonate and Salicylic Acid Followed by Cold Storage Synergistically Improves Red Colour Development and Health-Promoting Compounds in Blood Oranges. J. Plant Growth Regul. 2024, 43, 1657–1671. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Woodward, A.; Afrifa-Yamoah, E. Methyl jasmonate advances fruit ripening, colour development, and improves antioxidant quality of ‘Yoho’ and ‘Jiro’ persimmon. Food Chem. 2024, 459, 140360. [Google Scholar] [CrossRef]
- Ma, J.; Liu, S.; Zeng, J.; Zhang, Y.; Chang, W.; Meng, Z.; Zhou, Y.; Zhang, W.; Ding, X.; Pan, X.; et al. Comparative metabolome and transcriptome analyses reveal the role of MeJA in improving postharvest disease resistance and maintaining the quality of Rosa roxburghii fruit. Postharvest Biol. Technol. 2025, 220, 113314. [Google Scholar] [CrossRef]
- Lu, W.; Li, W.; Zhao, K.; Bai, X.; Zhang, Y.; Li, Q.; Xue, Z.; Wang, X.-X. Blue light irradiation combined with low-temperature storage further enhances postharvest quality of strawberries through improving antioxidant defense and cell wall metabolic activities. Food Chem. X 2025, 25, 102115. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, L.; Wang, Y.; Shi, Y.; Liu, Y.; Luo, A. Improvement of storage quality of ‘Hayward’ kiwifruit by MeJA combined with SA treatment through activation of phenylpropane metabolism. Sci. Hortic. 2023, 321, 112354. [Google Scholar] [CrossRef]
- Li, H.; Jia, W.; Li, R.; Zhao, B.; Li, W.; Shao, Y. The combined application of Debaryomyces hansenii and Bacillus atrophaeus inhibits disease development and enhances postharvest quality in litchi fruit by activating flavonoid metabolism. Biol. Control 2023, 187, 105357. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Guillén, F.; Díaz-Mula, H.M.; Martínez-Romero, D.; Serrano, M.; Valero, D. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 2. Improvement of fruit quality and antioxidant systems during postharvest storage. Postharvest Biol. Technol. 2014, 98, 115–122. [Google Scholar] [CrossRef]
- Xie, G.; Liu, N.; Zhang, Y.; Tan, S.; Xu, Y.; Luo, Z. Postharvest MeJA maintains the shelf quality of kiwifruit after cold storage by regulating antioxidant capacity and activating the disease resistance. Postharvest Biol. Technol. 2024, 211, 112827. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience HortSci 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Jia, R.; Li, X.; Wang, B.; Zhao, Z. Comparison of Fruit Texture and Storage Quality of Four Apple Varieties. Foods 2024, 13, 1563. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, T.; Zhou, J.; Huang, F.; Wang, Y.; Liu, Y.; Liu, Z.; He, W.; Li, M.; Lin, Y.; et al. Fruit sugar and organic acid composition and inheritance analysis in an intraspecific cross of Chinese cherry. LWT Food Sci. Technol. 2024, 198, 116101. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Liu, S. Evaluation of 1-methylcyclopropene (1-MCP) and low temperature conditioning (LTC) to control brown of Huangguan pears. Sci. Hortic. 2020, 259, 108738. [Google Scholar] [CrossRef]
- Men, C.; Wu, C.; Zhang, J.; Wang, Y.; Chen, M.; Liu, C.; Zheng, L. α-Lipoic acid treatment regulates enzymatic browning and nutritional quality of fresh-cut pear fruit by affecting phenolic and carbohydrate metabolism. Food Chem. 2024, 458, 140223. [Google Scholar] [CrossRef] [PubMed]
- Mattus-Araya, E.; Guajardo, J.; Herrera, R.; Moya-León, M.A. ABA Speeds Up the Progress of Color in Developing F. chiloensis Fruit through the Activation of PAL, CHS and ANS, Key Genes of the Phenylpropanoid/Flavonoid and Anthocyanin Pathways. Int. J. Mol. Sci. 2022, 23, 3854. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Cao, S.; Shang, H.; Yang, Z.; Zheng, Y. Methyl jasmonate reduces decay and enhances antioxidant capacity in Chinese bayberries. J. Agric. Food Chem. 2009, 57, 5809–5815. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, M.; Dai, R.; Liu, X.; Wang, C. Comparative Physiological and Transcriptome Analyses Reveal Mechanisms of Salicylic-Acid-Reduced Postharvest Ripening in ‘Hosui’ Pears (Pyrus pyrifolia Nakai). Plants 2023, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Feng, Y.; Yuan, S.; Zhao, X.; Wu, C.; Wang, C.; Xue, Z. Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: A review. Plant Mol. Biol. 2021, 107, 477–497. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.X. Regulation of fleshy fruit ripening: From transcription factors to epigenetic modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef]
- Zheng, X.; Mo, W.; Zuo, Z.; Shi, Q.; Chen, X.; Zhao, X.; Han, J. From Regulation to Application: The Role of Abscisic Acid in Seed and Fruit Development and Agronomic Production Strategies. Plant Physiol. Biochem. 2024, 25, 12024. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Liu, F.Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 12. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.; Cheng, Z.-M.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Ranganath, K.G. Pigments That Colour Our Fruits: An Overview. Erwerbs-Obstbau 2022, 64, 535–547. [Google Scholar] [CrossRef]
- Li, S.; Ou, C.; Wang, F.; Zhang, Y.; Ismail, O.; Elaziz, Y.S.G.A.; Edris, S.; Li, H.; Jiang, S. Ppbbx24-del mutant positively regulates light-induced anthocyanin accumulation in the ‘Red Zaosu’ pear (Pyrus pyrifolia White Pear Group). J. Integr. Agric. 2024. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Huang, C.; Yu, B.; Su, J.; Shu, Q.; Teng, Y. A Study on Coloration Physiology of Fruit in Two Red ChineseSand Pear Cultivars ‘Meirensu’ and ‘Yunhongli No.1’. Sci. Agric. Sin. 2010, 43, 1433–1440. [Google Scholar]
- An, J.P.; Zhang, X.W.; Liu, Y.J.; Wang, X.F.; You, C.X.; Hao, Y.J. ABI5 regulates ABA-induced anthocyanin biosynthesis by modulating the MYB1-bHLH3 complex in apple. J. Exp. Bot. 2021, 72, 1460–1472. [Google Scholar] [CrossRef]
- Li, D.; Luo, Z.; Mou, W.; Wang, Y.; Ying, T.; Mao, L. ABA and UV-C effects on quality, antioxidant capacity and anthocyanin contents of strawberry fruit (Fragaria ananassa Duch.). Postharvest Biol. Technol. 2014, 90, 56–62. [Google Scholar] [CrossRef]
- Ovadia, R.; Michal, O.-S.; Tatiana, K.; Yohanan, Z.; Amnon, L.; Lurie, S. Effects of plant growth regulators and high temperature on colour development in ‘Crimson Seedless’ grapes. J. Hortic. Sci. Biotechnol. 2013, 88, 387–392. [Google Scholar] [CrossRef]
- Lu, W.; Mao, L.; Chen, J.; Han, X.; Ren, X.; Ying, T.; Luo, Z. Interaction of abscisic acid and auxin on gene expression involved in banana ripening. Acta Physiol. Plant. 2018, 40, 46. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Luo, Z.; Mou, W.; Mao, L.; Ying, T. Comparative Transcriptome Analysis Reveals the Influence of Abscisic Acid on the Metabolism of Pigments, Ascorbic Acid and Folic Acid during Strawberry Fruit Ripening. PLoS ONE 2015, 10, e0130037. [Google Scholar] [CrossRef]
- Huang, C.; Yu, B.; Teng, Y.; Su, J.; Shu, Q.; Cheng, Z.; Zeng, L. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci. Hortic. 2009, 121, 149–158. [Google Scholar] [CrossRef]
- Shafiq, M.; Singh, Z.; Khan, A.S. Time of methyl jasmonate application influences the development of ‘Cripps Pink’ apple fruit colour. J. Sci. Food Agric. 2013, 93, 611–618. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Tang, W.; Chen, J.; Ge, Y.; Li, J. Concentration-dependent impacts of exogenous methyl jasmonate (MeJA) on chlorophyll degradation of apple fruit during ripening. Postharvest Biol. Technol. 2023, 203, 112398. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Marín-San Román, S.; Jofré, V.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Effects on chlorophyll and carotenoid contents in different grape varieties (Vitis vinifera L.) after nitrogen and elicitor foliar applications to the vineyard. Food Chem. 2018, 269, 380–386. [Google Scholar] [CrossRef]
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid During Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Qin, B.; Yin, Y.; An, W.; Mu, Z.; Cao, Y. ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol. 2019, 19, 317. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.-J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.-S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef]
- Huang, L.; Fan, J.; Han, C.; Du, C.; Wei, Z.; Du, D. Methods and instruments for the evaluation of food texture: Advances and perspectives. Food Res. Int. 2025, 208, 116162. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef]
- Kou, X.; Yang, S.; Chai, L.; Wu, C.; Zhou, J.; Liu, Y.; Xue, Z. Abscisic acid and fruit ripening: Multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci. Hortic. 2021, 281, 109999. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef]
- Zhai, Z.; Xiao, Y.; Wang, Y.; Sun, Y.; Peng, X.; Feng, C.; Zhang, X.; Du, B.; Zhou, X.; Wang, C.; et al. Abscisic acid-responsive transcription factors PavDof2/6/15 mediate fruit softening in sweet cherry. Plant Physiol. 2022, 190, 2501–2518. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Zhang, J.; Ge, Y.; Li, C.; Meng, K.; Li, J. Effects of methyl jasmonate on expression of genes involved in ethylene biosynthesis and signaling pathway during postharvest ripening of apple fruit. Sci. Hortic. 2018, 229, 157–166. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Liu, X.F.; Fu, B.L.; Zhang, Q.Y.; Tong, Y.; Wang, J.; Wang, W.Q.; Grierson, D.; Yin, X.R. Methyl Jasmonate Enhances Ethylene Synthesis in Kiwifruit by Inducing NAC Genes That Activate ACS1. J. Agric. Food Chem. 2020, 68, 3267–3276. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Zuo, J.; Gao, L.; Lv, J.; Wang, Q. Methyl jasmonate delays postharvest ripening and senescence in the non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2016, 120, 76–83. [Google Scholar] [CrossRef]
- Rana, S.S.; Pradhan, R.C.; Mishra, S. Variation in properties of tender jackfruit during different stages of maturity. J. Food Sci. Technol. 2018, 55, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, Z.; Zhang, Y.; Chai, L.; Yi, H.; Deng, X. An integrative analysis of the transcriptome and proteome of the pulp of a spontaneous late-ripening sweet orange mutant and its wild type improves our understanding of fruit ripening in citrus. J. Exp. Bot. 2014, 65, 1651–1671. [Google Scholar] [CrossRef]
- Cao, K.; Wei, Y.; Chen, Y.; Jiang, S.; Chen, X.; Wang, X.; Shao, X. PpCBF6 is a low-temperature-sensitive transcription factor that binds the PpVIN2 promoter in peach fruit and regulates sucrose metabolism and chilling injury. Postharvest Biol. Technol. 2021, 181, 111681. [Google Scholar] [CrossRef]
- Cantín, C.M.; Fidelibus, M.W.; Crisosto, C.H. Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol. Technol. 2007, 46, 237–241. [Google Scholar] [CrossRef]
- Ozturk, B.; Yıldız, K.; Ozkan, Y. Effects of Pre-Harvest Methyl Jasmonate Treatments on Bioactive Compounds and Peel Color Development of “Fuji” Apples. Int. J. Food Prop. 2015, 18, 954–962. [Google Scholar] [CrossRef]
- Ziosi, V.; Bonghi, C.; Bregoli, A.M.; Trainotti, L.; Biondi, S.; Sutthiwal, S.; Kondo, S.; Costa, G.; Torrigiani, P. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J. Exp. Bot. 2008, 59, 563–573. [Google Scholar] [CrossRef]
- Janoudi, A.; Flore, J.A. Effects of multiple applications of methyl jasmonate on fruit ripening, leaf gas exchange and vegetative growth in fruit trees. J. Hortic. Sci. Biotechnol. 2003, 78, 793–797. [Google Scholar] [CrossRef]
- Olivares, D.; Contreras, C.; Muñoz, V.; Rivera, S.; González-Agüero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 2017, 115, 286–297. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Yamamoto, L.Y.; de Assis, A.M.; Roberto, S.R.; Bovolenta, Y.R.; Nixdorf, S.L.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera × Vitis labrusca) for color improvement: Effects on color, phenolic composition and antioxidant capacity of their grape juice. Food Res. Int. 2015, 77, 572–583. [Google Scholar] [CrossRef]
- Masia, A. Superoxide dismutase and catalase activities in apple fruit during ripening and post-harvest and with special reference to ethylene. Physiol. Plant. 1998, 104, 668–672. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Superoxide Dismutase Activities in Senescing Apple Fruit (Malus domestica Borkh.). J. Food Sci. 1994, 59, 581–584. [Google Scholar] [CrossRef]
- Baker, J.E. Superoxide Dismutase in Ripening Fruits. Plant Physiol. 1976, 58, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 112314. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Z.; Wang, J.; Fu, Y.; Zhang, Z.; Khan, M.R.; Cong, X. Effect of exogenous melatonin on postharvest storage quality of passion fruit through antioxidant metabolism. LWT Food Sci. Technol. 2024, 194, 115835. [Google Scholar] [CrossRef]
- Wang, Y.S.; Tian, S.P.; Xu, Y.; Qin, G.Z.; Yao, H. Changes in the activities of pro- and anti-oxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20 °C. Postharvest Biol. Technol. 2004, 34, 21–28. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zou, X.; Li, S.; Tang, C.; Tang, H.; Zhang, Y. Postharvest Application of Abscisic Acid and Methyl Jasmonate on Fruit Quality of ‘Red Zaosu’ Pear. Agronomy 2025, 15, 1263. https://doi.org/10.3390/agronomy15061263
Wu Y, Zou X, Li S, Tang C, Tang H, Zhang Y. Postharvest Application of Abscisic Acid and Methyl Jasmonate on Fruit Quality of ‘Red Zaosu’ Pear. Agronomy. 2025; 15(6):1263. https://doi.org/10.3390/agronomy15061263
Chicago/Turabian StyleWu, Yuhao, Xin Zou, Shangyun Li, Chao Tang, Haoru Tang, and Yong Zhang. 2025. "Postharvest Application of Abscisic Acid and Methyl Jasmonate on Fruit Quality of ‘Red Zaosu’ Pear" Agronomy 15, no. 6: 1263. https://doi.org/10.3390/agronomy15061263
APA StyleWu, Y., Zou, X., Li, S., Tang, C., Tang, H., & Zhang, Y. (2025). Postharvest Application of Abscisic Acid and Methyl Jasmonate on Fruit Quality of ‘Red Zaosu’ Pear. Agronomy, 15(6), 1263. https://doi.org/10.3390/agronomy15061263





