Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experiment Set-Up and Experimental Design
2.3. Relative Chlorophyll Content and Stomatal Conductance
2.4. Determination of Photosynthetic Pigments
- Chl-a (µg/mL): 12.25 A663.2 − 2.79 A646.8
- Chl-b (µg/mL): 21.50 A646.8 − 5.10 A663.2
- Carotenoids (µg/mL): (1000 A470 − 1.82 Chl-a − 85.02 Chl-b)/198
2.5. Na+ and Nutrients Content
2.6. Determination of Proline Content
2.7. Lipid Peroxidation
2.8. Hydrogen Peroxide (H2O2)
2.9. Determination of Antioxidant Enzyme Activity
2.10. Data Analysis
3. Results
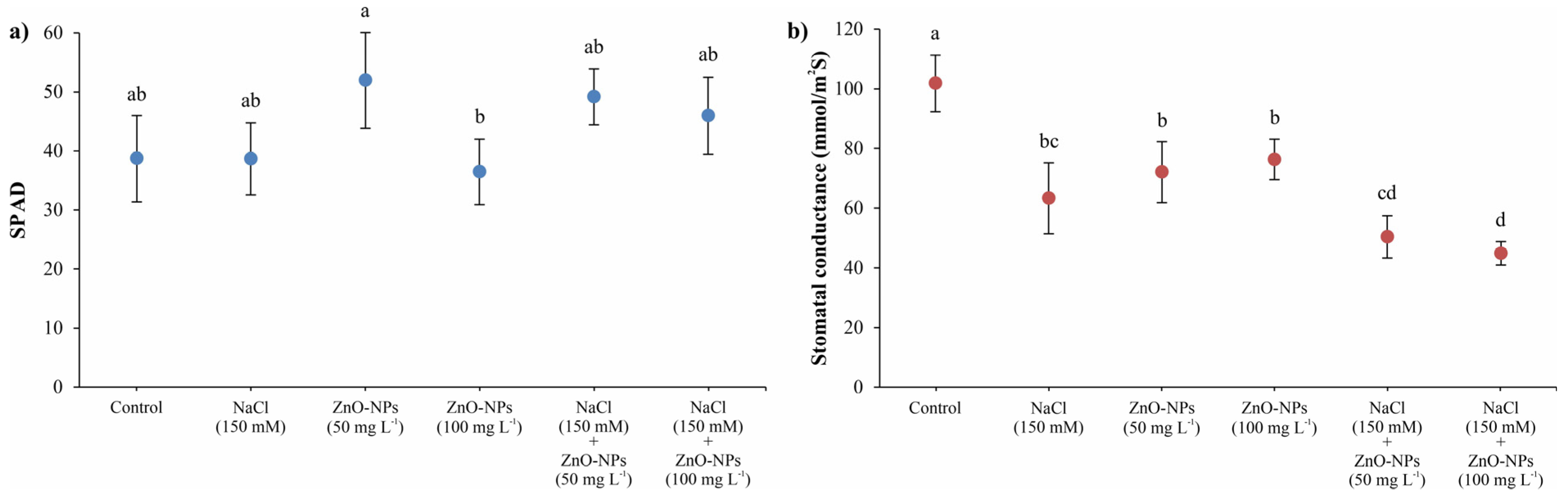
3.1. Relative Chlorophyll Content and Stomatal Conductance
3.2. Determination of Photosynthetic Pigments
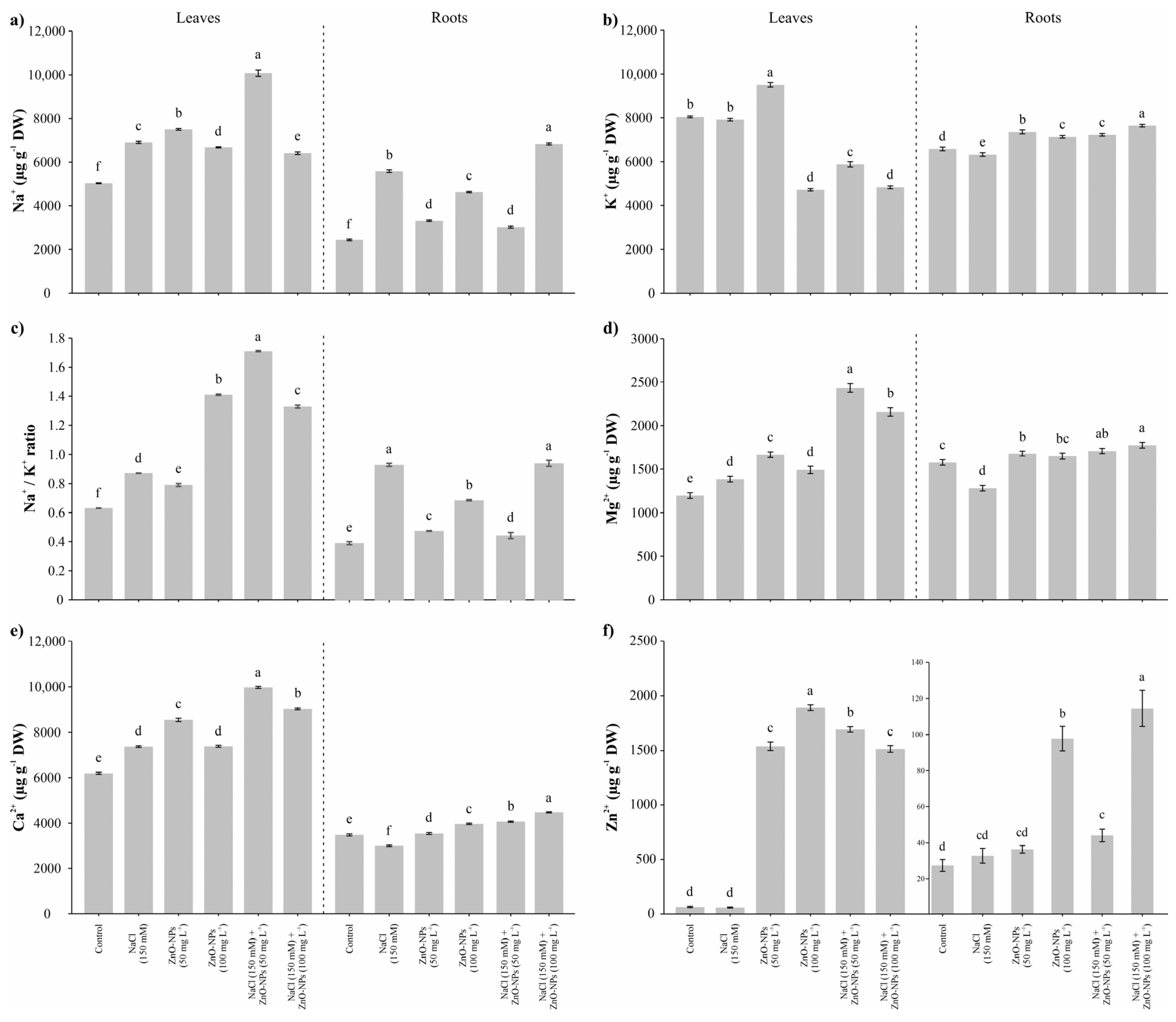
3.3. Na+ and Nutrients Content
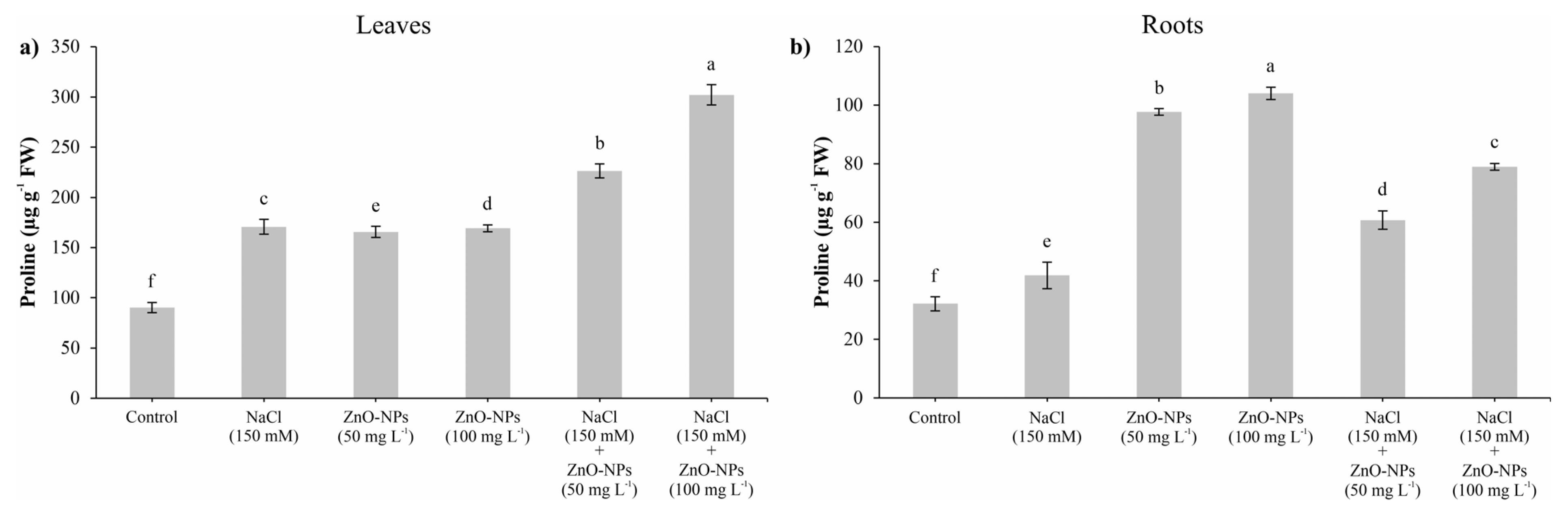
3.4. Determination of Proline Content
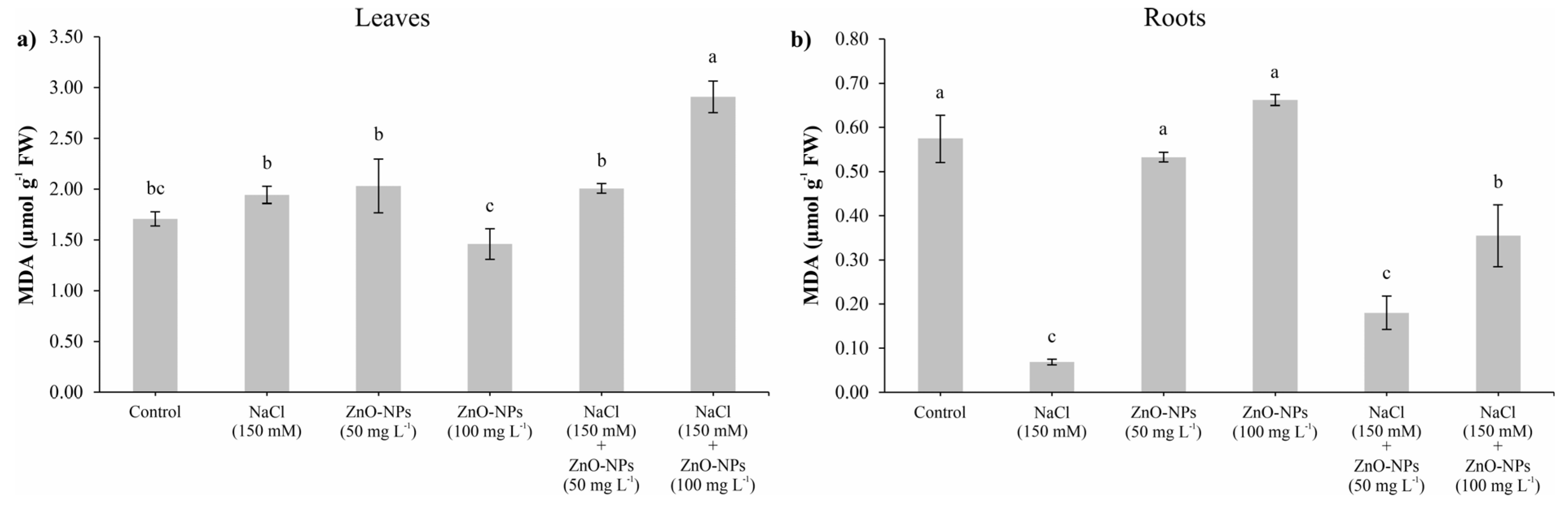
3.5. Lipid Peroxidation
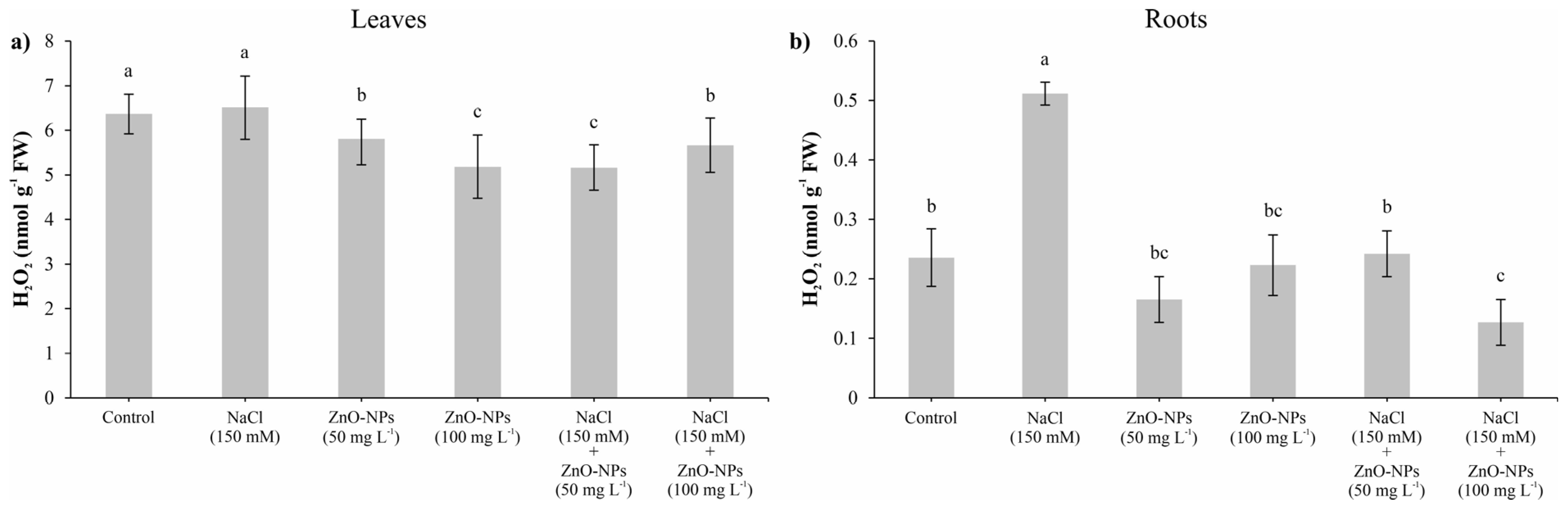
3.6. Hydrogen Peroxide (H2O2)
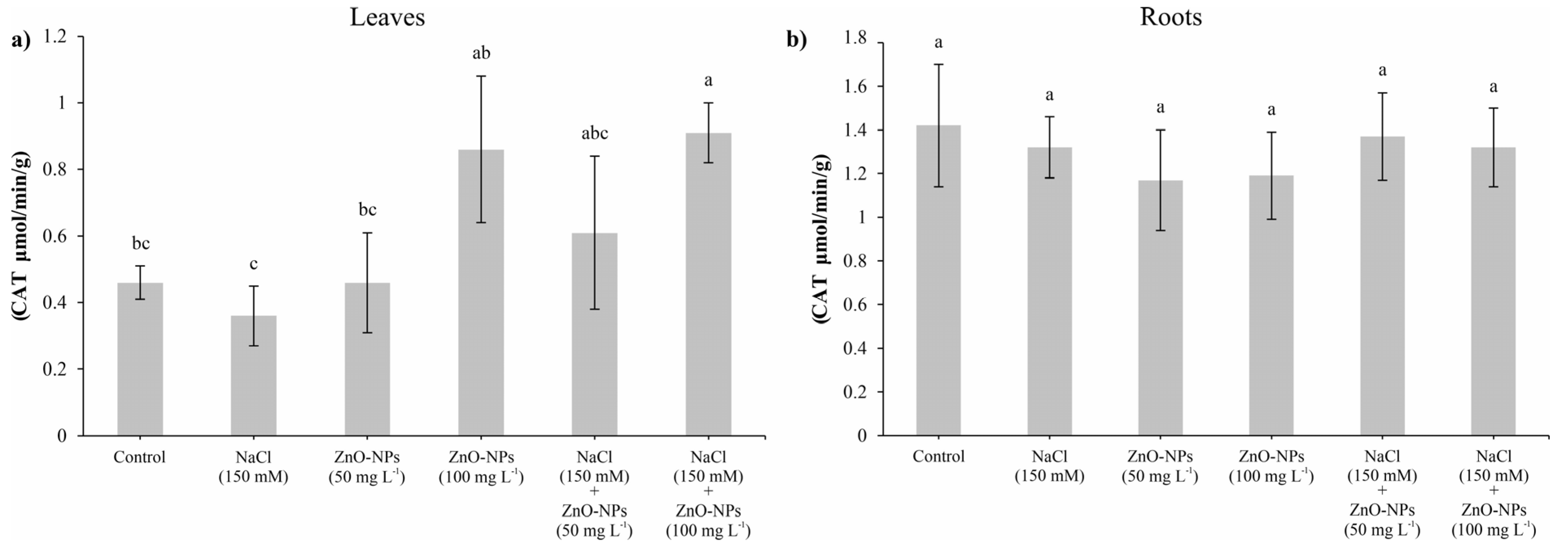
3.7. Determination of Antioxidant Enzyme Activity
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milla, M.E.; Oliva, S.M.; Leiva, S.T.; Collazos, R.; Gamarra, O.Á.; Barrena, M.Á.; Maicelo, J.L. Características morfológicas de variedades de café cultivadas en condiciones de sombra. Acta Agron. 2019, 68, 271–277. [Google Scholar] [CrossRef]
- Saito, M. Sustainable Coffee Production. In Coffee: Growing, Processing, Sustainable Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 384–390. ISBN 978-3-527-61962-7. [Google Scholar]
- International Coffee Organization (ICO). Monthly Coffee Market Report; International Coffee Organization (ICO): London, UK, 2024. [Google Scholar]
- dos Santos, T.B.; Budzinski, I.G.F.; Marur, C.J.; Petkowicz, C.L.O.; Pereira, L.F.P.; Vieira, L.G.E. Expresión de Tres Isoformas de La Galactinol Sintasa en Coffea arabica L. y Acumulación de Rafinosa y Estaquiosa en Respuesta al Estrés Abiótico. Plant Physiol. Biochem. 2011, 49, 441–448. [Google Scholar] [CrossRef]
- do Céu Silva, M.; Várzea, V.; Guerra-Guimarães, L.; Azinheira, H.G.; Fernandez, D.; Petitot, A.-S.; Bertrand, B.; Lashermes, P.; Nicole, M. Coffee Resistance to the Main Diseases: Leaf Rust and Coffee Berry Disease. Braz. J. Plant Physiol. 2006, 18, 119–147. [Google Scholar] [CrossRef]
- dos Santos, T.B.; da Silva Ferreira, M.F.; Marques, I.; Oliveira, S.C.; Zaidan, I.R.; Oliveira, M.G.; Rodrigues, W.P.; Ribas, A.F.; Guyot, R.; Ramalho, J.C.; et al. Current Challenges and Genomic Advances Towards the Development Resilient Coffee Genotypes to Abiotic Stresses. In Genomic Designing for Abiotic Stress Resistant Technical Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 41–69. ISBN 978-3-031-05706-9. [Google Scholar]
- Borgo, L.; Rabêlo, F.H.S.; Marchiori, P.E.R.; Guilherme, L.R.G.; Guerra-Guimarães, L.; de Resende, M.L.V. Impact of Drought, Heat, Excess Light, and Salinity on Coffee Production: Strategies for Mitigating Stress Through Plant Breeding and Nutrition. Agriculture 2025, 15, 9. [Google Scholar] [CrossRef]
- Kumar, A.; Naik, G.K.; Simmi, P.S.; Giridhar, P. Salinity and Drought Response Alleviate Caffeine Content of Young Leaves of Coffea canephora var. Robusta cv. S274. J. Appl. Biol. Biotechnol. 2015, 3, 50–60. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Figueirêdo, V.B.; de Faria, M.A.; da Silva, E.L. Crescimento inicial do cafeeiro irrigado com água salina e salinização do solo. Rev. Bras. Eng. Agríc. Ambient. 2006, 10, 50–57. [Google Scholar] [CrossRef]
- de Lima, R.B.; dos Santos, T.B.; Vieira, L.G.E.; de Lourdes Lúcio Ferrarese, M.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; de Oliveira Petkowicz, C.L. Salt Stress Alters the Cell Wall Polysaccharides and Anatomy of Coffee (Coffea arabica L.) Leaf Cells. Carbohydr. Polym. 2014, 112, 686–694. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Growth and Physiological Responses of Coffee (Coffea arabica L.) Seedlings Irrigated with Diluted Deep Sea Water. Afr. J. Agric. Res. 2018, 13, 311–320. [Google Scholar] [CrossRef]
- FAO. Soil Letters Salt-Affected Soils Are a Global Issue. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/8b5c687f-5a9b-4034-a4e2-0457066c1ae3/content (accessed on 14 May 2025).
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC Transcription Factors in Plant Abiotic Stress Responses. Biochim. Biophys. Acta 2012, 1819, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Chamoli, S.; Salvi, P.; Saxena, S.C. Fostering Nanoscience’s Strategies: A New Frontier in Sustainable Crop Improvement for Abiotic Stress Tolerance. Plant Nano Biol. 2023, 3, 100026. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with Zero Valent Iron (nZVI) Enhances Germination and Growth in Aromatic Rice Cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Nanoparticles Potentially Mediate Salt Stress Tolerance in Plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc Oxide Nanoparticle-Mediated Changes in Photosynthetic Efficiency and Antioxidant System of Tomato Plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of Zinc Oxide Nanoparticles Using Plant Leaf Extract against Urinary Tract Infection Pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram (Cicer arietinum) Seedlings Using Plant Agar Method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.C.; Sahi, S.V. Zinc Oxide Nanoparticles (ZnONPs) Alleviate Heavy Metal-Induced Toxicity in Leucaena Leucocephala Seedlings: A Physiochemical Analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Nazir, M.A.; Hasan, M.; Mustafa, G.; Tariq, T.; Ahmed, M.M.; Golzari Dehno, R.; Ghorbanpour, M. Zinc Oxide Nano-Fertilizer Differentially Effect on Morphological and Physiological Identity of Redox-Enzymes and Biochemical Attributes in Wheat (Triticum aestivum L.). Sci. Rep. 2024, 14, 13091. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; University of California, College of Agriculture, Agricultural Experiment Station: Berkeley, CA, USA, 1938; pp. 1884–1949. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Schmitt, M.; Boras, S.; Tjoa, A.; Watanabe, T.; Jansen, S. Aluminium Accumulation and Intra-Tree Distribution Patterns in Three Arbor Aluminosa (Symplocos) Species from Central Sulawesi. PLoS ONE 2016, 11, e0149078. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, B.; Sun, Z.; Zhu, C. Relationship Between Proline and Hg2+-Induced Oxidative Stress in a Tolerant Rice Mutant. Arch. Environ. Contam. Toxicol. 2009, 56, 723–731. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll Metabolism in Higher Plants. VII. Chlorophyll Degradation in Senescing Tobacco Leaves; Phenolic-Dependent Peroxidative Degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Las Nanopartículas de Óxido de Selenio y Zinc Modulan Los Procesos Moleculares y Morfofisiológicos Durante La Germinación de Semillas de Brassica napus Bajo Estrés Salino. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Haliloglu, K.; Tolga Cinisli, K.; Ozkan, G.; Ozturk, H.I.; Pour-Aboughadareh, A.; Poczai, P. Application of Zinc Oxide Nanoparticles and Plant Growth Promoting Bacteria Reduces Genetic Impairment under Salt Stress in Tomato (Solanum lycopersicum L. ‘Linda’). Agriculture 2020, 10, 521. [Google Scholar] [CrossRef]
- Mustafa, G.; Chaudhari, S.K.; Manzoor, M.; Batool, S.; Hatami, M.; Hasan, M. Zinc Oxide Nanoparticles Mediated Salinity Stress Mitigation in Pisum Sativum: A Physio-Biochemical Perspective. BMC Plant Biol. 2024, 24, 835. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, J.; Kalia, A.; Kuca, K. The Janus Face of Nanomaterials: Physiological Responses as Inducers of Stress or Promoters of Plant Growth? In Plant and Nanoparticles; Chen, J.-T., Ed.; Springer Nature: Singapore, 2022; pp. 395–426. ISBN 978-981-19-2503-0. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Jajoo, A. Changes in Photosystem II in Response to Salt Stress. In Ecophysiology and Responses of Plants Under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 149–168. ISBN 978-1-4614-4747-4. [Google Scholar]
- Wu, Z.-H.; Yang, C.-W.; Yang, M.-Y. Photosynthesis, Photosystem II Efficiency, Amino Acid Metabolism and Ion Distribution in Rice (Oryza sativa L.) in Response to Alkaline Stress. Photosynthetica 2014, 52, 157–160. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of Foliar Application of Zinc Sulfate and Zinc Nanoparticles in Coffee (Coffea arabica L.) Plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Cadenas-Pliego, G.; Morales-Díaz, A.B.; Trejo-Téllez, L.I.; Tortella, G.; Benavides-Mendoza, A. ZnO Nanoparticles as Potential Fertilizer and Biostimulant for Lettuce. Heliyon 2023, 9, e12787. [Google Scholar] [CrossRef]
- Yoshihara, S.; Yamamoto, K.; Nakajima, Y.; Takeda, S.; Kurahashi, K.; Tokumoto, H. Absorption of Zinc Ions Dissolved from Zinc Oxide Nanoparticles in the Tobacco Callus Improves Plant Productivity. Plant Cell Tissue Organ Cult. 2019, 138, 377–385. [Google Scholar] [CrossRef]
- Caffarri, S.; Tibiletti, T.; Jennings, R.C.; Santabarbara, S. A Comparison Between Plant Photosystem I and Photosystem II Architecture and Functioning. Curr. Protein Pept. Sci. 2014, 15, 296–331. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Bonnet, M.; Camares, O.; Veisseire, P. Effects of Zinc and Influence of Acremonium Lolii on Growth Parameters, Chlorophyll a Fluorescence and Antioxidant Enzyme Activities of Ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar] [CrossRef]
- Cherif, J.; Derbel, N.; Nakkach, M.; von Bergmann, H.; Jemal, F.; Lakhdar, Z.B. Analysis of in Vivo Chlorophyll Fluorescence Spectra to Monitor Physiological State of Tomato Plants Growing under Zinc Stress. J. Photochem. Photobiol. B Biol. 2010, 101, 332–339. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Ač, A.; Marek, M.V.; Kalina, J.; Urban, O. Differences in Pigment Composition, Photosynthetic Rates and Chlorophyll Fluorescence Images of Sun and Shade Leaves of Four Tree Species. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-S.; Li, P.; Cheng, L. Effects of High Temperature Coupled with High Light on the Balance between Photooxidation and Photoprotection in the Sun-Exposed Peel of Apple. Planta 2008, 228, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mlinarić, S.; Antunović Dunić, J.; Štolfa, I.; Cesar, V.; Lepeduš, H. High Irradiation and Increased Temperature Induce Different Strategies for Competent Photosynthesis in Young and Mature Fig Leaves. S. Afr. J. Bot. 2016, 103, 25–31. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of Proline on Antioxidant System in Leaves of Grapevine (Vitis vinifera L.) Exposed to Oxidative Stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Shi, H.; Zhang, X.; Xu, X. Responses of Reactive Oxygen Scavenging Enzymes, Proline and Malondialdehyde to Water Deficits among Six Secondary Successional Seral Species in Loess Plateau. PLoS ONE 2014, 9, e98872. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon Alleviates Salt and Drought Stress of Glycyrrhiza Uralensis Seedling by Altering Antioxidant Metabolism and Osmotic Adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline Accumulation in Plants: A Review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Mohsin, S.M.; Fujita, M. Comparative Physiological and Biochemical Changes in Tomato (Solanum lycopersicum L.) under Salt Stress and Recovery: Role of Antioxidant Defense and Glyoxalase Systems. Antioxidants 2019, 8, 350. [Google Scholar] [CrossRef]
- Queirós, F.; Rodrigues, J.A.; Almeida, J.M.; Almeida, D.P.F.; Fidalgo, F. Differential Responses of the Antioxidant Defence System and Ultrastructure in a Salt-Adapted Potato Cell Line. Plant Physiol. Biochem. 2011, 49, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Shan, R.; Shi, Y.; Li, H.; Xue, L.; Song, Y.; Zhao, T.; Zhu, S.; Chen, J.; Jiang, M. Zinc Oxide Nanoparticles Alleviate Salt Stress in Cotton (Gossypium hirsutum L.) by Adjusting Na+/K+ Ratio and Antioxidative Ability. Life 2024, 14, 595. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium Transport and Plant Salt Tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulación Del Impacto de La Salinidad En La Etapa Temprana de Plántula Mediante La Aplicación de Nanocebado de Óxido de Zinc En Semillas de Colza (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with Zinc Oxide Nanoparticles Improve Germination and Photosynthetic Performance in Wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.; Ganjewala, D.; Singhal, R.K.; Rajput, V.D.; Minkina, T.; Voloshina, M.; Srivastava, S.; Shrivastava, M. Effect of ZnO Nanoparticles on Growth and Biochemical Responses of Wheat and Maize. Plants 2021, 10, 2556. [Google Scholar] [CrossRef]
- Naseer, I.; Javad, S.; Iqbal, S.; Shah, A.A.; Alwutayd, K.; AbdElgawad, H. Deciphering the Role of Zinc Oxide Nanoparticles on Physiochemical Attributes of Zea Mays Exposed to Saline Conditions through Modulation in Antioxidant Enzyme Defensive System. S. Afr. J. Bot. 2023, 160, 469–482. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin, N.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between Nanoparticles and Plants: Phytotoxicity and Defense Mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]


| Treatment | P (µg g−1 DW) | C (%) | N (%) | |
|---|---|---|---|---|
| Leaves | Roots | |||
| Control | 152.35 ± 5.90 a | 139.94 ± 5.61 a | 42.00 ± 1.60 a | 2.71 ± 0.60 a |
| NaCl (150 mM) | 161.5 ± 11.90 a | 122.34 ± 18.75 a | 41.15 ± 2.14 a | 2.83 ± 0.92 a |
| ZnO-NPs (50 mg L−1) | 57.41 ± 11.21 b | 131.06 ± 12.57 a | 41.85 ± 1.75 a | 3.27 ± 0.41 a |
| ZnO-NPs (100 mg L−1) | 26.00 ± 8.13 c | 133.52 ± 12.30 a | 42.01 ± 1.20 a | 3.33 ± 0.64 a |
| NaCl (150 mM) + ZnO-NPs (50 mg L−1) | 48.56 ± 10.66 bc | 134.96 ± 20.20 a | 40.23 ± 3.07 a | 3.26 ± 0.36 a |
| NaCl (150 mM) + ZnO-NPs (100 mg L−1) | 34.84 ± 4.2 bc | 123.10 ± 11.32 a | 39.89 ± 4.03 a | 3.33 ± 0.85 a |
| CV (%) | 12.03 | 10.24 | 6.02 | 21.14 |
| p-value | <0.0001 | 0.571 | 0.821 | 0.753 |
| Treatment | Fe2+ (µg g−1 DW) | Cu2+ (µg g−1 DW) | Mn2+ (µg g−1 DW) | |||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| Control | 104.72 ± 20.99 b | 384.83 ± 18.87 a | 16.01 ± 3.36 ab | 7.18 ± 0.92 b | 23.65 ± 3.93 b | 9.45 ± 1.73 b |
| NaCl (150 mM) | 107.64 ± 11.15 b | 239.97 ± 10.58 b | 19.64 ± 3.43 ab | 19.57 ± 2.89 a | 30.35 ± 4.98 ab | 14.06 ± 2.61 ab |
| ZnO-NPs (50 mg L−1) | 132.21 ± 8.93 ab | 182.19 ± 12.03 d | 26.65 ± 5.01 a | 7.34 ± 1.68 b | 36.08 ± 6.04 ab | 9.72 ± 1.75 b |
| ZnO-NPs (100 mg L−1) | 127.32 ± 7.14 ab | 200.10 ± 4.97 cd | 23.74 ± 2.77 a | 19.47 ± 3.30 a | 32.96 ± 5.13 ab | 14.03 ± 2.07 ab |
| NaCl (150 mM) + ZnO-NPs (50 mg L−1) | 140.52 ± 5.56 a | 205.88 ± 5.36 cd | 27.29 ± 6.87 a | 7.38 ± 0.96 b | 43.56 ± 8.23 a | 10.18 ± 1.04 b |
| NaCl (150 mM) + ZnO-NPs (100 mg L−1) | 104.35 ± 3.33 b | 215.42 ± 10.06 bc | 9.80 ± 1.05 b | 21.85 ±3.21 a | 33.36 ± 2.88 ab | 16.17 ± 3.17 a |
| CV (%) | 9.28 | 4.75 | 20.28 | 17.28 | 16.39 | 17.7 |
| p-value | 0.005 | <0.0001 | 0.002 | <0.0001 | 0.018 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez-Mori, J.B.; Lapiz-Culqui, Y.K.; Huaman-Huaman, E.; Zuta-Puscan, M.; Oliva-Cruz, M. Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica? Agronomy 2025, 15, 1239. https://doi.org/10.3390/agronomy15051239
Meléndez-Mori JB, Lapiz-Culqui YK, Huaman-Huaman E, Zuta-Puscan M, Oliva-Cruz M. Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica? Agronomy. 2025; 15(5):1239. https://doi.org/10.3390/agronomy15051239
Chicago/Turabian StyleMeléndez-Mori, Jegnes Benjamín, Yoiner K. Lapiz-Culqui, Eyner Huaman-Huaman, Marileydi Zuta-Puscan, and Manuel Oliva-Cruz. 2025. "Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica?" Agronomy 15, no. 5: 1239. https://doi.org/10.3390/agronomy15051239
APA StyleMeléndez-Mori, J. B., Lapiz-Culqui, Y. K., Huaman-Huaman, E., Zuta-Puscan, M., & Oliva-Cruz, M. (2025). Can Zinc Oxide Nanoparticles Alleviate the Adverse Effects of Salinity Stress in Coffea arabica? Agronomy, 15(5), 1239. https://doi.org/10.3390/agronomy15051239








