Amino Acid Profile of Must and Aromatic Potential of 30 Minor Grape Varieties Grown in Alcalá de Henares (Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Climatology
2.3. Evaluation of Maturity Status
2.4. Amino Acid Determination
2.5. Data Analysis
3. Results
3.1. Characterization of Varieties
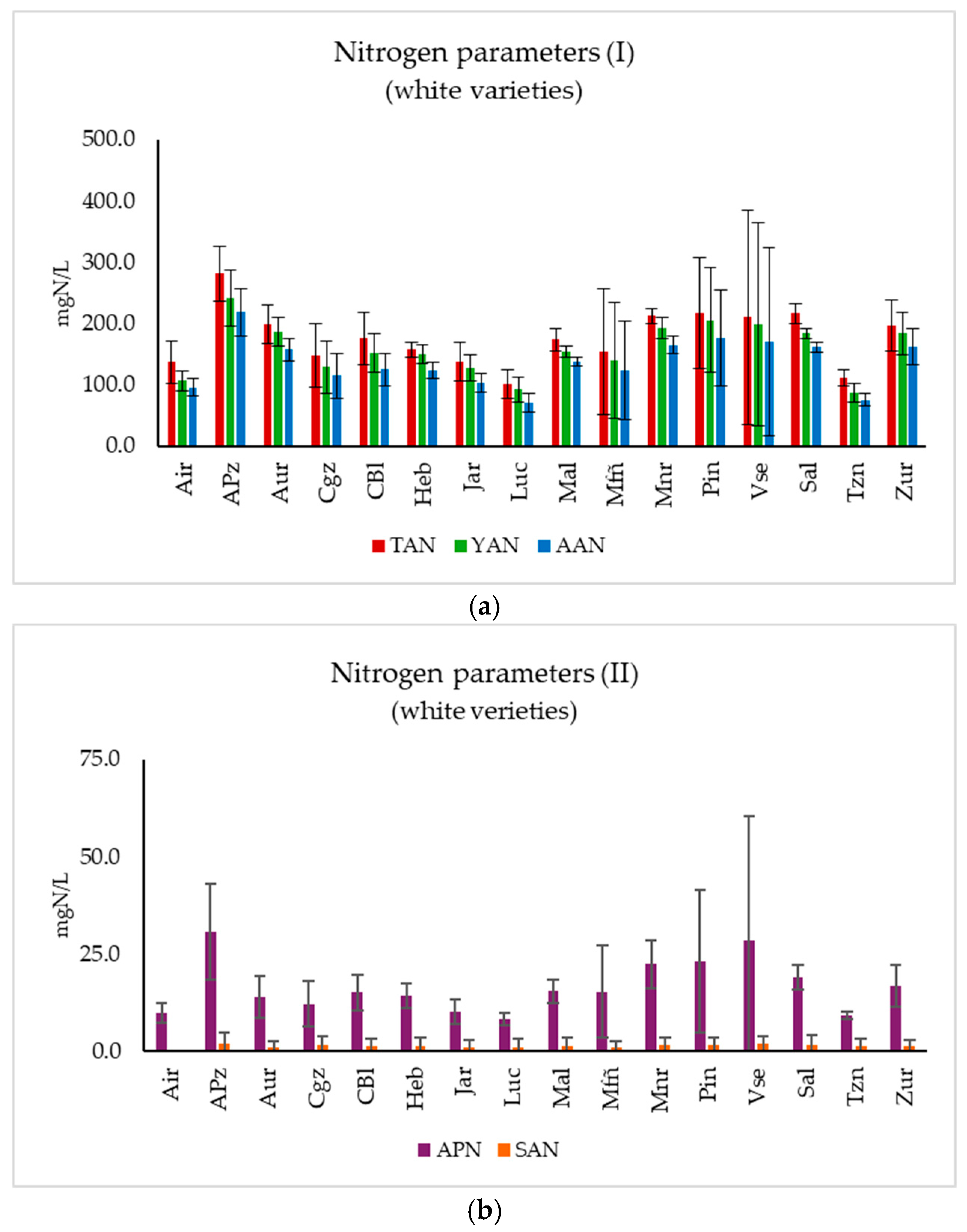
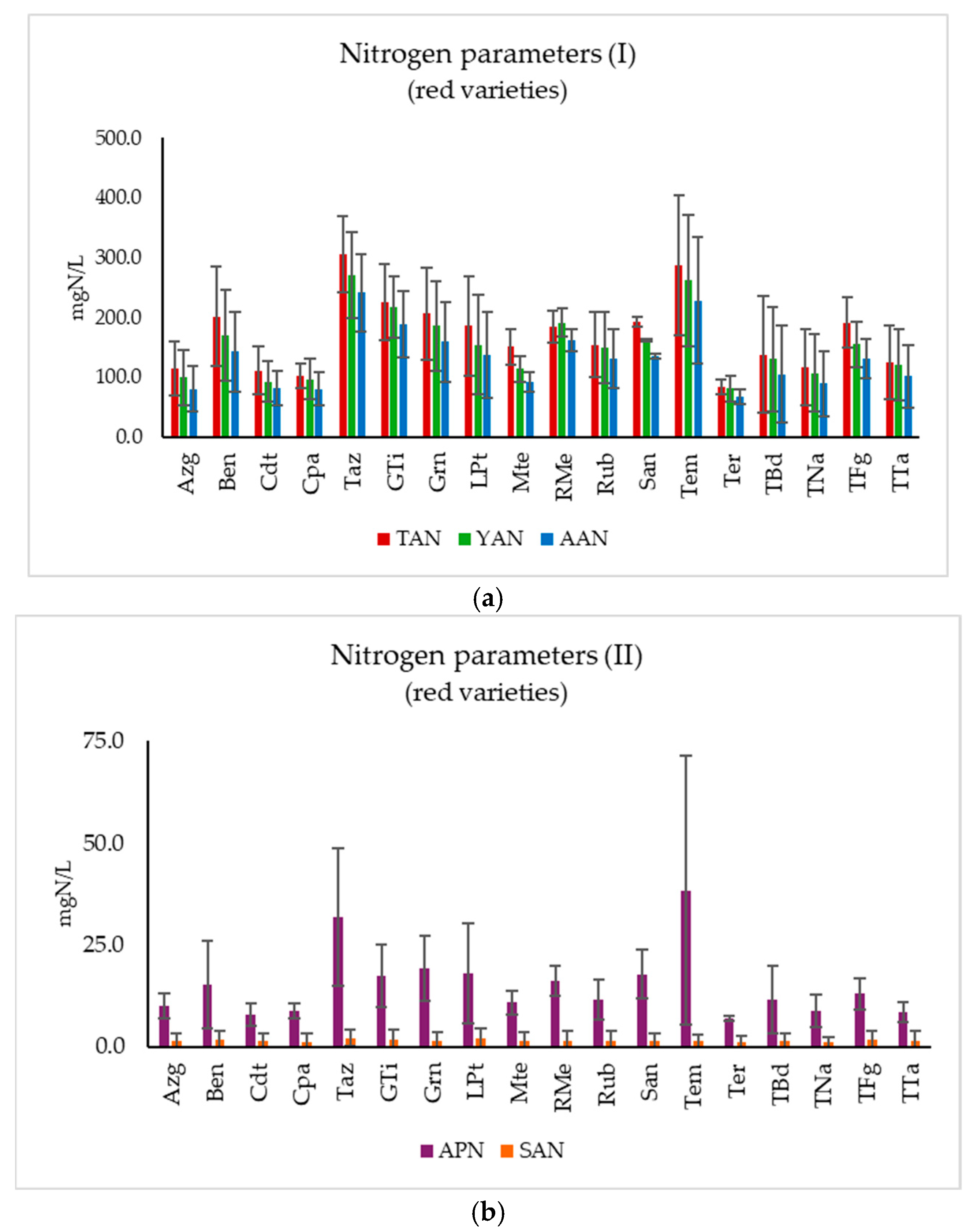
3.2. Nitrogen Parameters
3.2.1. White Varieties
3.2.2. Red Varieties
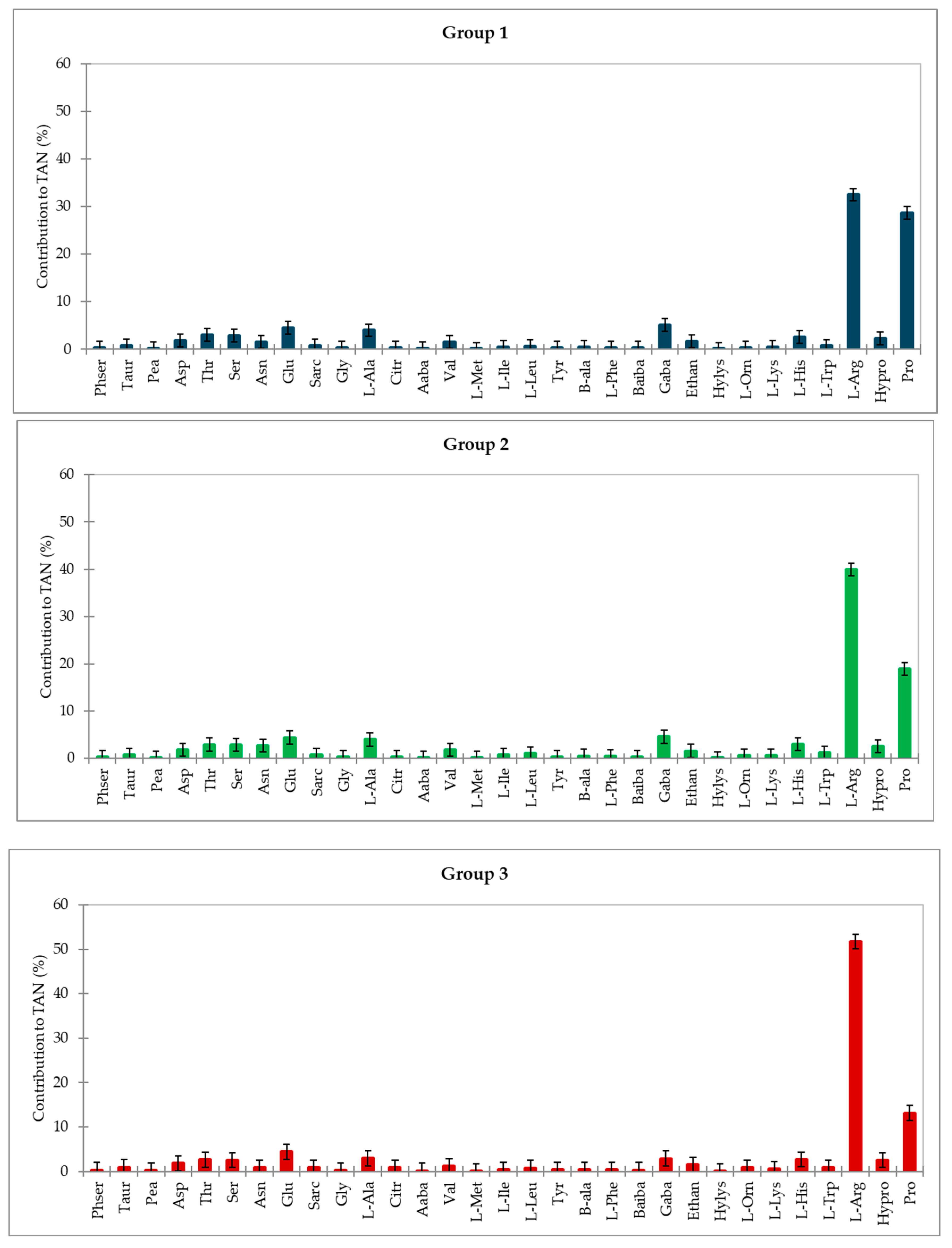
3.3. Amino Acid Profile
3.3.1. White Varieties
3.3.2. Red Varieties
3.4. Classification of Varieties by Amino Acids
3.4.1. White Varieties
3.4.2. Red Varieties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open-access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
| IMIDRA | Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentario |
| Id | Short identification name of each variety |
| Air | Airén |
| APz | Albillo del Pozo |
| Aur | Aúrea |
| Cgz | Cagarrizo |
| CBl | Castellana Blanca |
| Heb | Hebén |
| Jar | Jarrosuelto |
| Luc | Lucomol |
| Mal | Malvar |
| Mfñ | Marfileña |
| Mnr | Montonera |
| Pin | Pintada |
| Vse | Verdejo Serrano |
| Sal | Salvador |
| Tzn | Tortozón |
| Zur | Zurieles |
| Azg | Azargón |
| Ben | Benedicto |
| Cdt | Cadrete |
| Cpa | Crepa |
| Taz | Tazazonal |
| GTi | Garnacha Tinta |
| Grn | Granadera |
| LPt | Listán Prieto |
| Mte | Morate |
| RMe | Rayada Melonera |
| Rub | Rubeliza |
| San | Sanguina |
| Tem | Tempranillo |
| Ter | Terriza |
| TBd | Tinto Bastardo |
| TNa | Tinto de Navalcarnero |
| TFg | Tinto Fragoso |
| TTa | Tortozona Tinta |
| TAN (mgN/L) | Total amino acid nitrogen/total free amino acids |
| YAN (mgN/L) | Yeast assimilable nitrogen |
| AAN (mgN/L) | Assimilable AA concentration |
| APN (mgN/L) | Aromatic precursor nitrogen |
| SAN (mgN/L) | Nitrogen from S-containing AA |
| Phser | Phenylserine |
| Taur | Taurine |
| Pea | Phenylethylamine |
| Asp | Aspartic acid |
| Thr | Threonine |
| Ser | Serine |
| Asn | Asparagine |
| Glu | Glutamic acid |
| Sarc | Sarcosine |
| Gly | Glycine |
| L-Ala | Alanine |
| Citr | Citrulline |
| Aaba | a-aminobutyric acid |
| Val | Valine |
| L-Met | Methionine |
| L-Ile | Isoleucine |
| L-Leu | Leucine |
| Tyr | Tyrosine |
| B-ala | B-alanine |
| L-Phe | Phenylalanine |
| Baiba | B-aminobutyric acid |
| Gaba | y-aminobutyric acid |
| Ethan | Ethanolamine |
| Hylys | Hydroxylysine |
| L-Orn | Ornithine |
| L-Lys | Lysine |
| L-His | Histidine |
| L-Trp | Tryptophan |
| L-Arg | Arginine |
| Hypro | Hydroxyproline |
| Pro | Proline |
| Urea | Urea |
| Amm | Ammonium |
References
- Nassar, A.; Kliewer, W. Free Amino Acids in Various Parts of Vitis Vinifera at Different Stages of Development. Am. Soc. Hortic. Sci. 1966, 89, 281–294. [Google Scholar]
- Kliewer, W.M. Free Amino Acids and Other Nitrogenous Fractions in Wine Grapes. J. Food Sci. 1970, 35, 17–21. [Google Scholar] [CrossRef]
- Ough, C.S. Proline Content of Grapes and Wines. VITIS-J. Grapevine Res. 1968, 7, 321–331. [Google Scholar] [CrossRef]
- Christensen, P. Nutrient Level Comparisons of Leaf Petioles and Blades in Twenty-Six Grape Cultivars Over Three Years (1979 through 1981). Am. J. Enol. Vitic. 1984, 35, 124–133. [Google Scholar] [CrossRef]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; Hoj, P.B.; Heeswijck, R. Proline and Arginine Accumulation in Developing Berries of Vitis vinifera L. in Australian Vineyards: Influence of Vine Cultivar, Berry Maturity and Tissue Type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Huang, Z.; Ough, C.S. Effect of Vineyard Locations, Varieties, and Rootstocks on the Juice Amino Acid Composition of Several Cultivars. Am. J. Enol. Vitic. 1989, 40, 135–139. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Ancín-Azpilicueta, C.; Salinas, M.R. Study of the Evolution of Nitrogen Compounds during Grape Ripening. Application to Differentiate Grape Varieties and Cultivated Systems. J. Agric. Food Chem. 2009, 57, 2410–2419. [Google Scholar] [CrossRef]
- Lee, J.; Schreiner, R.P. Free Amino Acid Profiles from ‘Pinot Noir’ Grapes Are Influenced by Vine N-Status and Sample Preparation Method. Food Chem. 2010, 119, 484–489. [Google Scholar] [CrossRef]
- Oliva, J.; Garde-Cerdán, T.; Martínez-Gil, A.; Salinas, M.R.; Barba, A. Fungicide Effects on Ammonium and Amino Acids of Monastrell Grapes. Food Chem. 2011, 129, 1676–1680. [Google Scholar] [CrossRef]
- Vilanova, M.; Rodríguez, I.; Canosa, P.; Otero, I.; Gamero, E.; Moreno, D.; Talaverano, I.; Valdés, E. Variability in chemical composition of Vitis vinifera cv Mencía from different geographic areas and vintages in Ribeira Sacra (NW Spain). Food Chem. 2015, 169, 187–196. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Stevenson, D.S.; Gehringer, A. The Effect of NPK Fertilization on Element Uptake, Yield and Fruit Composition of Foch Grapes in British Columbia. Can. J. Plant Sci. 1987, 67, 511–520. [Google Scholar] [CrossRef]
- Bell, S.; Robson, A. Effect of nitrogen fertilization on growth, canopy density, and yield of Vitis vinifera L. cv. Cabernet Sauvignon. Am. J. Enol. Vitic. 1999, 50, 351–358. [Google Scholar] [CrossRef]
- Bell, A.A.; Ough, C.S.; Kliewer, W.M. Effects on Must and Wine Composition, Rates of Fermentation, and Wine Quality of Nitrogen Fertilization of Vitis Vinifera Var. Thompson Seedless Grapevines. Am. J. Enol. Vitic. 1979, 30, 124–129. [Google Scholar] [CrossRef]
- Maigre, D.; Aerny, J. Enherbement Permanent et Fumure Azotée Sur Cv. ‘Gamay’ Dans Le Valais Central. Rev. Suisse Vitic. Arbor. Hort. 2001, 33, 343–349. [Google Scholar]
- Spring, J.-L. Influence Du Type d’enherbement Sur Le Comportement de La Vigne et La Qualité Des Vins. Résultats d’un Essai Sur Chasselas Dans Le Basin Lémanique. 1. Résultats Agronomiques. Rev. Suisse Vitic. Arboric. Hortic. 2001, 33, 253–260. [Google Scholar]
- Kliewer, W.M.; Bogdanoff, C.; Benz, M. Responses of Thompson Seedless Grapevines Trained to Single and Divided Canopy Trellis Systems to Nitrogen Fertilisation. In Proceedings of the Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, USA, 18–19 June 1991; Rantz, J.M., Ed.; American Society for Enology and Viticulture: Davis, CA, USA, 1991; pp. 282–289. [Google Scholar]
- Miele, A.; Carbonneau, A.; Bouard, J. Composition En Acides Aminés Libres Des Feuilles Et Des Baies Du Cépage Cabernet Sauvignon. J. Int. Sci. Vigne Vin 2000, 34, 19–26. [Google Scholar]
- Canoura, C.; Kelly, M.T.; Ojeda, H. Effect of Irrigation and Timing and Type of Nitrogen Application on the Biochemical Composition of Vitis Vinifera L. Cv. Chardonnay and Syrah Grapeberries. Food Chem. 2018, 241, 171–181. [Google Scholar] [CrossRef]
- Niculcea, M.; Martinez-Lapuente, L.; Guadalupe, Z.; Sánchez-Díaz, M.; Morales, F.; Ayestarán, B.; Antolín, M.C. Effects of Water-Deficit Irrigation on Hormonal Content and Nitrogen Compounds in Developing Berries of Vitis vinifera L. Cv. Tempranillo. J. Plant Growth Regul. 2013, 32, 551–563. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Pérez-Magariño, S.; Del-Villar-Garrachón, V.; González-Huerta, C.; Moro Gonzalez, L.C.; Guadarrama Rodríguez, A.; Villanueva Sanchez, S.; Gallo González, R.; Martín de la Helguera, S. Study of the Effect of Vintage, Maturity Degree, and Irrigation on the Amino Acid and Biogenic Amine Content of a White Wine from the Verdejo Variety. J. Sci. Food Agric. 2014, 94, 2073–2082. [Google Scholar] [CrossRef]
- Perez-Harvey, J.; Witting, D. Influence of Soil Nitrogen Fertilisation and Artificial Shading on the Nitrogen and Potassium Levels in Leaves and Berries of Cabernet Sauvignon; AGRO Montpellier: Montpellier, France, 2001. [Google Scholar]
- Bruwer, F.A.; du Toit, W.; Buica, A. Nitrogen and Sulphur Foliar Fertilisation. S. Afr. J. Enol. Vitic. 2019, 40, 2. [Google Scholar] [CrossRef]
- Conradie, W.J. Timing of Nitrogen Fertilisation and the Effect of Poultry Manure on the Performance of Grapevines on Sandy Soil. IL Leaf Analysis, Juice Analysis and Wine Quality. S. Afr. J. Enol. Vitic. 2001, 22, 60–68. [Google Scholar] [CrossRef][Green Version]
- Soufleros, E.; Bouloumpasi, E.; Tsarchopoulos, C.; Biliaderis, C. Primary Amino Acid Profiles of Greek White Wines and Their Use in Classification According to Variety, Origin and Vintage. Food Chem. 2003, 80, 261–273. [Google Scholar] [CrossRef]
- Sponholz, W.R. Nitrogen Compounds in Grapes, Must, and Wine; American Society for Enology and Viticulture (ASEV): Davis, CA, USA, 1991. [Google Scholar]
- Rapp, A.; Versini, G. Influence of Nitrogen Compounds in Grapes on Aroma Compounds of Wines. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1659–1694. [Google Scholar]
- Bell, S.J.; Henschke, P.A. Implications of Nitrogen Nutrition for Grapes, Fermentation and Wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Guitart, A.; Orte, P.H.; Ferreira, V.; Peña, C.; Cacho, J. Some Observations About the Correlation Between the Amino Acid Content of Musts and Wines of the Chardonnay Variety and Their Fermentation Aromas. Am. J. Enol. Vitic. 1999, 50, 253–258. [Google Scholar] [CrossRef]
- Henschke, P.A.; Ough, C.S. Urea Accumulation in Fermenting Grape Juice. Am. J. Enol. Vitic. 1991, 42, 317–321. [Google Scholar] [CrossRef]
- Loulakakis, K.A.; Roubelakis-Angelakis, K.A. Nitrogen Assimilation in Grapevine. In Molecular Biology & Biotechnology of the Grapevine; Springer: Dordrecht, The Netherlands, 2001; pp. 59–85. [Google Scholar]
- Cabello, F.; Asensio, M.L.; Valde, E. Characterisation of Some Spanish White Grapevine Cultivars by Morphology and Amino Acid Analysis. Sci. Hortic. 2002, 93, 289–299. [Google Scholar]
- Bouzas-Cid, Y.; Falqué, E.; Orriols, I.; Trigo-Córdoba, E.; Díaz-Losada, E.; Fornos-Rivas, D.; Mirás-Avalos, J.M. Amino Acids Profile of Two Galician White Grapevine cultivars (Godello and Treixadura). Ciência Técnica Vitivinícola 2015, 30, 84–93. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between Varietal Amino Acid Profile of Grapes and Wine Aromatic Composition. Experiments with Model Solutions and Chemometric Study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef]
- García-Muñoz, S.; Asproudi, A.; Cabello, F.; Borsa, D. Aromatic Characterization and Enological Potential of 21 Minor Varieties (Vitis vinifera L.). Eur. Food Res. Technol. 2011, 233, 473–481. [Google Scholar] [CrossRef]
- Muñoz-Organero, G.; Espinosa, F.E.; Cabello, F.; Zamorano, J.P.; Urbanos, M.A.; Puertas, B.; Lara, M.; Domingo, C.; Puig-Pujol, A.; Valdés, M.E.; et al. Phenological Study of 53 Spanish Minority Grape Varieties to Search for Adaptation of Vitiviniculture to Climate Change Conditions. Horticulturae 2022, 8, 984. [Google Scholar] [CrossRef]
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Santos, J.A. Modelling Climate Change Impacts on Viticultural Yield, Phenology and Stress Conditions in Europe. Glob. Change Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef] [PubMed]
- Morales-Castilla, I.; de Cortázar-Atauri, I.G.; Cook, B.I.; Lacombe, T.; Parker, A.; van Leeuwen, C.; Nicholas, K.A.; Wolkovich, E.M. Diversity Buffers Winegrowing Regions from Climate Change Losses. Proc. Natl. Acad. Sci. USA 2020, 117, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.B.; Whetton, P.H.; Bhend, J.; Darbyshire, R.; Briggs, P.R.; Barlow, E.W.R. Earlier Wine-Grape Ripening Driven by Climatic Warming and Drying and Management Practices. Nat. Clim. Change 2012, 2, 259–264. [Google Scholar] [CrossRef]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Observed Trends in Winegrape Maturity in Australia. Glob. Change Biol. 2011, 17, 2707–2719. [Google Scholar] [CrossRef]
- Neethling, E.; Barbeau, G.; Bonnefoy, C.; Quénol, H. Change in Climate and Berry Composition for Grapevine Varieties Cultivated in the Loire Valley. Clim. Res. 2012, 53, 89–101. [Google Scholar] [CrossRef]
- Clingeleffer, P.R.; Davis, H.P. Assessment of Phenology, Growth Characteristics and Berry Composition in a Hot Australian Climate to Identify Wine Cultivars Adapted to Climate Change. Aust. J. Grape Wine Res. 2022, 28, 255–275. [Google Scholar] [CrossRef]
- Espinosa-Roldán, F.E.; García-Díaz, A.; Raboso, E.; Crespo, J.; Cabello, F.; Martínez de Toda, F.; Muñoz-Organero, G. Phenological Evaluation of Minority Grape Varieties in the Wine Region of Madrid as a Strategy for Adaptation to Climate Change. Horticulturae 2024, 10, 353. [Google Scholar] [CrossRef]
- Rustioni, L.; Maghradze, D.; Popescu, C.F.; Cola, G.; Abashidze, E.; Aroutiounian, R.; Brazão, J.; Coletti, S.; Cornea, V.; Dejeu, L.; et al. First Results of the European Grapevine Collections’ Collaborative Network: Validation of a Standard Eno-Carpological Phenotyping Method. Vitis-J. Grapevine Res. 2014, 53, 219–226. [Google Scholar]
- Valdés, E.; Vilanova, M.; Sabio, E.; Benalte, M.J. Clarifying Agents Effect on the Nitrogen Composition in Must and Wine during Fermentation. Food Chem. 2011, 125, 430–437. [Google Scholar] [CrossRef]
- Valdés, M.E.; Talaverano, M.I.; Moreno, D.; Prieto, M.H.; Mancha, L.A.; Uriarte, D.; Vilanova, M. Effect of the Timing of Water Deficit on the Must Amino Acid Profile of Tempranillo Grapes Grown under the Semiarid Conditions of SW Spain. Food Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Portu, J.; González-Arenzana, L.; López-Alfaro, I.; Santamaría, P. Study of the Effects of Proline, Phenylalanine, and Urea Foliar Application to Tempranillo Vineyards on Grape Amino Acid Content. Comparison with Commercial Nitrogen Fertilisers. Food Chem. 2014, 163, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Valdés, M.E.; Moreno, D.; Vilanova, M.; Yuste, J.; Montoro, A.; Talaverano, M.I.; Técnico, I.; Provincial, A. Composición Nitrogenada de Las Variedades Blancas ‘Airén’, ‘Cigüente’, ‘Moscatel de Alejandría’ y ‘Verdejo’, Cultivadas En España; Incidencia Del Régimen Hídrico; SECH (Sociedad Española de Ciencias Hortícolas): Córdoba, Spain, 2014; pp. 290–296. [Google Scholar]
- Ingledew, W.M.; Kunkee, R.E. Factors Influencing Sluggish Fermentations of Grape Juice. Am. J. Enol. Vitic. 1985, 36, 65–76. [Google Scholar] [CrossRef]
- Suter, B.; Destrac Irvine, A.; Gowdy, M.; Dai, Z.; van Leeuwen, C. Adapting Wine Grape Ripening to Global Change Requires a Multi-Trait Approach. Front. Plant Sci. 2021, 12, 624867. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Study of Must and Wine Amino Acids Composition after Seaweed Applications to Tempranillo Blanco Grapevines. Food Chem. 2020, 308, 125605. [Google Scholar] [CrossRef]


| White Varieties | Id 1 | Red Varieties | Id 1 |
|---|---|---|---|
| Airén | Air | Azargón | Azg |
| Albillo del Pozo | APz | Benedicto | Ben |
| Aúrea | Aur | Cadrete | Cdt |
| Cagarrizo | Cgz | Crepa | Cpa |
| Castellana Blanca | CBl | Tazazonal | Taz |
| Hebén | Heb | Garnacha Tinta | GTi |
| Jarrosuelto | Jar | Granadera | Grn |
| Lucomol | Luc | Listán Prieto | LPt |
| Malvar | Mal | Morate | Mte |
| Marfileña | Mfñ | Rayada Melonera | RMe |
| Montonera | Mnr | Rubeliza | Rub |
| Pintada | Pin | Sanguina | San |
| Verdejo Serrano | Vse | Tempranillo | Tem |
| Salvador | Sal | Terriza | Ter |
| Tortozón | Tzn | Tinto Bastardo | TBd |
| Zurieles | Zur | Tinto de Navalcarnero | TNa |
| Tinto Fragoso | TFg | ||
| Tortozona Tinta | TTa |
| Month | Minimum Average Temperature | Maximum Average Temperature | Average Temperature | Historical Serial (1957–2019) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2023 | 2020 | 2021 | 2022 | 2023 | 2020 | 2021 | 2022 | 2023 | Min Average | Max Average | Average | |
| January | 1.46 | −2.28 | −2.22 | −0.55 | 11.55 | 7.85 | 13.05 | 10.90 | 5.80 | 2.38 | 4.11 | 4.96 | −0.15 | 10.37 | 5.11 |
| Ferbuary | 2.58 | 4.33 | 0.40 | −1.53 | 16.22 | 14.73 | 16.12 | 13.44 | 9.07 | 9.36 | 7.73 | 5.40 | 0.55 | 12.50 | 6.53 |
| March | 5.02 | 2.58 | 5.30 | 3.80 | 16.66 | 16.79 | 14.02 | 18.58 | 10.58 | 9.70 | 9.62 | 11.13 | 2.64 | 16.23 | 9.43 |
| April | 8.59 | 6.52 | 5.13 | 5.50 | 18.47 | 18.17 | 18.10 | 24.13 | 13.39 | 12.06 | 11.59 | 15.38 | 4.82 | 18.33 | 11.58 |
| May | 11.35 | 8.88 | 10.60 | 9.00 | 26.01 | 24.10 | 27.41 | 23.98 | 18.80 | 16.76 | 19.73 | 16.64 | 8.25 | 23.06 | 15.66 |
| June | 13.31 | 13.85 | 14.71 | 15.46 | 28.88 | 28.95 | 32.16 | 29.09 | 21.69 | 21.85 | 24.33 | 22.20 | 12.54 | 28.91 | 20.73 |
| July | 18.17 | 15.41 | 18.98 | 17.47 | 35.76 | 33.01 | 37.35 | 35.00 | 27.63 | 24.82 | 28.97 | 26.93 | 15.04 | 32.90 | 23.97 |
| August | 16.20 | 16.59 | 18.67 | 17.68 | 32.83 | 34.17 | 34.69 | 35.46 | 24.81 | 25.57 | 27.10 | 27.08 | 14.72 | 32.50 | 23.61 |
| September | 13.15 | 13.55 | 12.83 | 14.05 | 27.38 | 26.24 | 27.55 | 26.22 | 20.32 | 19.46 | 20.44 | 19.88 | 11.86 | 27.64 | 19.75 |
| October | 6.28 | 7.70 | 11.38 | 11.13 | 19.49 | 22.41 | 24.46 | 22.86 | 12.69 | 14.54 | 17.53 | 16.47 | 7.81 | 20.63 | 14.22 |
| November | 5.48 | 1.51 | 5.30 | 5.49 | 15.84 | 13.84 | 16.09 | 15.60 | 10.09 | 7.24 | 10.64 | 10.26 | 3.08 | 14.13 | 8.60 |
| December | 1.57 | 2.12 | 5.73 | 0.16 | 10.78 | 13.58 | 12.77 | 11.83 | 6.30 | 7.26 | 9.02 | 5.35 | 0.54 | 10.78 | 5.66 |
| Month | Precipitation (mm) | Average Relative Humidity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | 2023 | 2020 | 2021 | 2022 | 2023 | |
| January | 12.40 | 37.50 | 9.80 | 14.60 | - | 80.00 | 66.00 | 72.00 |
| Ferbuary | 0.00 | 64.10 | 5.00 | 0.60 | 75.00 | 74.00 | 60.00 | 58.00 |
| March | 63.20 | 0.00 | 88.00 | 17.90 | 70.00 | 59.00 | 71.00 | 58.00 |
| April | 83.40 | 120.50 | 41.10 | 7.50 | 76.00 | 65.00 | 61.00 | 42.00 |
| May | 42.20 | 20.00 | 1.20 | 36.50 | 55.00 | 54.00 | 45.00 | 48.00 |
| June | 27.70 | 57.50 | 2.70 | 114.40 | 43.00 | 47.00 | 35.00 | 57.00 |
| July | 2.00 | 21.80 | 4.20 | 0.00 | 33.00 | 37.00 | 30.00 | 34.00 |
| August | 12.80 | 39.10 | 16.40 | 0.00 | 38.00 | 38.00 | 36.00 | 31.00 |
| September | 35.60 | 43.40 | 32.00 | 119.40 | 47.00 | 61.00 | 49.00 | 68.00 |
| October | 59.50 | 85.00 | 21.40 | 137.20 | 65.00 | 63.00 | 60.00 | 68.00 |
| November | 48.00 | 21.00 | 40.80 | 41.60 | 80.00 | 70.00 | 75.00 | 83.00 |
| December | 20.20 | 26.70 | 127.30 | 42.20 | 77.00 | 78.00 | 86.00 | 83.00 |
| White Varieties | °Brix ¹ | CV ² | pH ¹ | CV ² | Titratable Acidity ¹ | CV ² | Red Varieties | °Brix ¹ | CV ² | pH ¹ | CV ² | Titratable Acidity ¹ | CV ² |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Air | 20.70 | 0.07 | 3.66 | 0.03 | 3.98 | 0.11 | Azg | 19.50 | 0.05 | 3.72 | 0.05 | 2.88 | 0.31 |
| APz | 23.30 | 0.07 | 3.61 | 0.02 | 4.64 | 0.20 | Ben | 23.20 | 0.03 | 3.29 | 0.03 | 5.86 | 0.11 |
| Aur | 22.37 | 0.09 | 3.45 | 0.04 | 5.70 | 0.08 | Cdt | 22.60 | 0.05 | 3.58 | 0.06 | 3.50 | 0.22 |
| CBl | 22.50 | 0.04 | 3.23 | 0.03 | 5.63 | 0.05 | Cpa | 16.65 | 0.07 | 3.36 | 0.00 | 5.13 | 0.10 |
| Cgz | 21.47 | 0.06 | 3.60 | 0.04 | 4.35 | 0.06 | GTi | 22.27 | 0.03 | 3.38 | 0.03 | 4.42 | 0.26 |
| Heb | 21.40 | 0.06 | 3.68 | 0.09 | 4.58 | 0.09 | Grn | 19.50 | 0.02 | 3.52 | 0.01 | 4.50 | 0.08 |
| Jar | 23.37 | 0.07 | 3.46 | 0.05 | 4.73 | 0.30 | LPt | 23.30 | 0.02 | 3.92 | 0.01 | 2.73 | 0.01 |
| Luc | 22.80 | 0.05 | 3.59 | 0.07 | 5.10 | 0.28 | Mte | 21.63 | 0.05 | 3.43 | 0.04 | 5.50 | 0.14 |
| Mal | 22.10 | 0.04 | 3.63 | 0.07 | 3.56 | 0.05 | RMe | 22.10 | 0.06 | 3.42 | 0.04 | 5.15 | 0.24 |
| Mfñ | 21.55 | 0.06 | 3.96 | 0.08 | 3.26 | 0.54 | Rub | 22.55 | 0.07 | 3.57 | 0.00 | 3.75 | 0.19 |
| Mnr | 23.13 | 0.15 | 3.39 | 0.04 | 6.81 | 0.23 | San | 20.33 | 0.03 | 3.11 | 0.04 | 6.72 | 0.18 |
| Pin | 21.35 | 0.06 | 3.45 | 0.05 | 5.40 | 0.21 | Taz | 23.53 | 0.08 | 3.90 | 0.04 | 5.66 | 0.29 |
| Sal | 22.15 | 0.04 | 3.69 | 0.02 | 4.38 | 0.12 | Tem | 22.33 | 0.04 | 3.80 | 0.06 | 3.80 | 0.07 |
| Tzn | 22.50 | 0.00 | 3.57 | 0.01 | 4.64 | 0.21 | Ter | 17.95 | 0.07 | 3.45 | 0.07 | 4.95 | 0.41 |
| Vse | 23.00 | 0.06 | 3.52 | 0.04 | 4.94 | 0.26 | TBd | 24.30 | 0.03 | 3.54 | 0.09 | 4.00 | 0.18 |
| Zur | 21.77 | 0.03 | 3.69 | 0.01 | 3.35 | 0.11 | TNa | 20.50 | 0.04 | 3.80 | 0.00 | 3.63 | 0.15 |
| TFg | 23.87 | 0.02 | 3.47 | 0.03 | 5.06 | 0.08 | |||||||
| TTa | 22.43 | 0.07 | 3.34 | 0.03 | 5.82 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Roldán, F.E.; Valdés Sánchez, M.E.; Rico, R.P.; Moreno Cardona, D.; Martínez de Toda, F.; Muñoz-Organero, G. Amino Acid Profile of Must and Aromatic Potential of 30 Minor Grape Varieties Grown in Alcalá de Henares (Spain). Agronomy 2025, 15, 1111. https://doi.org/10.3390/agronomy15051111
Espinosa-Roldán FE, Valdés Sánchez ME, Rico RP, Moreno Cardona D, Martínez de Toda F, Muñoz-Organero G. Amino Acid Profile of Must and Aromatic Potential of 30 Minor Grape Varieties Grown in Alcalá de Henares (Spain). Agronomy. 2025; 15(5):1111. https://doi.org/10.3390/agronomy15051111
Chicago/Turabian StyleEspinosa-Roldán, Francisco Emmanuel, M. Esperanza Valdés Sánchez, Raquel Pavo Rico, Daniel Moreno Cardona, Fernando Martínez de Toda, and Gregorio Muñoz-Organero. 2025. "Amino Acid Profile of Must and Aromatic Potential of 30 Minor Grape Varieties Grown in Alcalá de Henares (Spain)" Agronomy 15, no. 5: 1111. https://doi.org/10.3390/agronomy15051111
APA StyleEspinosa-Roldán, F. E., Valdés Sánchez, M. E., Rico, R. P., Moreno Cardona, D., Martínez de Toda, F., & Muñoz-Organero, G. (2025). Amino Acid Profile of Must and Aromatic Potential of 30 Minor Grape Varieties Grown in Alcalá de Henares (Spain). Agronomy, 15(5), 1111. https://doi.org/10.3390/agronomy15051111











