Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Collection, Isolation and Preparation for Storage
2.2. Molecular Identification
2.3. Tested Volatile Compounds
2.4. Chemotaxis Assay
2.5. Statistical Analysis
3. Results
3.1. Molecular Identification
3.2. Nematode Chemoattraction Towards VOCs
Nematode Motility
3.3. Chemotaxis Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, H.K.; Gaugler, R. Entomopathogenic Nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Koppenhöffer, A.M.; Fuzy, E.M.; Crocker, R.; Gelernter, W.; Polavarapu, S. Pathogenicity of Steinernema scarabaei, Heterorhabditis bacteriophora and S. glaseri to Twelve White Grub Species. Biocontrol Sci. Technol. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An Investigation on the Chemotactic Responses of Different Entomopathogenic Nematode Strains to Mechanically Damaged Maize Root Volatile Compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef]
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic Nematodes. Curr. Biol. 2012, 22, R430–R431. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the intraguild competition between Oscheius spp. nematodes and entomopathogenic nem-atodes: Implications for their natural distribution in Swiss agricultural soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef]
- Pieterse, A.; Tiedt, L.R.; Malan, A.P.; Ross, J.L. First Record of Phasmarhabditis papillosa (Nematoda: Rhabditidae) in South Africa and Its Virulence Against the Invasive Slug, Deroceras panormitanum. Nematology 2017, 19, 1035–1050. [Google Scholar] [CrossRef]
- Laznik, Ž.; Majić, I.; Trdan, S.; Malan, A.; Pieterse, A.; Ross, J.L. Is Phasmarhabditis papillosa (Nematoda: Rhabditidae) a Possible Biological Control Agent Against the Spanish Slug, Arion vulgaris (Gastropoda: Arionidae)? Nematology 2020, 23, 577–585. [Google Scholar] [CrossRef]
- Lewis, E.E. Behavioural Ecology. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 205–223. [Google Scholar]
- Campbell, J.F.; Lewis, E.E.; Stock, S.P.; Nadler, S.; Kaya, H.K. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 2003, 35, 142–145. [Google Scholar]
- Laznik, Ž.; Križman, M.; Zekič, J.; Roškarič, M.; Trdan, S.; Urbanek Krajnc, A. Navigational Signals for Insect and Slug Parasitic Nematodes: The Role of Ascorbate–Glutathione System and Volatiles Released by Insect-Damaged Sweet Pepper Roots. Insects 2024, 15, 805. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J. Chem. Ecol. 2010, 36, 361–368. [Google Scholar] [CrossRef]
- Crespo, E.; Hordijk, C.A.; de Graff, R.M.; Samudrala, D.; Cristescu, S.M.; Harren, F.J.M.; van Dam, N.M. On-line detection of root-induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae. Phytochemistry 2012, 84, 68–77. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. Attraction behaviors of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to synthetic volatiles emitted by insect-damaged carrot roots. J. Pest Sci. 2016, 89, 597–606. [Google Scholar] [CrossRef]
- Jagodič, A.; Ipavec, N.; Trdan, S.; Laznik, Ž. Attraction Behaviors: Are Synthetic Volatiles, Typically Emitted by Insect-Damaged Brassica nigra Roots, Navigation Signals for Entomopathogenic Nematodes (Steinernema and Heterorhabditis)? BioControl 2017, 62, 515–524. [Google Scholar] [CrossRef]
- Grunseich, J.M.; Thompson, M.N.; Hay, A.A.; Gorman, Z.; Kolomiets, M.V.; Eubanks, M.D.; Helms, A.M. Risky roots and careful herbivores: Sustained herbivory by a root-feeding herbivore attenuates indirect plant defences. Funct. Ecol. 2020, 34, 1779–1789. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Göbel, C.; Francis, F.; Haubruge, E.; Wathelet, J.-P.; du Jardin, P.; Feussner, I.; Fauconnier, M.-L. Attacks by Piercing-Sucking Insect (Myzus persicae Sultzer) or a Chewing Insect (Leptinotarsa decemlineata Say) on Potato Plants (Solanum tuberosum L.) Induce Differential Changes in Volatile Compound Release and Oxylipin Synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef]
- Laznik, Ž.; Košir, I.J.; Rozman, L.; Kač, M.; Trdan, S. Preliminary Results of Variability in Mechanical-Induced Volatile Root-Emissions of Different Maize Cultivars. Maydica 2011, 56, 343–350. [Google Scholar]
- Hallem, E.A.; Dillman, A.R.; Hong, A.V.; Zhang, Y.; Yano, J.M.; DeMarco, S.F.; Sternberg, P.W. A Sensory Code for Host Seeking in Parasitic Nematodes. Curr. Biol. 2011, 21, 377–383. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.; Lewis, E.E.; Campbell, J.F.; Kim-Shapiro, D.B. Directional Movement of Entomopathogenic Nematodes in Response to Electrical Field: Effect of Species, Magnitude of Voltage, and Infective Juvenile Age. J. Invertebr. Pathol. 2012, 109, 34–40. [Google Scholar] [CrossRef]
- Rowson, B.; Turner, J.; Anderson, R.; Symondson, B. Slugs of Britain and Ireland; FSC Publications: Telford, UK, 2014. [Google Scholar]
- Hominick, W.M.; Briscoe, B.R.; del Pino, F.G.; Heng, J.; Hunt, D.J.; Kozodoy, E.; Mracek, Z.; Nguyen, K.B.; Reid, A.P.; Spiridonov, S.; et al. Biosystematics of Entomopathogenic Nematodes: Current Status, Protocols and Definitions. J. Helminthol. 1997, 71, 271–298. [Google Scholar] [CrossRef]
- Liu, J.; Berry, R.E.; Moldenke, A.F. Phylogenetic Relationships of Entomopathogenic Nematodes (Heterorhabditidae and Steinernematidae) Inferred from Partial 18S rRNA Gene Sequences. J. Invertebr. Pathol. 1997, 69, 246–252. [Google Scholar] [CrossRef]
- Weissteiner, S.; Huetteroth, W.; Kollmann, M.; Weißbecker, B.; Romani, R.; Schachtner, J.; Schütz, S. Cockchafer Larvae Smell Host Root Scents in Soil. PLoS ONE 2012, 7, e45827. [Google Scholar] [CrossRef]
- O’Halloran, D.M.; Burnell, A.M. An Investigation of Chemotaxis in the Insect Parasitic Nematode Heterorhabditis bacteriophora. Parasitology 2003, 127, 375–385. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory Neurons with Overlapping Functions Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef]
- Torrini, G.; Mazza, G.; Carletti, B.; Benvenuti, C.; Roversi, P.F.; Fanelli, E.; De Luca, F.; Troccoli, A.; Tarasco, E. Oscheius onirici sp. n. (Nematoda: Rhabditidae): A new entomopathogenic nematode from an Italian cave. Zootaxa 2015, 3937, 533–548. [Google Scholar] [CrossRef]
- Bui, T.H.; Desaeger, J. Volatile Compounds as Potential Bio-Fumigants Against Plant-Parasitic Nematodes—A Mini Review. J. Nematol. 2021, 53, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; Ran, Y.; Yang, H.; Mo, M.; Li, G. Volatile Metabolites from Brevundimonas diminuta and Nematicidal Esters Inhibit Meloidogyne javanica. Microorganisms 2023, 11, 966. [Google Scholar] [CrossRef]
- Anastasiadis, I.A.; Karanastasi, E. Factors affecting the efficacy of Brassica species and ryegrass (Lolium perenne L.) on root-knot nematode infestation of tomato. Commun. Agric. Appl. Biol. Sci. 2011, 76, 333–340. [Google Scholar]
- Roubtsova, T.V.; Yakovleva, E.; Bostock, R.M.; Subbotin, S.A.; Kravchenko, A.N.; Borneman, J.; Becker, J.O. Effect of Broccoli (Brassica oleracea) Tissue, Incorporated at Different Depths in a Soil Column, on Meloidogyne incognita. J. Nematol. 2007, 39, 111–117. [Google Scholar]
- Hiltpold, I.; Hibbard, B.E.; Wade French, B.; Turlings, T.C.J. Capsules Containing Entomopathogenic Nematodes as a Trojan Horse Approach to Control the Western Corn Rootworm. Plant Soil 2012, 358, 11–25. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S.; Šavli, K. Chemotactic Responses of Oscheius myriophilus to Mollusk Mucus. Agronomy 2024, 14, 3049. [Google Scholar] [CrossRef]

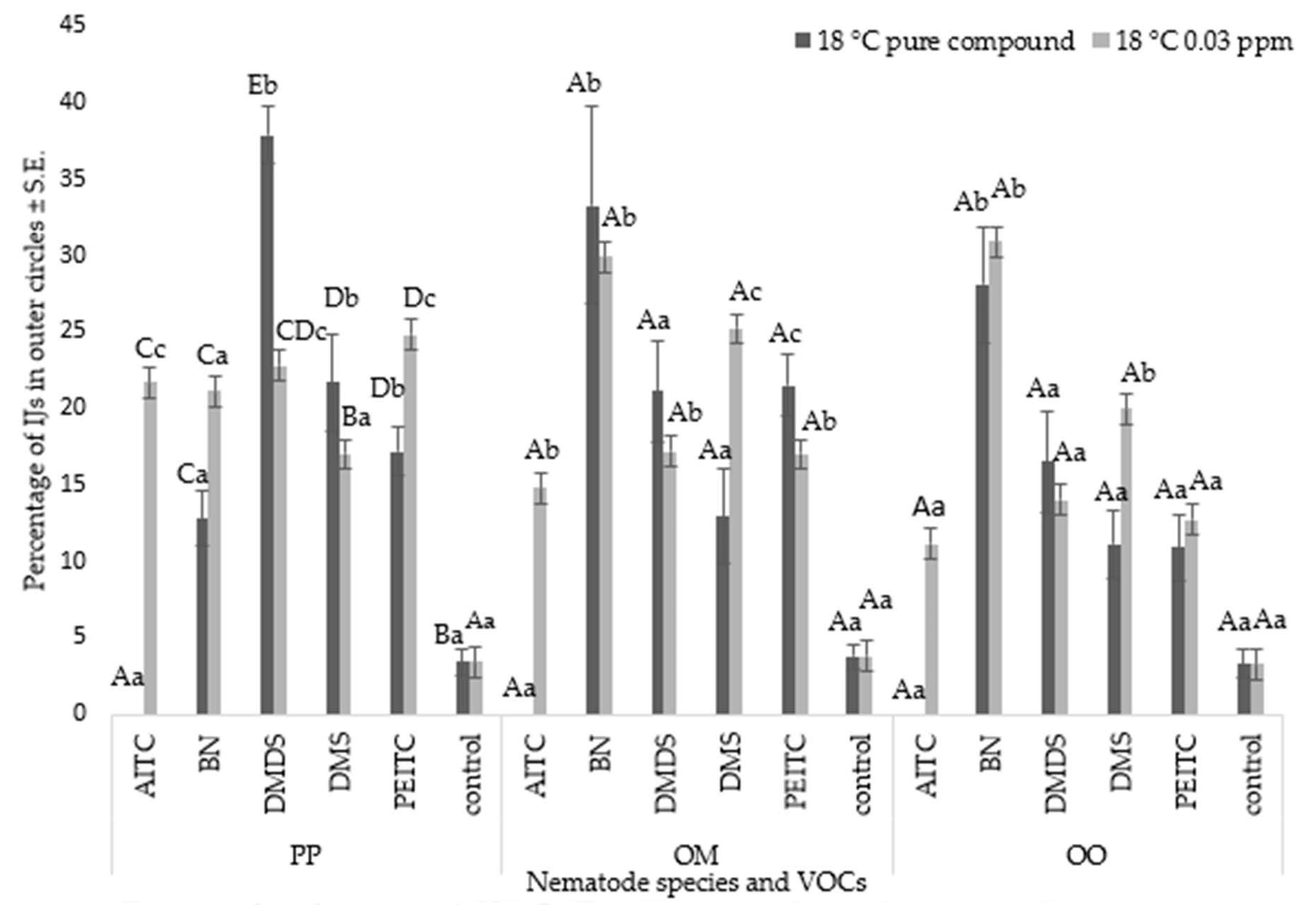
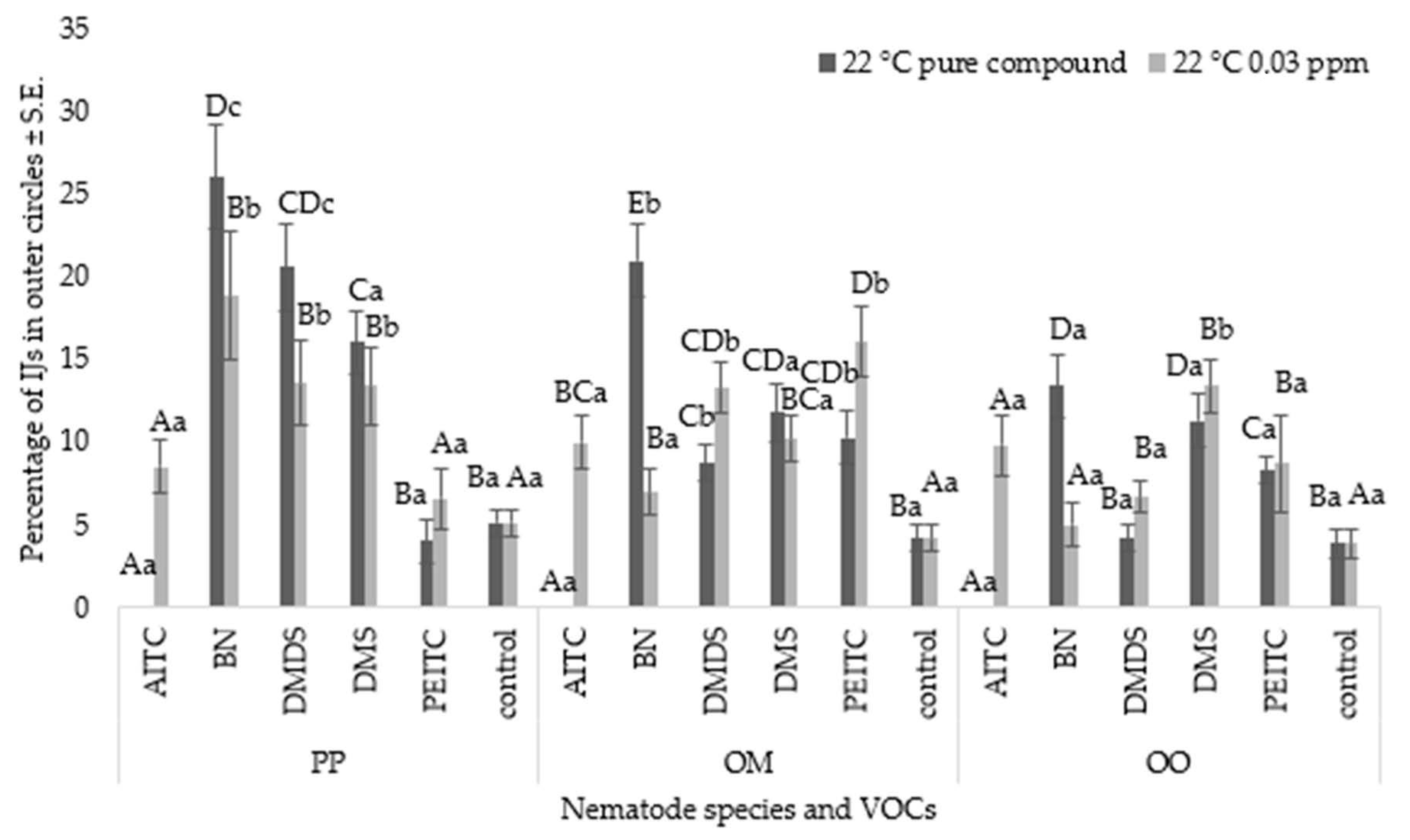
| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 1056.2 | 2 | 0.58 | 0.5584 |
| VOCs (V) | 23,659.5 | 5 | 5.23 | 0.0001 |
| VOCs concentration (C) | 56.3 | 1 | 0.06 | 0.8031 |
| Temperature (T) | 13,266.3 | 1 | 14.65 | 0.0001 |
| Temporal replication | 9090.7 | 9 | 1.12 | 0.3492 |
| Spatial replication | 2240.0 | 2 | 1.24 | 0.2451 |
| S × V | 2992.8 | 10 | 0.33 | 0.9729 |
| S × C | 2445.0 | 2 | 1.35 | 0.2599 |
| S × T | 1402.2 | 2 | 0.77 | 0.4615 |
| V × C | 14,199.4 | 5 | 3.14 | 0.0083 |
| V × T | 10,561.0 | 5 | 2.33 | 0.0409 |
| S × V × C | 16,593.1 | 10 | 1.83 | 0.0520 |
| S × V × T | 17,130.6 | 10 | 1.89 | 0.0434 |
| Residual | 583,594.6 | 662 | ||
| Total (Corrected) | 707,145.0 | 719 |
| Factor | Sum of Squares | Df | F | p |
|---|---|---|---|---|
| Nematode species (S) | 0.0083 | 2 | 0.55 | 0.5774 |
| VOCs (V) | 1.1279 | 5 | 29.84 | 0.0001 |
| VOCs concentration (C) | 0.0009 | 1 | 0.12 | 0.7263 |
| Temperature (T) | 0.1173 | 1 | 15.52 | 0.0001 |
| Temporal replication | 0.0786 | 9 | 1.16 | 0.3214 |
| Spatial replication | 0.0165 | 2 | 1.08 | 0.4312 |
| S × V | 0.4928 | 10 | 6.52 | 0.0001 |
| S × C | 0.0088 | 2 | 0.58 | 0.5592 |
| S × T | 0.0153 | 2 | 1.01 | 0.3644 |
| V × C | 0.0386 | 5 | 1.02 | 0.4049 |
| V × T | 0.6238 | 5 | 16.50 | 0.0001 |
| S × V × C | 0.1600 | 10 | 2.12 | 0.0214 |
| S × V × T | 0.6584 | 10 | 8.71 | 0.0001 |
| Residual | 6.4948 | 682 | ||
| Total (Corrected) | 8.6428 | 719 |
| VOCs | 18 °C | ||
|---|---|---|---|
| Pure Compound | |||
| Phasmarhabditis papillosa | Oscheius myriophilus | Oscheius onirici | |
| AITC | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Aa |
| BN | −0.02 ± 0.01 Aa | 0.22 ± 0.05 Db | 0.23 ± 0.03 Db |
| DMDS | 0.05 ± 0.01 Cb | −0.11 ± 0.03 Aa | 0.02 ± 0.01 Bb |
| DMS | 0.11 ± 0.00 Db | 0.04 ± 0.02 Ca | 0.03 ± 0.01 Aa |
| PEITC | 0.01 ± 0.01 Ba | 0.17 ± 0.03 Dc | 0.06 ± 0.01 Cb |
| Control | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Aa |
| 0.03 ppm | |||
| AITC | −0.05 ± 0.03 Aa | 0.00 ± 0.04 BCa | −0.04 ± 0.01 Aa |
| BN | 0.12 ± 0.03 CDa | 0.22± 0.04 Eb | 0.27 ± 0.04 Db |
| DMDS | 0.16 ± 0.01 Dc | −0.08 ± 0.02 Aa | 0.04 ± 0.04 BCb |
| DMS | −0.06 ± 0.03 Aa | 0.08 ± 0.02 Db | −0.07 ± 0.03 Aa |
| PEITC | 0.11 ± 0.05 Ca | 0.07 ± 0.04 Ca | 0.08 ± 0.02 Ca |
| Control | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Ba |
| 22 °C | |||
|---|---|---|---|
| VOCs | Pure Compound | ||
| Phasmarhabditis papillosa | Oscheius myriophilus | Oscheius onirici | |
| AITC | 0.00 ± 0.00 Ba | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa |
| BN | 0.10 ± 0.02 Cb | 0.08 ± 0.03 Cb | 0.00 ± 0.01 Aa |
| DMDS | 0.13 ± 0.03 Cb | 0.03 ± 0.02 BCa | 0.02 ± 0.01 Aa |
| DMS | −0.06 ± 0.03 Aa | 0.01 ± 0.01 ABb | 0.07 ± 0.01 Bc |
| PEITC | −0.03 ± 0.01 Aa | 0.04 ± 0.02 BCb | −0.01 ± 0.02 Aa |
| Control | 0.01 ± 0.01 Ba | 0.01 ± 0.01 ABa | 0.01 ± 0.01 Aa |
| 0.03 ppm | |||
| AITC | −0.04 ± 0.02 Aa | 0.01 ± 0.02 Ab | 0.03 ± 0.01 Bb |
| BN | 0.05 ± 0.03 CDb | 0.02 ± 0.02 ABab | 0.00 ± 0.01 Aa |
| DMDS | 0.02 ± 0.02 Ba | 0.07 ± 0.02 Bb | 0.01 ± 0.01 ABa |
| DMS | 0.06 ± 0.01 Db | 0.01 ± 0.01 Aa | 0.07 ± 0.01 Cb |
| PEITC | 0.00 ± 0.01 Ba | 0.02 ± 0.03 ABa | −0.01 ± 0.02 Aa |
| Control | 0.01 ± 0.01 BCa | 0.01 ± 0.01 Aa | 0.01 ± 0.01 ABa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laznik, Ž.; Tóth, T.; Ádám, S.; Trdan, S.; Majić, I.; Lakatos, T. Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots. Agronomy 2025, 15, 664. https://doi.org/10.3390/agronomy15030664
Laznik Ž, Tóth T, Ádám S, Trdan S, Majić I, Lakatos T. Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots. Agronomy. 2025; 15(3):664. https://doi.org/10.3390/agronomy15030664
Chicago/Turabian StyleLaznik, Žiga, Tímea Tóth, Szabolcs Ádám, Stanislav Trdan, Ivana Majić, and Tamás Lakatos. 2025. "Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots" Agronomy 15, no. 3: 664. https://doi.org/10.3390/agronomy15030664
APA StyleLaznik, Ž., Tóth, T., Ádám, S., Trdan, S., Majić, I., & Lakatos, T. (2025). Responses of Parasitic Nematodes to Volatile Organic Compounds Emitted by Brassica nigra Roots. Agronomy, 15(3), 664. https://doi.org/10.3390/agronomy15030664








