Quantitative Resolution of Phosphorus Sources in an Agricultural Watershed of Southern China: Application of Phosphate Oxygen Isotopes and Multiple Models
Abstract
1. Introduction
2. Materials and Methods
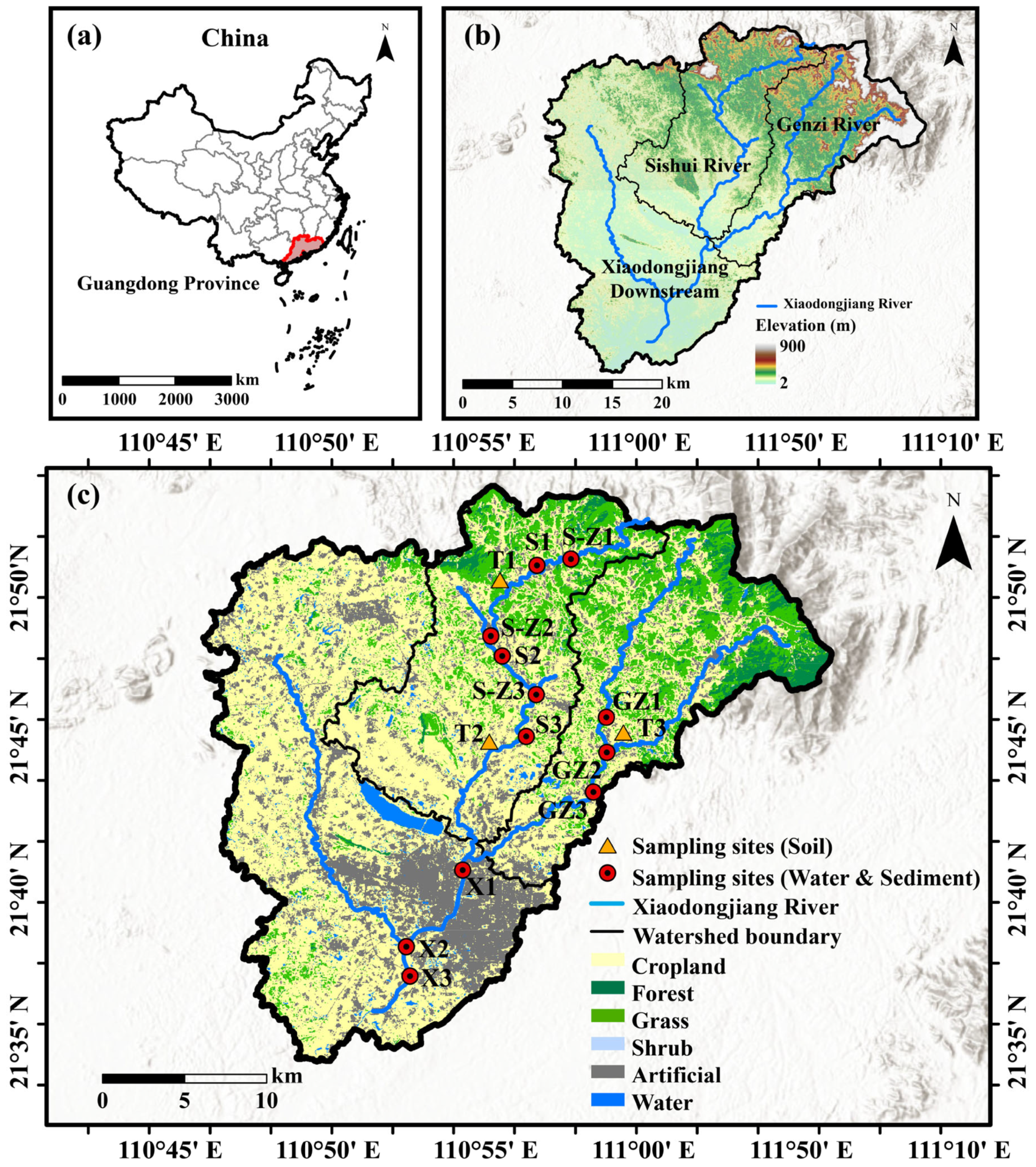
2.1. Study Area and Sampling Points
2.2. Sample Collection
2.3. Sample Preparation
2.4. Isotopic Composition Analysis
2.5. Stable Isotope Mixing Model
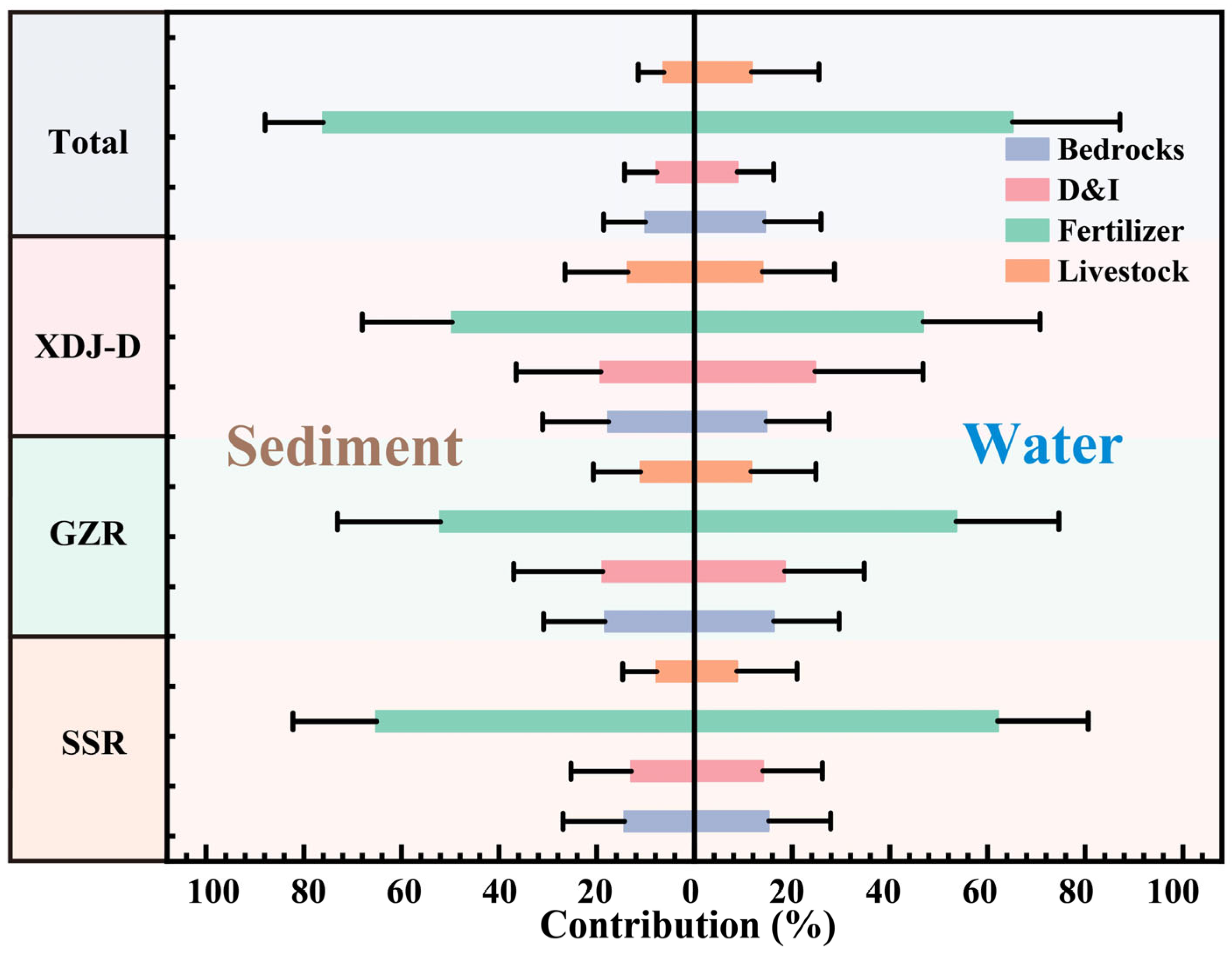
2.6. Sensitivity Analysis
3. Results
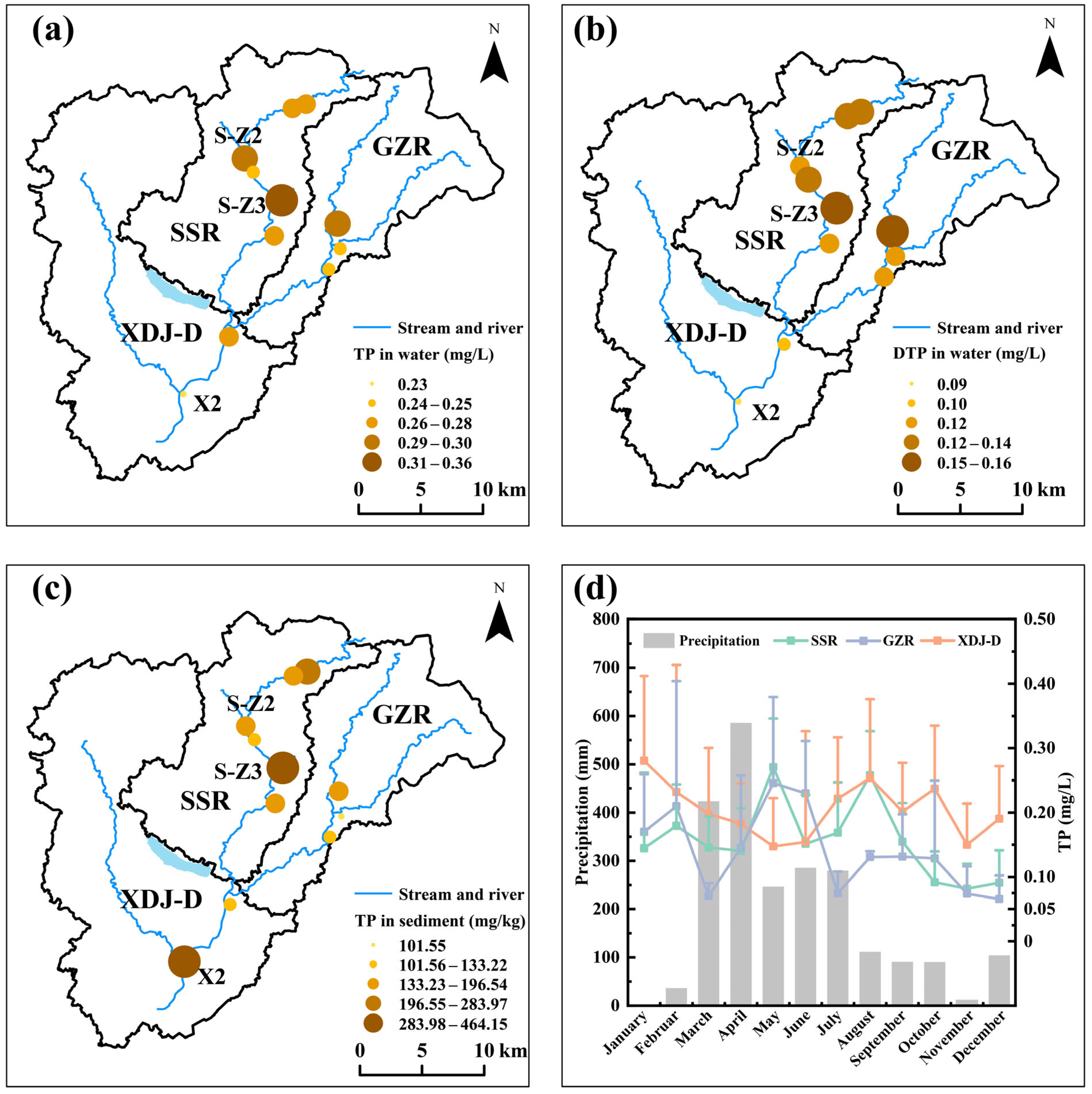
3.1. Spatio-Temporal Characteristics of Phosphorus in the XDJ Watershed
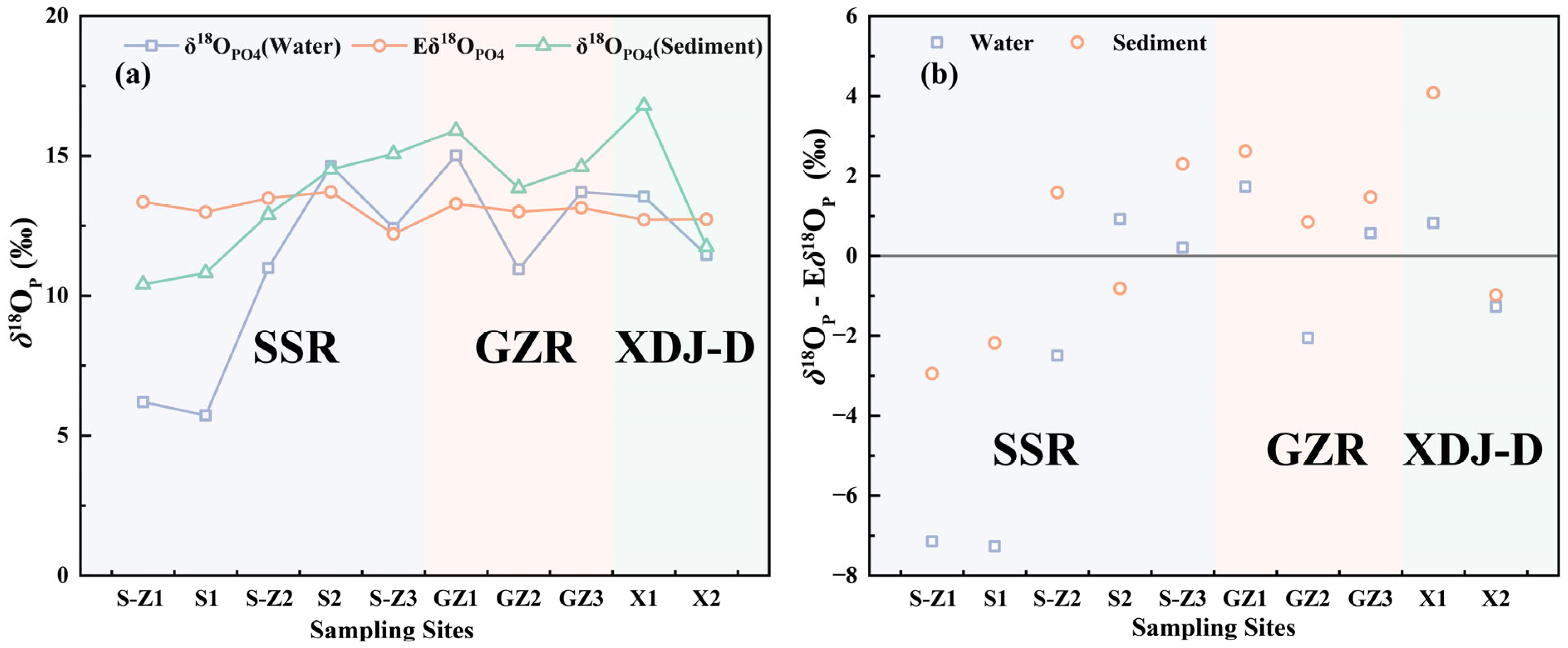
3.2. Characterization of δ18OP Distribution in Water Samples and Sediments from the XDJ Watershed and Feasibility Analysis of Tracer Sources
3.3. Deviation of δ18OP Values from Equilibrium Values in the XDJ Watershed
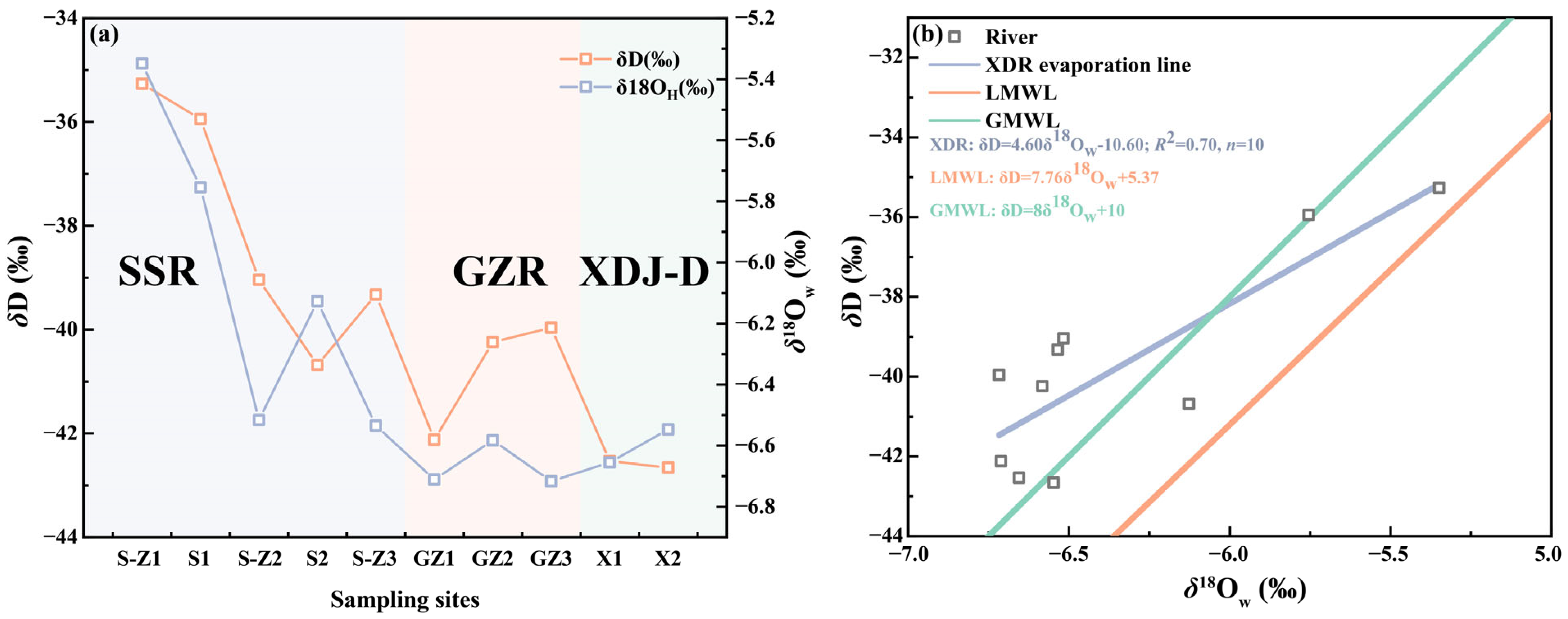
3.4. Spatial Distribution Characteristics of δD and δ18OW in the XDJ Watershed
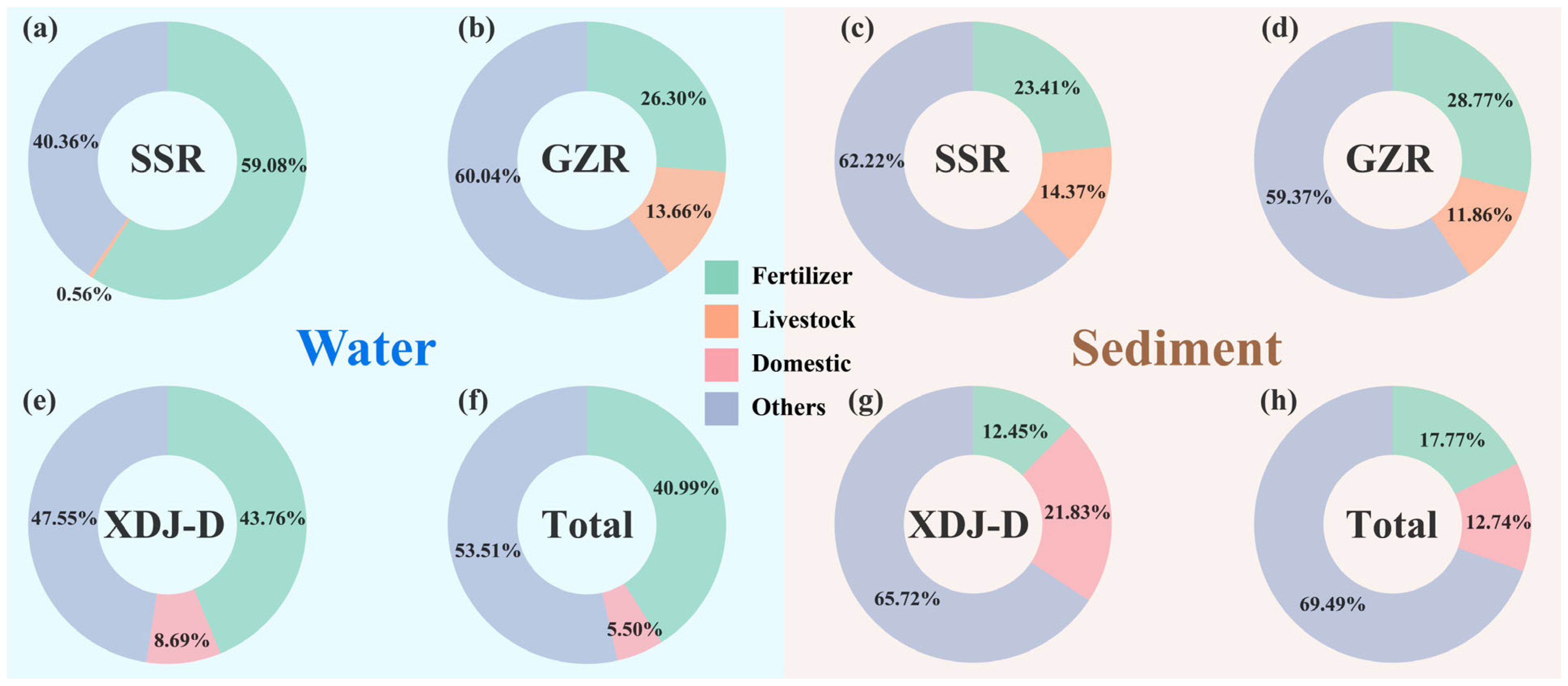
3.5. Analysis of Major Phosphorus Sources in the XDJ Watershed on Land-Use End-Source Mixing Modeling
3.6. Quantitative Analysis of Major Phosphorus Sources in the XDJ Watershed
4. Discussion
4.1. Indication of Phosphate and Its Isotopic Signature in Xiaodongjiang Watershed
4.2. Endogenous Control of River Phosphate
4.3. Exogenous Inputs of River Phosphate and Recommendations for Phosphorus Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, J.; Chen, J.; Jin, Z.; Ding, S.; Yang, X. Method for phosphate oxygen isotopes analysis in water based on in situ enrichment, elution, and purification. J. Environ. Manag. 2021, 279, 111618. [Google Scholar] [CrossRef] [PubMed]
- Karl, D.M. Phosphorus, the staff of life. Nature 2000, 406, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Alcamo, J.; Flörke, M.; Reder, K. Phosphorus loadings to the world’s largest lakes: Sources and trends. Glob. Biogeochem. Cycles 2018, 32, 617–634. [Google Scholar] [CrossRef]
- Gächter, R.; Wehrli, B. Ten years of artificial mixing and oxygenation: No effect on the internal phosphorus loading of two eutrophic lakes. Environ. Sci. Technol. 1998, 32, 3659–3665. [Google Scholar] [CrossRef]
- Luo, X.; Elrys, A.S.; Zhang, L.; Ibrahim, M.M.; Liu, Y.; Fu, S.; Yan, J.; Ye, Q.; Wen, D.; Hou, E. The global fate of inorganic phosphorus fertilizers added to terrestrial ecosystems. One Earth 2024, 7, 1402–1413. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Dong, L.; Zhang, J. Cleaner agricultural production in drinking-water source areas for the control of non-point source pollution in China. J. Environ. Manag. 2021, 285, 112096. [Google Scholar] [CrossRef]
- Ishida, T.; Uehara, Y.; Iwata, T.; Cid-Andres, A.P.; Asano, S.; Ikeya, T.; Osaka, K.i.; Ide, J.i.; Privaldos, O.L.A.; Jesus, I.B.B.D. Identification of phosphorus sources in a watershed using a phosphate oxygen isoscape approach. Environ. Sci. Technol. 2019, 53, 4707–4716. [Google Scholar] [CrossRef]
- Van Moorleghem, C.; De Schutter, N.; Smolders, E.; Merckx, R. The bioavailability of colloidal and dissolved organic phosphorus to the alga Pseudokirchneriella subcapitata in relation to analytical phosphorus measurements. Hydrobiologia 2013, 709, 41–53. [Google Scholar] [CrossRef]
- Linge, K.L.; Oldham, E.C. Control mechanisms for dissolved phosphorus and arsenic in a shallow lake. Appl. Geochem. 2004, 19, 1377–1389. [Google Scholar] [CrossRef]
- Cui, M.; Guo, Q.; Wei, R.; Tian, L. Human-driven spatiotemporal distribution of phosphorus flux in the environment of a mega river basin. Sci. Total Environ. 2021, 752, 141781. [Google Scholar] [CrossRef]
- Piantone, P.; Bodénan, F.; Derie, R.; Depelsenaire, G. Monitoring the stabilization of municipal solid waste incineration fly ash by phosphation: Mineralogical and balance approach. Waste Manag. 2003, 23, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Fernandes, L.S.; Pereira, M.; Cortes, R.; Pacheco, F. A framework model for investigating the export of phosphorus to surface waters in forested watersheds: Implications to management. Sci. Total Environ. 2015, 536, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.L.; Surridge, B.W.; Gooddy, D.C. Phosphate oxygen isotopes within aquatic ecosystems: Global data synthesis and future research priorities. Sci. Total Environ. 2014, 496, 563–575. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, L.; Zhao, C.; Du, C.; Zhang, J.; Sun, F.; Tekleab, T.Z.; Wei, R.; Fu, P.; Gooddy, D.C. Source partitioning using phosphate oxygen isotopes and multiple models in a large catchment. Water Res. 2023, 244, 120382. [Google Scholar] [CrossRef]
- Blake, R.E.; Alt, J.C.; Martini, A.M. Oxygen isotope ratios of PO4: An inorganic indicator of enzymatic activity and P metabolism and a new biomarker in the search for life. Proc. Natl. Acad. Sci. USA 2001, 98, 2148–2153. [Google Scholar] [CrossRef]
- Lecuyer, C.; Grandjean, P.; Sheppard, S.M. Oxygen isotope exchange between dissolved phosphate and water at temperatures≤ 135 C: Inorganic versus biological fractionations. Geochim. Cosmochim. Acta 1999, 63, 855–862. [Google Scholar] [CrossRef]
- Elsbury, K.E.; Paytan, A.; Ostrom, N.E.; Kendall, C.; Young, M.B.; McLaughlin, K.; Rollog, M.E.; Watson, S. Using oxygen isotopes of phosphate to trace phosphorus sources and cycling in Lake Erie. Environ. Sci. Technol. 2009, 43, 3108–3114. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, Y.; Qiao, Q.; Bai, L. Using a multi-isotope approach and isotope mixing models to trace and quantify phosphorus sources in the Tuojiang River, Southwest China. Environ. Sci. Technol. 2023, 57, 7328–7335. [Google Scholar] [CrossRef]
- Granger, S.J.; Heaton, T.H.; Pfahler, V.; Blackwell, M.S.; Yuan, H.; Collins, A.L. The oxygen isotopic composition of phosphate in river water and its potential sources in the Upper River Taw catchment, UK. Sci. Total Environ. 2017, 574, 680–690. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, H.; Dong, A.; Zhou, Y.; Huang, R.; Yin, H.; Zhang, L.; Liu, E.; Li, Q.; Jia, B. Tracing the sources of phosphorus in lake at watershed scale using phosphate oxygen isotope (δ18OP). Chemosphere 2022, 305, 135382. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, D.-H.; Kim, M.-S.; Rhee, H.-P.; Hur, J.; Shin, K.-H. Systematic tracing of nitrate sources in a complex river catchment: An integrated approach using stable isotopes and hydrological models. Water Res. 2023, 235, 119755. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tang, Y.; Xu, Y.; Zhang, S.; Ma, J.; Xiao, S.; Ji, D.; Yang, Z.; Liu, D. Using stable nitrogen and oxygen isotopes to identify nitrate sources in the Lancang River, upper Mekong. J. Environ. Manag. 2020, 274, 111197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, W.; Li, Y.; Zhang, J.; Xu, Z. Multiple isotopes reveal a hydrology dominated control on the nitrogen cycling in the Nujiang River Basin, the last undammed large river basin on the Tibetan Plateau. Environ. Sci. Technol. 2022, 56, 4610–4619. [Google Scholar] [CrossRef]
- Ren, X.; Yue, F.-J.; Tang, J.; Li, C.; Li, S.-L. Nitrate transformation and source tracking of rivers draining into the Bohai Sea using a multi-tracer approach combined with an optimized Bayesian stable isotope mixing model. J. Hazard. Mater. 2024, 463, 132901. [Google Scholar] [CrossRef]
- Yuan, B.; Guo, M.; Zhou, X.; Li, M.; Xie, S. Defining the sources and the fate of nitrate by using dual isotopes and a Bayesian isotope mixing model: Water–nitrate management in cascade dams of Lancang river. Sci. Total Environ. 2023, 886, 163995. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, L.; Wang, W.; Shi, R. Study on the Effect of Land-Use Change on Phosphorus Pollution In Water China, Jianjiang River, Huazhou Station Control Basin. Pol. J. Environ. Stud. 2022, 31, 2537–2547. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Liang, Z.; Yan, C.; Zhu, A.; Li, S.; Yang, Z.; He, H.; Gan, H.; Chen, J. Remobilization mechanism and release characteristics of phosphorus in saline sediments from the Pearl River Estuary (PRE), South China, based on high-resolution measurements. Sci. Total Environ. 2020, 703, 134411. [Google Scholar] [CrossRef]
- Chen, H.; Shi, R.; He, J.; Zheng, L.; Wang, W. Simulation Analysis of Runoff and Sediment Yield under the Change of Land-Use Type: A Case Study of the Upper Jianjiang River Basin, China. Pol. J. Environ. Stud. 2022, 31, 2527–2536. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, Q.; Li, C.; Li, Z.; Zhang, Y.; Li, F.; Shu, W.; Wang, D.; Hao, S. Phosphorus forms in sediments of Xiaodongjiang River and its environmental importance. J. Agric. Resour. Environ. 2024, 1–14. (In Chinese) [Google Scholar] [CrossRef]
- McLaughlin, K.; Silva, S.; Kendall, C.; Stuart-Williams, H.; Paytan, A. A precise method for the analysis of δ18O of dissolved inorganic phosphate in seawater. Limnol. Oceanogr. Methods 2004, 2, 202–212. [Google Scholar] [CrossRef]
- Gross, A.; Turner, B.L.; Goren, T.; Berry, A.; Angert, A. Tracing the sources of atmospheric phosphorus deposition to a tropical rain forest in Panama using stable oxygen isotopes. Environ. Sci. Technol. 2016, 50, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Jaisi, D.P.; Blake, R.E. Tracing sources and cycling of phosphorus in Peru Margin sediments using oxygen isotopes in authigenic and detrital phosphates. Geochim. Cosmochim. Acta 2010, 74, 3199–3212. [Google Scholar] [CrossRef]
- Pistocchi, C.; Tamburini, F.; Gruau, G.; Ferhi, A.; Trevisan, D.; Dorioz, J.-M. Tracing the sources and cycling of phosphorus in river sediments using oxygen isotopes: Methodological adaptations and first results from a case study in France. Water Res. 2017, 111, 346–356. [Google Scholar] [CrossRef]
- Longinelli, A.; Nuti, S. Revised phosphate-water isotopic temperature scale. Earth Planet. Sci. Lett. 1973, 19, 373–376. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Jin, Z.; Zhang, Y.; Xiao, J.; Li, F. Combining dual isotopes and a Bayesian isotope mixing model to quantify the nitrate sources of precipitation in Ningbo, East China. Sci. Total Environ. 2021, 778, 146297. [Google Scholar] [CrossRef]
- Tcaci, M.; Barbecot, F.; Hélie, J.-F.; Surridge, B.W.; Gooddy, D.C. A new technique to determine the phosphate oxygen isotope composition of freshwater samples at low ambient phosphate concentration. Environ. Sci. Technol. 2019, 53, 10288–10294. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, R.; Gan, Y.; Cai, L.; Freidenreich, A.; Wang, K.; Guo, T.; Wang, H. Multiple methods for the identification of heavy metal sources in cropland soils from a resource-based region. Sci. Total Environ. 2019, 651, 3127–3138. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, H.; Huang, L.; Cui, Z. Particulate organic carbon dynamics with sediment transport in the upper Yangtze River. Water Res. 2020, 184, 116193. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Jiang, Z.; Liu, S. Identifying sources of phosphorus in precipitation using phosphate oxygen isotope in a human and monsoon Co-affected embayment. Atmos. Environ. 2021, 244, 118008. [Google Scholar] [CrossRef]
- Somura, H.; Takeda, I.; Arnold, J.; Mori, Y.; Jeong, J.; Kannan, N.; Hoffman, D. Impact of suspended sediment and nutrient loading from land uses against water quality in the Hii River basin, Japan. J. Hydrol. 2012, 450, 25–35. [Google Scholar] [CrossRef]
- Wang, L.; Shang, S.; Liu, W.; She, D.; Hu, W.; Liu, Y. Hydrodynamic controls on nitrogen distribution and removal in aquatic ecosystems. Water Res. 2023, 242, 120257. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Gao, H.; Li, F.; Wu, K.; Zhang, Q.; Li, Z.; Mao, S.; Li, C.; Wang, J. River water quality evaluation and analysis of influencing factors in typical agricultural areas. J. Agric. Resour. Environ. 2023, 40, 583–597. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, M.M.; Wang, S.-m.; Chen, Y.-p.; Wu, J.-h.; Xue, L.-g.; Fan, T.T. Pollution status of the Yellow River tributaries in middle and lower reaches. Sci. Total Environ. 2020, 722, 137861. [Google Scholar] [CrossRef]
- Tong, Y.; Bu, X.; Chen, J.; Zhou, F.; Chen, L.; Liu, M.; Tan, X.; Yu, T.; Zhang, W.; Mi, Z. Estimation of nutrient discharge from the Yangtze River to the East China Sea and the identification of nutrient sources. J. Hazard. Mater. 2017, 321, 728–736. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Review of phosphate removal from water by carbonaceous sorbents. J. Environ. Manag. 2021, 287, 112245. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Zhao, H.; Wu, F. Phosphorus release characteristics of different trophic lake sediments under simulative disturbing conditions. J. Hazard. Mater. 2009, 161, 1551–1559. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, Y.; Xia, R.; Huang, G.; Qin, T.; Yan, D.; Chen, Y. Backwater makes the tributaries of large river becoming phosphorus “sink”. Water Res. 2024, 261, 122012. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Q.; Tian, L. Tracing phosphorus cycle in global watershed using phosphate oxygen isotopes. Sci. Total Environ. 2022, 829, 154611. [Google Scholar] [CrossRef]
- Liang, Y.; Blake, R. Oxygen isotope signature of Pi regeneration from organic compounds by phosphomonoesterases and photooxidation. Geochim. Cosmochim. Acta 2006, 70, 3957–3969. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Q.; Kukkadapu, R.K.; Liu, E.; Yu, J.; Fang, H.; Li, H.; Jaisi, D.P. Identifying sources and cycling of phosphorus in the sediment of a shallow freshwater lake in China using phosphate oxygen isotopes. Sci. Total Environ. 2019, 676, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, X.; Wang, H.; Fan, H.; Chen, Z.; Guo, Q.; Wang, Z.; Wu, C.; Canfield, D.E.; Zhang, S. Phosphate oxygen isotopes constrain Mesoproterozoic marine temperatures and the paucity of phosphorite. Chem. Geol. 2024, 644, 121831. [Google Scholar] [CrossRef]
- Zhong, Y. The Cause of Eutrophication and Phosphorus Source in Mianyuan River. Ph.D. Thesis, Chengdu University of Technology, Chengdu, China, 2019. [Google Scholar]
- He, X.; Han, W.; Chen, Q.; Zhang, Z.; Liu, C.; Zhang, Y.; Liu, Z.; Han, C.; Yao, Z.; Chen, K. Internal loading regulates the phosphorus concentration in a diversion input shallow lake in the semi-humid region of North China: Differentiated processes in littoral wetland and open water areas. Water Res. 2024, 257, 121680. [Google Scholar] [CrossRef]
- Li, S.; Arnscheidt, J.; Cassidy, R.; Douglas, R.; McGrogan, H.; Jordan, P. The spatial and temporal dynamics of sediment phosphorus attenuation and release in impacted stream catchments. Water Res. 2023, 245, 120663. [Google Scholar] [CrossRef]
- Ni, Z.; Li, Y.; Wang, S. Cognizing and characterizing the organic phosphorus in lake sediments: Advances and challenges. Water Res. 2022, 220, 118663. [Google Scholar] [CrossRef]
- Dorioz, J.M.; Cassell, E.; Orand, A.; Eisenman, K. Phosphorus storage, transport and export dynamics in the Foron River watershed. Hydrol. Process. 1998, 12, 285–309. [Google Scholar] [CrossRef]
- Luo, G. Review of waste phosphorus from aquaculture: Source, removal and recovery. Rev. Aquac. 2023, 15, 1058–1082. [Google Scholar] [CrossRef]
- Jayasiri, M.; Yadav, S.; Dayawansa, N.; Propper, C.R.; Kumar, V.; Singleton, G.R. Spatio-temporal analysis of water quality for pesticides and other agricultural pollutants in Deduru Oya river basin of Sri Lanka. J. Clean. Prod. 2022, 330, 129897. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Reiler, E.M.; Carazo, E.; Matarrita, J.; Muñoz, A.; Cedergreen, N. Influence of rice field agrochemicals on the ecological status of a tropical stream. Sci. Total Environ. 2016, 542, 12–21. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yang, F.; Feng, H.; Yu, Z.; Liu, C.; Wei, C.; Liang, T. Organic fertilizer reduced carbon and nitrogen in runoff and buffered soil acidification in tea plantations: Evidence in nutrient contents and isotope fractionations. Sci. Total Environ. 2021, 762, 143059. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.H.; Van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [PubMed]
- Mingus, K.A.; Liang, X.; Massoudieh, A.; Jaisi, D.P. Stable isotopes and Bayesian modeling methods of tracking sources and differentiating bioavailable and recalcitrant phosphorus pools in suspended particulate matter. Environ. Sci. Technol. 2018, 53, 69–76. [Google Scholar] [CrossRef]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef]
- Tian, L. The Research of Analysis Method of Soil Phosphate Oxygen Isotopic and Its Application in Agricultural Soils. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2017. [Google Scholar]
- Sun, Y.; Amelung, W.; Wu, B.; Haneklaus, S.; Maekawa, M.; Lücke, A.; Schnug, E.; Bol, R. ‘Co-evolution’ of uranium concentration and oxygen stable isotope in phosphate rocks. Appl. Geochem. 2020, 114, 104476. [Google Scholar] [CrossRef]
- Young, M.B.; McLaughlin, K.; Kendall, C.; Stringfellow, W.; Rollog, M.; Elsbury, K.; Donald, E.; Paytan, A. Characterizing the oxygen isotopic composition of phosphate sources to aquatic ecosystems. Environ. Sci. Technol. 2009, 43, 5190–5196. [Google Scholar] [CrossRef]
- Breaker, B.K. Phosphate-Oxygen Isotope ratios as a Tracer for Sources and Cycling of Phosphorus in the Illinois River Watershed; University of Arkansas: Fayetteville, AR, USA, 2009. [Google Scholar]
- Xue, K. The Impact of Phospahte Mining Activities on Surface Water Environment and Its Control Strategies—Insights from Phosphate Oxygen Isotope Tracing Technology. Master’s thesis, University of Chinese Academy of Sciences, Beijing, China, 2020. [Google Scholar]
- Liu, Y. Construction and Preliminary Application of Pretreatment Method System of Phosphate Oxygen Isotope Separation and Purification for Freshwater Lake Environmental Media. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Tan, J.; Gao, X.; Liu, S.; Li, C.; Zeng, L.; Wang, Y.; Wang, F.; Zhang, Q.; Chen, G. Quantitative Resolution of Phosphorus Sources in an Agricultural Watershed of Southern China: Application of Phosphate Oxygen Isotopes and Multiple Models. Agronomy 2025, 15, 663. https://doi.org/10.3390/agronomy15030663
Wang D, Tan J, Gao X, Liu S, Li C, Zeng L, Wang Y, Wang F, Zhang Q, Chen G. Quantitative Resolution of Phosphorus Sources in an Agricultural Watershed of Southern China: Application of Phosphate Oxygen Isotopes and Multiple Models. Agronomy. 2025; 15(3):663. https://doi.org/10.3390/agronomy15030663
Chicago/Turabian StyleWang, Dengchao, Jingwei Tan, Xinhua Gao, Shanbao Liu, Caole Li, Linghui Zeng, Yizhe Wang, Fan Wang, Qiuying Zhang, and Gang Chen. 2025. "Quantitative Resolution of Phosphorus Sources in an Agricultural Watershed of Southern China: Application of Phosphate Oxygen Isotopes and Multiple Models" Agronomy 15, no. 3: 663. https://doi.org/10.3390/agronomy15030663
APA StyleWang, D., Tan, J., Gao, X., Liu, S., Li, C., Zeng, L., Wang, Y., Wang, F., Zhang, Q., & Chen, G. (2025). Quantitative Resolution of Phosphorus Sources in an Agricultural Watershed of Southern China: Application of Phosphate Oxygen Isotopes and Multiple Models. Agronomy, 15(3), 663. https://doi.org/10.3390/agronomy15030663






