Forage Quality and Yield Enhancement via Wolfberry (Lycium barbarum L.)–Forage Intercropping System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Forage Growth and Yield Estimation
2.3. Forage Photosynthetic and Physiological Measurements
2.4. Forage Quality Estimation
2.5. Statistical Analysis
3. Results
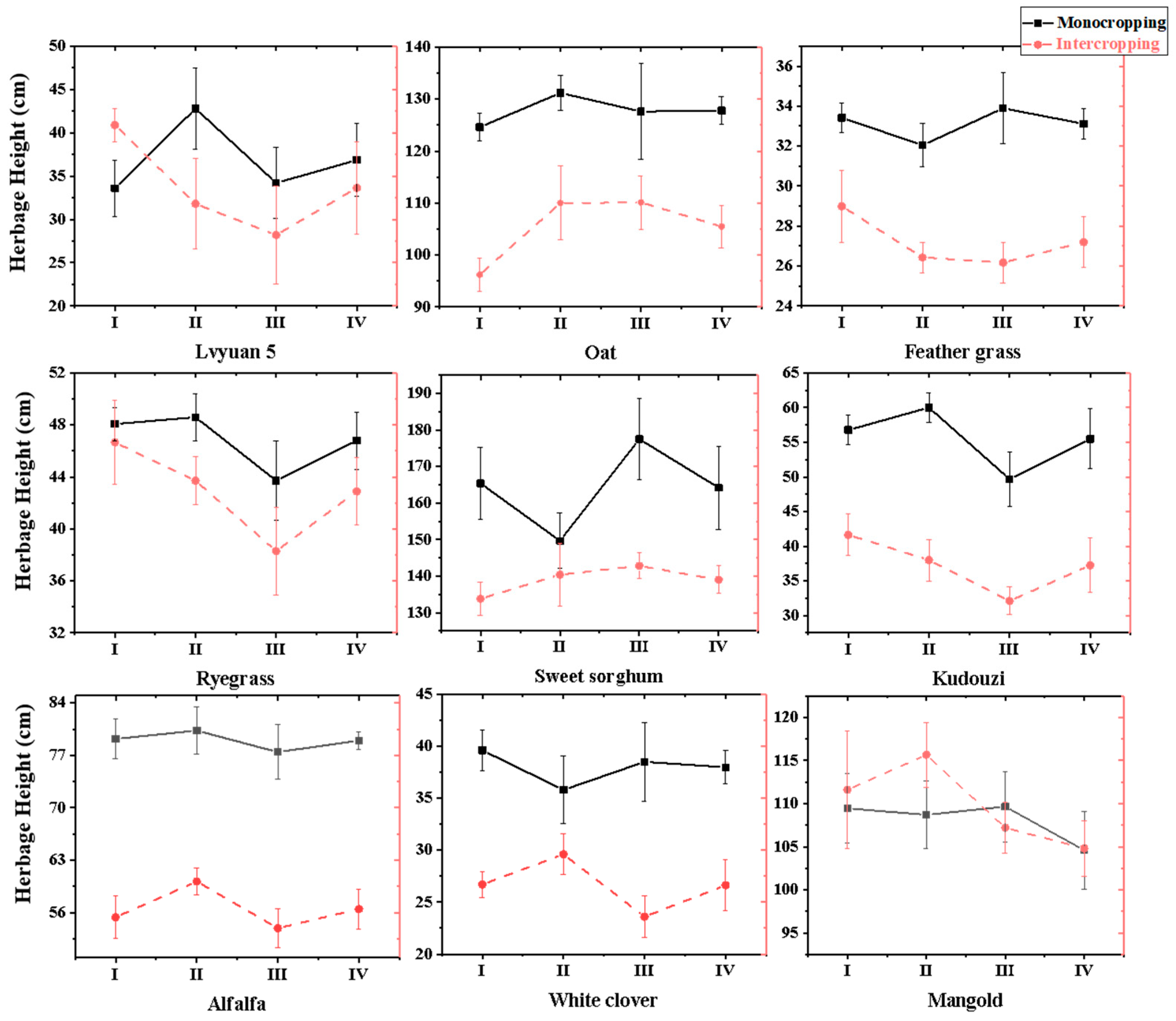
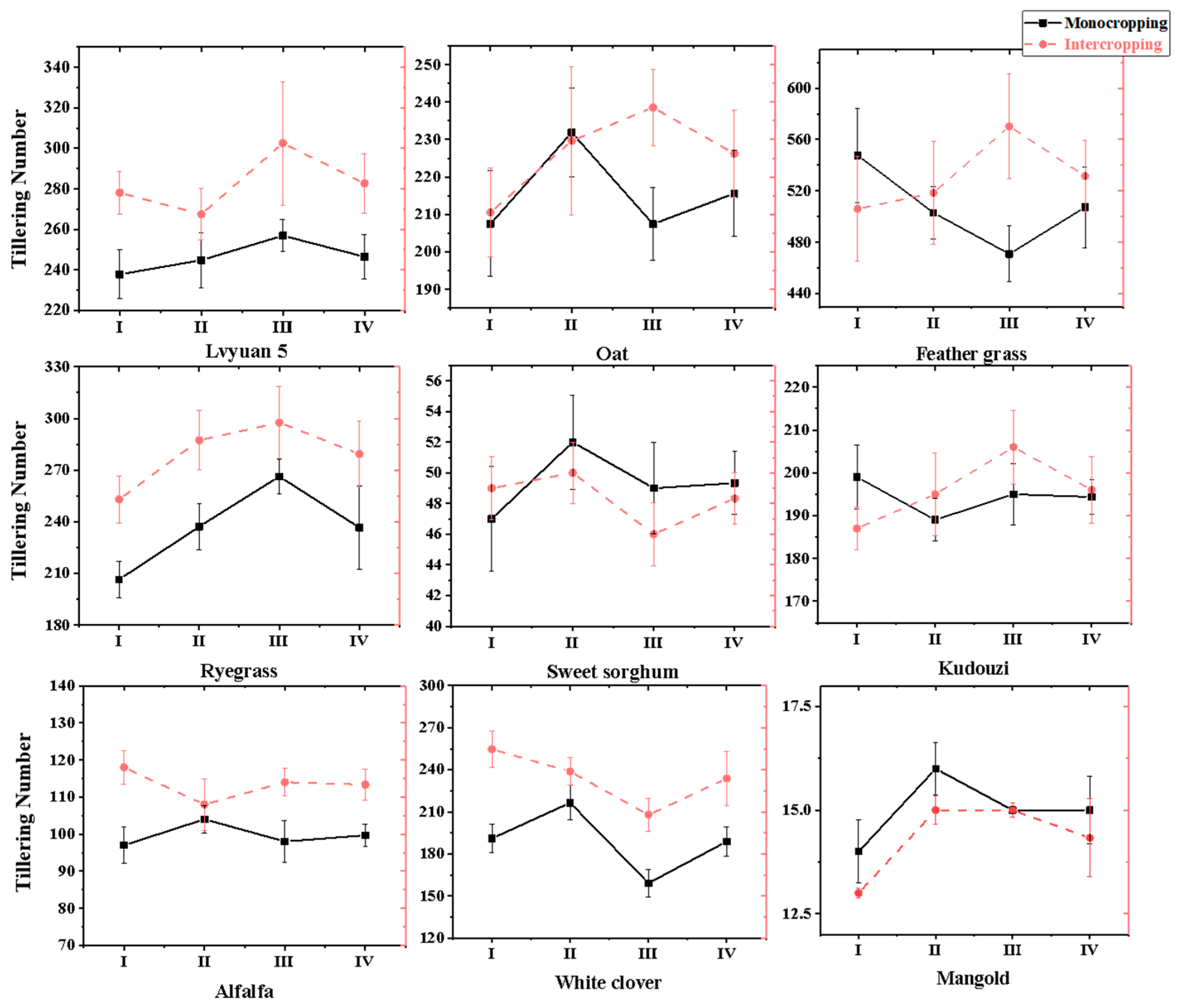
3.1. The Forage Morphological Indexes Under Different Wolfberry–Forage Intercropping Patterns
3.1.1. Influence on Forage Plant Height
3.1.2. The Effect on the Number of First Order Branches/Tillers of Forage
3.1.3. Effects on Leaf/Stem Ratio of Forage
3.1.4. Effects on Forage Yield and Dry–Fresh Ratio
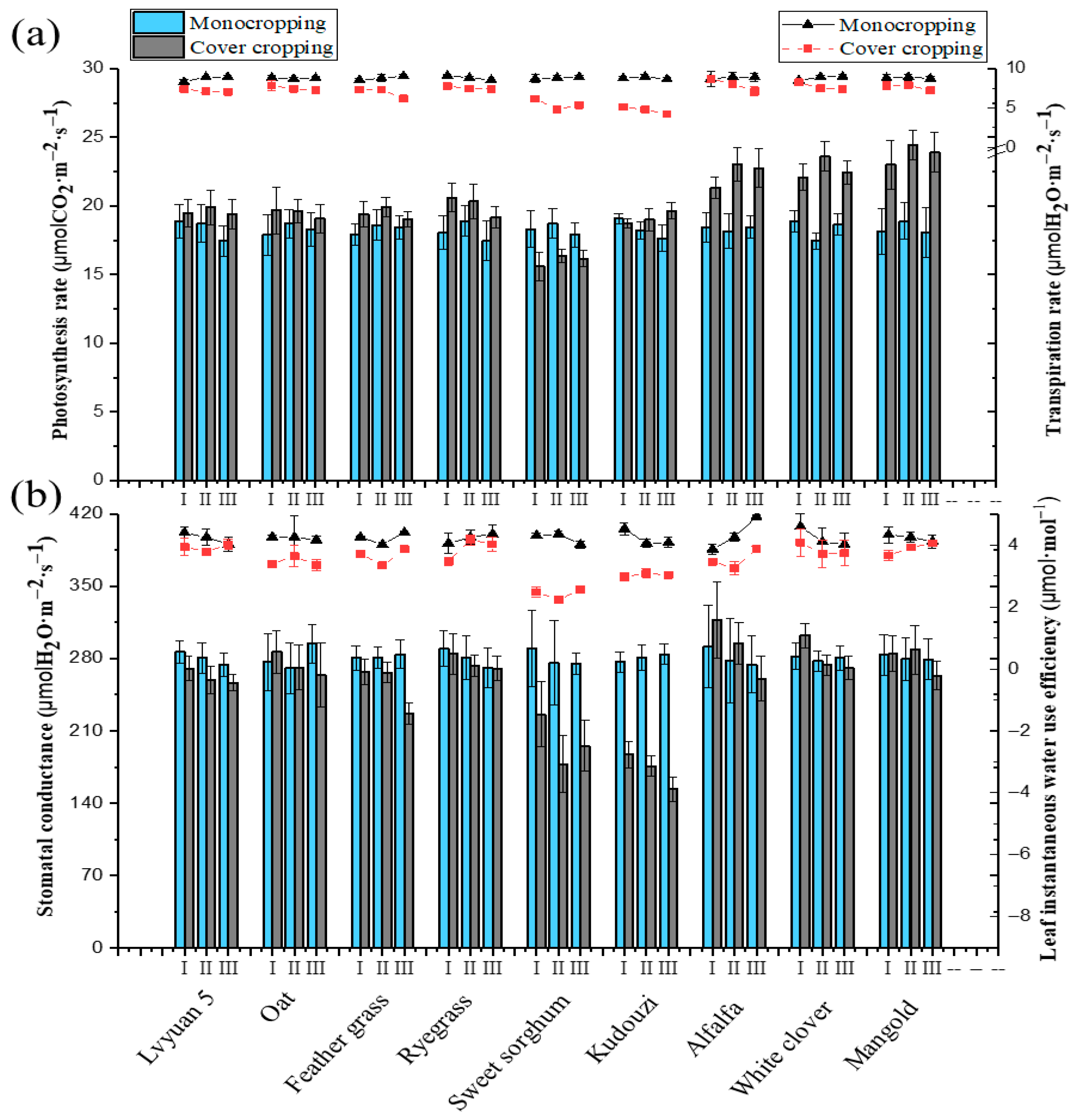
3.2. Effects of Different Wolfberry–Forage Intercropping Patterns on Forage Photosynthesis
3.3. Physiological Indexes of Forage by Different Wolfberry–Forage Intercropping Patterns
3.3.1. Aboveground Parts of Different Forage Types
3.3.2. Underground Parts of Different Forage Types
3.4. Nutrient Indexes of Forage by Different Wolfberry–Forage Intercropping Patterns
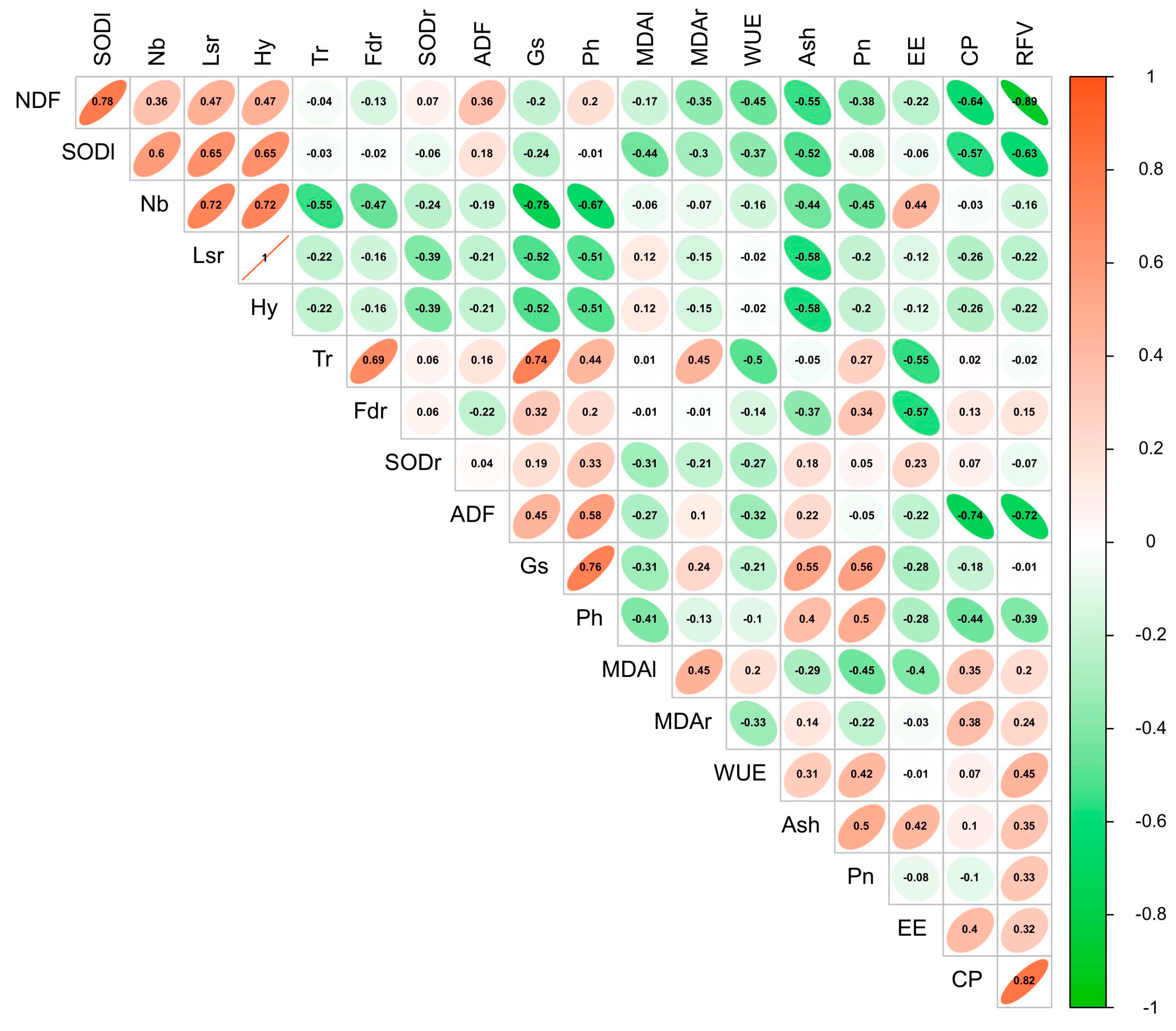
3.5. Factors Influencing Growth, Yield, and Quality of Forage
4. Discussion
4.1. Wolfberry–Forage Intercropping Affects Forage Crops Yield by Regulating Interspecific Competition
4.2. Wolfberry–Forage Intercropping Stimulates Forage Plant Growth by Enhancing the Overall Light Use Efficiency
4.3. Rational Wolfberry–Forage Intercropping Combinations Contribute to Improved Forage Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Zhu, L.; He, J.; Nan, X.; Chen, H.; Yang, L.; Jia, Q.; Yu, Z.; Wang, H.; Zhao, Y.; et al. Selecting appropriate forage cover crops to improve growth, yield, and fruit quality of wolfberry by regulation of photosynthesis and biotic stress resistance. Sci. Hortic. 2024, 337, 113470. [Google Scholar] [CrossRef]
- Crotty, F.V.; Stoate, C. The legacy of cover crops on the soil habitat and ecosystem services in a heavy clay, minimum tillage rotation. Food Energy Secur. 2019, 8, e00169. [Google Scholar] [CrossRef]
- Hamzei, J.; Seyyedi, M. Energy use and input–output costs for sunflower production in sole and intercropping with soybean under different tillage systems. Soil Tillage Res. 2016, 157, 73–82. [Google Scholar] [CrossRef]
- Hada, T.S.; Meena, L.K.; Pandey, P.P. Intercropping of mango and legumes for forage production: A review. Indian J. Agric. Allied Sci. 2021, 7, 3. [Google Scholar]
- Zhang, W.; Liu, G.; Sun, J.; Fornara, D.; Zhang, L.; Zhang, F.; Li, L. Temporal dynamics of nutrient uptake by neighboring plant species: Evidence from intercropping. Funct. Ecol. 2016, 31, 469–479. [Google Scholar] [CrossRef]
- Ali, A.; Mattsson, E. Individual tree size inequality enhances aboveground biomass in homegarden agroforestry systems in the dry zone of Sri Lanka. Sci. Total Environ. 2017, 575, 6–11. [Google Scholar] [CrossRef]
- Afrin, S.; Latif, A.; Banu, N.M.A.; Kabir, M.M.M.; Haque, S.S.; Ahmed, M.M.E.; Tonu, N.N.; Ali, M.P. Intercropping Empower Reduces Insect Pests and Increases Biodiversity in Agro-Ecosystem. Agric. Sci. 2017, 8, 1120–1134. [Google Scholar] [CrossRef]
- Knörzer, H.; Graeff-Hönninger, S.; Guo, B.; Wang, P.; Claupein, W. The Rediscovery of Intercropping in China: A Traditional Cropping System for Future Chinese Agriculture—A Review; Springer Nature: London, UK, 2009. [Google Scholar]
- Lv, Y.; Francis, C.; Wu, P.; Chen, X.; Zhao, X. Maize–Soybean Intercropping Interactions Above and Below Ground. Crop Sci. 2014, 54, 914–922. [Google Scholar] [CrossRef]
- Qin, D.Z.; Cui, W.F.; Chen, J.; Liu, J.; Qin, L.; Wang, L.P.; Zhao, Y.L.; Wang, L.H. Marginal effect of dry matter accumulation and nitrogen and phosphorus uptake and utilization in maize and soybean intercropping. Southwest China J. Agric. Sci. 2024, 37, 552–560. [Google Scholar]
- Gao, Y.; Duan, A.; Sun, J.; Li, F.; Liu, Z.; Liu, H.; Liu, Z. Crop coefficient and water use efficiency of winter wheat/spring maize strip intercropping. Field Crops Res. 2009, 111, 65–73. [Google Scholar] [CrossRef]
- Zhu, L.; He, J.; Tian, Y.; Li, X.; Li, Y.; Wang, F.; Qin, K.; Wang, J. Intercropping Wolfberry with Gramineae plants improves productivity and soil quality. Sci. Hortic. 2022, 292, 110632. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; He, J.; Zhou, X.; Wang, F.; Zhao, Y.; Liang, X.; Nan, X.; Li, Y.; Qin, K. Development of wolfberry–Forage Intercropping Patterns. Agronomy 2023, 13, 1365. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Jiao, L.; Jiao, L.; Hu, Y.; Wang, Y.; LI, H. Intercropping of Different Silage Maize Cultivars and Alfalfa. Acta Agron. Sin. 2006, 32, 125–130. [Google Scholar]
- Zhu, J.; van der Werf, W.; Anten, N.P.R.; Vos, J.; Evers, J.B. The contribution of phenotypic plasticity to complementary light capture in plant mixtures. New Phytol. 2015, 207, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W.; Yang, P.; Feng, B. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China—ScienceDirect. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, F.; Lei, J.; Lu, Y.; Guan, J. The Distribution and Growth Dynamics of Roots in Walnut-Alfalfa Intercropping Systems. Arid. Zone Res. 2015, 32, 504–508. [Google Scholar]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Liu, C.; Hu, T.; Li, Q.; Li, R.; Xie, C.; Wu, X. Investigation into the Photosynthetic Physiological and Ecological Adaptability of forage in Giant Eucalyptus Forest Intercropping Systems. Acta Prataculturae Sin. 2008, 17, 8. [Google Scholar]
- Wang, G.; Bei, S.; Li, J.; Bao, X.; Zhang, J.; Schultz, P.A.; Li, H.; Li, L.; Zhang, F.; Bever, J.D.; et al. Soil microbial legacy drives crop diversity advantage: Linking ecological plant–soil feedback with agricultural intercropping. J. Appl. Ecol. 2021, 58, 496–506. [Google Scholar] [CrossRef]
- Kong, C.-H.; Zhang, S.-Z.; Li, Y.-H.; Xia, Z.-C.; Yang, X.-F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; Yang, X.; Jin, Y.; Gao, T.; Yu, H.; Ma, H.; Qin, Z.; Zhao, L. Temporal and spatial variations of grassland desertification in Lingwu and Yanchi of Ningxia, China. In Proceedings of the 2013 Second International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Fairfax, VA, USA, 12–16 August 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Pei, B.; Zhang, G.C.; Zhang, S.Y. Effects of soil drought stress on photosynthesis and antioxidant enzyme activities of sea Buckthorn leaves. Acta Ecol. Sin. 2013, 33, 11. [Google Scholar]
- Yang, Q.; Guo, S.H. Plant Physiology and Biochemistry Experiment Course; China Agricultural Science and Technology Press: Beijing, China, 2010. [Google Scholar]
- Sun, Q.; Hu, J.J. Research Techniques in Plant Physiology; Northwest A&F University Press: Yangling, China, 2006. [Google Scholar]
- Ahmad, S.; Khan, P.A.; Verma, D.K.; Mir, N.H.; Sharma, A.; Wani, S.A. Forage production and orchard floor management through grass/legume intercropping in apple based agroforestry systems. Int. J. Chem. Stud. 2018, 6, 953–958. [Google Scholar]
- Gao, L.; Xu, H.; Bi, H.; Xi, W.; Bao, B.; Wang, X.; Bi, C.; Chang, Y. Intercropping Competition between Apple Trees and Crops in Agroforestry Systems on the Loess Plateau of China. PLoS ONE 2013, 8, e70739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, J.; Zheng, L.; Huang, J.; Yang, Y.; Li, X. Intercropping with marigold promotes soil health and microbial structure to assist in mitigating tobacco bacterial wilt. J. Plant Pathol. 2020, 102, 731–742. [Google Scholar] [CrossRef]
- Waha, K.; Dietrich, J.P.; Portmann, F.T.; Siebert, S.; Thornton, P.K.; Bondeau, A.; Herrero, M. Multiple cropping systems of the world and the potential for increasing cropping intensity. Glob. Environ. Chang. 2020, 64, 102131. [Google Scholar] [CrossRef]
- Giacomini, S.J.; Aita, C.; Vendruscolo, E. Dry matter, C/N ratio and nitrogen, phosphorus and potassium accumulation in mixed soil cover crops in Southern Brazil. Rev. Bras. De Ciência Solo 2003, 27, 325–334. [Google Scholar] [CrossRef]
- Chen, G.; Guo, L.; Ren, C.; Guo, L.; Zhao, G.; Hu, Y.; Zeng, Z. Effects of row spacing and intercropping on yield and quality of hay of Vicia sativa and Oat green. Acta Agron. Sin. 2011, 37, 2066–2074. [Google Scholar] [CrossRef]
- Yadeghari, L.Z.; Heidari, R.; Carapetian, J. The Influence of Cold Acclimation on Proline, Malondialdehyde (MDA), Total Protein and Pigments Contents in Soybean (Glycine max) Seedlings. J. Biol. Sci. 2007, 7, 1436–1441. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plant. 2010, 97, 104–110. [Google Scholar] [CrossRef]
- McKersie, B.D.; Chen, Y.; de Beus, M.; Bowley, S.R.; Bowler, C.; Inze, D.; D’Halluin, K.; Botterman, J. Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol. 1993, 103, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; González, E.M.; Minchin, F.R.; Webb, K.J.; Arrese-Igor, C.; Ramos, J.; Becana, M. Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiol. Plant. 2010, 115, 531–540. [Google Scholar] [CrossRef]
- Xu, Z.; Mi, W.; Mi, N.; Fan, X.; Zhou, Y.; Tian, Y. Comprehensive evaluation of soil quality in a desert steppe influenced by industrial activities in northern China. Sci. Rep. 2021, 11, 17493. [Google Scholar] [CrossRef]
- Huang, W.; Gfeller, V.; Erb, M. Root volatiles in plant-plant interactions II: Root terpenes from Centaurea stoebe modify Taraxacum officinale root chemistry and root herbivore growth. Cold Spring Harb. Lab. 2018, 42, 1964–1973. [Google Scholar]
- Huang, X.; Li, Y.; Yi, Q.; Ding, W. The Impact of Five Chemosensitive Compounds on the Enzymatic Activity of Ginseng Roots. Chin. Herbs 2010, 5, 117–121. [Google Scholar]
- Duan, Y.; Liu, X.; Wu, J.; Zhou, W.; Guo, X.; You, J.; Tang, T.; Wang, F.; Guo, J. The Impact of Intercropping Patterns on the Physiological Growth Characteristics and Rhizosphere Soil Physicochemical Properties of Coptis chinensis. J. Ecol. 2020, 39, 10. [Google Scholar]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology; Academic Press, Inc.: Cambridge, MA, USA, 1991. [Google Scholar]
- Isselstein, J.; Komainda, M.; Muto, P. Interaction of multispecies sward composition and harvesting management on forage yield and quality from establishment phase to the subsequent crop. Grass Forage Sci. 2022, 77, 89–99. [Google Scholar]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Lin, F.; Liu, X.; Tong, C.; Wu, Y. The Impact of Intercropping on Light Energy Utilization Efficiency and Productivity of Various Forage Crops. J. Appl. Ecol. 2019, 30, 11. [Google Scholar]
- Shao, Q.; Wang, H.; Guo, H.; Zhou, A.; Huang, Y.; Sun, Y.; Li, M. Effects of Shade Treatments on Photosynthetic Characteristics, Chloroplast Ultrastructure, and Physiology of Anoectochilus roxburghii. PLoS ONE 2014, 9, e85996. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crops Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Wu, P.; Gao, Y.; Yang, Q.; Shen, Y. Border row effects on light interception in wheat/maize strip intercropping systems. Field Crops Res. 2017, 214, 1–13. [Google Scholar] [CrossRef]
- Charbonnier, F.; le Maire, G.; Dreyer, E.; Casanoves, F.; Christina, M.; Dauzat, J.; Eitel, J.U.H.; Vaast, P.; Vierling, L.A.; Roupsard, O. Competition for light in heterogeneous canopies: Application of MAESTRA to a coffee (Coffea arabica L.) agroforestry system. AGR For. Meteorol 2013, 181, 152–169. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, X.; Schull, M.A.; Román-Colón, M.O.; Yao, T.; Wang, Z.; Zhang, Q.; Jupp, D.L.; Lovell, J.L.; Culvenor, D.S.; et al. Measuring effective leaf area index, foliage profile, and stand height in New England forest stands using a full-waveform ground-based lidar. Remote. Sens. Environ. 2011, 115, 2954–2964. [Google Scholar] [CrossRef]
- Riday, H.; Brummer, E.C.; Moore, K.J. Heterosis of Forage Quality in Alfalfa. Crop. Sci. 2002, 42, 1088–1093. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, F.; Wang, D.; Wei, Z.; Tang, C. Effects of broccoli and oat intercropping on yield and quality of forage. Chin. J. Grassl. 2020, 42, 127–135. [Google Scholar]
- Wu, Y.; Zhang, W.; Chen, M.; Wu, D. Production performance of different varieties of oat in Yangzhou area. Pratacultural Sci. 2018, 35, 6. [Google Scholar]
- Zhao, Y.; Liu, X.; Tong, C.; Wu, Y. Effect of root interaction on nodulation and nitrogen fixation ability of alfalfa in the simulated alfalfa/triticale intercropping in pots. Sci. Rep. 2020, 10, 1008–1010. [Google Scholar] [CrossRef] [PubMed]

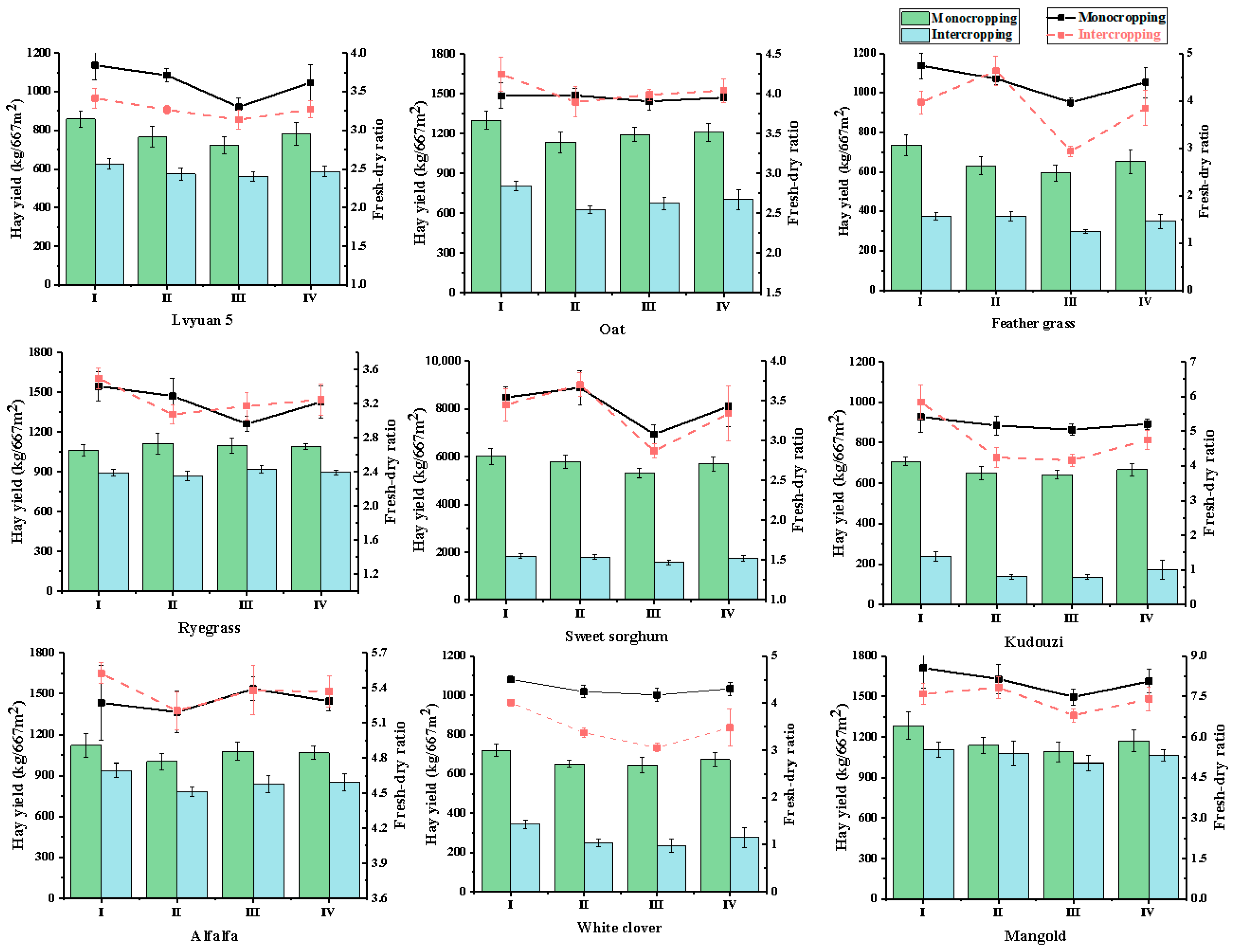
| Material | Cropping Pattern | Hay Yield (kg/667 m2) | Yield Change_I vs. M (%) | ||||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | Mean | Level 1 | Level 2 | ||
| Lvyuan 5 | M | 857.65 ± 39.71 | 765.09 ± 54.62 | 721.51 ± 44.58 | 781.41 ± 56.77 | −24.77 | 50.46 |
| I1 | 627.25 ± 27.66 | 574.01 ± 31.45 | 562.36 ± 24.99 | 587.87 ± 28.25 | |||
| I2 | 1254.51 ± 55.32 | 1148.0 ± 62.90 | 1124.71 ± 49.98 | 1175.74 ± 56.50 | |||
| Oat | M | 909.44 ± 68.42 | 795.01 ± 79.83 | 836.45 ± 56.16 | 846.97 ± 67.58 | −41.79 | 16.41 |
| I1 | 565.50 ± 34.99 | 440.34 ± 28.56 | 474.14 ± 47.68 | 493.33 ± 35.70 | |||
| I2 | 1131.00 ± 69.98 | 880.64 ± 57.12 | 948.28 ± 95.36 | 986.43 ± 71.39 | |||
| Feather grass | M | 441.34 ± 32.19 | 378.05 ± 25.66 | 356.15 ± 17.87 | 392.07 ± 60.21 | −46.43 | 7.15 |
| I1 | 225.88 ± 19.60 | 224.90 ± 17.80 | 178.97 ± 10.77 | 209.87 ± 36.48 | |||
| I2 | 451.76 ± 39.19 | 449.80 ± 25.66 | 357.94 ± 17.87 | 419.33 ± 72.94 | |||
| Ryegrass | M | 1062.29 ± 43.99 | 1113.67 ± 77.53 | 1100.19 ± 58.98 | 1092.05 ± 21.75 | −17.99 | 64 |
| I1 | 895.02 ± 21.76 | 870.70 ± 33.67 | 920.76 ± 31.07 | 895.49 ± 20.44 | |||
| I2 | 1790.03 ± 43.52 | 1741.40 ± 67.34 | 1841.51 ± 64.12 | 1790.98 ± 40.88 | |||
| Sweet sorghum | M | 3005.47 ± 230.6 | 2893.30 ± 270.62 | 2652.23 ± 89.55 | 2850.33 ± 194.76 | −69.65 | −39.29 |
| I1 | 915.54 ± 87.94 | 893.49 ± 74.69 | 786.61 ± 107.44 | 865.18 ± 72.61 | |||
| I2 | 1831.08 ± 175.88 | 1787.98 ± 149.38 | 1573.22 ± 214.88 | 1730.36 ± 125.22 | |||
| Kudouzi | M | 707.64 ± 20.56 | 650.07 ± 34.69 | 642.64 ± 19.88 | 666.78 ± 29.05 | −74.32 | −48.63 |
| I1 | 238.07 ± 23.88 | 138.51 ± 10.71 | 137.19 ± 9.88 | 171.26 ± 47.25 | |||
| I2 | 476.14 ± 47.76 | 277.03 ± 21.42 | 274.38 ± 19.76 | 342.51 ± 94.49 | |||
| Alfalfa | M | 1120.92 ± 85.19 | 1001.74 ± 57.31 | 1077.16 ± 66.34 | 1066.61 ± 49.22 | −20.07 | 59.87 |
| I1 | 938.80 ± 53.88 | 780.99 ± 35.46 | 837.94 ± 60.17 | 852.58 ± 65.25 | |||
| I2 | 1877.60 ± 107.76 | 1561.98 ± 70.92 | 1675.88 ± 120.34 | 1705.15 ± 130.51 | |||
| White clover | M | 720.14 ± 29.77 | 652.51 ± 18.69 | 646.79 ± 37.54 | 673.14 ± 33.31 | −58.97 | −17.94 |
| I1 | 345.07 ± 22.19 | 247.76 ± 19.80 | 235.79 ± 33.77 | 276.21 ± 48.94 | |||
| I2 | 690.13 ± 44.38 | 495.53 ± 39.60 | 471.58 ± 67.54 | 552.41 ± 97.87 | |||
| Mangold | M | 1282.72 ± 101.54 | 1137.80 ± 59.78 | 1090.26 ± 73.99 | 1170.26 ± 81.85 | −9.03 | 81.94 |
| I1 | 1107.49 ± 56.12 | 1080.14 ± 87.19 | 1006.19 ± 54.26 | 1064.61 ± 42.79 | |||
| I2 | 2214.99 ± 112.24 | 2160.27 ± 174.38 | 2012.38 ± 108.52 | 2129.21 ± 85.58 | |||
| Cropping Pattern | Treatment | Leaf | Root | ||
|---|---|---|---|---|---|
| MDA (mg g−1 FW min−1) | SOD (Ug g−1 FW min−1) | MDA (mg g−1 FW min−1) | SOD (Ug g−1 FW min−1) | ||
| Monocropping | Lvyuan 5 | 0.22 ± 0.010 ijk | 0.16 ± 0.027 def | 0.73 ± 0.007 i | 108.42 ± 10.491 d |
| Oat | 0.24 ± 0.008 ijk | 0.19 ± 0.009 bcd | 0.83 ± 0.061 df | 100.02 ± 12.323 ef | |
| Feather grass | 0.19 ± 0.012 jk | 0.38 ± 0.064 a | 0.77 ± 0.033 ghi | 92.27 ± 2.828 g | |
| Ryegrass | 0.25 ± 0.004 ijk | 0.17 ± 0.018 cde | 0.78 ± 0.012 gh | 135.34 ± 3.535 c | |
| Sweet sorghum | 0.21 ± 0.011 jk | 0.20 ± 0.026 b | 0.75 ± 0.049 hi | 141.64 ± 12.642 a | |
| Kudouzi | 1.43 ± 0.039 e | 0.11 ± 0.012 gh | 0.82 ± 0.015 df | 116.57 ± 7.071 c | |
| Alfalfa | 1.22 ± 0.081 f | 0.13 ± 0.005 fg | 1.61 ± 0.137 a | 90.26 ± 4.264 g | |
| White clover | 2.58 ± 0.239 a | 0.12 ± 0.001 g | 0.83 ± 0.072 df | 69.27 ± 2.828 i | |
| Mangold | 0.15 ± 0.000 k | 0.20 ± 0.011 b | 0.59 ± 0.021 j | 133.92 ± 12.727 b | |
| Intercropping | Wolfberry–Lvyuan 5 | 0.31 ± 0.016 hi | 0.14 ± 0.024 ef | 0.74 ± 0.017 hi | 103.42 ± 11.627 e |
| Wolfberry–oat | 0.25 ± 0.022 ijk | 0.20 ± 0.001 bc | 0.84 ± 0.098 d | 100.81 ± 8.485 ef | |
| Wolfberry–feather grass | 0.45 ± 0.003 g | 0.36 ± 0.027 a | 0.81 ± 0.029 dfg | 88.06 ± 9.005 g | |
| Wolfberry–ryegrass | 0.25 ± 0.073 ijk | 0.11 ± 0.015 g | 0.79 ± 0.042 fgh | 101.78 ± 10.056 ef | |
| Wolfberry–sweet sorghum | 0.39 ± 0.007 gh | 0.20 ± 0.043 bc | 0.76 ± 0.009 hi | 88.38 ± 8.478 g | |
| Wolfberry–kudouzi | 2.03 ± 0.040 c | 0.15 ± 0.016 ef | 1.03 ± 0.092 c | 118.55 ± 10.556 c | |
| Wolfberry–alfalfa | 1.54 ± 0.045 d | 0.11 ± 0.016 ghi | 1.23 ± 0.048 b | 90.24 ± 4.242 g | |
| Wolfberry–white clover | 2.23 ± 0.008 b | 0.16 ± 0.031 de | 0.84 ± 0.046 d | 90.56 ± 13.334 g | |
| Wolfberry–mangold | 0.06 ± 0.004 l | 0.20 ± 0.044 bc | 0.58 ± 0.012 j | 81.41 ± 4.242 h | |
| L.S.D. (5%) | |||||
| Cropping Pattern | Treatment | CP | NDF | ADF | Ash | EE | RFV |
|---|---|---|---|---|---|---|---|
| Monocropping | Lvyuan 5 | 10.41 ± 0.89 k | 52.17 ± 3.97 g | 38.32 ± 1.92 cd | 9.94 ± 0.98 e | 4.84 ± 0.34 cd | 104.41 ± 7.66 gh |
| Oat | 11.83 ± 0.76 h | 54.37 ± 3.19 f | 40.75 ± 1.56 b | 10.88 ± 0.12 cd | 4.41 ± 0.14 de | 97.79 ± 3.98 i | |
| Feather grass | 10.57 ± 0.35 jk | 70.72 ± 3.77 a | 34.36 ± 2.89 f | 4.58 ± 0.12 i | 3.64 ± 0.12 f | 81.73 ± 4.62 k | |
| Ryegrass | 11.32 ± 0.88 g | 47.43 ± 2.19 i | 24.87 ± 1.43 j | 13.46 ± 0.45 a | 6.81 ± 0.22 b | 136.36 ± 9.36 a | |
| Sweet sorghum | 7.87 ± 0.21 l | 66.75 ± 5.21 c | 45.02 ± 1.95 a | 11.52 ± 0.87 b | 2.75 ± 0.06 g | 75.02 ± 3.22 l | |
| Kudouzi | 20.89 ± 1.78 a | 56.68 ± 1.98 e | 28.61 ± 1.64 h | 6.38 ± 0.32 h | 4.12 ± 0.11 ef | 109.32 ± 4.51 e | |
| Alfalfa | 19.26 ± 0.65 b | 45.86 ± 2.06 j | 36.54 ± 1.44 e | 10.47 ± 0.17 d | 2.76 ± 0.67 g | 122.59 ± 7.90 b | |
| White clover | 14.14 ± 0.33 de | 53.45 ± 3.42 f | 30.59 ± 1.65 g | 7.36 ± 0.09 g | 1.15 ± 0.04 h | 113.25 ± 5.07 d | |
| Mangold | 13.38 ± 0.12 ef | 53.43 ± 2.99 f | 26.65 ± 1.77 i | 9.79 ± 0.13 e | 2.90 ± 0.07 g | 118.63 ± 4.11 c | |
| Intercropping | Wolfberry–Lvyuan 5 | 11.09 ± 0.66 ij | 51.77 ± 3.01 gh | 38.53 ± 1.57 cd | 9.72 ± 0.39 e | 5.12 ± 0.78 c | 105.81 ± 5.62 fg |
| Wolfberry–oat | 12.97 ± 0.23 fg | 52.30 ± 2.66 g | 39.23 ± 2.01 bc | 10.63 ± 1.00 d | 4.50 ± 0.19 de | 103.77 ± 4.77 h | |
| Wolfberry–feather grass | 11.24 ± 0.91 i | 69.22 ± 3.29 b | 34.11 ± 2.34 f | 4.58 ± 0.16 i | 3.89 ± 0.07 f | 83.76 ± 4.61 j | |
| Wolfberry–ryegrass | 12.56 ± 1.06 de | 46.01 ± 1.86 j | 24.13 ± 0.99 j | 12.09 ± 1.12 a | 7.46 ± 0.64 a | 141.73 ± 7.87 a | |
| Wolfberry–sweet sorghum | 7.96 ± 0.09 l | 64.52 ± 3.42 d | 44.31 ± 2.33 a | 11.30 ± 0.80 bc | 2.81 ± 0.03 g | 78.41 ± 3.42 l | |
| Wolfberry–kudouzi | 20.52 ± 0.89 a | 57.42 ± 3.19 e | 29.22 ± 1.90 h | 6.61 ± 0.23 h | 4.01 ± 0.04 ef | 107.15 ± 4.80 f | |
| Wolfberry–alfalfa | 18.47 ± 0.28 c | 47.55 ± 2.17 i | 38.09 ± 2.09 d | 10.70 ± 0.78 d | 2.58 ± 0.12 g | 115.88 ± 4.42 c | |
| Wolfberry–white clover | 13.96 ± 0.68 de | 54.11 ± 3.21 f | 30.89 ± 2.01 g | 7.90 ± 0.39 f | 1.14 ± 0.09 h | 111.46 ± 3.17 d | |
| Wolfberry–mangold | 14.42 ± 1.32 d | 50.79 ± 4.06 h | 26.86 ± 1.65 i | 8.02 ± 0.64 f | 1.22 ± 0.07 h | 124.50 ± 6.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhu, L.; Qiao, G.; Nan, X.; Wang, F.; Wang, Y.; Yu, Z.; Qu, R.; Wang, H.; Li, Y.; et al. Forage Quality and Yield Enhancement via Wolfberry (Lycium barbarum L.)–Forage Intercropping System. Agronomy 2025, 15, 2660. https://doi.org/10.3390/agronomy15112660
Li R, Zhu L, Qiao G, Nan X, Wang F, Wang Y, Yu Z, Qu R, Wang H, Li Y, et al. Forage Quality and Yield Enhancement via Wolfberry (Lycium barbarum L.)–Forage Intercropping System. Agronomy. 2025; 15(11):2660. https://doi.org/10.3390/agronomy15112660
Chicago/Turabian StyleLi, Ruitao, Lizhen Zhu, Gaixia Qiao, Xiongxiong Nan, Fang Wang, Yali Wang, Zelong Yu, Rong Qu, Hao Wang, Yu Li, and et al. 2025. "Forage Quality and Yield Enhancement via Wolfberry (Lycium barbarum L.)–Forage Intercropping System" Agronomy 15, no. 11: 2660. https://doi.org/10.3390/agronomy15112660
APA StyleLi, R., Zhu, L., Qiao, G., Nan, X., Wang, F., Wang, Y., Yu, Z., Qu, R., Wang, H., Li, Y., & Gu, X. (2025). Forage Quality and Yield Enhancement via Wolfberry (Lycium barbarum L.)–Forage Intercropping System. Agronomy, 15(11), 2660. https://doi.org/10.3390/agronomy15112660






