Urea-N Activated Biochar Effectively Suppresses CO2 and N2O Emissions from Farmland Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
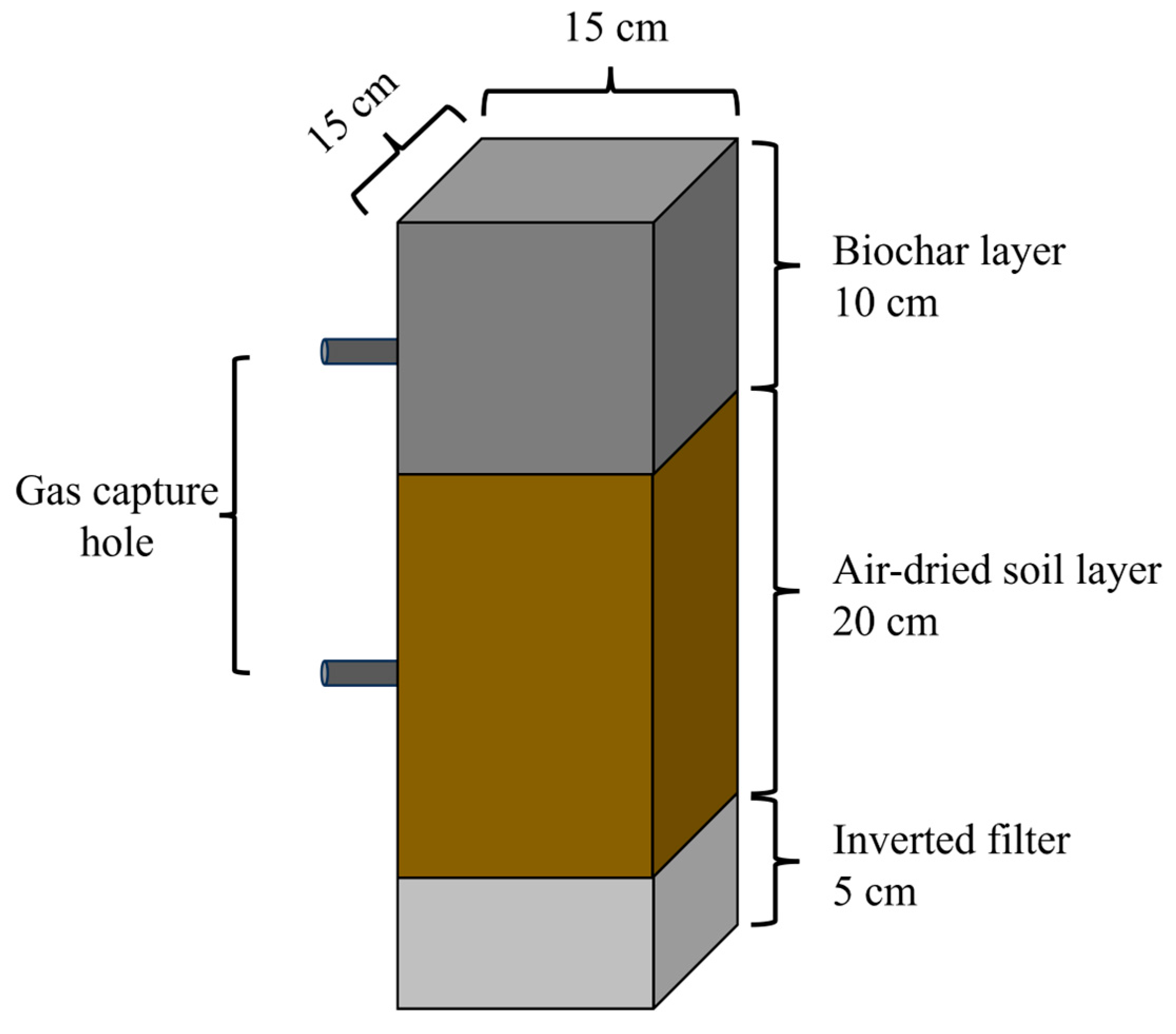
2.2. Biochar Preparation and Experimental Design
2.3. Gas Sample Collection and Analysis
2.3.1. Gas Emissions from a Soil Profile
2.3.2. Surface Emissions
2.4. Soil Sample Collection and Analyses
2.5. SEM and RDA
2.6. Statistical Analysis
3. Result
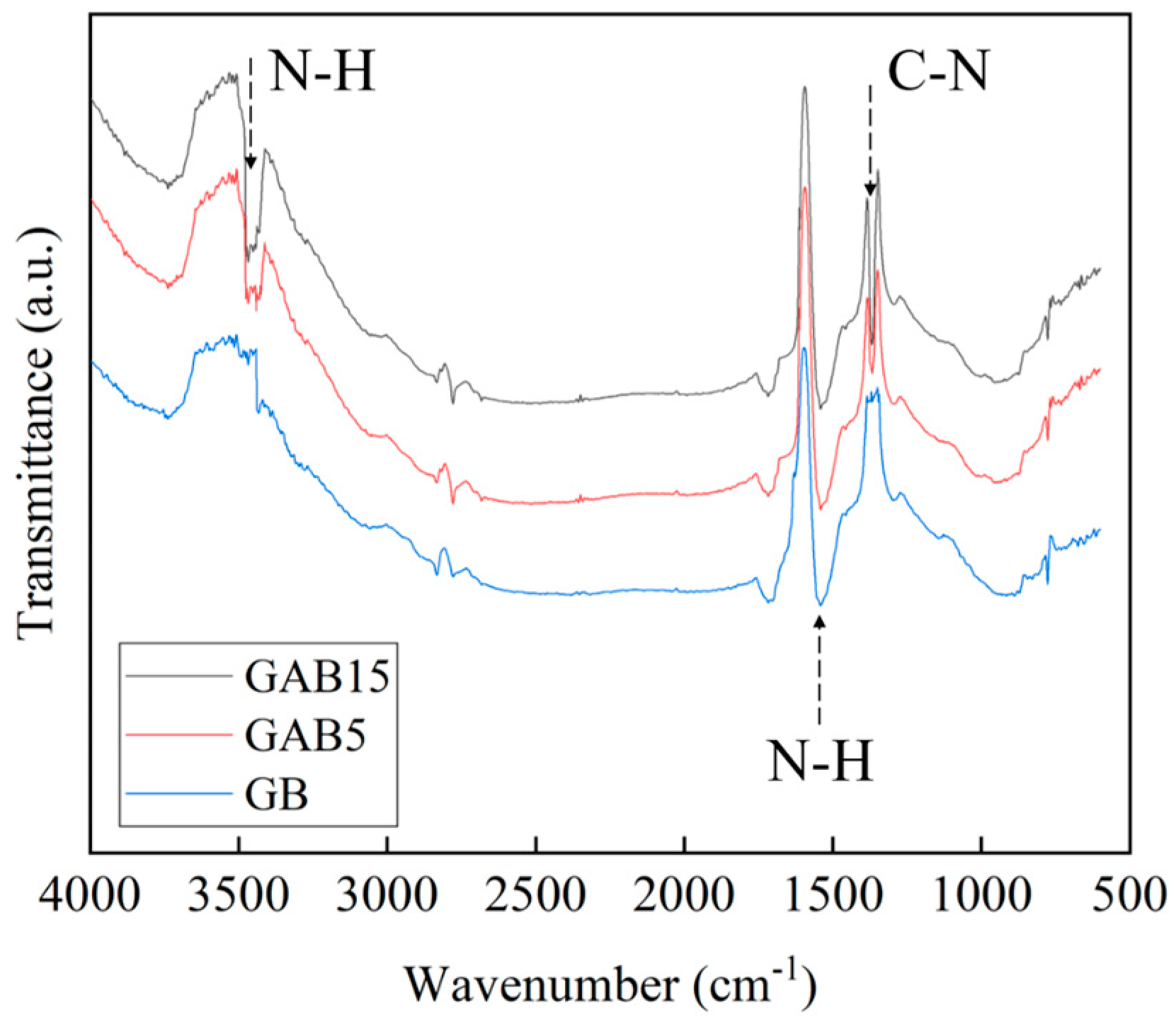
3.1. Chemical and Physical Properties of Biochar
3.2. Soil Physical Properties
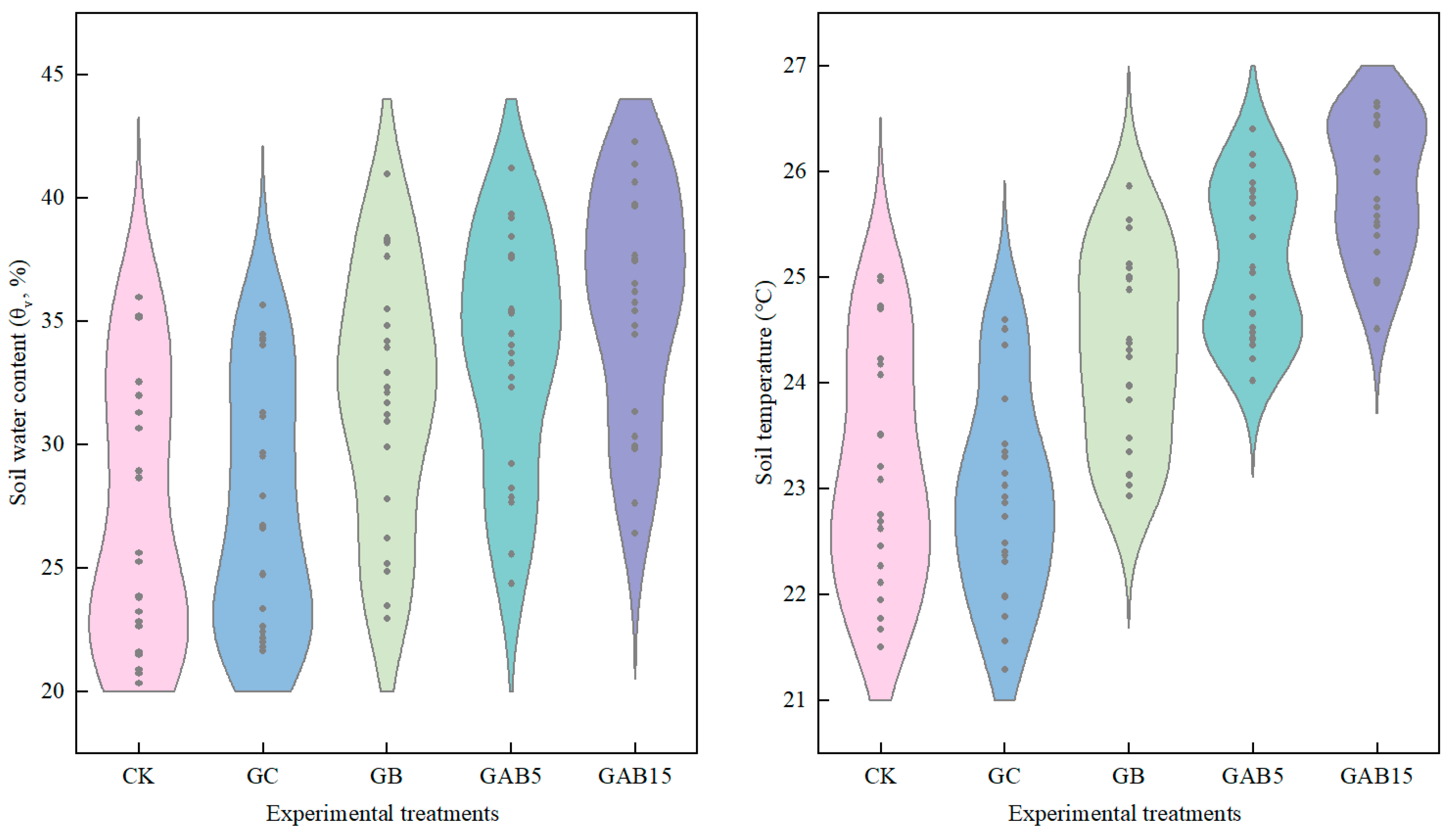
3.2.1. Soil Moisture and Temperature
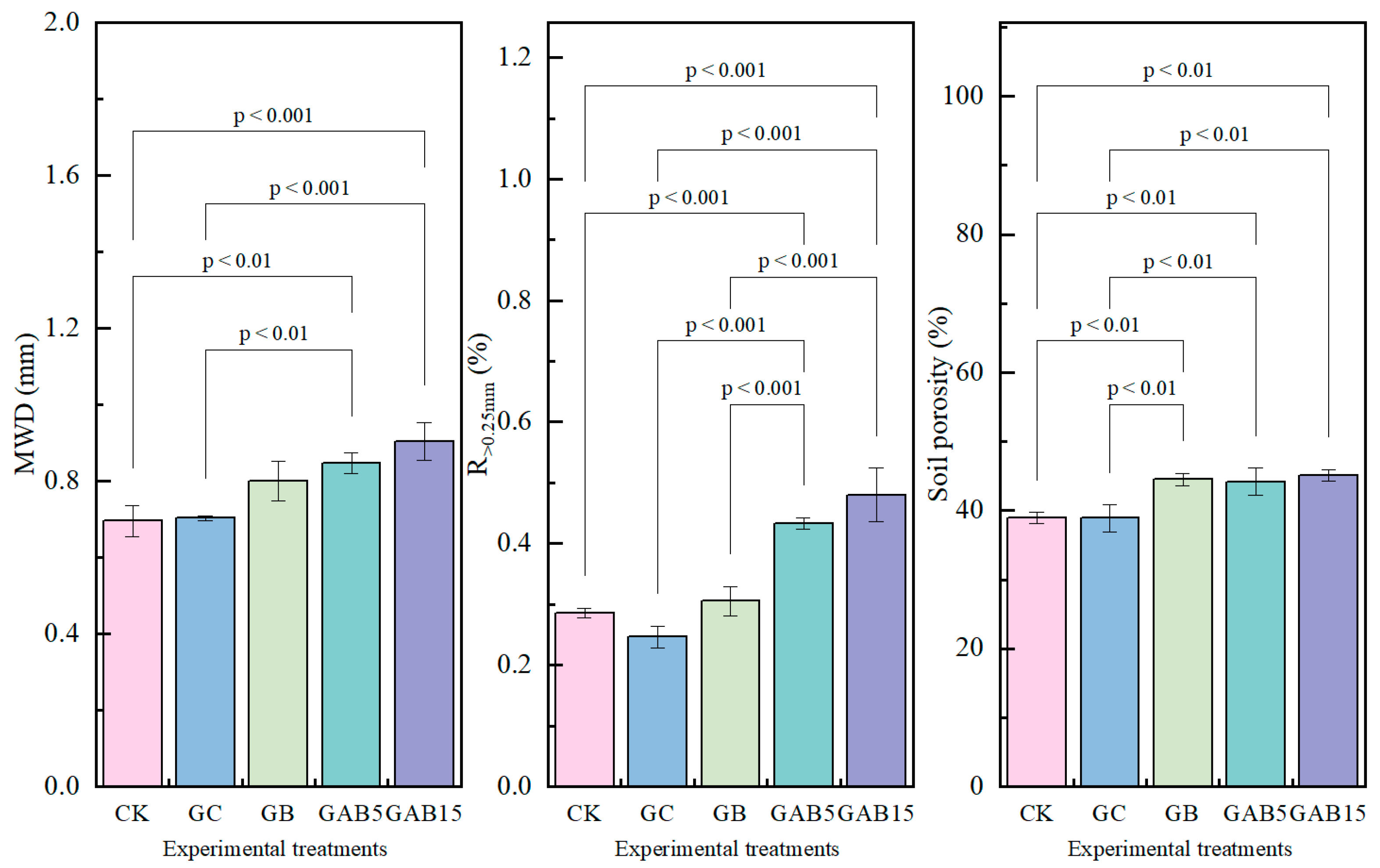
3.2.2. Soil Aggregate Stability
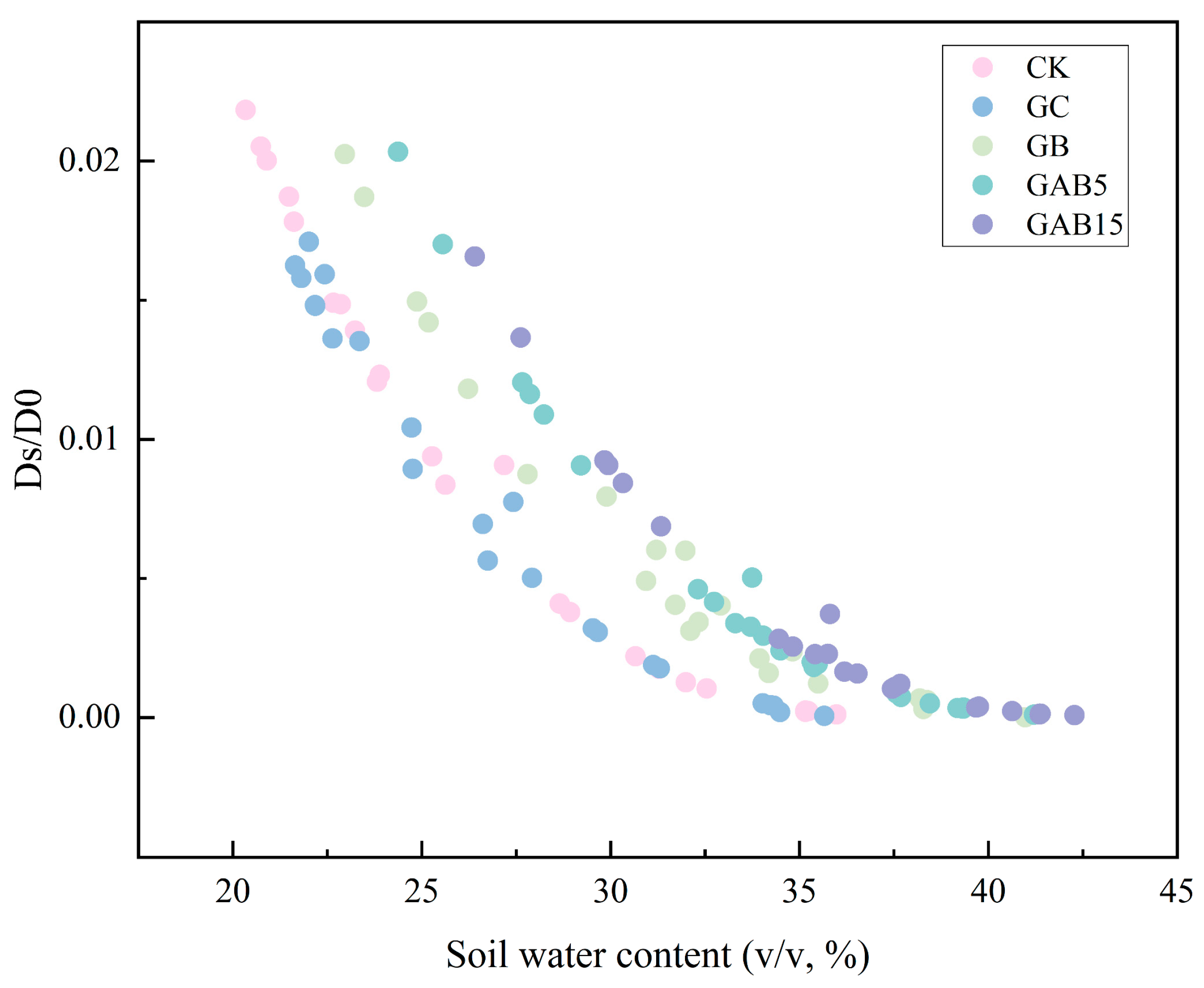
3.2.3. Ds/D0 and Soil Water Content
3.3. Soil Chemical Properties
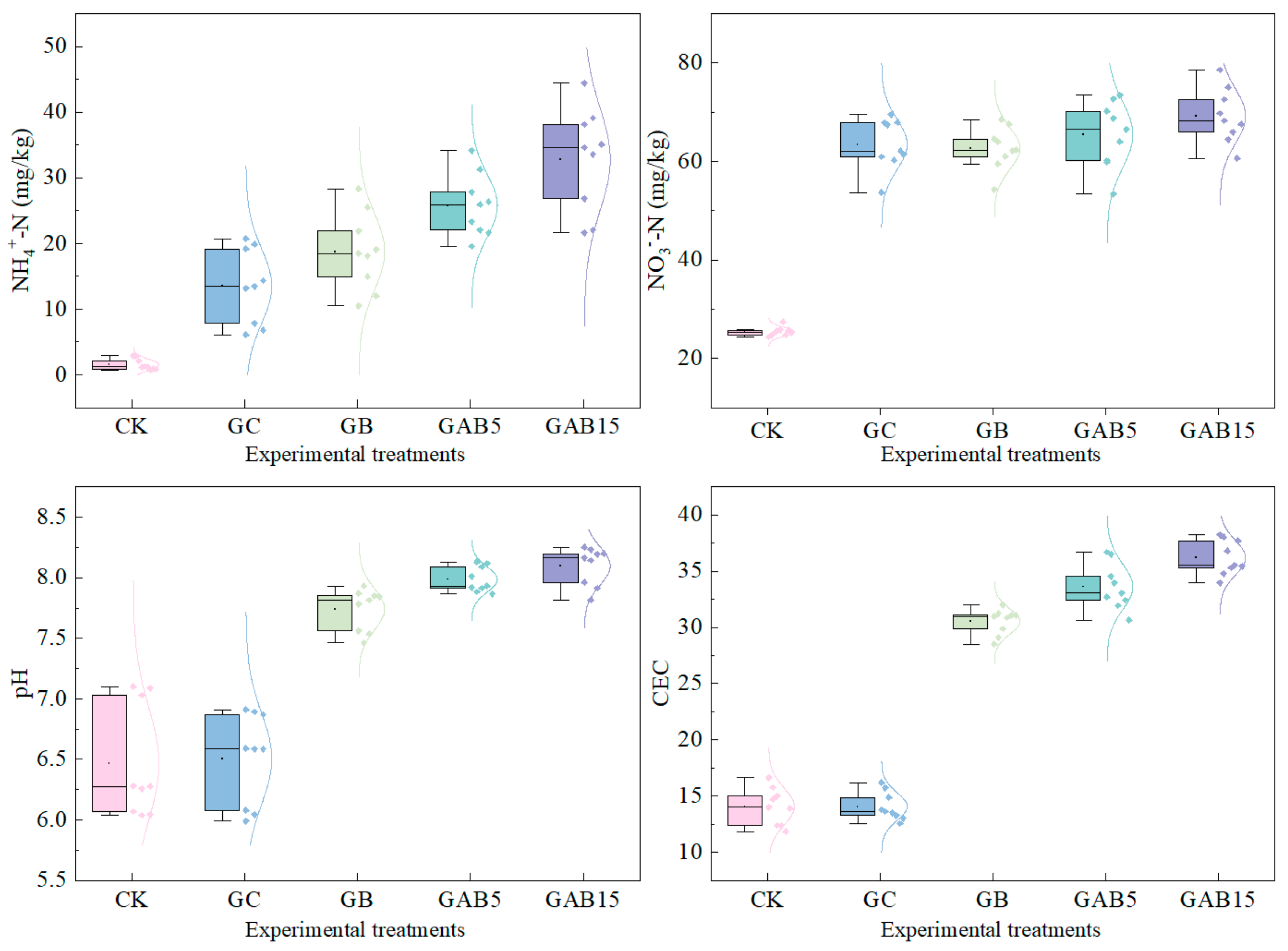
3.3.1. Soil pH and CEC
3.3.2. Soil NH4+-N and NO3−-N
3.4. Soil Enzymatic Activities and Microbial Biomass
3.4.1. Soil Enzymatic Activities
3.4.2. Microbial Biomass
3.5. The Dynamic of CO2 and N2O
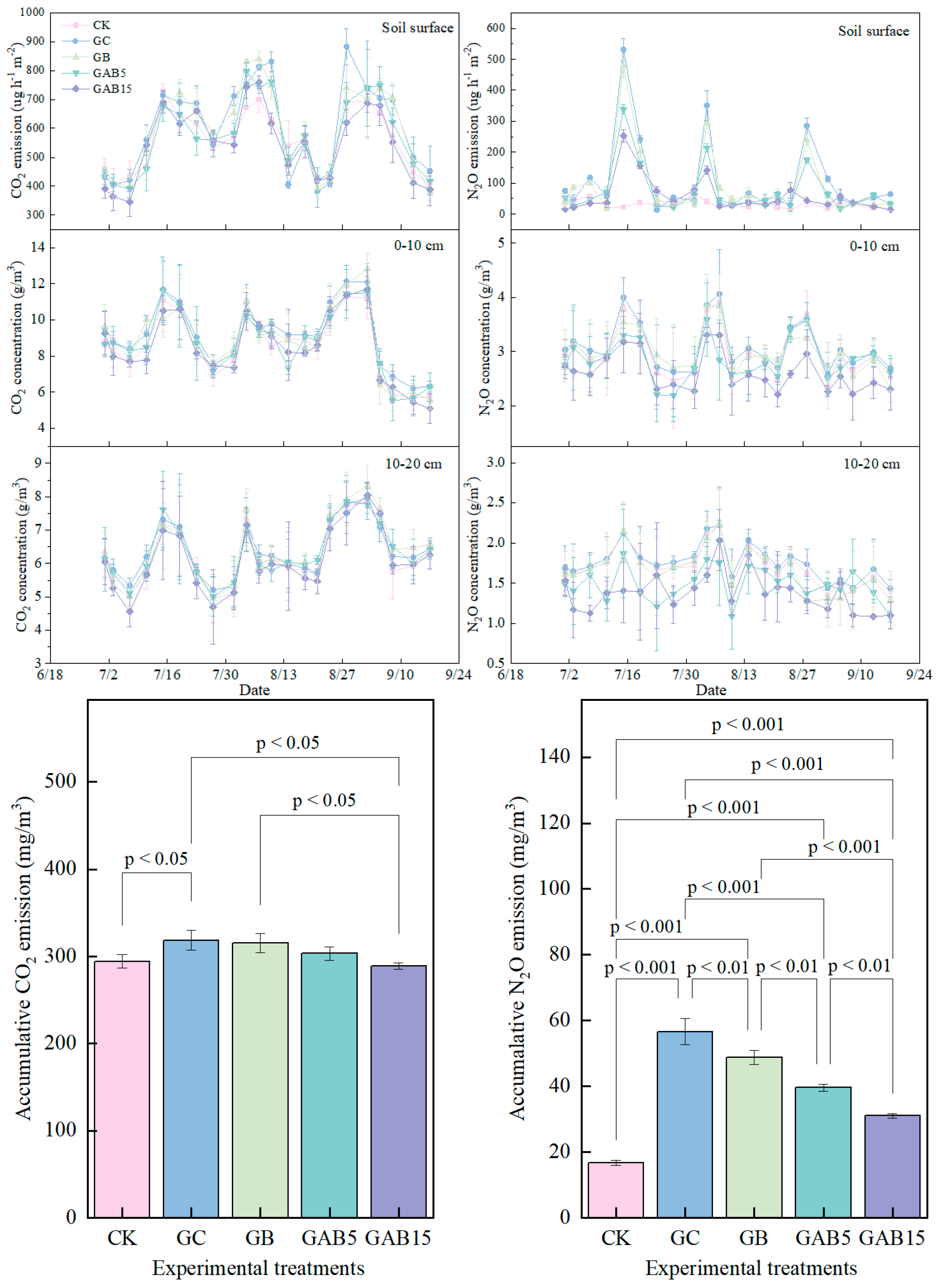
3.5.1. The Emission of CO2 and N2O in the Soil Surface
3.5.2. The Accumulative CO2 and N2O in the Soil Profile
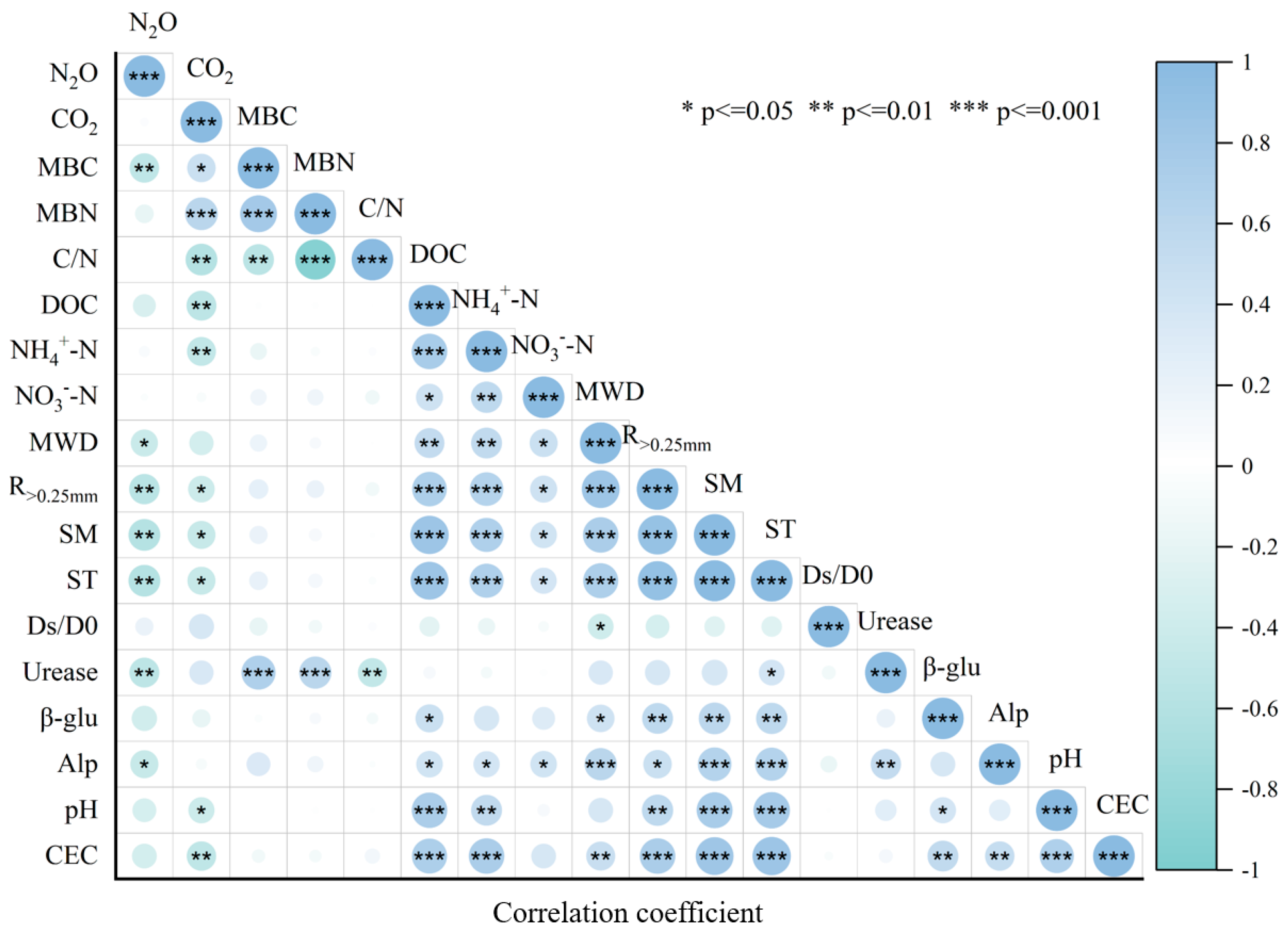
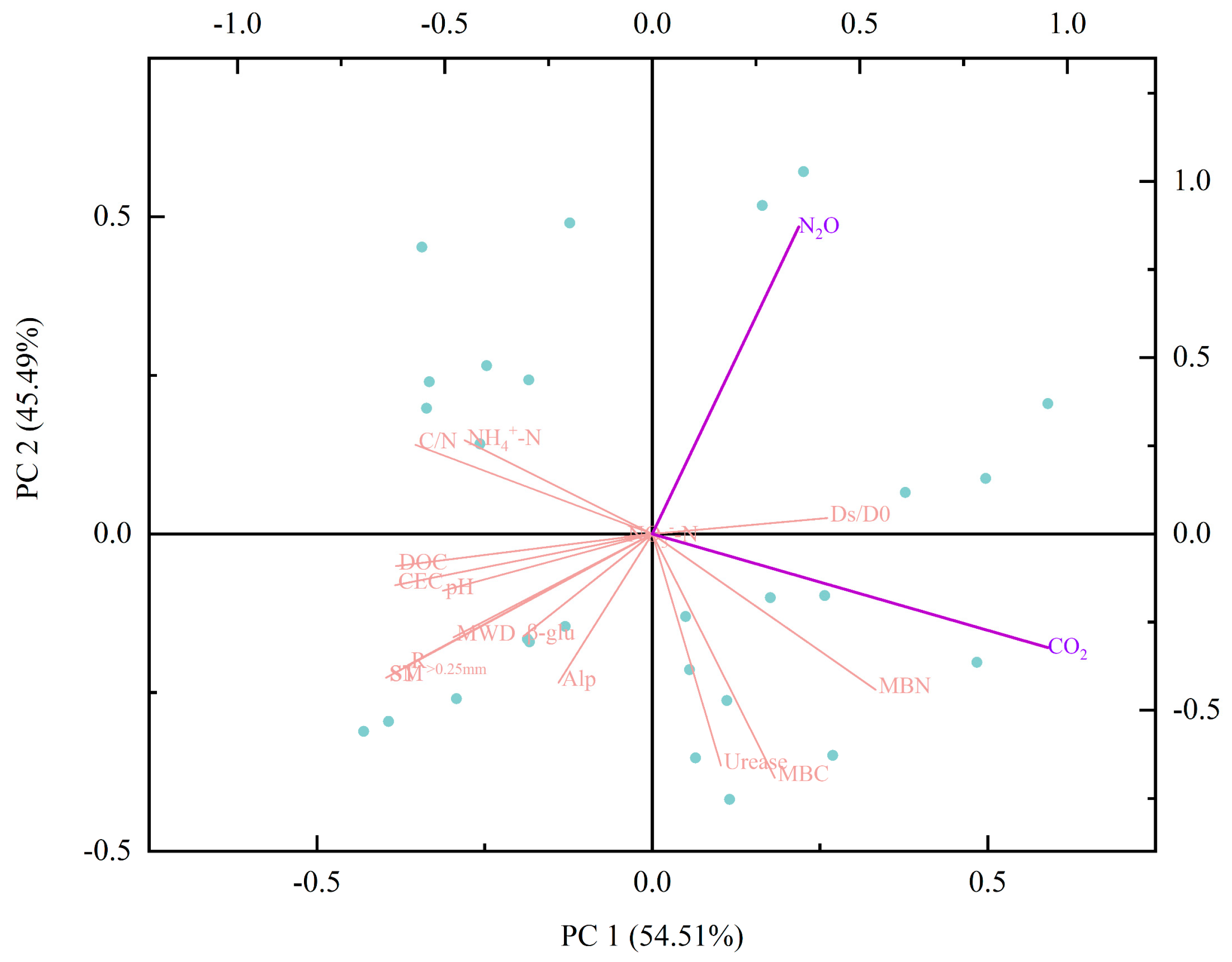
3.6. Redundancy Analysis of Soil CO2 and N2O Emission
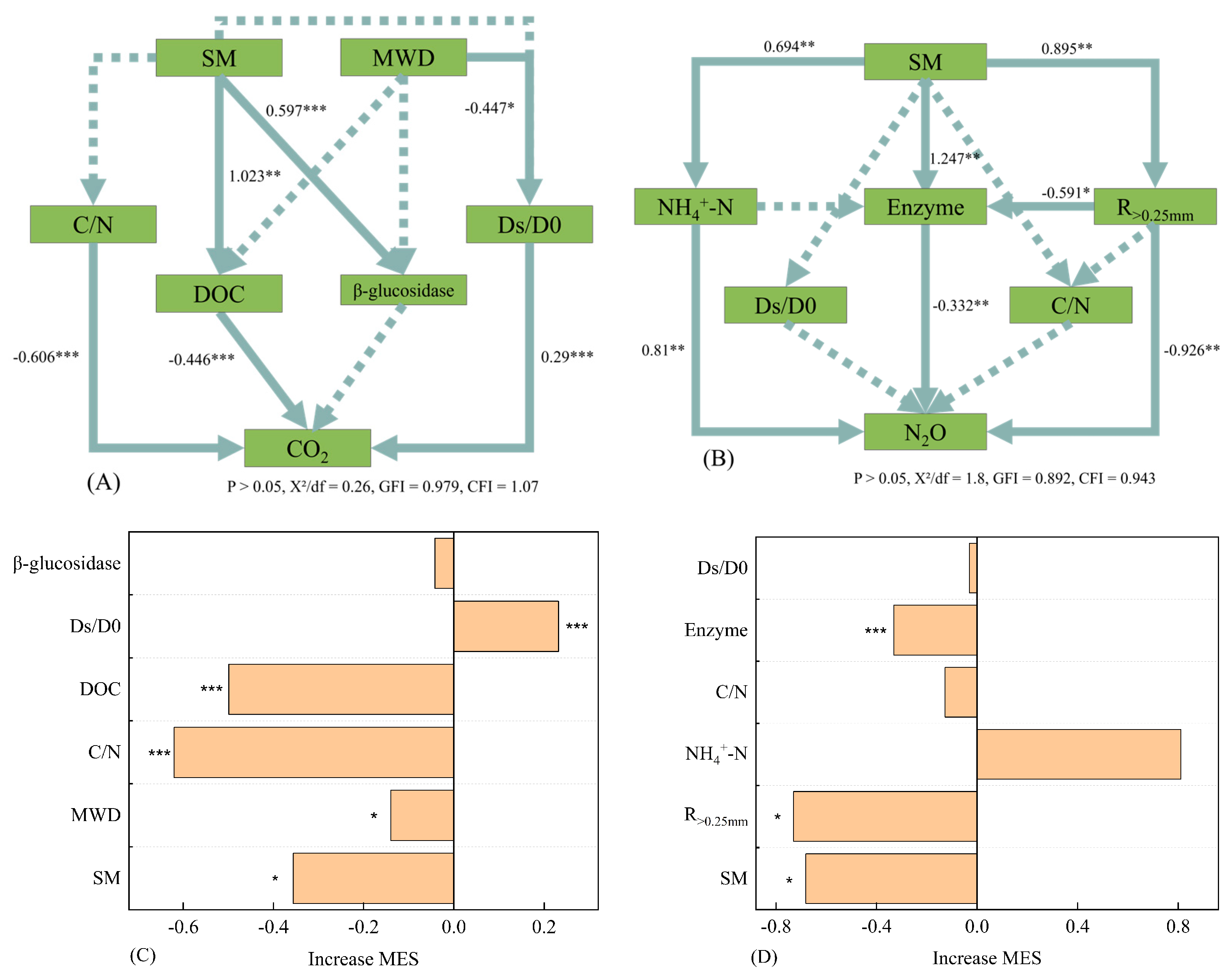
3.7. SEM of Soil CO2 and N2Oemission
4. Discussion
4.1. The Mechanism of Soil CO2 Emission
4.2. The Mechanism of Soil N2O Emissions
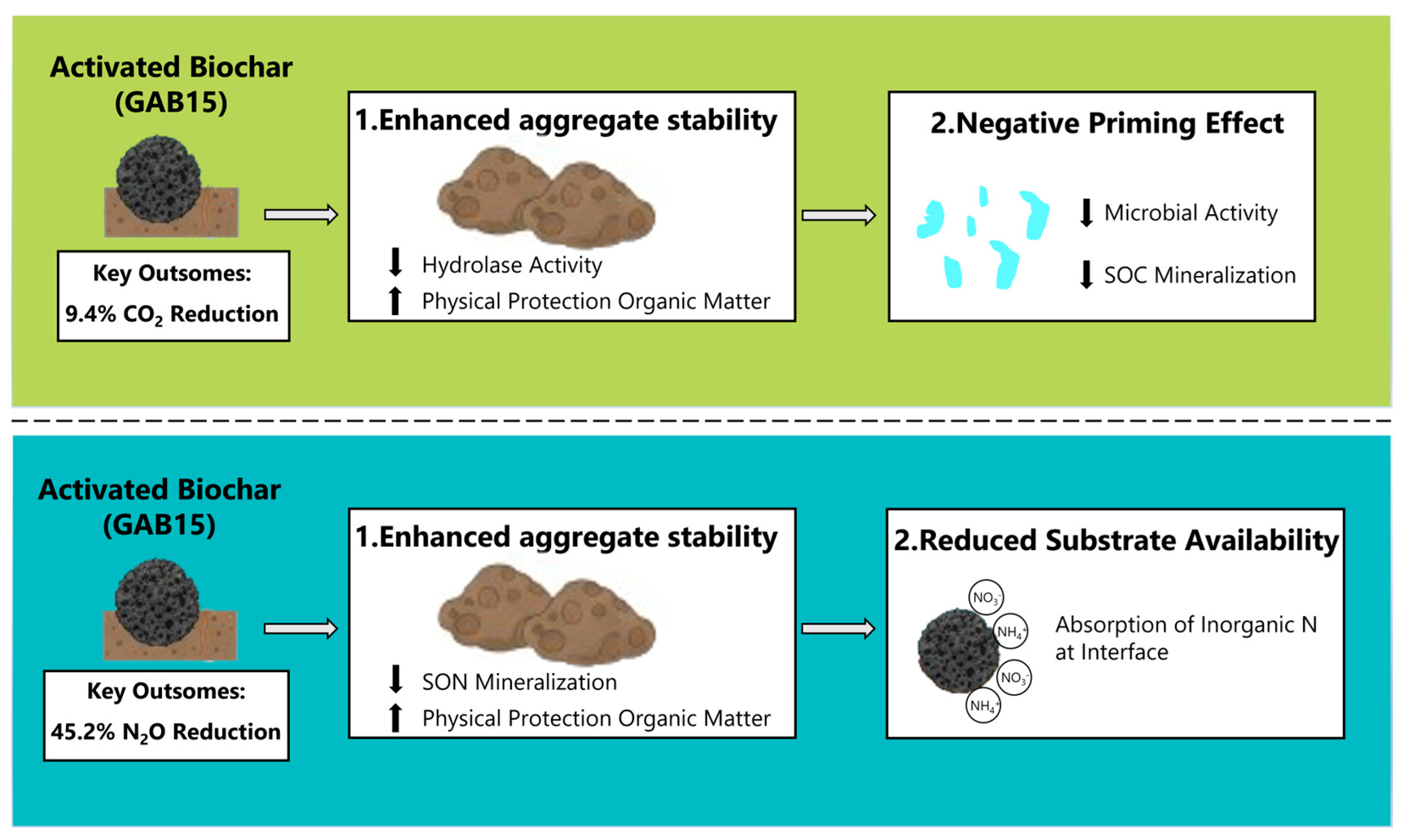
4.3. Practical Implications and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qi, L.; Pokharel, P.; Chang, S.X.; Zhou, P.; Niu, H.; He, X.; Wang, Z.; Gao, M. Biochar application increased methane emission, soil carbon storage and net ecosystem carbon budget in a 2-year vegetable–rice rotation. Agric. Ecosyst. Environ. 2020, 292, 106831. [Google Scholar] [CrossRef]
- Han, J.; Zhang, A.; Kang, Y.; Han, J.; Yang, B.; Hussain, Q.; Wang, X.; Zhang, M.; Khan, M.A. Biochar promotes soil organic carbon sequestration and reduces net global warming potential in apple orchard: A two-year study in the loess plateau of China. Sci. Total Environ. 2021, 803, 150035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mai, H.; Qiu, Q.; Zhu, Y.; Long, J.; Chen, S.; Chen, Y. The responses of c, n, p and stoichiometric ratios to biochar and vermicompost additions differ from alfalfa and a mine soil. Agriculture 2023, 13, 1954. [Google Scholar] [CrossRef]
- Qi, L.; Pokharel, P.; Ni, C.; Gong, X.; Zhou, P.; Niu, H.; Wang, Z.; Gao, M. Biochar changes thermal activation of greenhouse gas emissions in a rice–lettuce rotation microcosm experiment. J. Clean. Prod. 2020, 247, 119148. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Z.; Xiao, Y.; Yu, L.; Gao, X.; Wang, C.; Li, B.; Tao, Q.; Xu, Q.; Gao, M. Increases of temperature response for CO2 emission in a biochar-amended vegetable field soil. Environ. Sci. Pollut. Res. 2021, 29, 50895–50905. [Google Scholar] [CrossRef]
- Lv, L.; Huang, S.; Zhou, H. Performance investigation of biochar/paraffin composite phase change materials in latent heat storage systems: Feasibility of biochar as a thermal conductivity enhancer. J. Energy Storage 2024, 91, 112106. [Google Scholar] [CrossRef]
- Rijk, I.; Ekblad, A.; Dahlin, A.S.; Enell, A.; Larsson, M.; Leroy, P.; Kleja, D.B.; Tiberg, C.; Hallin, S.; Jones, C. Biochar and peat amendments affect nitrogen retention, microbial capacity and nitrogen cycling microbial communities in a metal and polycyclic aromatic hydrocarbon contaminated urban soil. Sci. Total Environ. 2024, 936, 173454. [Google Scholar] [CrossRef]
- Zhong, L.; Gu, Z.; Song, Y.; Wang, H.; Cai, X.; Xu, X. Biochar reduces N2O emission from fertilized cropland soils: A meta-analysis. Carbon Res. 2024, 4, 31. [Google Scholar] [CrossRef]
- Valkama, E.; Tzemi, D.; Esparza-Robles, U.R.; Syp, A.; O’Toole, A.; Maenhout, P. Effectiveness of soil management strategies for mitigation of N2O emissions in European arable land: A meta-analysis. Eur. J. Soil Sci. 2024, 5, 75. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, T.; Zhang, G.; Bahn, M.; Mo, F. Biochar strategy for long-term N2O emission reduction: Insights into soil physical structure and microbial interaction. Soil Biol. Biochem. 2025, 202, 109685. [Google Scholar] [CrossRef]
- Wu, D.; Senbayram, M.; Zang, H.; Ugurlar, F.; Blagodatskaya, E. Effect of biochar origin and soil pH on greenhouse gas emissions from sandy and clay soils. Appl. Soil Ecol. 2018, 129, 121–127. [Google Scholar] [CrossRef]
- Wang, B.; Gao, Y.; Lai, X.; LLuo Zhang, X.; Hu, D.; Shen, Z.; Hu, S.; Zhang, L. The effects of biochar derived from feedstock with different Si and Al concentration on soil N2O and CO2 emissions. Environ. Pollut. 2023, 317, 120731. [Google Scholar] [CrossRef] [PubMed]
- Aamer, M.; Shaaban, M.; Hassan, M.U.; Guoqin, H.; Ying, L.; Ying, T.H. Biochar mitigates the N2O emissions from acidic soil by increasing the nosZ and nirK gene abundance and soil pH. J. Environ. Manag. 2020, 255, 109891. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Palacios, J.H.; Jeanne, T.; Hogue, R.; Dubé, P.; Limousy, L.; Raghavan, V. Effect of six engi-neered biochars on GHG emissions from two agricultural soils: A short-term incubation study. Geoderma 2018, 327, 73–84. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Wang, Z.; Zhang, J.; Li, P.; Su, L. Effects of biochar and nitrogen fertilizer on microbial communities, CO2 emissions, and organic carbon content in soil. Sci. Rep. 2025, 15, 9789. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, W. Response mechanisms of agricultural soil biochemical properties and CO2 emissions to field application of modified biochar. J. Soils Sediments 2024, 24, 1194–1205. [Google Scholar] [CrossRef]
- Bovsun, M.A.; Castaldi, S.; Nesterova, O.V.; Semal, V.A.; Sakara, N.A.; Brikmans, A.V.; Khokhlova, A.I.; Karpenko, T.Y. Effect of biochar on soil CO2 fluxes from agricultural field experiments in Russian Far East. Agronomy 2021, 11, 1559. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adetunji, A.T. Agro-residue biochar and N fertilizer addition mitigates CO2-C emission and stabilized soil organic carbon pools in a rain-fed agricultural cropland. Int. Soil Water Conserv. Res. 2021, 9, 76–86. [Google Scholar] [CrossRef]
- Lee, S.I.; Park, H.J.; Jeong, Y.J.; Seo, B.S.; Kwak, J.H.; Yang, H.I.; Choi, W.J. Biochar-induced reduction of N2O emission from East Asian soils under aerobic conditions: Review and data analysis. Environ. Pollut. 2021, 291, 118154. [Google Scholar] [CrossRef]
- Alkiewicz, A.; Kalinichenko, K.; Kubaczyński, A.; Brzezińska, M.; Bieganowski, A. Usage of biochar for mitigation of CO2 emission and enhancement of CH4 consumption in forest and orchard Haplic Luvisol (Siltic) soils. Appl. Soil Ecol. 2020, 156, 103711. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef]
- Case, S.D.; McNamara, N.P.; Reay, D.S.; Stott, A.W.; Grant, H.K.; Whitaker, J. Biochar suppresses N2O emissions while maintaining N availability in a sandy loam soil. Soil Biol. Biochem. 2015, 81, 178–185. [Google Scholar] [CrossRef]
- Bai, J.; Moreira, B.R.D.A.; Bai, Y.; Nadar, C.G.; Feng, Y.; Yadav, S. Assessing biochar’s impact on greenhouse gas emissions, microbial biomass, and enzyme activities in agricultural soils through meta-analysis and machine learning. Sci. Total Environ. 2025, 963, 178541. [Google Scholar] [CrossRef]
- Gabetto, F.P.; Tenelli, S.; Netto-Ferreira, J.B.; Martins Junior, J.; Almeida, O.A.C.; Cosenza, M.L.; Cosenza, M.L.; Cosenza, M.; Carvalho, J.L.N. Biochar from crop residues mitigates N2O emissions and increases carbon content in tropical soils. Biofuels Bioprod. Biorefin. 2025, 19, 759–775. [Google Scholar] [CrossRef]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, M. Does biochar accelerate the mitigation of greenhouse gaseous emissions from agricultural soil?—A global meta-analysis. Environ. Res. 2021, 202, 111789. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.A.A.; Ni, L.; Shaghaleh, H.; Elsadek, E.; Hamoud, Y.A. Effect of carbon con-tent in wheat straw biochar on N2O and CO2 emissions and pakchoi productivity under different soil moisture conditions. Sustainability 2023, 15, 5100. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Novak, J. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Dandan, L.; Zhurong, W.; Ze, W.; Zhenghua, H.; Shuyun, Y. Assessment of the straw and biochar application on greenhouse gas emissions and yield in paddy fields under intermittent and controlled irrigation patterns. Agric. Ecosyst. Environ. 2024, 359, 108745. [Google Scholar] [CrossRef]
- Jia, F.; Xuhui, Z.; Yanghui, H.; Ruiqiang, L.; Yixian, Y.; Guiyao, Z.; Hongyang, C.; Lingyan, Z.; Yuling, F.; Hosseini, B.S. Co-application of biochar and organic amendments on soil greenhouse gas emissions: A meta-analysis. Sci. Total Environ. 2023, 897, 166171. [Google Scholar] [CrossRef]
- Jinze, B.; Jiajie, S.; Danyang, C.; Zhihao, Z.; Qi, Y.; Guangxin, R.; Xinhui, H.; Xiaojiao, W.; Chengjie, R.; Gaihe, Y.; et al. Biochar combined with n fertilization and straw return in wheat-maize agroecosystem: Key practices to enhance crop yields and minimize carbon and nitrogen footprints. Agric. Ecosyst. Environ. 2023, 347, 108366. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Zhang, J.; Liu, H.; Wang, H.; Zhang, J. Enhanced CO2 absorption in amine-based carbon capture aided by coconut shell-derived nitrogen-doped biochar. Sep. Purif. Technol. 2024, 353, 128451. [Google Scholar] [CrossRef]
- Huan, Z.; Zifang, W.; Hai, L.; Houjun, X.; Ming, G. Water-stable aggregates and aggregate-associated organic carbon after two years of biochar application. Arch. Agron. Soil Sci. 2023, 69, 2218–2232. [Google Scholar] [CrossRef]
- Hematimatin, N.; Igaz, D.; Aydın, E.; Horák, J. Biochar application regulating soil inorganic nitrogen and organic carbon content in cropland in the central europe: A seven-year field study. Biochar 2024, 6, 14. [Google Scholar] [CrossRef]
- Wu, J.; Teng, B.; Zhong, Y.; Duan, X.; Gong, L.; Guo, W.; Qi, P.; Haider, F.U.; Cai, L. Enhancing soil aggregate stability and organic carbon in northwestern China through straw, biochar, and nitrogen supplementation. Agronomy 2024, 14, 899. [Google Scholar] [CrossRef]
- Ojeda, G.; Gil, J.M.; Mattana, S.; Bachmann, J.; Quenea, K.; Sobral, A.J. Biochar ageing effects on soil respiration, biochar wettability and gaseous CO2 adsorption. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 11. [Google Scholar] [CrossRef]
- Thers, H.; Abalos, D.; Dörsch, P.; Elsgaard, L. Nitrous oxide emissions from oilseed rape cultivation was unaffected by flash pyrolysis biochar of different type, rate and field ageing. Sci. Total Environ. 2020, 724, 138140. [Google Scholar] [CrossRef]
- Liao, X.; Kang, H.; Haider, G.; Wang, W.; Malghani, S. The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: A meta-analysis. Geoderma 2022, 411, 115692. [Google Scholar] [CrossRef]
- Baraket, F.; González, M.; Brahim, N.; Roca, N.; Mbarek, H.; Świtoniak, M.; Chaker, R.; Sánchez bellón, Á.; Rigane, H.; Gargouri, K.; et al. Short and long-term effect of land use and management on soil organic carbon stock in semi-desert areas of north Africa Tunisia. Agriculture 2021, 11, 1267. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Faheem, M.; Wang, A.; Zhang, F.; Li, Q.; Zhu, G. One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol–urea–formaldehyde resin for co 2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Ouassim, B.; Ahmed, S.; Fouad, G.; Ouafae, A.; Mouad, D.; Tarik, C. CO2 capture using n-containing nanoporous activated carbon obtained from argan fruit shells. J. Environ. Chem. Eng. 2018, 6, 1995–2002. [Google Scholar] [CrossRef]
- Zhang, L. Preparation of Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2019. [Google Scholar]
- Ismail, I.S.; Singh, G.; Smith, P.; Kim, S.; Vinu, A. Oxygen functionalized porous activated biocarbons with high surface area derived from grape marc for enhanced capture of CO2 at elevated-pressure. Carbon 2020, 160, 113–124. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Qun, J.; Lei, W.; Ying, Z. Koh-activated porous biochar with high specific surface area for adsorptive removal of chromium (vi) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2020, 401, 123292. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, W.; Jin, Z.; Chen, L.; Jiang, X. One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S. Fuel 2021, 289, 119932. [Google Scholar] [CrossRef]
- Hisse, D.; Bessa, I.A.; Silva, L.P.; da Silva, A.F.; Araujo, J.R.; Archanjo, B.S.; Soares, A.V.H.; Passos, F.B.; Carneiro, J.W.M.; dos Santos, T.C.; et al. Microporous Nitrogen-Doped Activated Biochars Derived from Corn: Use of Husk Waste and Urea for CO2 Capture. J. Braz. Chem. Soc. 2024, 35, e-20240034. [Google Scholar] [CrossRef]
- Yuan, X.; Suvarna, M.; Lim, J.Y.; Pérez-Ramírez, J.; Wang, X.; Ok, Y.S. Active learning-based guided synthesis of engineered biochar for CO2 capture. Environ. Sci. Technol. 2024, 58, 6628–6636. [Google Scholar] [CrossRef]
- Gao, X.P.; Rajendran, N.; Tenuta, M.; Dunmola, A.; Burton, D.L. Greenhouse gas accumulation in the soil profile is not always related to surface emissions in a prairie pothole agricultural landscape. Soil Sci. Soc. Am. J. 2014, 78, 805–817. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, D.; Shah, A.; Huang, J.; Zhang, L.; Núez-Delgado, A.; Han, T.; Du, J.; Ali, S.; Sial, T.A. The impact of pristine and modified rice straw biochar on the emission of greenhouse gases from a red acidic soil. Environ. Res. 2022, 208, 112676. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Yang, J.S.; Yao, R.J.; Chen, X.B.; Wang, X.P. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Methods in Applied Soil Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1995; pp. 569–576. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Marshall, T.J. The diffusion of gases through porous media. J. Soil Sci. 1959, 10, 79–82. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Zhang, J.; Wang, C.; Zhou, S. Long term comparison of ghg emissions and crop yields in response to direct straw or biochar incorporation in rice-wheat rotation systems: A 10-year field observation. Agric. Ecosyst. Environ. 2024, 374, 109188. [Google Scholar] [CrossRef]
- Yang, F.; Lee, X.; Theng, B.K.G.; Wang, B.; Cheng, J.; Wang, Q. Effect of biochar addition on short-term N2O and CO2 emissions during repeated drying and wetting of an anthropogenic alluvial soil. Environ. Geochem. Health 2017, 39, 635–647. [Google Scholar] [CrossRef]
- Yangzhou, X.; Ying, L.; Khan, N.N.; Nanthi, B.; Ling, Z.; Siyu, Z.; Jianming, X.; Bin, Y.; Yuan, L. Biochar addition increased soil bacterial diversity and richness: Large-scale evidence of field experiments. Sci. Total Environ. 2023, 893, 164961. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Du, X.; Cai, Y.; Peng, J.; Zhang, S.; Liu, F. Characteristics of greenhouse gas emissions from constructed wetlands vegetated withmyriophyllum aquatic: The effects of influent c/n ratio and microbial responses. Water 2024, 16, 308. [Google Scholar] [CrossRef]
- Wenzhu, Z.; Lan, W.; Qinglong, L.; Honghong, L.; Jingchun, T. Adsorption of pcbs on microplastics mitigated greenhouse gas emission by changing c/n metabolism in freshwater sediment. J. Clean. Prod. 2024, 434, 12. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Shangguan, Z. Combined biochar and nitrogen fertilization at appropriate rates could balance the leaching and availability of soil inorganic nitrogen. Agric. Ecosyst. Environ. 2019, 276, 21–30. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Qing, J.; Li, J.; Xue, J.; Yan, B.; Chen, G.; Kang, X.; Rui, Y. Biochar can reduce N2O production potential from rhizosphere of fertilized agricultural soils by suppressing bacterial denitrification. Eur. J. Soil Biol. 2022, 109, 103391. [Google Scholar] [CrossRef]
- Mangalassery, S.; Sjogersten, S.; Sparkes, D.L.; Sturrock, C.J.; Mooney, S.J. The effect of soil aggregate size on pore structure and its consequence on emission of greenhouse gases. Soil Tillage Res. 2013, 132, 39–46. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, N.; Zhao, J.; Shaaban, M.; Hu, R. Crop straw incorporation mediates the impacts of soil aggregate size on greenhouse gas emissions. Geoderma 2021, 401, 115342. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, R.; Zhang, C.; Wang, L.; Wang, H.; Wang, X. Role of vermicompost and biochar in soil quality improvement by promoting bupleurum falcatum l. Nutrient absorption. Soil Use Manag. 2023, 39, 1600–1617. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Ma, J.; Zhang, X.; Wang, C.; Zhou, S. Effect of straw biochar application on soil carbon, greenhouse gas emissions and nitrogen leaching: A vegetable crop rotation field experiment. Soil Use Manag. 2022, 39, 729–741. [Google Scholar] [CrossRef]
- Davys, D.; Rayns, F.; Charlesworth, S.; Lillywhite, R. The effect of different biochar characteristics on soil nitrogen transformation processes: A review. Sustainability 2023, 15, 16446. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Yoshida, S.; Okabe, A.; Nishida, K. Impact of clod (aggregate) size on evaporation rate and soil moisture profile during the drying process. Eur. J. Soil Sci. 2023, 74, e13420. [Google Scholar] [CrossRef]
- Xuping, Y.; Ding, J.; Xiaoxue, C.; Chuan, Y.; Shuang, W.; Zhixia, H.; Sivakumar, E. Adsorption properties of seaweed-based biochar with the greenhouse gases (CO2, CH4, N2O) through density functional theory. Biomass Bioenergy 2022, 163, 106519. [Google Scholar] [CrossRef]
- Zepeng, L.; Yueyao, H.; Jie, Z.; Ying, G.; Liping, Z.; Ping, Y.; Tongtong, Z.; Xingyu, H.; Meng, W.; Hui, G. Enhanced adsorption of congo red from urea/calcium chloride co-modified biochar: Performance, mechanisms and toxicity assessment. Bioresour. Technol. 2023, 388, 129783. [Google Scholar] [CrossRef]
- Hy, A.; Cf, B.; Yi, M.B.; Yd, B.; Jian, L.C. Long-term ditch-buried straw return increases functionality of soil microbial communities. Catena 2021, 202, 202. [Google Scholar] [CrossRef]
| Treatment | BET Surface Aera (m2 g−1) | Pore Volume (Micropore) (m3 g−1 10−6) | Micropore Diameter (m 10−9) | Ash (%) | N (%) | O (%) | pH | EC (mS cm−1) |
|---|---|---|---|---|---|---|---|---|
| GAB15 | 1251 | 0.582 | 0.566 | 18.5 | 1.1 | 15.4 | 8.86 | 1.65 |
| GAB5 | 1091 | 0.501 | 0.569 | 47.81 | 1.98 | 13.84 | 9.01 | 2.41 |
| GB | 324 | 0.11 | 0.584 | 57.93 | 3.99 | 10.7 | 10.51 | 3.82 |
| Urease (mg NH4+-N g−1 24 h−1) | β-Glucosidase (nmol MUB g−1 h−1) | Alkaline Phosphatase (mg Phenol g−1 24 h−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DAS20 | DAS40 | DAS60 | DAS20 | DAS40 | DAS60 | DAS20 | DAS40 | DAS60 | |
| GAB15 | 0.211 b ± 0.006 | 0.341 a ± 0.043 | 0.246 bc ± 0.020 | 127.78 ab ± 12.99 | 128.76 ab ± 1.57 | 126.96 ab ± 12.08 | 0.584 bc ± 0.030 | 0.661 b ± 0.050 | 0.617 b ± 0.013 |
| GAB5 | 0.241 ab ± 0.011 | 0.328 a ± 0.015 | 0.326 ab ± 0.022 | 131.19 ab ± 5.53 | 138.19 a ± 9.37 | 132.53 ab ± 6.56 | 0.629 b ± 0.048 | 0.713 b ± 0.041 | 0.647 b ± 0.045 |
| GB | 0.254 a ± 0.022 | 0.345 a ± 0.063 | 0.365 a ± 0.021 | 145.36 a ± 8.02 | 140.43 a ± 8.04 | 143.51 a ± 4.75 | 0.794 a ± 0.048 | 0.855 a ± 0.056 | 0.833 a ± 0.045 |
| GC | 0.207 b ± 0.003 | 0.359 a ± 0.025 | 0.272 bc ± 0.046 | 111.72 b ± 10.76 | 117.35 b ± 6.44 | 114.27 b ± 5.59 | 0.462 cd ± 0.04 | 0.568 bc ± 0.056 | 0.549 b ± 0.102 |
| CK | 0.158 c ± 0.010 | 0.220 b ± 0.022 | 0.226 c ± 0.038 | 66.86 c ± 8.38 | 78.15 c ± 5.40 | 71.50 c ± 10.31 | 0.436 d ± 0.056 | 0.486 c ± 0.051 | 0.465 b ± 0.051 |
| MBC (g kg 10−3) | MBN (g kg 10−3) | C/N | DOC (g kg 10−3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAS20 | DAS40 | DAS60 | DAS20 | DAS40 | DAS60 | DAS20 | DAS40 | DAS60 | DAS20 | DAS40 | DAS60 | |
| GAB15 | 147.33 b ± 8.6 | 171.15 ab ± 5.3 | 160.7 a ± 5.4 | 20.00 b ± 1.2 | 33.35 c ± 0.8 | 22.2 bc ± 0.8 | 7.4 ab ± 0.3 | 5.1 b ± 0.1 | 7.2 a ± 0.0 | 161.01 a ± 4.8 | 158.28 a ± 5.0 | 153.98 a ± 3.4 |
| GAB5 | 146.12 b ± 8.5 | 179.66 a ± 6.1 | 168.8 a ± 6.1 | 24.06 a ± 2.9 | 36.39 b ± 1.5 | 25.6 ab ± 1.0 | 6.2 c ± 0.9 | 4.9 b ± 0.1 | 6.6 b ± 0.0 | 148.19 b ± 4.6 | 141.84 b ± 7.1 | 138.19 b ± 8.6 |
| GB | 141.77 b ± 7.1 | 179.24 a ± 6.3 | 168.4 a ± 6.1 | 20.97 b ± 0.9 | 39.41 a ± 3.0 | 27.8 a ± 3.3 | 6.8 bc ± 0.1 | 4.6 bc ± 0.4 | 6.2 b ± 0.8 | 135.28 c ± 6.1 | 131.34 b ± 5.3 | 125.88 bc ± 5.8 |
| GC | 159.2 a ± 2.4 | 166.23 b ± 2.2 | 132.4 b ± 4.1 | 20.27 b ± 1.6 | 21.32 e ± 1.4 | 20.9 bc ± 1.4 | 7.9 a ± 0.6 | 7.8 a ± 0.5 | 6.4 b ± 0.2 | 118.28 d ± 3.4 | 111.52 c ± 9.4 | 115.19 c ± 5.5 |
| CK | 80.3 c ± 6.8 | 90.99 c ± 4.0 | 80.43 c ± 3.8 | 19.37 b ± 1.2 | 28.03 d ± 1.5 | 16.9 c ± 1.3 | 4.2 d ± 0.3 | 3.3 d ± 0.3 | 4.8 c ± 0.6 | 62.56 e ± 14.1 | 65.12 d ± 12.4 | 64.65 d ± 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zheng, Y.; Liu, X.; Liu, D.; Cao, C.; Li, K.; Lu, P.; Yang, P.; Wang, H.; Zheng, C.; et al. Urea-N Activated Biochar Effectively Suppresses CO2 and N2O Emissions from Farmland Soil. Agronomy 2025, 15, 2655. https://doi.org/10.3390/agronomy15112655
Wang X, Zheng Y, Liu X, Liu D, Cao C, Li K, Lu P, Yang P, Wang H, Zheng C, et al. Urea-N Activated Biochar Effectively Suppresses CO2 and N2O Emissions from Farmland Soil. Agronomy. 2025; 15(11):2655. https://doi.org/10.3390/agronomy15112655
Chicago/Turabian StyleWang, Xiao, Yudong Zheng, Xuetong Liu, Dan Liu, Caiyun Cao, Kejiang Li, Ping Lu, Peiling Yang, Huiguang Wang, Chunlian Zheng, and et al. 2025. "Urea-N Activated Biochar Effectively Suppresses CO2 and N2O Emissions from Farmland Soil" Agronomy 15, no. 11: 2655. https://doi.org/10.3390/agronomy15112655
APA StyleWang, X., Zheng, Y., Liu, X., Liu, D., Cao, C., Li, K., Lu, P., Yang, P., Wang, H., Zheng, C., & Dang, H. (2025). Urea-N Activated Biochar Effectively Suppresses CO2 and N2O Emissions from Farmland Soil. Agronomy, 15(11), 2655. https://doi.org/10.3390/agronomy15112655






